What Was the Reason for the Durable Effect of Sr31 against Wheat Stem Rust?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
- Spring common wheat T. aestivum with the identified Sr31—cvs. Kavkaz, Seri 82, Bacanora (=Kauz’s’), PWB343 and a near-isogenic line of cv. Thatcher (RL-6078, NIL-THATCHER-Lr26,Sr31-ST-1-25[000]) (TcLr26/Sr31).
- Spring common wheat that is susceptible to stem rust—standard cvs. Pamyati Azieva (medium-early) and Duet (medium-ripe); wheat that is universally susceptible to leaf diseases —cv. Chernyava 13.
- Winter rye, S. cereale—cvs. Petkus, Siberia, Irina, Vavilovskayja universalnaya, Zilant, Tyumenka, Evrika, line 22/18 (originated in the Omsk Agrarian Scientific Center—Omsk ASC, from combination Jubileynayja 25 × Tetra short). According to the information provided in the Plant Genetic Resources Database of Federal Research Center N.I. Vavilov All-Russian Institute of Plant Genetic Resources (VIR, St. Petersburg, Russia) cv. Petkus, it is present in the pedigrees of rye cultivars and line 22/18 (http://db.vir.nw.ru/virdb (accessed on 1 September 2022))
2.2. Infection Material
2.3. Estimation of Stem Rust Development in the Field and Laboratory Conditions
2.4. Cytological Studies
- − The proportion of germinated spores with growing tubes of the total number of spores (germinated and non-germinated) on the plant (in %);
- − The proportion of growing tubes developed appressoria of the total number of germinated spores with growing tubes (in %);
- − The proportion of appressoria on stomata from their total number on plant surface (in %);
- − The proportion of appressoria germinated into stomata (formed substomal vesicles) from the total number on the stomata (%).
3. Results
3.1. Estimation of Stem Rust Development in the Field and Laboratory
3.2. Results of Cytological Research of Pgt Interactions with Rye and Wheat
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| Abbreviations in text | |
| cv. | cultivar |
| HMC | haustorial mother cell |
| HR | hypersensitive reaction |
| IT | infection type |
| Lr | leaf rust resistance gene |
| NHR | nonhost resistance |
| NIL | near-isogenic line |
| Pgt | Puccinia graminis f. sp. tritici |
| ROS | reactive oxygen species |
| Sr | stem rust resistance gene |
| Tc | cultivar Thatcher |
| Yr | stripe rust resistance gene |
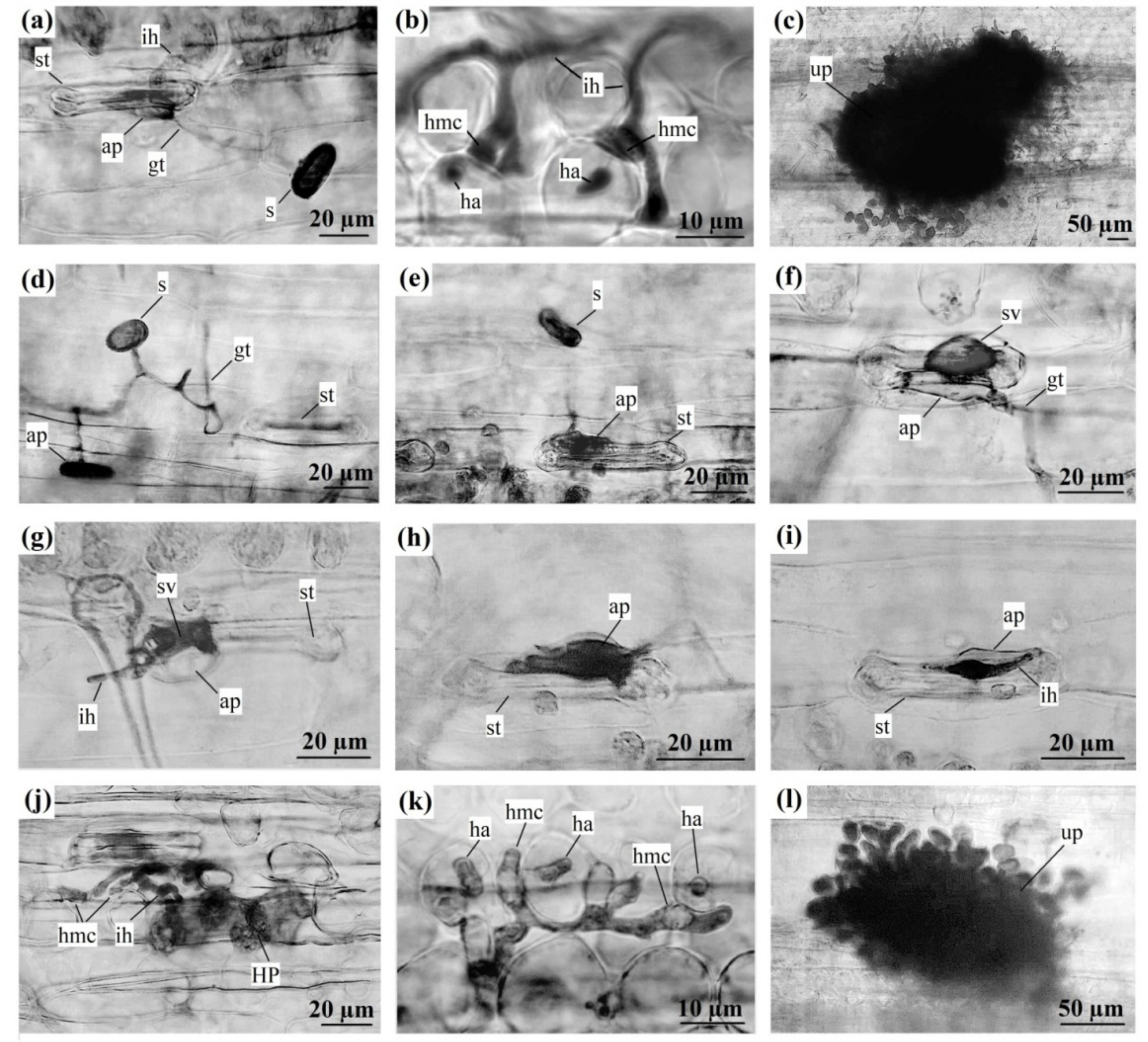
| Abbrevations to Figure 2 | |
| ap | appressorium |
| gt | growing tube |
| ha | haustoria |
| hmc | haustorial mother cell |
| HR | hypersensitive reaction |
| ih | infection hypha |
| s | urediniospore |
| st | stoma |
| sv | substomal vesicle |
| up | urediniopustule |
References
- USDA. World Agricultural Production; USDA Foreign Agricultural Service: Washington, DC, USA, 2016; p. 20250. [Google Scholar]
- Singh, R.P.; Singh, P.K.; Rutkoski, J.; Hodson, D.P.; He, X.; Jorgensen, L.N.; Hovmoller, M.S.; Huerta-Espino, J. Disease impact on wheat yield potential and prospects of genetic control. Annu. Rev. Phytopathol. 2016, 54, 303–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knott, D.R. Can losses from wheat stem rust be eliminated in North America. Crop Sci. 1971, 11, 97–99. [Google Scholar] [CrossRef]
- German, S.E.; Barcellos, A.; Chaves, M.; Kohli, M.; Campos, P.; de Viedma, L. The situation of common wheat rusts in the Southern Cone of America and perspectives for control. Aust. J. Agric. Res. 2007, 58, 620–630. [Google Scholar] [CrossRef]
- Park, R.L. Stem rust of wheat in Australia. Aust. J. Agric. Res. 2007, 58, 558–566. [Google Scholar] [CrossRef]
- Singh, R.P.; Hodson, D.P.; Jin, Y.; Lagudah, E.S.; Ayliffe, M.A.; Bhavani, S.; Rouse, M.N.; Pretorius, Z.A.; Szabo, L.J.; Huerta-Espino, J.; et al. Emergence and spread of new races of wheat stem rust fungus: Continued threat to food security and prospects of genetic control. Phytopathology 2015, 10, 872–884. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.P.; Hodson, D.P.; Huerta-Espino, J.; Jin, Y.; Njau, P.; Wanyera, R.; Herrera-Foessel, S.; Ward, R. Will stem rust destroy the world’s wheat crop. Adv. Agron. 2008, 98, 272–309. [Google Scholar] [CrossRef]
- Stakman, E.C.; Piemeisel, F.J. Biologic forms of Puccinia graminis on cereals and grasses. J. Agric. Res. 1917, 10, 429–495. [Google Scholar]
- Stakman, E.C.; Stewart, D.M.; Loegering, W.Q. Identification of Physiological Races of Puccinia gramini sf. sp. tritici; USDA: Washington, DC, USA, 1962; p. 617. [Google Scholar]
- Campbell, C.L.; Long, D.L. The Campaign to Eradicate the Common Barberry in the United States. In Stem Rust of Wheat. From Ancient Enemy to Modern Foe; Peterson, P.D., Ed.; APS Press: St. Paul, MN, USA, 2001; pp. 16–50. [Google Scholar]
- Flath, K.; Miedaner, T.; Olivera, P.D.; Rouse, M.N.; Jin, Y. Genes for wheat stem rust resistance postulated in German cultivars and their efficacy in seedling and adult-plant field tests. Plant Breed. 2018, 1, 12. [Google Scholar] [CrossRef]
- Zeller, F.J.; Hsam, S.L.K. Broadening the Genetic Variability of Cultivated Wheat by Utilizing Rye Chromatin. In Proceedings of the Sixth International Wheat Genetics Symposium, Kyoto, Japan, 28 November–3 December 1983; pp. 161–173. [Google Scholar]
- Singh, R.P.; Hodson, D.P.; Huerta-Espino, J.; Jin, Y.; Bhavani, S.; Njau, P.; Herrera-Foessel, S.; Singh, P.K.; Singh, S.; Govindan, V. The emergence of Ug99 races of the stem rust fungus is a threat to world wheat production. Annu. Rev. Phytopathol. 2011, 49, 465–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pretorius, Z.A.; Bender, C.M.; Visser, B.; Terefe, T. First report of a Puccinia graminis f. sp. tritici race virulent to the Sr24 and Sr31 wheat stem rust resistance genes in South Africa. Plant Dis. 2010, 94, 784. [Google Scholar] [CrossRef]
- Bhattacharya, S. Deadly new wheat disease threatens Europe’s crops. Nature 2017, 542, 145–146. [Google Scholar] [CrossRef] [PubMed]
- Tsushima, A.; Lewis, C.M.; Flath, K.; Kildea, S.; Saunders, D.G.O. Wheat stem rust recorded for the first time in decades in Ireland. Plant Pathol. 2022, 71, 890–900. [Google Scholar] [CrossRef] [PubMed]
- Sibikeev, S.N.; Markelova, T.S.; Baukenova, E.A.; Druzhin, A.E. Likely threat of the spread of race Ug99 of Puccinia graminis f. sp. tritici on wheat in southeastern Russia. Russ. Agric. Sci. 2016, 42, 145–148. [Google Scholar] [CrossRef]
- Lapochkina, I.F.; Baranova, O.A.; Gainullin, N.R.; Volkova, G.V.; Gladkova, E.V.; Kovaleva, E.O.; Osipova, A.V. The development of winter wheat lines with several genes for resistance to Puccinia graminis Pers. f. sp. tritici for use in breeding programs in Russia. Vavilov J. Genet. Breed. 2018, 22, 676–684. [Google Scholar] [CrossRef] [Green Version]
- Shamanin, V.P.; Pototskaya, I.V.; Shepelev, S.S.; Pozherukova, V.E.; Salina, Å.A.; Skolotneva, Å.S.; Hodson, D.; Hovmøller, M.; Patpour, M.; Morgounov, A.I. Stem rust in Western Siberia—Race composition and effective resistance genes. Vavilov J. Genet. Breed. 2020, 24, 131–138. [Google Scholar] [CrossRef] [Green Version]
- Olivera, P.; Newcomb, M.; Szabo, L.J.; Rouse, M.; Johnson, J.; Gale, S.; Luster, D.G.; Hodson, D.; Cox, J.A.; Burgin, L.; et al. Phenotypic and genotypic characterization of race TKTTF of Puccinia graminis f. sp. tritici that caused a wheat stem rust epidemic in southern Ethiopia in 2013–2014. Phytopathology 2015, 105, 917–928. [Google Scholar] [CrossRef] [Green Version]
- Patpour, M.; Hovmøller, M.S.; Rodriguez-Algaba, J.; Randazzo, B.; Villegas, D.; Shamanin, V.P.; Berlin, A.; Flath, K.; Czembor, P.; Hanzalova, A.; et al. Wheat Stem Rust Back in Europe: Diversity, Prevalence and Impact on Host Resistance. Front. Plant Sci. 2022, 13, 882440. [Google Scholar] [CrossRef]
- Kelbin, V.N.; Skolotneva, E.S.; Salina, E.A. Challenges and prospects for developing genetic resistance in common wheat against stem rust in Western Siberia. Vavilov J. Genet. Breed. 2020, 24, 821–828. [Google Scholar] [CrossRef]
- Skolotneva, Å.S.; Kelbin, V.N.; Morgunov, A.I.; Boiko, N.I.; Shamanin, V.P.; Salina, E.A. Races composition of the Novosibirsk population of Puccinia graminis f. sp. tritici. Micol. Phytopatol. 2020, 54, 49–58. [Google Scholar] [CrossRef]
- Jin, Y.; Szabo, L.J.; Pretorius, Z.A.; Singh, R.P.; Ward, R.; Fetch, T., Jr. Detection of virulence to resistance gene Sr24 with in race TTKS of Puccinia gramini sf. sp. tritici. Plant Dis. 2008, 92, 923–926. [Google Scholar] [CrossRef] [Green Version]
- Skolotneva, E.S.; Kelbin, V.N.; Shamanin, V.P.; Boyko, N.I.; Aparina, V.A.; Salina, E.A. The gene Sr38 for bread wheat breeding in Western Siberia. Vavilov J. Genet. Breed. 2021, 25, 740–745. [Google Scholar] [CrossRef]
- Baranova, O.A.; Sibikeev, S.N.; Druzhin, A.E. Molecular identification of the stem rust resistance genes in the introgression lines of spring bread wheat. Vavilov J. Genet. Breed. 2019, 23, 296–303. [Google Scholar] [CrossRef] [Green Version]
- Kolmer, J. Leaf Rust of Wheat: Pathogen Biology, Variation and Host Resistance. Forests 2013, 4, 70–84. [Google Scholar] [CrossRef] [Green Version]
- Prasad, P.; Bhardwaj, S.C.; Savadi, S.; Kashyap, P.L.; Gangwar, O.P.; Khan, H.; Singh, R.; Singh, S.B.; Kumar, S. Population distribution and differentiation of Puccinia graminis tritici detected in the Indian subcontinent during 2009–2015. Crop Protect. 2018, 108, 128–136. [Google Scholar] [CrossRef]
- Brar, G.S.; Fetch, T.; McCallum, B.D.; Hucl, P.J.; Kutcher, H.R. Virulence Dynamics and Breeding for Resistance to Stripe, Stem, and Leaf Rust in Canada Since 2000. Plant Dis. 2019, 103, 2981–2995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.; Zang, C.; Zhang, Y.; Xu, Y.; Wang, S.; Li, T.; Gao, L. Characterization of wheat monogenic lines with known Sr genes and wheat cultivars for resistance to three new races of Puccinia gramini sf. sp. tritici in China. J. Integr. Agric 2022, in press. [Google Scholar] [CrossRef]
- Genievskaya, Y.; Turuspekov, Y.; Rsaliyev, A.; Abugalieva, S. Genome-wide association mapping for resistance to leaf, stem, and yellow rusts of common wheat under field conditions of South Kazakhstan. Peer J. 2020, 8, e9820. [Google Scholar] [CrossRef]
- Parlevliet, J.E. What is Durable Resistance, a General Outline. In Durability of Disease Resistance; Jacobs, T., Parlevliet, J.E., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1993; pp. 23–39. [Google Scholar] [CrossRef]
- Johnson, R. Genetic Background of Durable Resistance. In Durable Resistance in Crops; Lamberti, F., Waller, J.M., Vander Graaff, N.A., Eds.; Plenum Press: New York, NY, USA, 1983; pp. 152–163. [Google Scholar]
- Parlevliet, J.E. Durability of resistance against fungal, bacterial and viral pathogens; present situation. Euphytica 2002, 124, 147–156. [Google Scholar] [CrossRef]
- Niks, R.E. How Specific is Non-Hypersensitive Host and Nonhost Resistance of Barley to Rust and Mildew Fungi? J. Integr. Agric. 2014, 13, 244–254. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.-Y.; Lee, H.-A.; Seo, E.; Lee, J.; Kim, S.-B.; Oh, S.; Choi, E.; Choi, E.; Lee, S.E.; Choi, D. Current Understandings of Plant Nonhost Resistance. Mol. Plant Microbe Interact. 2017, 30, 5–15. [Google Scholar] [CrossRef]
- Andreev, L.N.; Plotnikova, Y.M. Wheat Rust: Cytology and Physiology; Science: Moscow, Russia, 1989; 304p. [Google Scholar]
- Niks, R.E. Nonhost plant species as donors for resistance to pathogens with narrow host range. I. Determination of nonhost status. Euphytica 1987, 36, 841–852. [Google Scholar] [CrossRef]
- Heath, M.C. Nonhost resistance and nonspecific plant defenses. Curr. Opin. Plant Biol. 2000, 3, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Heath, M.C. Apoptosis, programmed cell death and the hypersensitive response. Eur. J. Plant Pathol. 1998, 104, 117–124. [Google Scholar] [CrossRef]
- Brown, J.F.; Shipton, W.A.; White, N.H. The relationship between hypersensitive tissue and resistance in wheat seedlings infected with Puccinia graminis tritici. Ann. Appl. Biol. 1966, 58, 279–290. [Google Scholar]
- Manocha, M.S. Fine structure of rust haustoria in susceptible and resistant wheat. Indian Phytopathol. 1966, 19, 159–161. [Google Scholar]
- Samborski, D.J.; Kim, W.K.; Rohringer, R.; Howes, N.K.; Baker, R.J. Histological studies on host-cell necrosis conditioned by the Sr6 gene for resistance in wheat to stem rust. Can. J. Bot. 1977, 55, 1445–1452. [Google Scholar] [CrossRef]
- Harder, D.E.; Rohringer, R.; Samborski, D.I.; Rimmer, S.R.; Kim, W.K.; Chong, J. Electron microscopy of susceptible and resistant near isogenic (Sr6/Sr6) lines of wheat infected by Puccinia graminis tritici. I. The host-pathogen interface in the compatible (Sr6/P6) interaction. Can. J. Bot. 1978, 56, 2955–2967. [Google Scholar] [CrossRef]
- Rohringer, R.; Kim, W.K.; Samborski, D.J. A histological study of interactions between avirulent races of stem rust and wheat containing resistance genes Sr5, Sr6, Sr8, or Sr22. Can. J. Bot. 1979, 57, 324–331. [Google Scholar] [CrossRef]
- Salcedo, A.; Rutter, W.; Wang, S.C.; Akhunova, A.; Bolus, S.; Chao, S.M.; Anderson, N.; De Soto, M.F.; Rouse, M.; Szabo, L.; et al. Variation in the AvrSr35 gene determines Sr35 resistance against wheat stem rust race Ug99. Science 2017, 358, 1604–1606. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.P.; Upadhyaya, N.M.; Ortiz, D.; Sperschneider, J.; Li, F.; Bouton, C.; Breen, S.; Dong, C.M.; Xu, B.; Zhang, X.X.; et al. Loss of AvrSr50 by somatic exchange in stem rust leads to virulence for Sr50 resistance in wheat. Science 2017, 358, 1607–1610. [Google Scholar] [CrossRef] [Green Version]
- Roelfs, A.P.; Singh, R.P.; Saari, E.E. Rust Diseases of Wheat: Concepts and Methods of Disease Management; International Maize and Wheat Improvement Center: Mexico City, Mexico, 1992. [Google Scholar]
- Koyshybaev, M. Wheat Diseases; FAO: Ankara, Turkey, 2018; 394p. [Google Scholar]
- Roelfs, A.P.; Martens, J.W. An international system of nomenclature for Puccinia graminis f. sp. tritici. Phytopathology 1988, 78, 526–533. [Google Scholar] [CrossRef] [Green Version]
- Plotnikova, L.Y.; Meshkova, L.V. Evolution of cytophysiological relationships between leaf rust causal agent and common wheat in the process of overcoming of resistance determined by the gene Lr19. Mikol. Fitopatol. 2009, 43, 343–357. [Google Scholar]
- Huerta-Espino, J.; Singh, R.P.; Germán, S.S.; McCallum, B.D.; Park, R.F.; Chen, W.Q.; Bhardwaj, S.C.; Goyeau, H. Global Status of Wheat Leaf Rust Caused by Puccinia triticina. Euphytica 2011, 179, 143–160. [Google Scholar] [CrossRef]
- Hovmøller, M.S.; Walter, S.; Bayles, R.; Hubbard, A.; Flath, K.; Sommerfeldt, N.; Leconte, M.; Czembor, P.; Rodriguez-Algaba, J.; Thach, T.; et al. Replacement of the European wheat yellow rust population by new races from the centre of diversity in the near-Himalayan region. Plant Pathol. 2016, 65, 402–411. [Google Scholar] [CrossRef] [Green Version]
- Prank, M.; Kenaley, S.C.; Bergstrom, G.C.; Acevedo, M.; Mahowald, N.M. Climate change impacts the spread potential of wheat stem rust, a significant crop disease. Environ. Res. Lett. 2019, 14, 124053. [Google Scholar] [CrossRef] [Green Version]
- Rsaliyev, A.S.; Rsaliyev, S.S. Principal approaches and achievements in studying race composition of wheat stem rust. Vavilov J. Genet. Breed. 2018, 22, 967–977. [Google Scholar] [CrossRef]
- Rosseyeva, L.P.; Belan, I.A.; Meshkova, L.V.; Blokhina, N.P.; Lozhnikova, L.F.; Osadchaya, T.S.; Trubacheyeva, N.V.; Pershina, L.A. Breeding spring soft wheat for resistance to stem rust in west Siberia. J. Altai State Agrar. Univ. 2017, 7, 5–12. [Google Scholar]
- Voronkova, A.A.; Dubonosov, T.S.; Panarin, I.V. The Reasons of Epidemics of Leaf Rust in the Krasnodar Region. In Cereal Rust; Kolos: Moscow, Russia, 1975; pp. 80–88. (In Russian) [Google Scholar]
- Sharma, A.; Srivastava, P.; Mavi, G.S.; Kaur, S.; Kaur, J.; Bala, R.; Singh, T.P.; Sohu, V.S.; Chhuneja, P.; Bains, N.S.; et al. Resurrection of Wheat Cultivar PBW343 Using Marker-Assisted Gene Pyramiding for Rust Resistance. Front. Plant Sci. 2021, 12, 570408. [Google Scholar] [CrossRef]
- Van Baarlen, P.; van Belkum, A.; Summerbell, R.C.; Crous, P.W.; Thomma, B.P.H.J. Molecular mechanisms of pathogenicity: How do pathogenic microorganisms develop cross-kingdom host jumps? FEMS Microbiol. Rev. 2007, 31, 239–277. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Hutton, S.; Whitaker, V. Mini Review: Potential Applications of Non-host Resistance for Crop Improvement. Front. Plant Sci. 2016, 7, 997. [Google Scholar] [CrossRef] [Green Version]
- Ellingboe, A.H. Genetics of Host-Parasite Interactions. In Encyclopedia of Plant Physiology; Heitefuss, R., Williams, P.H., Eds.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1976; pp. 761–778. [Google Scholar]
- Heath, M.C. Reaction of Nonsuscepts to fungal pathogens. Curr. Opin. Plant Biol. 1980, 18, 211–236. [Google Scholar] [CrossRef]
- Heath, M.C. Signalling between pathogenic rust fungi and resistant or susceptible host plants. Ann. Bot. 1997, 80, 713–720. [Google Scholar] [CrossRef] [Green Version]
- Jones, J.D.G.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gill, U.S.; Lee, S.; Mysore, K.S. Host versus nonhost resistance: Distinct wars with similar arsenals. Phytopathology 2015, 105, 580–587. [Google Scholar] [CrossRef]
- Schulze-Lefert, P.; Panstruga, R. A molecular evolutionary concept connecting nonhost resistance, pathogen host range, and pathogen speciation. Trends Plant Sci. 2011, 16, 117–125. [Google Scholar] [CrossRef]
- Ayliffe, M.; Jin, Y.; Kang, Z.; Persson, M.; Steffenson, B.; Wang, S.; Leung, H. Determining the basis of nonhost resistance in rice to cereal rusts. Euphytica 2011, 179, 33–40. [Google Scholar] [CrossRef]
- Plotnikova, L.Y. Influence of the surface features and physiological reactions of non-host species on the development of cellular structures of rust fungi. Tcitologija 2008, 50, 439–446. [Google Scholar]
- Cheng, Y.; Yao, J.; Zhang, H.; Huang, L.; Kang, Z. Cytological and molecular analysis of nonhost resistance in rice to wheat powdery mildew and leaf rust pathogens. Protoplasma 2015, 252, 1167–1179. [Google Scholar] [CrossRef]
- Plotnikova, L.Y.; Seryukov, G.M.; Shvarts, Y.K. Cytophysiological resistance mechanisms to leaf rust in Wheat-Agropyron hybrids created on the base of Agropyron elongatum. Mikol. Fitopatol. 2011, 45, 443–454. (In Russian) [Google Scholar]
- Vaz Patto, M.C.; Niks, R.E. Leaf wax layer may prevent appressorium differentiation but does not influence orientation of the leaf fungus Puccinia hordei on Hordeum chilense leaves. Eur. J. Plant Pathol. 2001, 107, 795–803. [Google Scholar] [CrossRef]
- Elmhirst, J.F.; Heath, M.C. Interactions of the bean rust and cowpea rust fungi with species of the Phaseolus-Vignaplant complex. I. Fungal growth and development. Can. J. Bot. 1987, 65, 1096–1107. [Google Scholar] [CrossRef]
- Plotnikova, L.Y.; Knaus, J.K.; Meshkova, L.V. Cytophysiological features of expression of leaf rust resistance genes transferred to common wheat from wild cereals. Mikol. Fitopatol. 2007, 41, 362–372. [Google Scholar]
- Plotnikova, L.Y.; Shtubey, T.Y. Influence of salicylic and succinic acids on the cytophysiological reactions of wheat infected by brown rust. Tcitologija 2009, 51, 43–52. [Google Scholar]
- Plotnikova, L.Y. Effect of benzothiadiazole, an inducer of systemic acquired resistance, on the pathogenesis of wheat brown rust. Rus. J. Plant Phys. 2009, 56, 517–526. [Google Scholar] [CrossRef]
- Anker, C.C.; Niks, R.E. Prehaustorial resistance to the wheat leaf rust fungus Puccinia triticina in Triticum monococcum (s.s.). Euphytica 2001, 117, 209–215. [Google Scholar] [CrossRef]
- Plotnikova, L.Y.; Pozherukova, V.Y.; Mitrofanova, O.P.; Degtyarev, A.I. The Effect of Oxidative Burst Suppression or Induction on the Interaction between Brown Rust Fungus and Timopheevi Wheat. Appl. Biochem. Microbiol. 2016, 52, 61–70. [Google Scholar] [CrossRef]
- Schulze-Lefert, P.; Panstruga, R. Establishment of Biotrophy by Parasitic Fungi and Reprogramming of Host Cells for Disease Resistance. Annu. Rev. Phytopathol. 2003, 41, 641–667. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Zhang, S. MAPK cascades in plant disease resistance signaling. Annu. Rev. Phytopathol. 2013, 51, 245–266. [Google Scholar] [CrossRef] [PubMed]
- Tucker, S.L.; Talbot, N.J. Surface attachment and pre-penetration stage development by plant pathogenic fungi. Annu. Rev. Phytopathol. 2001, 39, 385–417. [Google Scholar] [CrossRef]
- Deising, H.; Nicholson, R.L.; Haug, M.; Howard, R.J.; Mendgen, K. Adhesion pad formation and the involvement of cutinase and esterases in the attachment of uredospores to the host cuticle. Plant Cell 1992, 9, 1101–1111. [Google Scholar] [CrossRef] [Green Version]
- Tsuba, M.; Katagiri, C.; Takeuchi, Y.; Takada, Y.; Yamaoka, N. Chemical factors of the leaf surface involved in the morphogenesis of Blumeria graminis. Physiol. Mol. Plant Pathol. 2002, 60, 51–57. [Google Scholar] [CrossRef]
- Hansjakob, A.; Bischof, S.; Bringmann, G.; Riederer, M.; Hildebrandt, U. Very-long-chain aldehydes promote in vitro prepenetration processes of Blumeria graminis in a dose- and chain length-dependent manner. New Phytol. 2010, 188, 1039–1054. [Google Scholar] [CrossRef] [PubMed]
- Uppalapati, S.R.; Ishiga, Y.; Doraiswamy, V.; Bedair, M.; Mittal, S.; Chen, J.; Nakashima, J.; Tang, Y.; Tadege, M.; Ratet, P.; et al. Loss of abaxial leaf epicuticular wax in Medicago truncatula irg1/palm1 mutants results in reduced spore differentiation of anthracnose and nonhost rust pathogens. Plant Cell 2012, 24, 353–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heath, M.C. Influence of carbohydrates on the induction of haustoria of the cowpea rust fungus in vitro. Exp. Mycol. 1990, 14, 84–88. [Google Scholar] [CrossRef]
- Mendgen, K.; Struck, C.; Voegele, R.T.; Hahn, M. Biotrophy and rust huastoria. Physiol. Plant Pathol. 2000, 56, 141–145. [Google Scholar] [CrossRef] [Green Version]
- Mendgen, K.; Hahn, M. Plant infection and the establishment of fungal biotrophy. Trends Plant Sci. 2002, 7, 352–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendgen, K.; Wiesel, S.G.R.; Jux, A.; Hoffmann, J.; Boland, W. Volatiles modulate the development of plant pathogenic rust fungi. Planta 2006, 224, 1353–1361. [Google Scholar] [CrossRef] [Green Version]
- Kolmer, J.A. Genetics of resistance to wheat leaf rust. Annu. Rev. Phytopathol. 1996, 34, 435–455. [Google Scholar] [CrossRef]
- Plotnikova, L.Y.; Meshkova, L.V. Immunological features of action of the wheat resistance to leaf rust gene Lr23. I. Phenotypic appearance and components of partial resistance. Mikol. Fitopatol. 2013, 47, 56–59. [Google Scholar]
- Plotnikova, L.Y. Immunological features of action of the wheat resistance to leaf rust gene Lr23. II. Cytophysiological aspects. Mikol. Fitopatol. 2013, 47, 60–65. [Google Scholar]
- Rajaraman, J.; Douchkov, D.; Hensel, G.; Stefanato, F.L.; Gordon, A.; Ereful, N.; Caldararu, O.F.; Petrescu, A.; Kumlehn, J.; Boyd, L.A.; et al. An LRR/Malectin Receptor-like Kinase Mediates Resistance to Non-Adapted and Adapted Powdery Mildew Fungi in Barley and Wheat. Front. Plant Sci. 2016, 7, 1836. [Google Scholar] [CrossRef] [Green Version]
- Jafary, H.; Albertazzi, G.; Marcel, T.C.; Niks, R.E. High diversity of genes for nonhost resistance of barley to heterologous rust fungi. Genetics 2008, 178, 2327–2339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, P.; Garrood, J.M.; Shen, Q.H.; Smith, P.H.; Boyd, L.A. The genetics of non-host disease resistance in wheat to barley yellow rust. Theor. Appl. Genet. 2004, 109, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Jafary, H.; Szabo, L.J.; Niks, R.E. Innate nonhost immunity in barley to different heterologous rust fungi is controlled by sets of resistance genes with different and overlapping specificities. Mol. Plant Microbe Interact. 2006, 19, 1270–1279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dracatos, P.M.; Nansamba, M.; Berlin, A.; Park, R.F.; Niks, R.E. Isolate specificity and polygenic inheritance of resistance in barley to the heterologous rust pathogen Puccinia graminis f. sp. avenae. Phytopathology 2016, 106, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, R.A.; Yamazaki, Y.; Dubcovsky, J.; Rogers, W.J.; Morris, C.; Appel, S.; Xia, X.C. Catalogue of Gene Symbols for Wheat. In Proceedings of the 12th International Wheat Genetics Symposium, Yokohama, Japan, 7–14 September 2013. [Google Scholar]
- McIntosh, R.A.; Dubcovsky, J.; Rogers, W.J.; Xia, X.C.; Raupp, W.J. Catalogue of Gene Symbols for Wheat: 2020 Supplement. Annu. Wheat Newsl. 2020, 66, 109–128. [Google Scholar]

| Cultivar, Line | Field, IT & Severity | Laboratory, IT * | ||
|---|---|---|---|---|
| 2018 | 2019 | 2020 | ||
| Triticumaestivum | ||||
| Pamyati Azieva | 100S | 100S | 50S | 4 |
| Duet | 80S | 70MS | 20S | 4 |
| Chernyava 13 | 100S | 100S | 50S | 4 |
| TcLr26/Sr31 | 5M | 0 | 0 | 0 |
| Kavkaz | 10MR | 0 | 0 | 0 |
| Seri 82 | 5MR | 0 | 0 | 0 |
| Bacanora | 5M | 0 | 0 | ;1 |
| PWB343 | 30MS | 20MS | 10MS | ;, 2−, 3+ |
| Secale sereale | ||||
| Petkus | 0 | 0 | - | 0 |
| Siberia | 0 | 0 | - | 0 |
| Irina | 0 | 5MR | - | ; |
| Line 22/18 | 0 | 0 | - | ; |
| Vavilovskayja universalnaya | 0 | 0 | - | 0 |
| Tyumenka | 0 | 0 | - | 0 |
| Zilant | 0 | 0 | - | ; |
| Evrika | 0 | 0 | - | 0 |
| Cultivarline Isolate | Pamyati Azieva—Control | TcLr26/Sr31 | Kavkaz | Seri 82 | Bacanora | PWB343 |
|---|---|---|---|---|---|---|
| 1 | 4 | 0 | 0 | 0 | 0 | 0 |
| 2 | 4 | 0 | 0 | ; | ; | 3+ |
| 3 | 4 | 0 | 0 | 0 | 0 | 0 |
| 4 | 4 | 0 | 0 | ; | ; | 0 |
| 5 | 4 | 0 | 0 | 0 | 0 | 0 |
| 6 | 4 | 0 | 0 | 0 | 0 | 0 |
| 7 | 4 | 0 | 0 | 0 | ; | 1 |
| 8 | 3 | 0 | 0 | ; | 0 | 0 |
| 9 | 4 | 0 | 0 | ; | 0 | 0 |
| 10 | 3 | 0 | 0 | 0 | ;1 | 2 |
| 11 | 4 | 0 | 0 | ; | ;1 | 2 |
| 12 | 3 | 0 | 0 | 0 | 1, 2 | 3+ |
| 13 | 4 | ; | ; | 0 | ;, 2, 3+ | 3+ |
| 14 | 4 | 0 | 0 | ; | 0 | 0 |
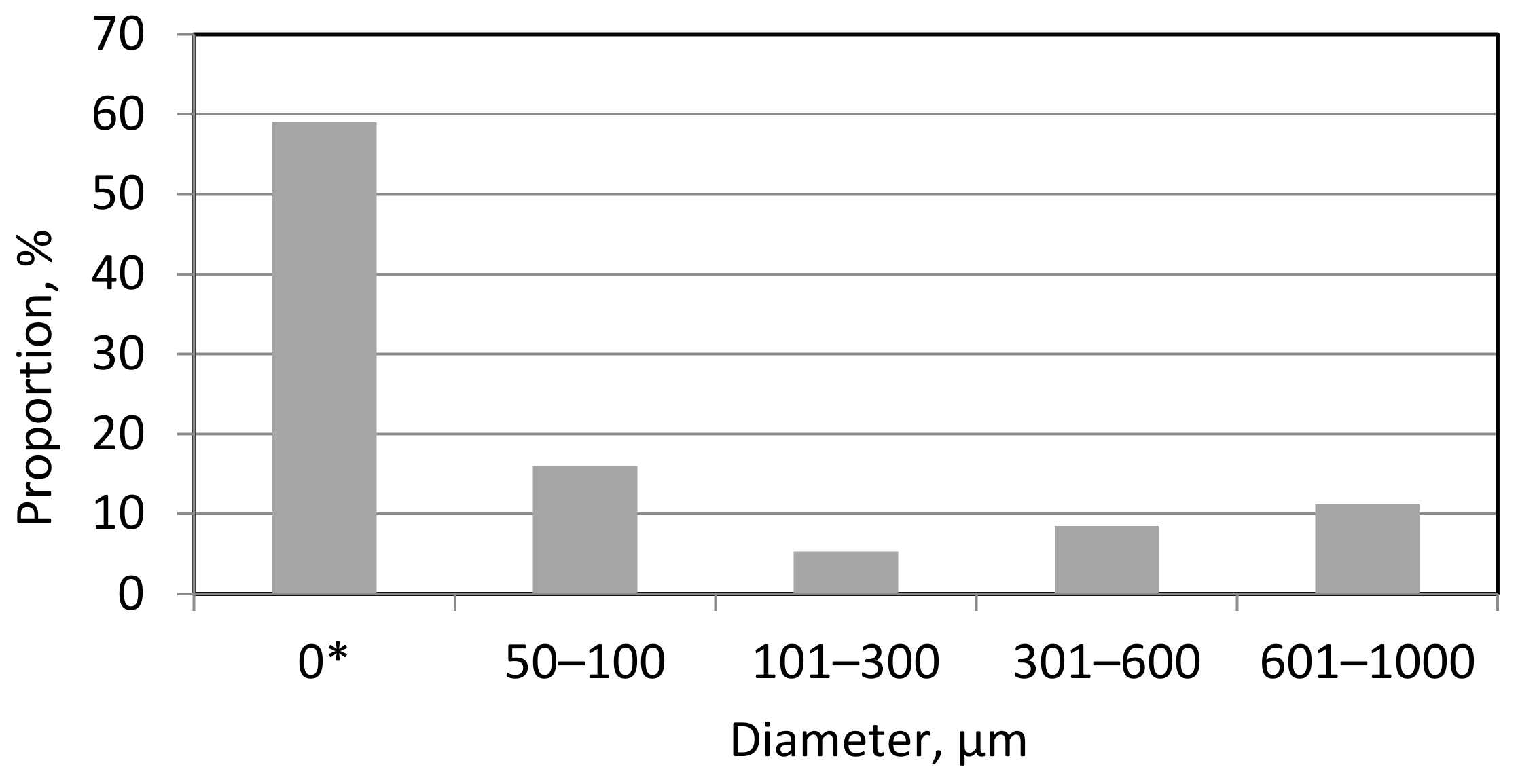
| Caltivar, Line | Violation of Spore Adhesion ** | Proportion of Germinated Spores, % | Proportion of Growing Tubes with Appressoria, % | Proportion of Appressoria, % | |
|---|---|---|---|---|---|
| On Stomata from the Total Number | Germinated from the Number on the Stomata | ||||
| Pamyati Azieva—control | - | 93.1 ± 3.9 | 85.1 ± 2.7 | 88.0 ± 3.2 | 83.2 ± 1.0 |
| Petkus | + | 62.7 ± 1.3 | 17.8 ± 1.2 | 43.8 ± 2.1 | 29.1 ± 2.1 |
| Siberia | ++ | 46.9 ± 1.2 | 59.3 ± 2.4 | 24.2 ± 2.0 | 38.4 ± 2.4 |
| Irina | + | 57.4 ± 1.2 | 25.0 ± 1.3 | 34.5 ± 1.9 | 12.5 ± 1.7 |
| Line 22/18 | - | 61.2 ± 1.3 | 34.2 ± 1.9 | 25.7 ± 1.9 | 26.6 ± 2.4 |
| Vavilovskayja universalnaya | + | 62.5 ± 1.4 | 31.4 ± 1.6 | 18.2 ± 1.3 | 30.6 ± 2.1 |
| Tyumenka | ++ | 46.3 ± 1.3 | 61.5 ± 2.0 | 30.1 ± 1.8 | 43.7 ± 2.5 |
| Zilant | + | 59.1 ± 1.2 | 49.6 ± 1.8 | 34.6 ± 1.7 | 29.3 ± 2.1 |
| Evrika | - | 65.8 ± 1.5 | 32.8 ± 1.7 | 45.1 ± 2.1 | 27.2 ± 2.1 |
| Cultivar, Line | Isolate | IT | Proportion of Germinated Spores, % | Proportion of Growing Tubes with Appressoria, % | Proportion of Appressoria, % | |
|---|---|---|---|---|---|---|
| On Stomata of the Total Number | Germinated from the Number on the Stomata | |||||
| Pamyati Azieva—control | 1 | 4 | 93.0 ± 3.9 | 85.0 ± 2.7 | 88.0 ± 3.2 | 83.0 ± 1.0 |
| 10 | 3+ | 88.9 ± 3.5 | 83.1 ± 2.9 | 82.5 ± 3.5 | 89.1 ± 2.3 | |
| 11 | 4 | 92.1 ± 2.6 | 80.9 ± 3.1 | 85.1 ± 1.9 | 92.3 ± 1.8 | |
| 13 | 4 | 88.5 ± 2.3 | 87.9 ± 2.5 | 90.2 ± 2.3 | 94.3 ± 2.1 | |
| TcLr26/Sr31 | 1 | 0 | 84.2 ± 1.2 | 45.9 ± 1.9 | 65.3 ± 1.6 | 8.9 ± 1.1 |
| 10 | 0 | 88.3 ± 1.6 | 56.9 ± 1.4 | 61.8 ± 1.4 | 12.1 ± 1.5 | |
| 11 | 0 | 86.5 ± 1.3 | 60.4 ± 1.2 | 71.4 ± 1.2 | 13.3 ± 1.4 | |
| Seri 82 | 1 | 0 | 86.4 ± 1.0 | 41.5 ± 1.2 | 76.2 ± 0.9 | 10.3 ± 1.3 |
| 10 | 0 | 83.5 ± 1.7 | 65.2 ± 2.8 | 67.8 ± 2.8 | 31.2 ± 2.8 | |
| 11 | ; | 88.6 ± 0.7 | 78.4 ± 1.8 | 85.1 ± 0.9 | 7.5 ± 1.0 | |
| Bacanora | 1 | 0 | 87.3 ± 1.2 | 56.1 ± 1.7 | 68.2 ± 1.4 | 33.1 ± 1.5 |
| 10 | 0 | 88.8 ± 1.2 | 60.3 ± 1.6 | 85.0 ± 0.9 | 7.6 ± 0.9 | |
| 11 | ;1 | 91.8 ± 1.2 | 33.4 ± 2.1 | 65.5 ± 2.8 | 23.3 ± 2.9 | |
| 13 | ;, 2, 3+ | 84.0 ± 1.5 | 82.4 ± 2.1 | 91.7 ± 1.2 | 35.4 ± 1.3 | |
| PWB343 | 1 | 0 | 89.1 ± 1.3 | 68.9 ± 1.7 | 72.4 ± 1.1 | 41.2 ± 1.4 |
| 10 | 2 | 98.2 ± 2.1 | 79.3 ± 1.6 | 84.6 ± 1.8 | 59.4 ± 2.5 | |
| 11 | 3+ | 94.5 ± 2.5 | 83.4 ± 2.1 | 77.6 ± 1.3 | 66.4 ± 2.1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plotnikova, L.; Pozherukova, V.; Knaub, V.; Kashuba, Y. What Was the Reason for the Durable Effect of Sr31 against Wheat Stem Rust? Agriculture 2022, 12, 2116. https://doi.org/10.3390/agriculture12122116
Plotnikova L, Pozherukova V, Knaub V, Kashuba Y. What Was the Reason for the Durable Effect of Sr31 against Wheat Stem Rust? Agriculture. 2022; 12(12):2116. https://doi.org/10.3390/agriculture12122116
Chicago/Turabian StylePlotnikova, Lyudmila, Violetta Pozherukova, Valeria Knaub, and Yuryi Kashuba. 2022. "What Was the Reason for the Durable Effect of Sr31 against Wheat Stem Rust?" Agriculture 12, no. 12: 2116. https://doi.org/10.3390/agriculture12122116
APA StylePlotnikova, L., Pozherukova, V., Knaub, V., & Kashuba, Y. (2022). What Was the Reason for the Durable Effect of Sr31 against Wheat Stem Rust? Agriculture, 12(12), 2116. https://doi.org/10.3390/agriculture12122116








