The Effects of Light Treatments on Growth and Flowering Characteristics of Oncidesa Gower Ramsey ‘Honey Angel’ at Different Growth Stages
Abstract
:1. Introduction
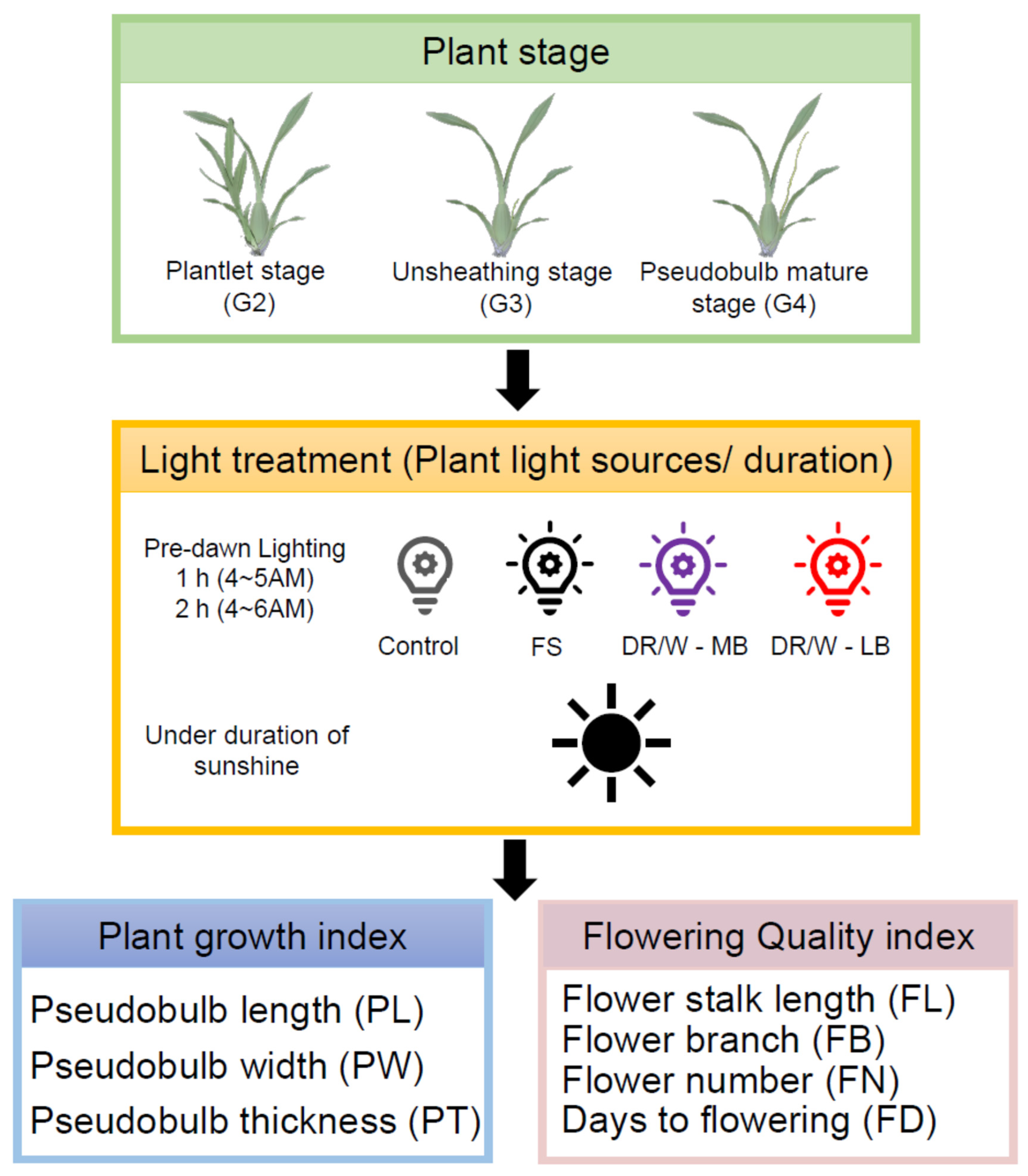
2. Materials and Methods
2.1. Plant Materials and Cultural Practices
2.2. Supplemental Light Treatment (SL)
- Control treatment was natural light in the greenhouse, with no artificial light source added;
- Full spectrum (FS) LED (EL-B05L1200-DSM-D4890W, >350 μmol·m−2·s−1at 30 cm, 400–700nm, SOLIDLITE, Taipei, Taiwan) for 1 h (subsequently referred to as FS-1) and 2 h (subsequently referred to as FS-2);
- Deep red/white (DR/W)—medium blue (MB) (Philips Green Power LED, Philips, The Netherlands), primary light colors being DR/W with typical PPFD 800 m−2 s−1, power consumption 285W, 400–750nm, and efficacy 2.8 μmol J−1, for 1 h (subsequently referred to as MB-1) and 2 h (subsequently referred to as MB-2) period;
- Deep red/white (DR/W)—low blue (LB) (Philips Green Power LED, Philips, The Netherlands), primary light colors being DR/W with typical PPFD 800 m−2 s−1, power consumption 275W, 350–750nm, and efficacy 2.9 μmol J−1, for 1 h (subsequently referred to as LB-1) and 2 h (subsequently referred to as LB-2).
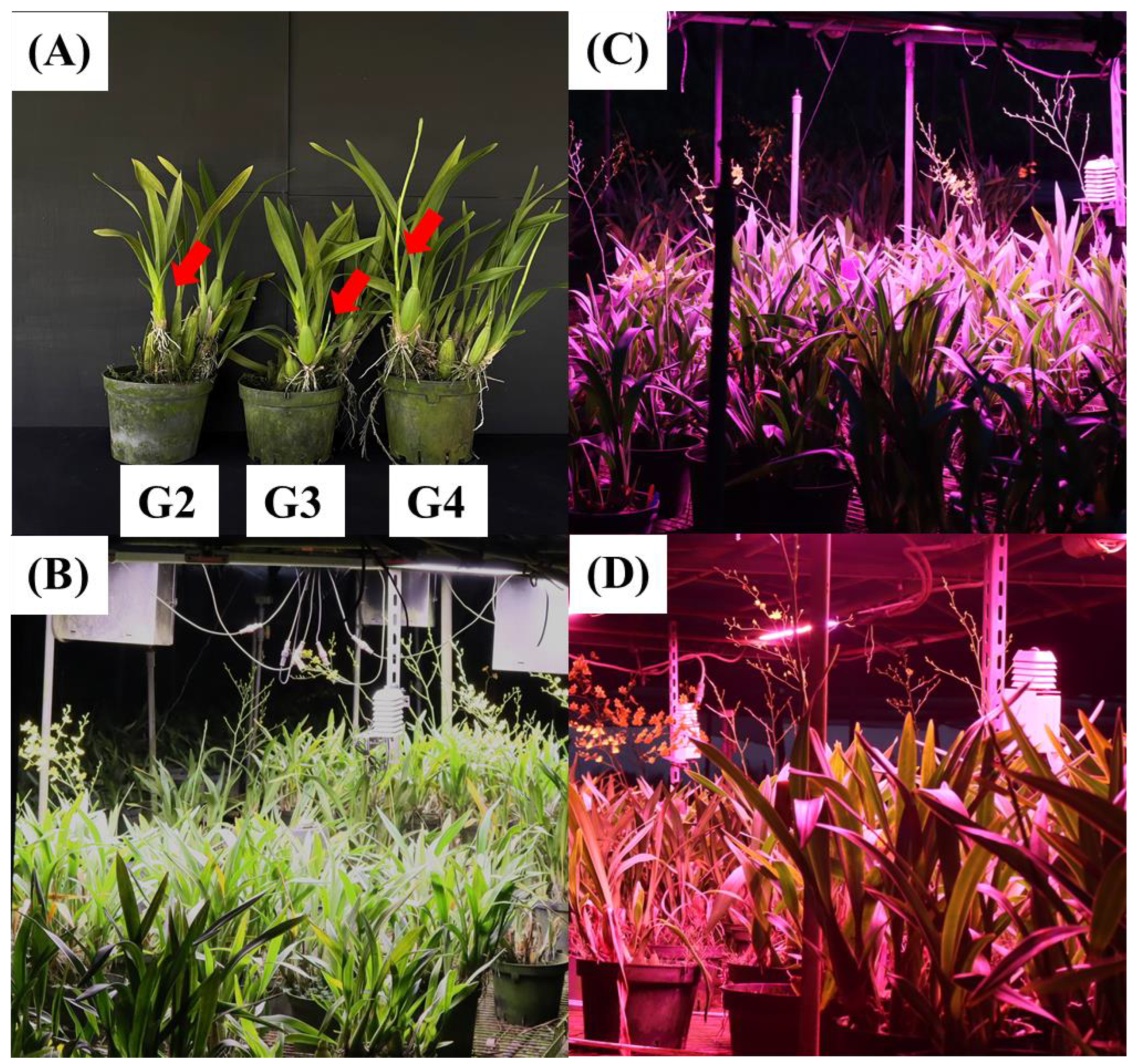
2.3. The Assessments of Plant Growth and Flowering Quality
- Pseudobulb length (PL) was measured with a Vernier caliper as the long axis (mm) between the base and top of a pseudobulb.
- Pseudobulb width (PW) was measured with a Vernier caliper as the short axis (girth, mm) of a pseudobulb.
- Pseudobulb thickness (PT) was measured as the maximum thickness (mm) of a pseudobulb.
- Inflorescence length (FL) was measured with a Vernier caliper as the length (mm) between the base and top of a flower stalk.
- Number of branches (FB) was recorded as all the branches of a flower stalk.
- Number of florets (FN) was recorded as all the florets (unopened buds and opened florets) of a flower stalk.
- Days to flowering (FD) was recorded as the number of days from the start of light treatment until flower harvest.
2.4. Statistical Analysis
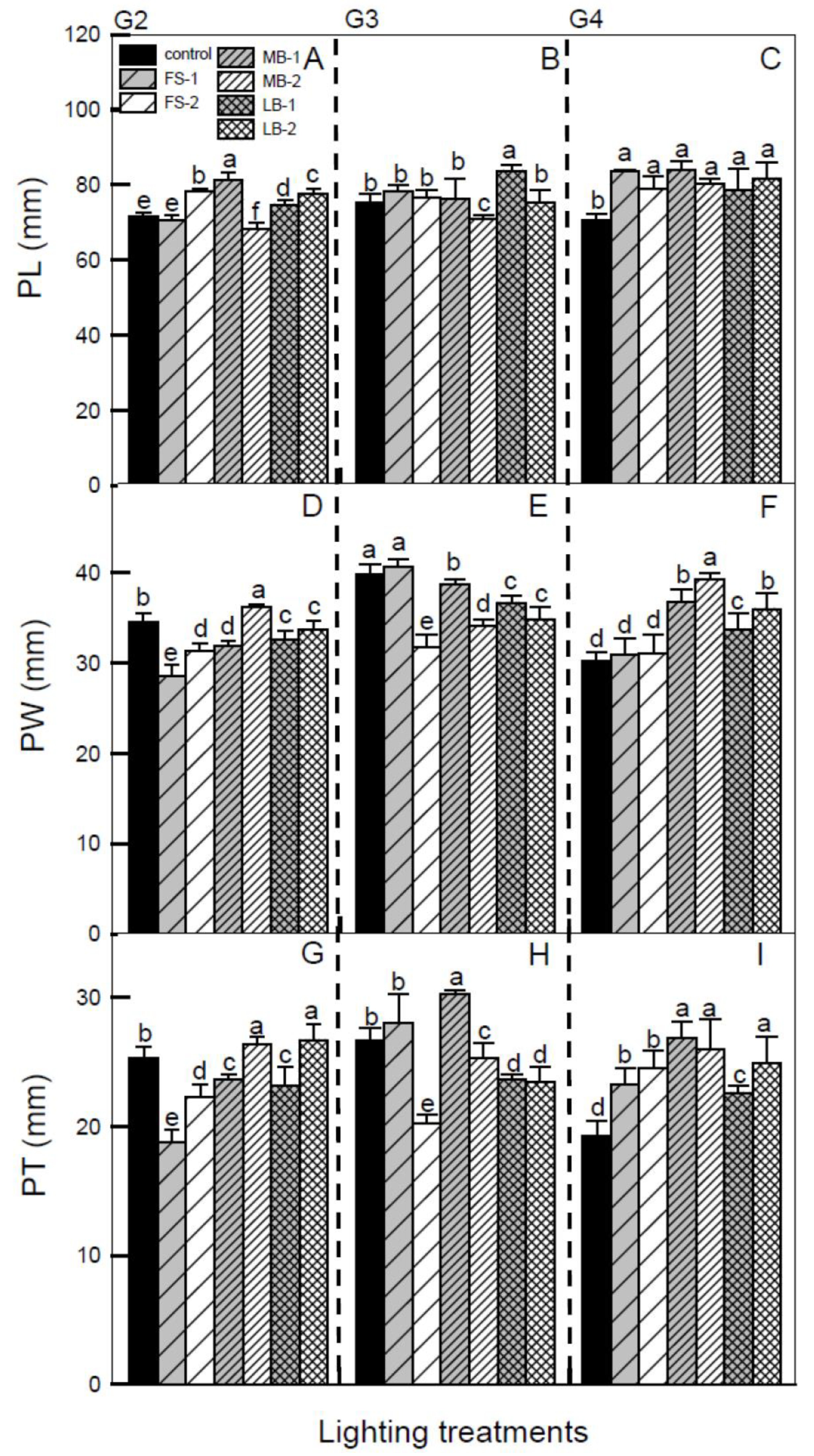
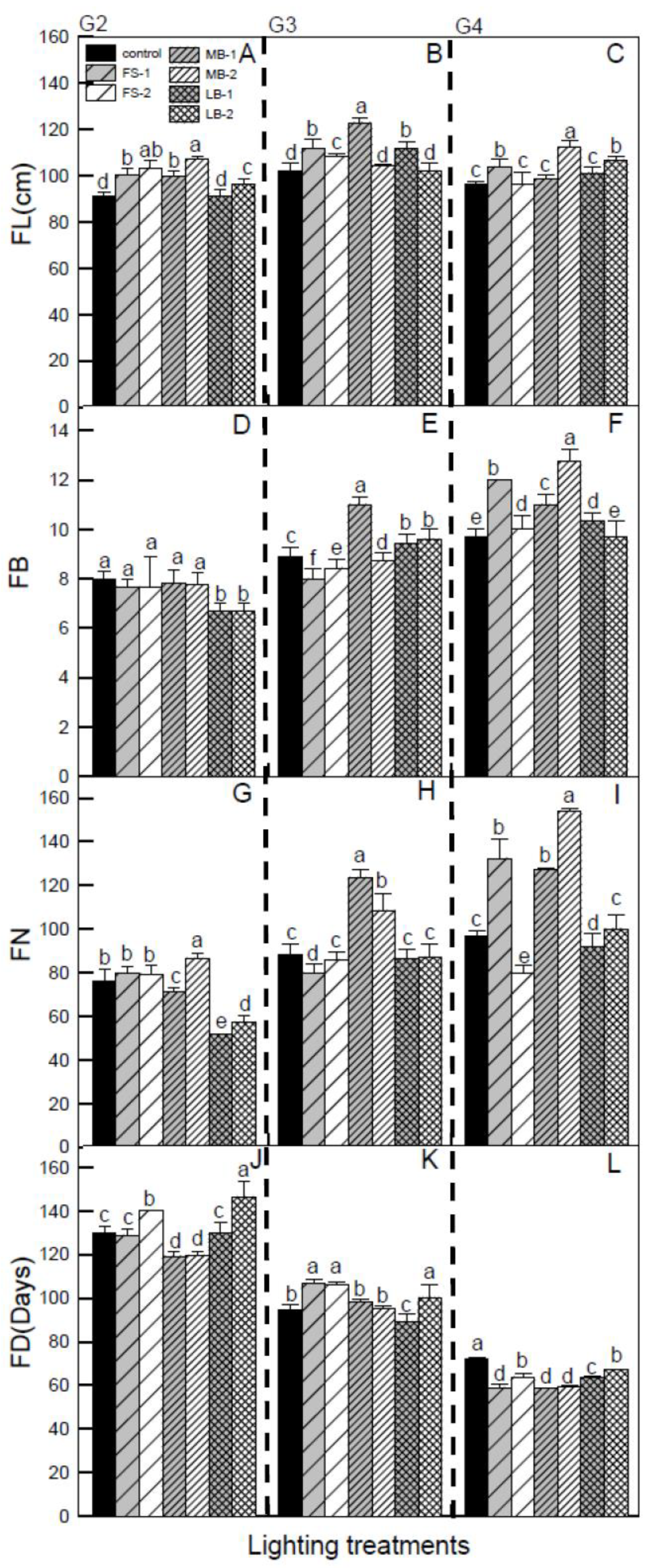
3. Results
4. Discussion
4.1. Effects of Different LEDs on HA Pseudobulb Growth at Different Growing Stages
4.2. Influences of Different LEDs on Flower Quality in HA at Different Growing Stages
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chase, M.W.; Cameron, K.M.; Freudenstein, J.V.; Pridgeon, A.M.; Salazar, G.; Van den Berg, C.; Schuiteman, A. An updatedclassification of Orchidaceae. Bot. J. Linn. Soc. 2015, 177, 151–174. [Google Scholar] [CrossRef]
- Zhang, D.; Zhao, X.W.; Li, Y.Y.; Ke, S.J.; Yin, W.L.; Lan, S.; Liu, Z.J. Advances and prospects of orchid research and industrialization. Hortic. Res. 2022, 9, uhac220. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.M.; Lin, K.H.; Huang, M.Y.; Chen, C.I.; Hsueh, M.L.; Wang, C.W.; Yeh, K.W. Growth and Flowering Characteristics of Oncidium Gower Ramsey Varieties under Various Fertilizer Management Treatments in Response to Light Intensities. Agronomy 2021, 11, 2549. [Google Scholar] [CrossRef]
- De, L.C. Good Agricultural Practices of Oncidium orchids. Biot. Res. Today 2022, 4, 233–237. [Google Scholar]
- Lin, H.; Chang, Y.C.A. Carbohydrate Changes in the Pseudobulbs of Oncidesa Gower Ramsey ‘Honey Angel’ during Vegetative and Reproductive Growth. HortScience 2023, 58, 114–121. [Google Scholar] [CrossRef]
- Blanchard, M.G.; Erik, S.R. Intermittent light from a rotating high-pressure sodium lamp promotes flowering of long-day plants. HortScience 2010, 45, 236–241. [Google Scholar] [CrossRef]
- Wang, S.L.; Viswanath, K.K.; Tong, C.G.; An, H.R.; Jang, S.; Chen, F.C. Floral Induction and Flower Development of Orchids. Front. Plant Sci. 2019, 10, 1258. [Google Scholar] [CrossRef]
- Chatield, S.P.; Stirnberg, P.; Forde, B.G.; Leyser, O. The hormonal regulation of axillary bud growth in Arabidopsis. Plant J. 2000, 24, 159–169. [Google Scholar] [CrossRef]
- McArtney, S.; Obermiller, J.D. Effect of notching, 6-benzyladenine, and 6-benzyladenine plus gibberellin A4+A7 on budbreak and shoot development from paradormant buds on the leader of young apple trees. Technol. Prod. Rep. 2015, 25, 233–237. [Google Scholar] [CrossRef]
- Zheng, L.; He, H.; Song, W. Application of light-emitting diodes and the effect of light quality on horticultural crops: A review. HortScience 2019, 54, 1656–1661. [Google Scholar] [CrossRef]
- Li, J.F.; Yi, C.Y.; Zhang, C.R.; Pan, F.; Xie, C.; Zhou, W.; Zhou, C. Effects of light quality on leaf growth and photosynthetic fluorescence of Brasenia schreberi seedlings. Heliyon 2021, 7, e06082. [Google Scholar] [CrossRef]
- De, L.C. Good agricultural practices of commercial orchids. Vigyan Varta 2020, 1, 53–64. [Google Scholar]
- De, L.C.; Sailo, N.; Singh, D.R. Growth and developmental aspects in commercial orchids. Floric. Sci. 2018, 2, 6–15. [Google Scholar]
- Hew, C.S.; Yong, J.W.H. Growth and photosynthesis of Oncidium ‘Goldiana’. J. Hort. Sci. 1994, 69, 809–819. [Google Scholar] [CrossRef]
- Johkan, M.; Shoji, K.; Goto, F.; Hashida, S.N.; Yoshihara, T. Blue light-emitting diode light irradiation of seedlings improves seedling quality and growth after transplanting in red leaf lettuce. HortScience 2010, 45, 1809–1814. [Google Scholar] [CrossRef]
- Hernandez, R.; Kubota, C. Physiological responses of cucumber seedlings under different blue and red photon flux ratios using LEDs. Environ. Exp. Bot. 2016, 29, 66–74. [Google Scholar] [CrossRef]
- Lazzarin, M.; Meisenburg, M.; Meijer, D.; van Ieperen, W.; Marcelis, L.F.M.; Kappers, I.F.; van der Krol, A.R.; van Loon, J.J.A.; Dicke, M. LEDs make it resilient: Effects on plant growth and defense. Trends Plant Sci. 2021, 26, 496–508. [Google Scholar] [CrossRef]
- Cavallaro, V.; Pellegrino, A.; Muleo, R.; Forgione, I. Light and plant growth regulators on in vitro proliferation. Plants 2022, 11, 844. [Google Scholar] [CrossRef]
- Paradiso, R.; Meinen, E.; Snel, J.F.; De Visser, P.; Van Ieperen, W.; Hogewoning, S.W.; Marcelis, L.F.M. Spectral dependence of photosynthesis and light absorptance in single leaves and canopy in rose. Sci. Hortic. 2021, 127, 548–554. [Google Scholar] [CrossRef]
- Smith, H.L.; McAusland, L.; Murchie, E.H. Don’t ignore the green light: Exploring diverse roles in plant processes. J. Exp. Bot. 2017, 68, 2099–2110. [Google Scholar] [CrossRef]
- Chin, D.C.; Shen, C.H.; SenthilKumar, R.; Yeh, K.W. Prolonged exposure to elevated temperature induces floral transition via up-regulation of cytosolic ascorbate peroxidase 1 and subsequent reduction of the ascorbate redox ratio in Oncidium hybrid orchid. Plant Cell Physiol. 2014, 55, 2164–2176. [Google Scholar] [CrossRef] [PubMed]
- Dodd, A.N.; Salathia, N.; Hall, A.; Kévei, E.; Tóth, R.; Nagy, F.; Hibberd, J.M.; Millar, A.J.; Webb, A.A. Plant circadian clocks increase photosynthesis, growth, survival, and competitive advantage. Science 2005, 309, 630–633. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Manivannan, A.; Wei, H. Light Quality-Mediated Influence of Morphogenesis in Micro propagated Horticultural Crops: A Comprehensive Overview. BioMed. Res. Int. 2022, 2022, 4615079. [Google Scholar] [CrossRef]
- Paradiso, R.; Proietti, S. Light-Quality Manipulation and Photomorphogenesis in Greenhouse Horticulture: The State of the Art and the Opportunities of Modern LED Systems. J. Plant Growth Regul. 2022, 41, 742–780. [Google Scholar] [CrossRef]
- Sakurako, H.; Shota, Y.; Haruki, K.; Saashia, F.; Shigekazu, K.; Ken-Ichiro, S.; Atsushi, T. A BLUS1 kinase signal and a decrease in intercellular CO2 concentration are necessary for stomatal opening in response to blue light. Plant Cell 2021, 33, 1813–1827. [Google Scholar]
- Song, Y.; Shang, W.; Ma, D.; Wang, Z.; He, S.; Shi, L.; Shen, Y.; He, D.; Wang, E.; Wang, X. Effect on the Growth and Photosynthetic Characteristics of Anthurium andreanum (‘Pink Champion’,‘Alabama’) under Hydroponic Culture by Different LED Light Spectra. Horticulturae 2022, 8, 389. [Google Scholar] [CrossRef]
- Izzo, L.G.; Mele, B.H.; Vitale, L.; Vitale, E.; Arena, C. The role of monochromatic red and blue light in tomato early photomorphogenesis and photosynthetic traits. Environ. Exp. Bot. 2020, 179, 104195. [Google Scholar] [CrossRef]
- Yousef, A.F.; Ali, M.M.; Rizwan, H.M.; Gad, A.G.; Chen, F. Light quality and quantity affect graft union formation of tomato plants. Sci. Rep. 2021, 11, 9870. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.S.; Murthy, H.N.; Heo, J.W.; Hahn, E.J.; Park, K.Y. The effect of light quality on the growth and development of in vitro cultured Doritaenops is plants. Acta Physiol. Plant. 2008, 30, 339–343. [Google Scholar] [CrossRef]
- Liu, M.; Xu, Z.; Yang, Y.; Feng, Y. Effects of different spectral lights on Oncidium PLBs induction, proliferation, and plant regeneration. Plant Cell Tissue Organ Cult. 2011, 106, 1–10. [Google Scholar]
- Lin, Y.; Li, J.; Li, B.; He, T.; Chun, Z. Effects of light quality on growth and development of protocorm-like bodies of Dendrobium officinale in vitro. Plant Cell Tissue Organ Cult. 2011, 105, 329–335. [Google Scholar] [CrossRef]
- Cybularz-Urban, T.; Hanus-Fajerska, E.; Bach, A. Callus induction and organogenesis in vitro of Cattleya from protocorm-like bodies (PLBs) under different light conditions. Acta Sci. Pol. Hortorum Cultus 2015, 14, 19–28. [Google Scholar]
- Suarez-Lopez, P.; Wheatley, K.; Robson, F.; Onouchi, H.; Valverde, F.; Coupland, G. Constans mediates between the circadian clock and the control of flowering in Arabidopsis. Nature 2001, 410, 1116–1120. [Google Scholar] [CrossRef] [PubMed]

| Trait | Main and Interaction Effects | |||||
|---|---|---|---|---|---|---|
| L | G | L × G | ||||
| F and p Values with Significance | ||||||
| F | p | F | p | F | p | |
| PL (mm) | 5.81 | 0.00001 **** | 7.21 | 0.001 ** | 2.68 | 0.005 ** |
| PW (mm) | 4.63 | 0.0005 *** | 15.69 | 0.00001 **** | 6.44 | 0.00001 **** |
| PT (mm) | 4.33 | 0.0009 *** | 3.18 | 0.05 * | 5.87 | 0.00001 **** |
| FL(cm) | 7.02 | 0.00001 **** | 24.10 | 0.00001 **** | 4.43 | 0.00001 **** |
| FB | 3.95 | 0.001 ** | 76.44 | 0.00001 **** | 4.80 | 0.00001 **** |
| FN | 19.38 | 0.00001 **** | 83.21 | 0.00001 **** | 6.82 | 0.00001 **** |
| FD (day) | 9.49 | 0.00001 **** | 836.2 | 0.00001 **** | 4.95 | 0.00001 **** |
| Trait | Main and Interaction Effects | |||||
|---|---|---|---|---|---|---|
| T | S | T × S | ||||
| F and p Values with Significance | ||||||
| F | p | F | p | F | p | |
| PL (mm) | 0.86 | 0.43 NS | 2.57 | 0.11 NS | 5.55 | 0.005 ** |
| PW (mm) | 4.24 | 0.02 * | 0.10 | 0.75 NS | 0.85 | 0.43 NS |
| PT (mm) | 4.66 | 0.01 * | 0.01 | 0.91 NS | 0.92 | 0.40 NS |
| FL(cm) | 3.35 | 0.04 * | 0.71 | 0.40 NS | 0.04 | 0.96 NS |
| FB | 3.35 | 0.04 * | 0.38 | 0.53 NS | 0.10 | 0.91 NS |
| FN | 9.01 | 0.0003 *** | 0.02 | 0.89 NS | 1.03 | 0.36 NS |
| FD (day) | 0.29 | 0.75 NS | 0.04 | 0.85 NS | 0.29 | 0.75 NS |
| Trait | Main and Interaction Effect | |||||
|---|---|---|---|---|---|---|
| T | S | T × S | ||||
| F and p Values with Significance | ||||||
| F | p | F | p | F | p | |
| PL (mm) | 0.69 | 0.51 NS | 0.44 | 0.51 NS | 33.52 | 0.00001 **** |
| PW (mm) | 5.68 | 0.009 ** | 6.24 | 0.02 * | 0.61 | 0.55 NS |
| PT (mm) | 9.58 | 0.0008 *** | 10.87 | 0.003 ** | 0.075 | 0.93 NS |
| FL(cm) | 5.88 | 0.008 ** | 4.62 | 0.042 NS | 0.29 | 0.75 NS |
| FB | 2.18 | 0.13 NS | 0.001 | 0.97 NS | 0.001 | 0.99 NS |
| FN | 10.19 | 0.0005 *** | 2.03 | 0.17 NS | 1.08 | 0.35 NS |
| FD (day) | 12.13 | 0.0002 *** | 7.25 | 0.01 * | 2.13 | 0.14 NS |
| Trait | Main and Interaction Effect | |||||
|---|---|---|---|---|---|---|
| T | S | T × S | ||||
| F and p Values with Significance | ||||||
| F | p | F | p | F | p | |
| PL (mm) | 1.82 | 0.19 NS | 3.79 | 0.07 NS | 0.64 | 0.54 NS |
| PW (mm) | 0.11 | 0.89 NS | 23.86 | 0.00001 **** | 4.11 | 0.03 * |
| PT (mm) | 5.63 | 0.01 * | 14.50 | 0.0009 *** | 3.56 | 0.04 * |
| FL(cm) | 2.46 | 0.10 NS | 18.82 | 0.0001 *** | 3.37 | 0.04 * |
| FB | 9.27 | 0.0006 *** | 2.83 | 0.10 NS | 7.80 | 0.002 ** |
| FN | 23.74 | 0.00001 **** | 0.42 | 0.52 NS | 2.19 | 0.13 NS |
| FD (day) | 10.93 | 0.0002 *** | 1.27 | 0.27 NS | 4.11 | 0.02 * |
| Trait | Main and Interaction Effect | |||||
|---|---|---|---|---|---|---|
| T | S | T × S | ||||
| F and p Values with Significance | ||||||
| F | p | F | p | F | p | |
| PL (mm) | 0.17 | 0.84 NS | 0.37 | 0.55 NS | 0.74 | 0.49 NS |
| PW (mm) | 8.79 | 0.002 ** | 1.27 | 0.27 NS | 0.30 | 0.74 NS |
| PT (mm) | 1.56 | 0.23 NS | 0.41 | 0.53 NS | 0.45 | 0.64 NS |
| FL(cm) | 1.61 | 0.22 NS | 2.29 | 0.14 NS | 6.01 | 0.009 ** |
| FB | 7.28 | 0.004 ** | 0.55 | 0.47 NS | 7.68 | 0.003 ** |
| FN | 29.96 | 0.00001 **** | 1.41 | 0.25 NS | 24.35 | 0.00001 **** |
| FD (day) | 18.12 | 0.00001 **** | 13.09 | 0.002 ** | 2.10 | 0.15 NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, C.-M.; Wang, C.-W.; Huang, M.-Y.; Chen, C.-I.; Lin, K.-H.; Shen, C.-P. The Effects of Light Treatments on Growth and Flowering Characteristics of Oncidesa Gower Ramsey ‘Honey Angel’ at Different Growth Stages. Agriculture 2023, 13, 1937. https://doi.org/10.3390/agriculture13101937
Chang C-M, Wang C-W, Huang M-Y, Chen C-I, Lin K-H, Shen C-P. The Effects of Light Treatments on Growth and Flowering Characteristics of Oncidesa Gower Ramsey ‘Honey Angel’ at Different Growth Stages. Agriculture. 2023; 13(10):1937. https://doi.org/10.3390/agriculture13101937
Chicago/Turabian StyleChang, Chia-Man, Ching-Wen Wang, Meng-Yuan Huang, Chung-I Chen, Kuan-Hung Lin, and Chih-Pei Shen. 2023. "The Effects of Light Treatments on Growth and Flowering Characteristics of Oncidesa Gower Ramsey ‘Honey Angel’ at Different Growth Stages" Agriculture 13, no. 10: 1937. https://doi.org/10.3390/agriculture13101937
APA StyleChang, C.-M., Wang, C.-W., Huang, M.-Y., Chen, C.-I., Lin, K.-H., & Shen, C.-P. (2023). The Effects of Light Treatments on Growth and Flowering Characteristics of Oncidesa Gower Ramsey ‘Honey Angel’ at Different Growth Stages. Agriculture, 13(10), 1937. https://doi.org/10.3390/agriculture13101937







