The Use of Genetic Material of Tall Wheatgrass to Protect Common Wheat from Septoria Blotch in Western Siberia
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
- (1)
- four accessions of Th. ponticum from N.V. Tsitsin’s Main Botanical Garden (Moscow, Russia), including two originated from Russia (one of them a parent for the WWHs), the USA and Africa;
- (2)
- introgressive lines of spring common wheat with genetic material of the Th. ponticum (in 2015–2016–130, in 2017–2019–93 lines);
- (3)
- four cultivars of spring common wheat viz. Niva 2, Chernyava 13, Golubkovskaya, Sonata susceptible to Septoria diseases used for backcrosses;
- (4)
- standard cultivars of spring common wheat viz. Pamyati Azieva (medium-early), Duet (medium-ripe), Serebristaya (medium-late).
2.2. Methods
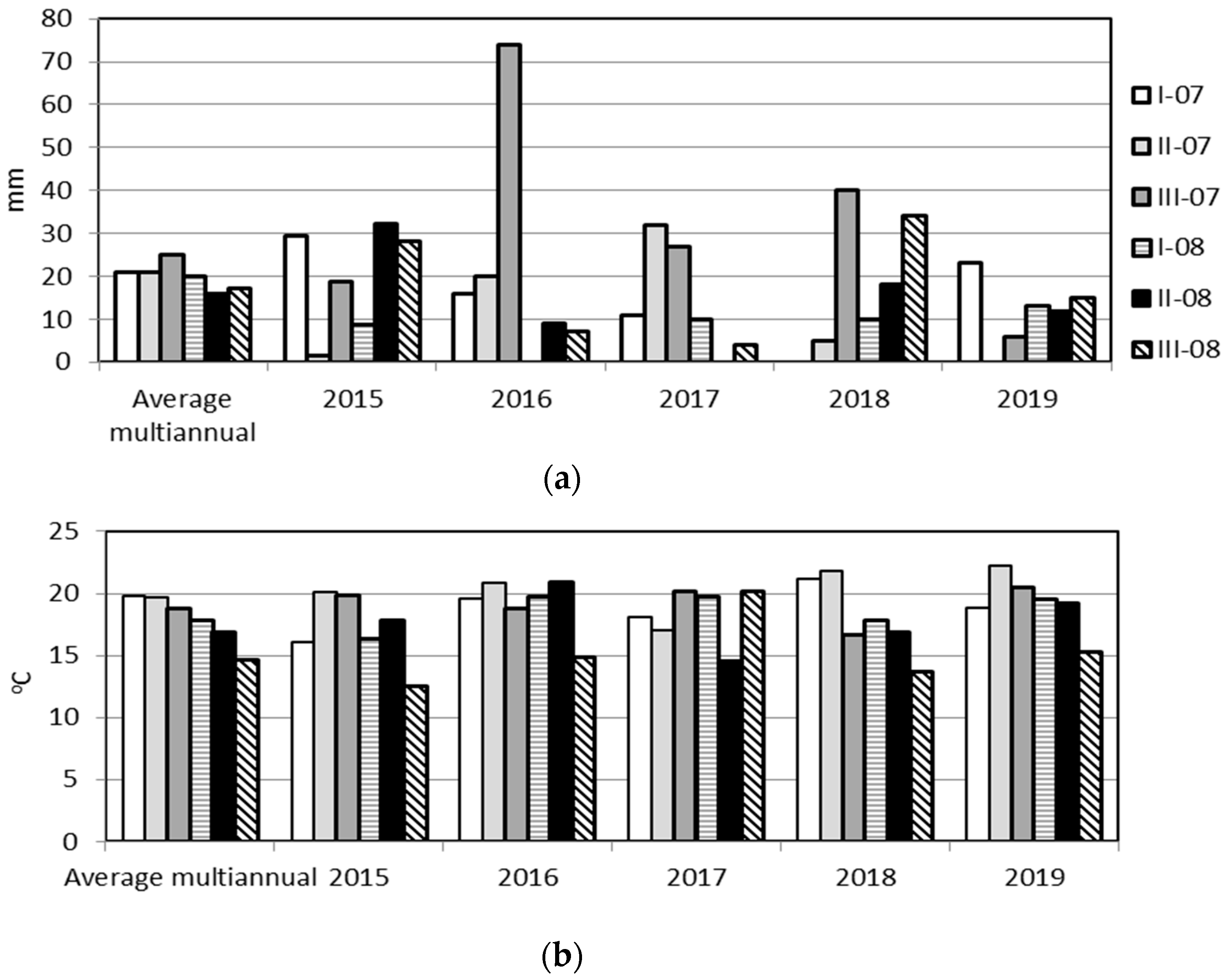
2.3. Environmental Conditions
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- USDA Foreign Agricultural Service. World Agricultural Production; USDA Foreign Agricultural Service: Washington, DC, USA, 2016; p. 20250.
- Singh, R.P.; Singh, P.K.; Rutkoski, J.; Hodson, D.P.; He, X.; Jorgensen, L.N.; Hovmoller, M.S.; Huerta-Espino, J. Disease impact on wheat yield potential and prospects of genetic control. Annu. Rev. Phytopathol. 2016, 54, 303–322. [Google Scholar] [CrossRef]
- Fones, H.; Gurr, S. The impact of Septoria tritici blotch disease on wheat: An EU perspective. Fungal Genet. Biol. 2015, 79, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Arif, M.A.R.; Arseniuk, E.; Börner, A. Genetic variability for resistance to fungal pathogens in bread wheat. Czech J. Genet. Plant Breed. 2023, 59, 23–32. [Google Scholar] [CrossRef]
- O’Driscoll, A.; Kildea, S.; Doohan, F.; Spink, J.; Mullins, E. The wheat–Septoria conflict: A new front opening up? Trends Plant Sci. 2014, 19, 602–610. [Google Scholar] [CrossRef]
- Pereira, D.; Croll, D.; Brunnera, P.C.; McDonald, B.A. Natural selection drives population divergence for local adaptation in a wheat pathogen. Fungal Genet. Biol. 2020, 141, 103398. [Google Scholar] [CrossRef]
- Bakulina, A.V.; Kharina, A.V.; Shirokikh, A.A. Septoria tritici and Stagonospora nodorum blotch of wheat: Genetic control of host resistance (review). Theor. Appl. Ecol. 2020, 2, 26–35. [Google Scholar] [CrossRef]
- Sanin, S.S.; Pakholkova, E.V.; Sanina, A.A. Epidemiology of winter wheat leaf blotch: Formation of infectious potentials of pathogens. Plant Prot. Quar./Zashchita I Karantin Rastenij 2018, 5, 25–29. (In Russian) [Google Scholar]
- Eyal, Z.; Scharen, A.L.; Prescott, J.M.; Ginkel, M. The Septoria Diseases of Wheat: Concepts and Methods of Disease Management; CIMMYT: Mexico, Brazil, 1987; 46p. [Google Scholar]
- Zhan, J.; Mundt, C.; McDonald, B. Sexual reproduction facilitates the adaptation of parasites to antagonistic host environments: Evidence from empirical study in the wheat—Mycosphaerella graminicola system. Int. J. Parasitol. 2007, 37, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, S.B.; M’Barek, S.; Dhillon, B. Finished genome of the fungal wheat pathogen Mycosphaerella graminicola reveals dispensome structure, chromosome plasticity, and stealth pathogenesis. PLoS Genet. 2011, 7, e1002070. [Google Scholar] [CrossRef]
- Siah, A.; Bomble, M.; Tisserant, B.; Cadalen, T.; Holvoet, M.; Hilbert, J.L.; Halama, P.; Reignault, P. Genetic structure of Zymoseptoria tritici in northern France at region, field, plant, and leaf layer scales. Phytopathology 2018, 108, 1114–1123. [Google Scholar] [CrossRef] [PubMed]
- Sanin, S.S.; Korneva, L.G.; Polyakova, T.M. Forecast of risk of development of epiphytoties of a Septoria leaf blotch of leaves and ear of wheat. Plant Prot. Quar./Zashchita I Karantin Rastenij 2015, 3, 33–36. (In Russian) [Google Scholar]
- McDonald, B.A.; Mundt, C.C. How knowledge of pathogen population biology informs management of Septoria tritici blotch. Phytopathology 2016, 106, 948–955. [Google Scholar] [CrossRef] [PubMed]
- Hassine, M.; Siah, A.; Hellin, P.; Cadalen, T.; Halama, P.; Hilbert, J.-L.; Hamada, W.; Baraket, M.; Yahyaoui, A.; Legreve, A.; et al. Sexual reproduction of Zymoseptoria tritici on durum wheat in Tunisia revealed by presence of airborne inoculum, fruiting bodies and high levels of genetic diversity. Fungal Biol. 2019, 123, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.K.; Chartrain, L.; Lasserre-Zuber, P.; Saintenac, C. Genetics of resistance to Zymoseptoria tritici and applications to wheat breeding. Fungal Genet. Biol. 2015, 79, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Hayden, E.J.; Bratulic, S.; Koenig, I.; Ferrada, E.; Wagner, A. The effects of stabilizing and directional selection on phenotypic and genotypic variation in a population of RNA enzymes. J. Mol. Evol. 2014, 78, 101–108. [Google Scholar] [CrossRef] [PubMed]
- McDonald, M.C.; Solomon, P.S. Just the surface: Advances in the discovery and characterization of necrotrophic wheat effectors. Curr. Opin. Microbiol. 2018, 46, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Faris, J.D.; Friesen, T.L. Plant genes hijacked by necrotrophic fungal pathogens. Curr. Opin. Plant Biol. 2020, 56, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Rocafort, M.; Fudal, I.; Mesarich, C.H. Apoplastic effector proteins of plant-associated fungi and oomycetes. Curr. Opin. Plant Biol. 2020, 56, 9–19. [Google Scholar] [CrossRef]
- Peters Haugrud, A.R.; Zhang, Z.; Richards, J.K.; Friesen, T.L.; Faris, J.D. Genetics of variable disease expression conferred by inverse gene-for-gene interactions in the wheat-Parastagonospora nodorum pathosystem. Plant Physiol. 2019, 180, 420–434. [Google Scholar] [CrossRef]
- Nazarova, L.N.; Korneva, L.G.; Zhokhova, T.P. Epidemiological situation with Septoria disease of wheat in 2001-2009. Plant Prot. Quar./Zashchita I Karantin Rastenij 2010, 10, 18–20. (In Russian) [Google Scholar]
- Zeleneva, J.; Afanasenko, O.; Soodnikova, V. Species composition of causative agents of wheat diseases in the Central Chernozem region. Bull. Michurinsk State Agrar. Univ./Vestn. Michurinskogo Gos. Agrar. Univ. 2019, 3, 58–63. (In Russian) [Google Scholar]
- Toropova, E.Y.; Kazakova, O.A.; Piskarev, V.V. Septoria blotch epidemic process on spring wheat varieties. Vavilov J. Genet. Breed. 2020, 24, 139–148. [Google Scholar] [CrossRef]
- Koshybaev, M. Wheat Diseases; UN FAO: Ankara, Turkey, 2018; p. 365. (In Russian) [Google Scholar]
- Pakholkova, E.V.; Sal’nicova, N.N.; Kurkova, N.A. Genetic structure of regional populations of Mycosphaerella graminicola (Septoria tritici), the Septoria leaf blotch agent of wheat. Agric. Biol. 2016, 51, 722–730. [Google Scholar] [CrossRef]
- Upelniek, V.P.; Belov, V.I.; Ivanova, L.P.; Dolgova, S.P.; Demidov, A.S. Heritage of academician N.V. Tsitsin: State-of-the-art and potential of the collection of intermediate wheat x couch-grass hybrids. Vavilov J. Genet. Breed. 2012, 16, 667–674. (In Russian) [Google Scholar]
- Salina, E.; Adonina, I.; Badaeva, E.; Kroupin, P.; Stasyuk, A.; Leonova, I.; Shishkina, A.; Divashuk, M.; Starikova, E.; Khuat, T.; et al. Thinopyrum intermedium chromosome in bread wheat cultivars as a source of genes conferring resistance to fungal diseases. Euphytica 2015, 204, 91–101. [Google Scholar] [CrossRef]
- Chen, Q.; Conner, R.L.; Laroche, A.; Thomas, J.B. Genome analysis of Thinopyrum intermedium and Thinopyrum ponticum using genomic in situ hybridization. Genome 1998, 41, 580–586. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, Y.; Wang, R.R.-C. Characterization of genomes and chromosomes in partial amphiploids of the hybrid Triticum aestivum × Thinopyrum ponticum by in situ hybridization, isozyme analysis, and RAPD. Genome 1996, 39, 1062–1071. [Google Scholar] [CrossRef]
- Kolmer, T.; Flowers, T.; Munns, R. Use of wild relatives to improve salt tolerance in wheat. J. Exp. Bot. 2006, 57, 1059–1078. [Google Scholar] [CrossRef]
- Taeb, M.; Koebner, R.; Forster, B. Genetic variation for waterlogging tolerance in the Triticeae and the chromosomal location of genes conferring waterlogging tolerance in Thinopyrum elongatum. Genome 1993, 36, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Baker, L.; Grewal, S.; Yang, C.; Hubbart-Edwards, S.; Scholefield, D.; Ashling, S.; Burridge, A.; Przewieslik-Allen, A.; Wilkinson, P.; King, I.; et al. Exploiting the genome of Thinopyrum elongatum to expand the gene pool of hexaploid wheat. Theor. Appl. Genet. 2020, 133, 2213–2226. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Cu, Y.; Zhang, Y.; Wang, H.; Bao, Y.; Li, X. Molecular cytogenetic identification of three rust-resistant wheat-Thinopyrum ponticum partial amphiploids. Mol. Cytogenet. 2018, 11, 27. [Google Scholar] [CrossRef]
- Yang, G.; Boshoff, W.; Li, H.; Pretorius, Z.; Luo, Q.; Li, B.; Li, Z.; Zheng, Q. Chromosomal composition analysis and molecular marker development for the novel Ug99-resistant wheat—Thinopyrum ponticum translocation line WTT34. Theor. Appl. Genet. 2021, 134, 1587–1599. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, X.L.; Hou, Y.L.; Cai, J.J.; Shen, X.R.; Zhou, T.T.; Xu, H.H.; Ohm, H.W.; Wang, H.W.; Li, A.F.; et al. High-density mapping of the major FHB resistance gene Fhb7 derived from Thinopyrum ponticum and its pyramiding with Fhb1 by marker-assisted selection. Theor. Appl. Genet. 2015, 128, 2301–2316. [Google Scholar] [CrossRef]
- Oliver, R.E.; Xu, S.S.; Stack, R.W.; Friesen, T.L.; Jin, Y.; Cai, X. Molecular cytogenetic characterization of four partial wheat–Thinopyrum ponticum amphiploids and their reaction to Fusarium head blight, tan spot, and Stagonospora nodorum blotch. Theor. Appl. Genet. 2006, 112, 1473–1479. [Google Scholar] [CrossRef]
- Plotnikova, L.Y.; Sagendykova, A.T.; Kuzmina, S.P. Estimation of ecological plasticity and resistance to the leaf rust of introgressive lines of common wheat with Agropyron elongatum genes. Agrar. Russ./Agrar. Ross. 2016, 9, 5–13. (In Russian) [Google Scholar]
- Plotnikova, L.Y.; Sagendykova, A.T.; Ignatyeva, E.Y. Defence of bread wheat with the Tall Wheatgrass genes while accelerating the physiological specialization of the causative agent of stem rust. Omsk SAU Bulletin/Vestn. Omsk. GAU 2021, 4, 35–44. [Google Scholar] [CrossRef]
- Plotnikova, L.Y.; Sagendykova, A.T.; Berezhkova, G.A. Perspective introgressive lines of soft spring wheat with Agropyron elongatum genes sustainable to Septorious in Western Siberia. Kazan SAU Bull./Vestn. Kazan. GAU 2017, 3, 39–45. (In Russian) [Google Scholar] [CrossRef]
- Plotnikova, L.Y.; Seryukov, G.M.; Shvarts, Y.K. Cytophysiological resistance mechanisms to leaf rust in wheat-Agropyron hybrids created on the base of Agropyron elongatum. Mycol. Phytopathol./Mikol. I Fitopatol. 2011, 45, 443–454. (In Russian) [Google Scholar]
- Plotnikova, L.Y.; Aidosova, A.T.; Rispekova, A.N.; Myasnikov, A.U. Introgressive lines of common wheat with genes of wheat grass Agropyron elongatum resistant to leaf diseases in the south West Siberia. Omsk SAU Bull./Vestn. Omsk. GAU 2014, 4, 3–7. (In Russian) [Google Scholar]
- James, W.C. An illustrated series of assessment diseases preparation and usage. Can. Plant Dis. Surv. 1971, 51, 36–65. [Google Scholar]
- National Standard of the Russian Federation. Wheat. Specifications. GOST R 52554-2006. Available online: https://internet-law.ru/gosts/gost/2644. (accessed on 1 November 2022).
- Pakholkova, E.V. Speed of development of leaf-stem infections of grain crops. Plant Prot. Quar./Zashchita I Karantin Rastenij 2015, 3, 39–40. (In Russian) [Google Scholar]
- Kharina, A.V.; Shcheshegova, T.K. Search for the parent material of spring soft wheat resistant to Septoria tritici blotch and analysis of the trait inheritance. Agric. Sci. Euro North East/Agrar. Nauka Evro-Sev. Vost. 2021, 22, 212–222. [Google Scholar] [CrossRef]
- Belan, I.A.; Rosseeva, L.P.; Blokhina, N.P.; Grigoriev, Y.P.; Mukhina, Y.V.; Trubacheeva, N.V.; Pershina, L.A. Resource potential of soft spring wheat varieties for the conditions of Western Siberia and Omsk region (analytical review). Agric. Sci. Euro North East/Agrar. Nauka Evro-Sev. Vost. 2021, 22, 449–465. [Google Scholar] [CrossRef]
- Shamanin, V.; Salina, E.; Wanyera, R.; Zelenskiy, Y.; Olivera, P.; Morgounov, A. Genetic diversity of spring wheat from Kazakhstan and Russia for resistance to stem rust UG99. Euphytica 2016, 212, 287–296. [Google Scholar] [CrossRef]
- Rsaliyev, A.S.; Gultyaeva, E.I.; Shaydayuk, E.L.; Kovalenko, N.M.; Moldazhanova, R.A.; Pahratdinova, Z.U. Chracteristic of perspective common spring wheat accessions for resistance to foliar diseases. Plant Biotechnol. Breed. 2019, 2, 14–23. [Google Scholar] [CrossRef]
- Lendenmann, M.H.; Croll, D.; Palma-Guerrero, J.; Stewart, E.L.; McDonald, B.A. QTL mapping of temperature sensitivity reveals candidate genes for thermal adaptation and growth morphology in the plant pathogenic fungus Zymoseptoria tritici. Heredity 2016, 116, 384–394. [Google Scholar] [CrossRef]
- Milus, E.A.; Kristensen, K.; Hovmoller, M.S. Evidence for increased aggressiveness in a recent widespread strain of Puccinia striiformis f. sp. tritici causing stripe rust of wheat. Phytopathology 2009, 99, 89–94. [Google Scholar] [CrossRef]
- Babkenova, S.A.; Babkenov, A.T.; Pakholkova, E.V.; Kanafin, B.K. Pathogenic complexity of Septoria spot disease of wheat in northern Kazakhstan. Plant Sci. Today 2020, 7, 601–606. [Google Scholar] [CrossRef]
- Francki, M.G. Improving Staganospora nodorum resistance in wheat: A Review. Crop Sci. 2013, 53, 355–365. [Google Scholar] [CrossRef]
- Francki, M.G.; Walker, E.; Li, D.A.; Forrest, K. High-density SNP mapping reveals closely linked QTL for resistance to Staganospora nodurum blotch (SNB) in flag leaf and glume of hexaploid wheat. Genome 2018, 61, 145–149. [Google Scholar] [CrossRef]
- Amelin, A.V.; Chekalin, E.I.; Zaikin, V.V.; Mazalov, V.I.; Gorodov, V.T.; Ikusov, R.A. Biochemical indicators of quality of grain in modern varieties of spring wheat. Bull. Agric. Sci./Vestn. Agrar. Nauk. 2019, 2, 3–11. [Google Scholar] [CrossRef]
- Sanin, S.S.; Zhokhova, T.P. Influence of diseases and plant protection means on wheat grain quality. Plant Prot. Quar./Zashchita I Karantin Rastenij 2012, 11, 16–20. (In Russian) [Google Scholar]
- Castro, A.C.; Simon, M.R. The impact of Septoria tritici blotch in bread making quality among Argentinean wheat cultivars. J. Cereal Sci. 2017, 77, 259–265. [Google Scholar] [CrossRef]

| Line | Origin |
|---|---|
| 2/2015 | S5 [WWH × B4 Lutescens 444] |
| 9/2015 | S5 [(WWH × Lutescens 444) × B2 Chernyava 13] |
| 37/2015 | S5 [(WWH × B3 Lutescens 444) × Chernyava 13] |
| 6/2015, 7/2015, 10/2015, 31/2017 | S5 [WWH × B4 Chernyava 13] |
| 374/2015, 314/2017, 322/2017, 337/2017 | S5 [WWH × B5 Chernyava 13] |
| 24/2015 | S5 [WWH × B5 Sonata] |
| 94/2015 | S5 [WWH × Niva 2) × B4 Golubkovskaya] |
| Sample | Average Vegetation Period, Days | Severity, % | Frequency of Occurrence, Z. tritici/P. nodorum, %/ % | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2015 | 2016 | 2017 | 2018 | 2019 | ||||||||||
| Leaf | Ear | Leaf | Ear | Leaf | Ear | Leaf | Ear | Leaf | Ear | 2017 | 2018 | 2019 | ||
| Th. ponticum | Perennial | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - | - | - |
| Medium-early | ||||||||||||||
| Pamyati Azieva—standard | 77 ± 3.5 | 50 | 25 | 75 | 50 | 75 | 50 | 60 | 50 | 60 | 50 | 52.5/47.5 | 37.5/62.5 | 57.5/43.5 |
| Chernyava 13 | 77 ± 3.3 | 50 | 25 | 75 | 25 | 75 | 50 | 50 | 60 | 60 | 75 | 57.5/42.5 | 52.5/47.5 | 62.5/37.5 |
| 2/2015 | 77 ± 3.9 | 50 | 25 | 75 | 50 | 5 | 10 | 10 | 10 | 20 | 10 | 5.0/95.0 | 5.0/95.0 | 5.0/95.0 |
| 6/2015 | 73 ± 3.7 | 10 | 5 | 50 | 0 | 10 | 0 | 5 | 10 | 10 | 10 | 100/0.0 | 95.0/5.0 | 100/0.0 |
| 7/2015 | 75 ± 3.6 | 25 | 10 | 75 | 25 | 10 | 0 | 10 | 5 | 10 | 10 | 100/0.0 | 92.5/7.5 | 100/0.0 |
| 9/2015 | 76 ± 3.3 | 50 | 25 | 75 | 50 | 75 | 30 | 25 | 20 | 50 | 30 | 67.5/32.5 | 52.5/47.5 | 67.5/32.5 |
| 10/2015 | 77 ± 3.5 | 10 | 5 | 50 | 10 | 60 | 30 | 20 | 20 | 50 | 30 | 62.5/37.5 | 57.5/42.5 | 65.0/35.0 |
| 94/2015 | 76 ± 3.6 | 10 | 5 | 25 | 10 | 10 | 10 | 10 | 20 | 5 | 10 | 5.0/95.0 | 5.0/95.0 | 10.0/90.0 |
| 322/2017 | 76 ± 2.8 | - | - | - | - | 10 | 0 | 10 | 20 | 5 | 10 | 100/0.0 | 92.5/7.5 | 100/0.0 |
| 337/2017 | 76 ± 3.1 | - | - | - | - | 20 | 10 | 20 | 20 | 20 | 5 | 77.5/22.5 | 67.5/32.5 | 82.5/17.5 |
| 374/2015 | 77 ± 3.4 | 10 | 25 | 75 | 25 | 10 | 0 | 10 | 10 | 20 | 10 | 100/0.0 | 92.5/7.5 | 100/0.0 |
| Medium-ripe | ||||||||||||||
| Duet—standard | 82 ± 4.4 | 5 | 25 | 75 | 25 | 60 | 50 | 50 | 60 | 75 | 50 | 70.0/30.0 | 62.5/37.5 | 77.5/22.5 |
| Sonata | 79 ± 3.9 | 10 | 25 | 75 | 25 | 60 | 50 | 40 | 40 | 75 | 50 | 52.5/47.5 | 47.5/52.5 | 62.5/37.5 |
| 31/2017 | 79 ± 38 | - | - | - | - | 5 | 0 | 5 | 5 | 10 | 5 | 100/0.0 | 90.0/10.0 | 100/0.0 |
| 314/2017 | 79 ± 3.6 | - | - | - | - | 10 | 5 | 10 | 5 | 20 | 10 | 75.0/25.0 | 65.0/35.0 | 82.5/17.5 |
| 24/2017 | 80 ± 3.8 | - | - | - | - | 10 | 10 | 10 | 20 | 5 | 5 | 5.0/95.0 | 5.0/95.0 | 10.0/90.0 |
| 37/2015 | 80 ± 3.9 | 25 | 25 | 75 | 5 | 20 | 10 | 20 | 20 | 20 | 10 | 70.0/30.0 | 62.5/37.5 | 72.5/27.5 |
| Medium-late | ||||||||||||||
| Serebristaya—standard | 87 ± 4.4 | 10 | 10 | 50 | 10 | 50 | 50 | 50 | 60 | 75 | 40 | 87.5/12.5 | 77.5/22.5 | 90.0/10.0 |
| Niva 2 | 84 ± 4.1 | 10 | 25 | 75 | 50 | 50 | 50 | 50 | 60 | 75 | 50 | 18.5/81.5 | 12.5/87.5 | 15.0/85.0 |
| Golubkovskaya | 85 ± 2.1 | 25 | 25 | 60 | 25 | 75 | 50 | 50 | 50 | 50 | 40 | 51.0/49.0 | 45.5/54.5 | 57.5/42.5 |
| Average ratio | - | - | - | - | - | - | - | - | - | - | - | 532/46.8 | 45.1/54.9 | 59.8/40.2 |
| Cultivar, Line | Grain Yield g/m2 | |||
|---|---|---|---|---|
| 2017 | 2018 | 2019 | Average | |
| Medium-early | ||||
| Pamyati Azieva-standard | 481 | 393 | 390 | 421 ± 30 |
| Chernyava 13 | 453 | 362 | 372 | 396 ± 29 |
| 2/2015 | 652 * | 582 * | 575 * | 603 ± 25 |
| 6/2015 | 470 | 410 * | 406 * | 429 ± 21 |
| 7/2015 | 441 | 393 | 404 * | 413 ± 15 |
| 9/2015 | 549 * | 416 * | 435 * | 467 ± 42 |
| 10/2015 | 577 * | 472 * | 480 * | 510 ± 34 |
| 94/2015 | 497 * | 427 * | 403 | 442 ± 28 |
| 322/2017 | 665 * | 619 * | 594 * | 626 ± 21 |
| 337/2017 | 598 * | 490 * | 471 * | 520 ± 40 |
| 374/2015 | 627 * | 595 * | 564 * | 595 ± 18 |
| SSD0.05 | 15.6 | 11.3 | 13.3 | - |
| Medium-ripe | ||||
| Duet-standard | 541 | 377 | 472 | 463 ± 48 |
| Sonata | 372 | 305 | 330 | 336 ± 29 |
| 31/2017 | 572 * | 423 * | 507 * | 501 ± 43 |
| 314/2017 | 594 * | 586 * | 528 * | 569 ± 21 |
| 24/2017 | 395 | 348 | 371 | 371 ± 14 |
| 37/2015 | 541 | 468 * | 492 * | 500 ± 21 |
| SSD0.05 | 16.2 | 14.4 | 12.1 | - |
| Medium-late | ||||
| Serebristaya-standard | 414 | 310 | 387 | 370 ± 31 |
| Niva 2 | 372 | 305 | 330 | 336 ± 20 |
| Golubkovskaya | 355 | 315 | 325 | 332 ± 12 |
| SSD0.05 | 10.1 | 14.6 | 12.6 | - |
| Cultivar, Line | Weight of 1000 Grains, g | Protein Content, % | Crude Gluten, % | Hectoliter Weight of Grain, g/L | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2017 | 2018 | 2019 | Average | 2017 | 2018 | 2019 | Average | 2017 | 2018 | 2019 | Average | 2017 | 2018 | 2019 | Average | |
| Medium-early | ||||||||||||||||
| Pamyati Azieva-standard | 35.8 | 37.3 | 28.2 | 33.8 ± 2.8 | 16.4 | 14.2 | 14.8 | 15.1 ± 0.7 | 27.7 | 31.5 | 30.7 | 30.0 ± 1.2 | 758 | 751 | 720 | 743 ± 12 |
| Chernyava 13 | 37.2 | 38.1 | 35.9 | 37.1 ± 0.6 | 14.5 | 12.5 | 13.0 | 13.3 ± 0.6 | 25.1 | 23.4 | 23.0 | 23.8 ± 0.6 | 731 | 746 | 761 * | 746 ± 9 |
| 2/2015 | 38.6 * | 40.3 * | 42.4 * | 40.4 ± 1.1 | 16.2 | 15.8 * | 16.4 * | 16.1 ± 0.2 | 32.5 * | 29.4 | 30.8 | 30.9 ± 0.9 | 750 | 721 | 728 | 733 ± 9 |
| 6/2015 | 38.8 * | 42.5 * | 40.9 * | 40.7 ± 1.1 | 19.6 * | 15.0 | 14.8 | 16.5 ± 1.6 | 32.9 * | 28.4 | 29.1 | 30.1 ± 1.4 | 785 * | 761 | 730 | 759 ± 16 |
| 7/2015 | 32.9 | 34.2 | 36.9 * | 34.7 ± 1.2 | 17.5 * | 14.7 * | 16.2 * | 16.1 ± 0.8 | 33.9 * | 28.8 | 29.3 | 30.7 ± 1.6 | 842 * | 821 * | 778 * | 814 ± 19 |
| 9/2015 | 35.0 | 38.1 | 36.1 * | 36.4 ± 0.9 | 16.4 | 15.1 * | 13.1 | 14.9 ± 1.0 | 32.8 * | 29.6 | 26.3 | 29.6 ± 1.9 | 794 * | 761 | 792 * | 782 ± 11 |
| 10/2015 | 39.3 * | 38.3 | 40.2 * | 39.3 ± 0.5 | 13.7 | 13.3 | 15.1 | 14.0 ± 0.5 | 27.7 | 26.2 | 28.8 | 27.6 ± 0.8 | 737 | 757 | 718 | 737 ± 11 |
| 94/2015 | 30.7 | 33.7 | 33.1 * | 32.5 ± 0.9 | 16.0 | 15.8 * | 16.8 * | 16.2 ± 0.3 | 30.8 * | 29.7 | 29.9 | 30.1 ± 0.3 | 852 * | 820 * | 805 * | 826 ± 14 |
| 322/2017 | 44.2 * | 48.8 * | 41.3 * | 44.8 ± 2.2 | 16.1 | 15.6 * | 16.3 * | 16.0 ± 0.2 | 32.3 * | 28.8 | 32.0 * | 31.0 ± 1.1 | 652 | 682 | 759 * | 698 ± 32 |
| 337/2017 | 41.6 * | 39.7 * | 43.5 * | 41.6 ± 1.1 | 15.6 | 14.9 * | 16.2 * | 15.6 ± 0.4 | 32.1 * | 28.6 | 32.7 * | 31.1 ± 1.3 | 705 | 715 | 744 * | 721 ± 12 |
| 374/2015 | 41.3 * | 39.2 * | 40.4 * | 39.8 ± 0.5 | 15.2 | 15.5 * | 14.7 | 15.1 ± 0.2 | 31.3 * | 29.8 | 28.7 | 29.9 ± 0.8 | 753 | 788 | 768 * | 770 ± 10 |
| SSD0.05 | 1.9 | 1.8 | 2.1 | - | 0.4 | 0.4 | 0.3 | - | 0.6 | 0.5 | 0.3 | - | 15 | 19 | 21 | - |
| Medium-ripe | ||||||||||||||||
| Duet-standard | 34.5 | 43.8 | 37.2 | 38.5 ± 2.8 | 16.6 | 15.2 | 15.4 | 15.7 ± 0.4 | 31.8 | 30.0 | 30.6 | 30.8 ± 0.5 | 758 | 698 | 693 | 716 ± 21 |
| Sonata | 36.9 * | 35.3 | 36.4 | 36.2 ± 0.5 | 14.5 | 13.0 | 13.9 | 13.8± 0.4 | 28.2 | 26.8 | 27.5 | 27.5 ± 0.4 | 735 | 749 * | 753 * | 746 ± 5 |
| 31/2017 | 52.0 * | 44.6 * | 39.0 * | 45.2 ± 3.8 | 15.0 | 14.6 | 15.6 | 15.1 ± 0.3 | 29.7 | 28.1 | 31.5 * | 29.8 ± 1.0 | 698 | 730 * | 747 * | 725 ± 14 |
| 314/2017 | 46.4 * | 46.2 * | 42.0 * | 44.9 ± 1.4 | 15.5 | 14.7 | 15.6 | 15.3 ± 0.3 | 30.4 | 29.2 | 31.5 * | 30.4 ± 0.7 | 658 | 718 * | 736 * | 704 ± 24 |
| 24/2017 | 39.1 * | 40.3 | 38.7 | 39.4 ± 0.5 | 16.4 | 15.9 * | 16.2 * | 16.2 ± 0.1 | 30.6 | 29.8 | 32.1 * | 30.8 ± 0.7 | 741 | 720 * | 772 * | 744 ± 15 |
| 37/2015 | 48.1 * | 44.2 | 40.9 * | 44.4 ± 2.1 | 16.2 | 15.2 | 16.9 * | 16.1 ± 0.5 | 32.3 | 30.5 * | 33.5 * | 32.1 ± 0.9 | 709 | 739 * | 757 * | 735 ± 14 |
| SSD0.05 | 1.8 | 1.5 | 1.6 | - | 0.3 | 0.5 | 0.4 | - | 0.6 | 0.4 | 0.7 | - | 21 | 17 | 19 | - |
| Medium-late | ||||||||||||||||
| Serebristaya-standard | 36.3 | 36.8 | 30.6 | 34.6 ± 2.0 | 13.8 | 13.0 | 14.2 | 13.7 ± 0.4 | 27.7 | 25.8 | 26.2 | 26.6 ± 0.6 | 736 | 730 | 768 | 745 ± 12 |
| Niva 2 | 37.7 | 34.1 | 33.2 * | 35.0 ± 1.4 | 13.5 | 12.5 | 12.9 | 13.0 ± 0.3 | 25.0 | 23.5 | 24.2 | 24.2 ± 0.4 | 778 * | 751 * | 743 | 757 ± 11 |
| Golubkovskaya | 35.2 | 32.0 | 315 | 32.9 ± 1.2 | 13.1 | 12.6 | 12.9 | 12.9 ± 0.1 | 24.9 | 24.0 | 23.5 | 24.1 ± 0.4 | 761 * | 775 * | 784 | 773 ± 7 |
| SSD0.05 | 1.5 | 1.6 | 1.3 | - | 0.4 | 0.3 | 0.5 | - | 0.5 | 0.6 | 0.6 | - | 19 | 15 | 25 | - |
| r - leaf damage - ear damage | −0.51 * −0.15 | −0.29 * −0.45 * | −0.19 −0.49 * | - | −0.29 * −0.18 | +0.17 −0.28 * | −0.23 −0.33 * | - | +0.09 −0.24 * | −0.15 −0.44 * | −0.28 * −0.35 * | - | +0.35 * +0.11 | −0.28 * +0.23 | +0.09 +0.30 * | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plotnikova, L.; Sagendykova, A.; Pozherukova, V. The Use of Genetic Material of Tall Wheatgrass to Protect Common Wheat from Septoria Blotch in Western Siberia. Agriculture 2023, 13, 203. https://doi.org/10.3390/agriculture13010203
Plotnikova L, Sagendykova A, Pozherukova V. The Use of Genetic Material of Tall Wheatgrass to Protect Common Wheat from Septoria Blotch in Western Siberia. Agriculture. 2023; 13(1):203. https://doi.org/10.3390/agriculture13010203
Chicago/Turabian StylePlotnikova, Lyudmila, Ainura Sagendykova, and Violetta Pozherukova. 2023. "The Use of Genetic Material of Tall Wheatgrass to Protect Common Wheat from Septoria Blotch in Western Siberia" Agriculture 13, no. 1: 203. https://doi.org/10.3390/agriculture13010203
APA StylePlotnikova, L., Sagendykova, A., & Pozherukova, V. (2023). The Use of Genetic Material of Tall Wheatgrass to Protect Common Wheat from Septoria Blotch in Western Siberia. Agriculture, 13(1), 203. https://doi.org/10.3390/agriculture13010203








