A Cucumber Photosynthetic Rate Prediction Model in Whole Growth Period with Time Parameters
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Methods
2.3. Data Preprocessing Method
2.4. Method of Photosynthetic Rate Prediction Model
- W = an weight vector
- φ(X) = a nonlinear mapping function
- b = a constant.
- C = the penalty factor
- and = a pair of relaxation factors.
- and = dual variables
- K(Xi,Xj) = φ(Xi)Tφ(Xj) = the kernel function.
2.5. Data Preprocessing Method
2.6. SVR Model Parameter Optimization
2.7. Data Processing Methods
3. Results and Discussion
3.1. Experimental Results
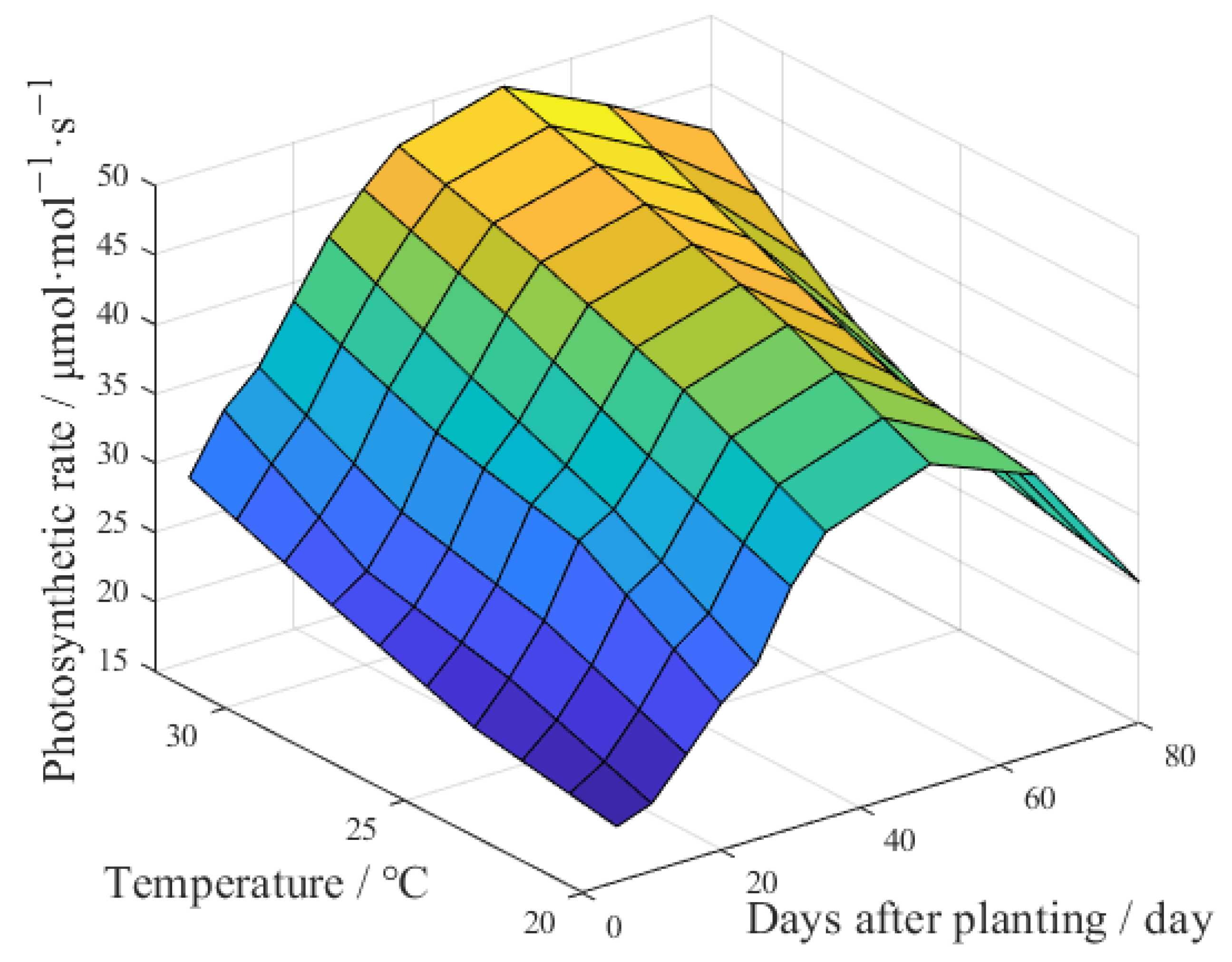
3.1.1. Effects of Environmental Factors on Photosynthetic Rate
3.1.2. Effects of Growing Time on Photosynthetic Rate
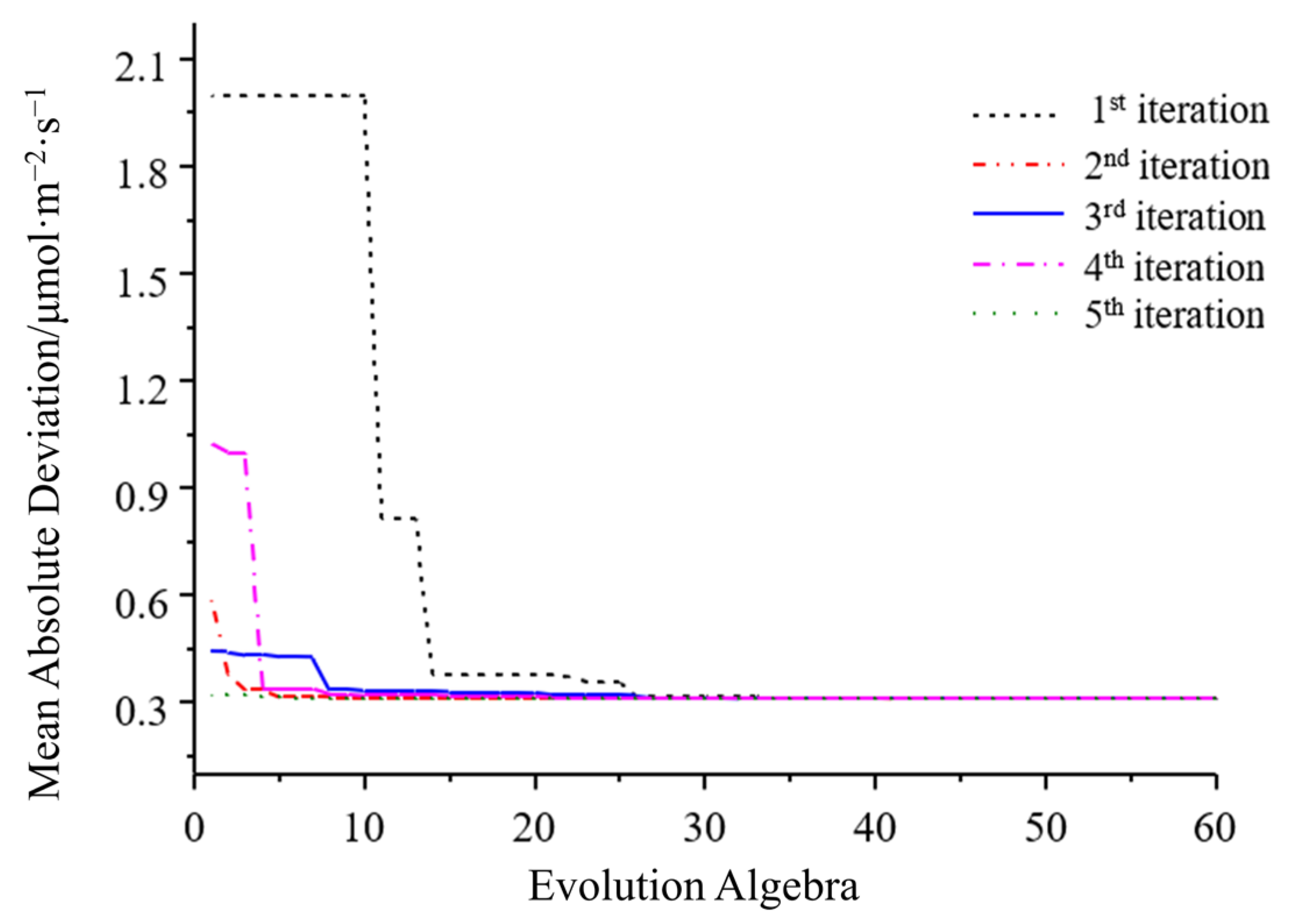
3.2. SVR Algorithm Optimization Results
3.2.1. Optimal Kernel Function Acquisition
3.2.2. Optimal C and Gamma Parameters Combination Acquisition
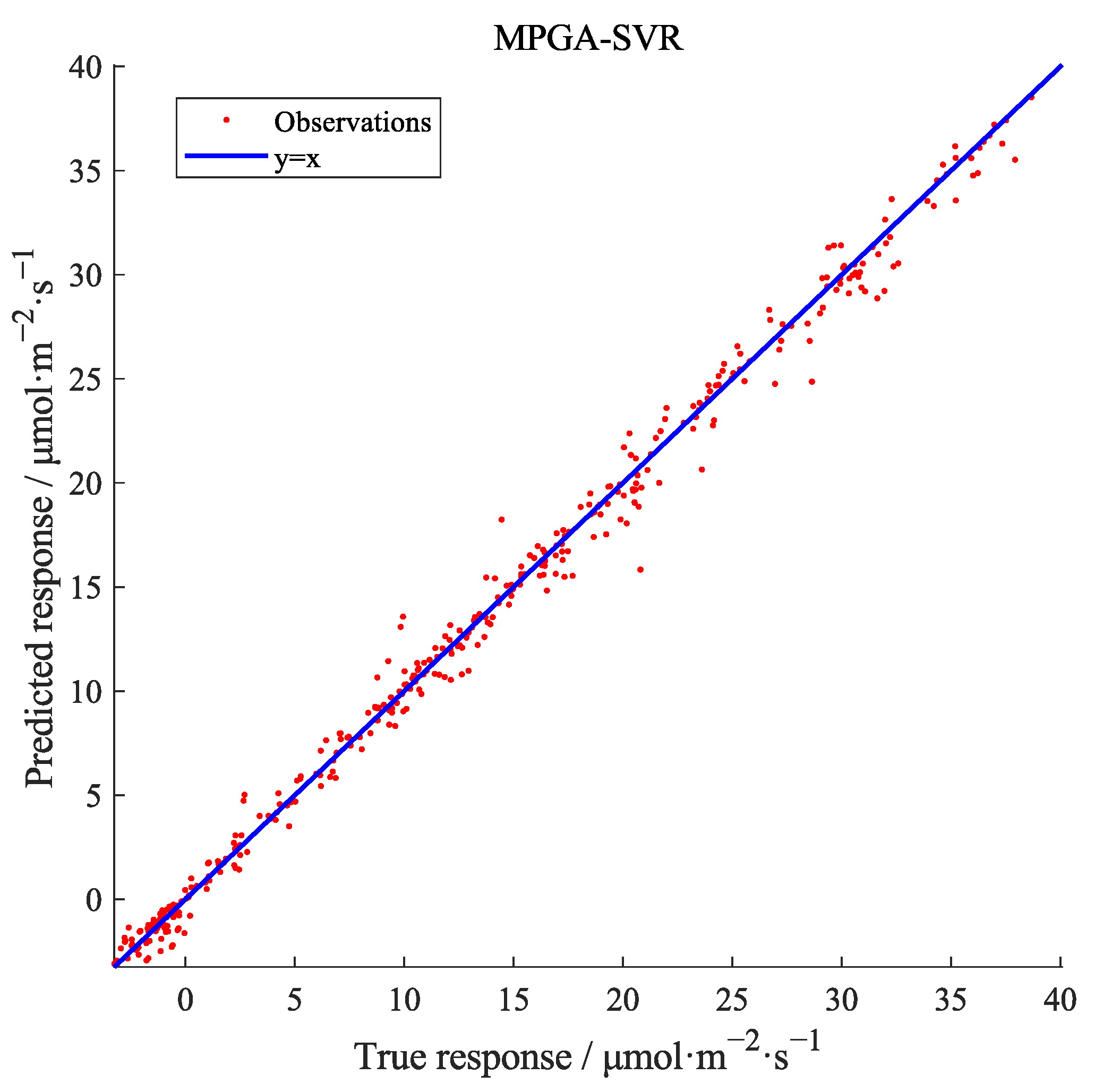
3.3. Model Validation
4. Conclusions
- (1)
- In terms of parameter optimization of SVR algorithm, MPGA algorithm overcomes the premature convergence phenomenon commonly seen in standard genetic algorithm through global optimization, and can obtain higher model accuracy than GridSearchCV algorithm;
- (2)
- Model verification results show that the R2 of the test set of the MPGA-SVR model is 0.998, and the MAD is 0.280 μmol·m−2·s−1. Its evaluation results are better than the models built by GridsearchCV-SVR algorithm, BPNN, and NLR method. At the same time, it shows that the modeling with growing days could well reflect the change of photosynthetic rate under different growth stages in the whole growth period, and the accuracy of the model could meet the needs of practical application.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hernández-Morales, C.A.; Luna-Rivera, J.M.; Perez-Jimenez, R. Design and deployment of a practical IoT-based monitoring system for protected cultivations. Comput. Commun. 2022, 186, 51–64. [Google Scholar] [CrossRef]
- Xie, J.; Yu, J.; Chen, B.; Feng, Z.; Li, J.; Zhao, C.; Lyu, J.; Hu, L.; Gan, Y.; Siddique, K.H.M. Facility Cultivation Systems: A Chinese Model for the Planet. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2017; Volume 145, pp. 1–42. [Google Scholar]
- Xu, Y.; Li, J.; Wan, J. Agriculture and crop science in China: Innovation and sustainability. Crop J. 2017, 5, 95–99. [Google Scholar] [CrossRef]
- Iqbal, M.; Mahmooduzzafar; Nighat, F.; Khan, P.R. Photosynthetic, metabolic and growth responses ofTriumfetta rhomboideato coal-smoke pollution at different stages of plant ontogeny. J. Plant Interact. 2010, 5, 11–19. [Google Scholar] [CrossRef]
- Jamil, F.; Ibrahim, M.; Ullah, I.; Kim, S.; Kahng, H.K.; Kim, D.-H. Optimal smart contract for autonomous greenhouse environment based on IoT blockchain network in agriculture. Comput. Electron. Agric. 2022, 192, 106573. [Google Scholar] [CrossRef]
- Jo, W.J.; Shin, J.H. Development of a transpiration model for precise tomato (Solanum lycopersicum L.) irrigation control under various environmental conditions in greenhouse. Plant Physiol. Biochem. 2021, 162, 388–394. [Google Scholar] [CrossRef]
- Xin, P.; Zhang, H.; Hu, J.; Shao, Z.; Peters, R.T. CO2 Control system design based on optimized regulation model. Appl. Eng. Agric. 2019, 35, 377–388. [Google Scholar] [CrossRef]
- Kaiser, E.; Morales, A.; Harbinson, J.; Kromdijk, J.; Heuvelink, E.; Marcelis, L.F. Dynamic photosynthesis in different environmental conditions. J. Exp. Bot. 2015, 66, 2415–2426. [Google Scholar] [CrossRef]
- Johnson, I.R.; Thornley, J.H.; Frantz, J.M.; Bugbee, B. A model of canopy photosynthesis incorporating protein distribution through the canopy and its acclimation to light, temperature and CO2. Ann. Bot. 2010, 106, 735–749. [Google Scholar] [CrossRef]
- Whitehead, W.F.; Singh, B.P. Leaf Age Affects Gas Exchange in Okra. HortScience 1995, 30, 1017–1019. [Google Scholar] [CrossRef]
- Zhang, S.B.; Hu, H.; Li, Z.R. Variation of photosynthetic capacity with leaf age in an alpine orchid, Cypripedium flavum. Acta Physiol. Plant. 2008, 30, 381–388. [Google Scholar] [CrossRef]
- Fan, X.X.; Xu, Z.G.; Liu, X.Y.; Tang, C.M.; Wang, L.W.; Han, X.L. Effects of light intensity on the growth and leaf development of young tomato plants grown under a combination of red and blue light. Sci. Hortic. 2013, 153, 50–55. [Google Scholar] [CrossRef]
- Sun, J.L.; Sui, X.L.; Huang, H.Y.; Wang, S.H.; Wei, Y.X.; Zhang, Z.X. Low Light Stress Down-Regulated Rubisco Gene Expression and Photosynthetic Capacity During Cucumber (Cucumis sativus L.) Leaf Development. J. Integr. Agric. 2014, 13, 997–1007. [Google Scholar] [CrossRef]
- Ding, X.T.; Jiang, Y.P.; Zhang, Z.H.; Jin, H.J.; Zhang, H.M.; Yu, J.Z. The change of cucumber growth and development in glasshouse. Acta Agric. Shanghai 2013, 29, 36–39. [Google Scholar]
- Li, P.; Zhou, J.; Wang, J.; Fu, W. Exponential sine equation for predicting cucumber growth process in greenhouses. J. Jiangsu Univ. 2009, 30, 325–329. [Google Scholar]
- Chen, W.Y. Predicting photosynthetic rate of sunflowers using back propagation neural network based on uniform design. Afr. J. Agric. Res. 2011, 6, 5817–5821. [Google Scholar] [CrossRef]
- Jung, D.H.; Shin, J.H.; Cho, Y.Y.; Son, J.E. Development of a two-variable spatial leaf photosynthetic model of irwin mango grown in greenhouse. Prot. Hortic. Plant Fact. 2015, 24, 161–166. [Google Scholar] [CrossRef]
- Hu, J.; Xin, P.; Zhang, S.; Zhang, H.; He, D. Model for tomato photosynthetic rate based on neural network with genetic algorithm. Int. J. Agric. Biol. Eng. 2019, 12, 179–185. [Google Scholar] [CrossRef]
- Xin, P.; Zhang, H.; Hu, J.; Wang, Z.; Zhang, Z. An Improved Photosynthesis Prediction Model Based on Artificial Neural Networks Intended for Cucumber Growth Control. Appl. Eng. Agric. 2018, 34, 769–787. [Google Scholar] [CrossRef]
- Yang, X.; Xu, H.; Shao, L.; Li, T.; Wang, Y.; Wang, R. Response of photosynthetic capacity of tomato leaves to different LED light wavelength. Environ. Exp. Bot. 2018, 150, 161–171. [Google Scholar] [CrossRef]
- Abdollahpour, S.; Kosari-Moghaddam, A.; Bannayan, M. Prediction of wheat moisture content at harvest time through ANN and SVR modeling techniques. Inf. Process. Agric. 2020, 7, 500–510. [Google Scholar] [CrossRef]
- Jiandong, M.; Juan, L. Dust particle size distribution inversion based on the multi population genetic algorithm. Terr. Atmos. Ocean. Sci. 2014, 25, 791. [Google Scholar]
- Zhu, H.; Jiao, L.; Pan, J. Multi-population genetic algorithm for feature selection. In Proceedings of the Advances in Natural Computation, Berlin/Heidelberg, Germany, 24–28 September 2006; pp. 480–487. [Google Scholar]
- Chang, C.C.; Lin, C.J. Libsvm: A library for support vector machines. ACM Trans. Intell. Syst. Technol. 2011, 2, 1–27. [Google Scholar] [CrossRef]
- Gao, P.; Tian, Z.; Lu, Y.; Lu, M.; Zhang, H.; Wu, H.; Hu, J. A decision-making model for light environment control of tomato seedlings aiming at the knee point of light-response curves. Comput. Electron. Agric. 2022, 198, 107103. [Google Scholar] [CrossRef]
- Krupa, Z.; Oquist, G.; Gustafsson, P. Photoinhibition of photosynthesis and growth responses at different light levels in psbA gene mutants of the cyanobacterium Synechococcus. Physiol. Plant. 1991, 82, 1–8. [Google Scholar] [CrossRef]
- Igamberdiev, A.U.; Kleczkowski, L.A. Optimization of CO2 fixation in photosynthetic cells via thermodynamic buffering. Biosystems 2011, 103, 224–229. [Google Scholar] [CrossRef]
- Kirschbaum, M.U.F. The sensitivity of C3 photosynthesis to increasing CO2 concentration: A theoretical analysis of its dependence on temperature and background CO2 concentration. Plant Cell Environ. 1994, 17, 747–754. [Google Scholar] [CrossRef]
- Galmes, J.; Capo-Bauca, S.; Niinemets, U.; Iniguez, C. Potential improvement of photosynthetic CO2 assimilation in crops by exploiting the natural variation in the temperature response of Rubisco catalytic traits. Curr. Opin. Plant Biol. 2019, 49, 60–67. [Google Scholar] [CrossRef]
- Aguera, E.; Ruano, D.; Cabello, P.; de la Haba, P. Impact of atmospheric CO2 on growth, photosynthesis and nitrogen metabolism in cucumber (Cucumis sativus L.) plants. J. Plant Physiol. 2006, 163, 809–817. [Google Scholar] [CrossRef]
- Xue, W.; Li, X.; Lin, L.; Wang, Y.; Li, L. Effects of short time heat stress on photosystem II, Rubisco activities and oxidative radicals in Alhagi sparsifolia. Chin. J. Plant Ecol. 2011, 35, 441–451. [Google Scholar] [CrossRef]
- Pettersen, R.I.; Torre, S.; Gislerød, H.R. Effects of leaf aging and light duration on photosynthetic characteristics in a cucumber canopy. Sci. Hortic. 2010, 125, 82–87. [Google Scholar] [CrossRef]
- Ming, Z.; Piyao, Z.; Ruifang, W. Studies on the dynamic changes of the photosynthetic rates of the leaves duration the course of the growth and development of summer-sown corn. Acta Agron. Sin. 1992, 18, 337–343. [Google Scholar]
- Bertamini, M.; Nedunchezhian, N. Leaf age effects on chlorophyll, Rubisco, photosynthetic electron transport activities and thylakoid membrane protein in field grown grapevine leaves. J. Plant Physiol. 2002, 159, 799–803. [Google Scholar] [CrossRef]
- Elliott, L.; Ingham, D.B.; Mera, N.S. A multi-population genetic algorithm approach for solving ill-posed problems. Comput. Mech. 2004, 33, 254–262. [Google Scholar] [CrossRef]
- Huang, Y.; Ma, X.; Su, S.; Tang, T. Optimization of train operation in multiple interstations with multi-population genetic algorithm. Energies 2015, 8, 14311–14329. [Google Scholar] [CrossRef]
- Hu, J.; Gao, P.; Chen, D.; Li, B.; Jing, H.; Zhang, H. Establishment of photosynthetic rate prediction model for eggplant leaves fused with dark fluorescence parameters. Trans. Chin. Soc. Agric. Mach. 2020, 51, 328–336. [Google Scholar]
- Lindström, L.I.; Pellegrini, C.N.; Aguirrezábal, L.A.N.; Hernández, L.F. Growth and development of sunflower fruits under shade during pre and early post-anthesis period. Field Crops Res. 2006, 96, 151–159. [Google Scholar] [CrossRef]


| Photon Flux Density (μmol·m−2·s−1) | Temperature (°C) | CO2 Concentration (μmol·mol−1) | |
|---|---|---|---|
| Range | 0–1800 | 20–32 | 600–1500 |
| Step size | / | 4 | 300 |
| Growth Stage | Initial Flowering Stage to Initial Fruiting Stage (Day) | Full Fruiting Stage to Final Fruiting Stage (Day) |
|---|---|---|
| Time range | 35 | 36–85 |
| Sampling interval | 5 | 15 |
| Kernel Function | Training Set | Test Set | ||
|---|---|---|---|---|
| RMSE (μmol·m−2·s−1) | R2 | RMSE (μmol·m−2·s−1) | R2 | |
| linearity | 2.222 | 0.866 | 4.417 | 0.874 |
| polynomial | 1.138 | 0.934 | 1.801 | 0.860 |
| RBF | 0.428 | 0.997 | 0.851 | 0.995 |
| Sigmoid | 2.571 | 0.824 | 5.111 | 0.832 |
| Optimization Algorithms | Optimal C | Optimal Gamma | E1 (μmol·m−2·s−1) 1 | E2 (μmol·m−2·s−1) 2 |
|---|---|---|---|---|
| GridsearchCV | 64 | 1 | 0.284 | 0.407 |
| MPGA | 79.268 | 0.937 | 0.193 | 0.280 |
| Models | MAD (μmol·m−2·s−1) | MAE (μmol·m−2·s−1) | R2 | |||
|---|---|---|---|---|---|---|
| Training Set | Test Set | Training Set | Test Set | Training Set | Test Set | |
| NLR | 1.923 | 2.307 | 8.798 | 10.115 | 0.824 | 0.707 |
| BPNN | 0.470 | 0.534 | 2.850 | 3.353 | 0.997 | 0.996 |
| GS-SVR | 0.335 | 0.407 | 2.354 | 2.809 | 0.996 | 0.997 |
| MPGA-SVR | 0.228 | 0.280 | 2.008 | 2.462 | 0.998 | 0.998 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Z.; Wan, X.; Lei, W.; Yuan, K.; Lu, M.; Li, B.; Gao, P.; Wu, H.; Hu, J. A Cucumber Photosynthetic Rate Prediction Model in Whole Growth Period with Time Parameters. Agriculture 2023, 13, 204. https://doi.org/10.3390/agriculture13010204
Wei Z, Wan X, Lei W, Yuan K, Lu M, Li B, Gao P, Wu H, Hu J. A Cucumber Photosynthetic Rate Prediction Model in Whole Growth Period with Time Parameters. Agriculture. 2023; 13(1):204. https://doi.org/10.3390/agriculture13010204
Chicago/Turabian StyleWei, Zichao, Xiangbei Wan, Wenye Lei, Kaikai Yuan, Miao Lu, Bin Li, Pan Gao, Huarui Wu, and Jin Hu. 2023. "A Cucumber Photosynthetic Rate Prediction Model in Whole Growth Period with Time Parameters" Agriculture 13, no. 1: 204. https://doi.org/10.3390/agriculture13010204
APA StyleWei, Z., Wan, X., Lei, W., Yuan, K., Lu, M., Li, B., Gao, P., Wu, H., & Hu, J. (2023). A Cucumber Photosynthetic Rate Prediction Model in Whole Growth Period with Time Parameters. Agriculture, 13(1), 204. https://doi.org/10.3390/agriculture13010204






