Abstract
Particle film forming materials were introduced as a solution to reduce the effects of excessive solar irradiation on plant photosynthesis. Covering plant leaves with particle films leads to plant bio-stimulant-like effects: increased protection against abiotic stress and increased fruit quality. We used zeolites and diatomaceous earth carriers for foliar fertilizer, known for their application as particle film-forming material. The aim of this paper was to investigate the plant bio-stimulant-like effects of this combined two-in-one product on the yield of apple trees and their photosynthetic pigments and fruit quality. The experiments were conducted for two years, 2021 and 2022, which had different agroclimatic patterns: 2021 had a deficit of precipitation, whereas 2022 was warmer by more than +4.8 °C compared to the recorded average temperature. Applying particle film-forming material and foliar fertilizer reduces the degradation of the photosynthetic pigments by drought and excessive solar radiation by 25–30%. In the year with a deficit of precipitation there was an increased yield by an additional 11.56–12.38% and the fruit quality similarly increased. Such effects of these combined two-in-one products were limited in 2022 as the temperature was higher than normal by several degrees.
1. Introduction
Plant bio-stimulants are a class of agricultural input defined by their main effects: enhancing/benefiting nutrient uptake and nutrient use efficiency, protecting plants against abiotic stress by increasing plant tolerance to and improving crop quality [1,2]. Plant bio-stimulants are classified as microbial and non-microbial plant bio-stimulants [1,3]. The non-microbial plant bio-stimulants are complex organic mixtures, such as humic and fulvic acids [4,5], seaweed extracts [6,7,8], protein hydrolysates [9,10], microalgae lysates [11,12,13,14] and inorganic elements recognized for their beneficial effects on plants, such as (soluble) silicon [15,16].
In recent decades, particle film-forming materials have been introduced as a short-term solution to reduce the effects of excessive heat and light on plant photosynthesis [17]. Several materials have been proposed for use in the formation of particle films on leaves, such as kaolin [18], zeolites [19], or diatomaceous earth [20]. Particle film-forming materials are used to cover the leaves of plants in order to mitigate solar radiation, mainly infrared (IR) and ultraviolet (UV) [17], generating a porous film that promotes gas exchange through the stomata [21]. Particle film-forming materials protect plants against abiotic stress, such as overheating and solar injury, improving the quality of the fruit [18,22,23].
The ideal film-forming materials are (nano)porous [17]. The combination of a (nano)porous structure, active surface with high sorption capacity for ionic forms of plant nutrients and reversible dehydration have been used to produce controlled-release fertilizers that can be applied to the soil [24,25,26]. Such formulations release mineral nutrients in a stimuli-controlled manner, e.g., driven by concentrations in the soil solutions [27,28]. (Nano)porous materials can also determine additional effects, such as soil improvement and enhanced nutrient use efficiency [27,29,30]. Zeolites are well-known nano-carriers for soil-applied fertilizers [31,32]. Diatomaceous earth has also proven to be an efficient (nano)carrier for soil fertilizer [33]. Until now, to the best of our knowledge, the use of (nano)porous materials that form films on leaves as carriers for foliar fertilizer was primarily carried out by our group.
We proposed the utilization of the (nano)porous structure of siliceous natural nanomaterials, e.g., zeolites and diatomaceous earth, as carriers for foliar fertilizers applied to stone fruits trees, apricot and peach [34]. A combined treatment reduces the leaf temperature by up to 4.5 °C. The anti-transpirant effect of the particle film enhanced water use efficiency by up to 30%. The nutritional effects of the foliar fertilizers on the yield were amplified by the film-forming (nano)porous material by up to 8.1%. The quality of peaches and apricots increased after applying the combined film-forming particle materials and foliar fertilizers [34].
Foliar fertilizers promote the plant bio-stimulant-like effects of particle film-forming materials. As already discussed, covering the plant leaves with particle films leads to increased protection against abiotic stress and increased fruit quality [18,22,23]. Plant physiology has also been modified using film-forming material treatments, as demonstrated by the higher fixation rate of carbon dioxide, improved stomatal conductance and increased water-use efficiency [34,35,36]. Such effects are similar to that of plant bio-stimulants.
This paper aimed to test the plant bio-stimulant-like effects of the newly developed combination of siliceous natural nanomaterials (SNNMs), diatomaceous earth, natural zeolites and foliar fertilizer on apple trees, as a representative of seed fruit trees. The studied effects were related to enhanced tolerance to abiotic stress, e.g., photosynthesis maintained while under abiotic stress conditions, as well as yield increases and improved fruit quality. An additional objective of the study was to determine the plant bio-stimulant-like effects of the newly developed two-in-one product on an experimental field with continental temperate conditions with higher summer average temperatures.
2. Materials and Methods
2.1. Plant Material and Experimental Site
The plant materials used were apples, Malus domestica, cv. Idared, planted at a distance of 4 × 4 m, respectively, with 625 apple trees per ha. The experiments were performed during 2021 and 2022 in the orchard of “Vasili Adamachi” experimental farm, Faculty of Horticulture, “Ion Ionescu de la Brad” Iasi University of Life Sciences. This orchard is located in northeastern Romania, Iasi county. The apple orchard from the “Vasili Adamachi” farm is a non-irrigated/rain-feed orchard.
The geographical coordinates were the following: 47°10′40–57″ North latitude, 27°30′25–42″ East longitude. The altitude of the orchard is 214 m. The apple orchard at the “Vasili Adamachi” farm is established on chernozem soil. The main characteristics of the soil are presented in Table 1.

Table 1.
The main characteristics of the soil from the orchard of “Vasili Adamachi” experimental farm, Faculty of Horticulture, “Ion Ionescu de la Brad”, Iasi University of Life Sciences.
The average values of the multi-annual (1975–2015) temperature, sunshine daily duration, total precipitations and wind speed for the apple orchard at the “Vasili Adamachi” farm are 9.6 °C, 7.3 h, 554.8 mm and, 4.8 m.s−1, respectively. The agrometeorological data for 2020–2021 and 2021–2022 are presented in Tables S1 and S2. In the vegetation period of the 2020–2021 agricultural year (April, May, June, July and August), the level of average monthly temperatures (+17.6 °C) varied slightly around the thermal norm of the region (+17.3 °C). In the 2021 vegetation period (April, May, June, July and August), the precipitation level was lower (254.2 mm), with a decrease of 83.3 mm compared to normal (337.5 mm), despite the highlighted periods of significant excessive rainfall in the summer of 2021. The period 2021–2022 was warmer by more than +4.8 °C than the recorded average temperature, reaching +14.5 °C for a total of 12 months. In the vegetation period, the average temperature was +6.0 °C higher than the multiyear average. Regarding precipitation, the vegetation period of 2022 was dryer by 75.0 mm compared to the period’s average. Still, April and May 2022 recorded double precipitation compared to the monthly normal (average) and managed to (partially) temper atmospheric and pedological drought.
2.2. Preparation and Analysis of Foliar Fertilizer with Siliceous Natural Nanomaterials (SNNMs) Carriers
Two types of siliceous natural nanomaterial, originating from Romanian quarries, were used: natural zeolites from the Rupea quarry (Zeolites Production, Rupea, Brașov) and diatomaceous earth (DE) from Pătârlagele (Sibiciu de Sus) quarry (Industriile de Diatomit, Pătârlagele, Buzău, Romania). The natural zeolites from the Rupea quarry are from the clinoptilolite type zeolites class—around 85% is clinoptilolite [37]. Sibiciu de Sus DE is formed by frustules of diatoms from the Aulacoseira (66%) and Actinocyclus genera [38]. The crystalline silica accounts for less than 0.9% and the amorphous silica content is around 85% [39]. The SNNMs were prepared and activated according to the process presented in our previous paper [34]. Briefly, the following steps were followed for the generation of the SNNMs: crushing, washing, drying at 105 °C for 24 h, milling in a planetary ball mill and activation. The zeolites were activated by using a thermal treatment, the first step at 200 °C for 2 h and a second activation treatment at 250 °C for 1 h [37]. The activation of DE was performed by using an acid treatment at room temperature (0.1 M HCl for 2 h, ratio 1 g DE to 10 mL HCl solution), followed by the treatment with a basic solution, with 10 mL NaOH 0.1 M and a final washing with pure water. The washed DE was dried at 105 °C for 24 h.
Two fertilizers with activated SNNMs were prepared, NanoFert Z (with zeolites) and NanoFert D (with diatomaceous earth)The foliar fertilizers were NPK 5:25:3, with micro-elements. This foliar fertilizer’s composition was selected as optimal for apple trees. The preparation process was undertaken based on the process presented in our previous paper [34]. Briefly, the soluble microelement sources, ammonium phosphate and potassium nitrate were homogenized for 15 min, mixed with chelated microelements (with EDTA—Cu, Fe, Mn, Zn) plus Boron (B) and Molybdenum (Mo), followed by a second homogenization for 15 min and grinding. The resulting composition was mixed with activated SNNMs. The same proportion of mixing SNNMs and foliar fertilizer as previously reported [34] was used: 3 kg activated SNNMs to 1 kg of foliar fertilizer mixture. The preparation process of the new two-in-one products, particle film-forming and foliar fertilizer are presented in Figure 1.
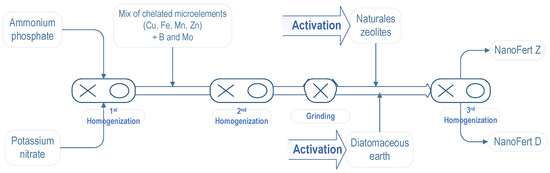
Figure 1.
Presentation of the preparation process of two-in-one products, particle film-forming and foliar fertilizer, NanoFert Z (with natural zeolites) and NanoFert D (with diatomaceous earth) foliar fertilizers. Modified from Moale et al. [34]. Copyright 2021, by the authors.
Standard methods were used to analyze the resulting foliar fertilizer formulation. Standard EN SR EN 15476/2009 was used to determine the total nitrogen. The ISO 6598:1996 method was used for the gravimetric determination of phosphorus (P) after the extraction. The EN 15477:2009 method was used for the determination of potassium (K). The determination of the copper, iron, manganese and zinc was performed according to EN 16965/2018 by flame atomic absorption spectrometry (FAAS) using an ICE 3300 atomic absorption spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). Boron was determined according to the EN 17041/2018 standard, using spectrometry with azo-methine-H. All of the analyses were performed in a laboratory certified according to ISO 17025 for fertilizer analysis based on the above-mentioned standards. The calibration curves for the microelements were carried out using certified reference materials (Certipur® ICP, Merck Group, Darmstadt, Germany).
2.3. Application of Foliar Fertilizer with SNNMs
The experiments were performed according to a completely randomized block design schedule with four treatments and four replications per treatment and year (each replicate was conducted in five individual trees). The volume applied for each fruit tree was 2 L, corresponding to a normalized spraying volume per ha of 1250 L (per ha being 625 apple trees). The following treatments were applied: control, sprayed with water; foliar fertilizer NanoFert D, concentration 1.6% (equivalent to 5 kg/ha foliar fertilizer and 15 kg/ha diatomaceous earth); foliar fertilizer NanoFert Z concentration 1.6% (equivalent to 5 kg/ha foliar fertilizer and 15 kg/ha Rupea natural zeolites); foliar fertilizer NPK 7:30:4, with micro-elements, concentration 0.4% (equiv. to 5 kg/ha foliar fertilizer). Three treatments were applied each year, one during flowering termination, with a BBCH scale of 69 and two during the fruit growing stages, with a BBCH scale of 73–78. The first treatment, during flower termination, was applied at phenological stage 69, the second treatment at stage 73, the second fruit fall and the third treatment at phenological stage 78, with the fruit about 80% of its final size [40]. The treatments were performed with a backpack mistblower (SR 420, Stihl, Waiblingen, Germany) and conducted in the morning, between 7.00 and 10.00 when the air temperature was lower than 23 °C.
2.4. Photosynthetic Pigment Analysis
The leaf samples were collected at noon and immediately shock-frozen in liquid nitrogen, freeze-dried and stored at −20 °C until analysis. Dimethyl sulphoxide (DMSO, Merck Group, Darmstadt, Germany) was used to extract photosynthetic pigments, chlorophyll and carotenoids. The extraction was performed using excess Mg2+ ions in the solution [41]. Briefly, the leaf powder was mixed in microcentrifuge tubes with 1 mL DMSO and 100 mg of magnesium hydroxide carbonate crystals. After incubation at 65 °C in the dark for 2 h, the samples were cooled and centrifuged at an RCF of 3500× g. Then, 250 µL of the extracts were pipetted into a microwell plate. The optical density of the extract was determined using a microplate reader (CLARIOstar, BMG Labtech, Ortenberg, Germany) at the following wavelengths: 480 nm (carotenoids), 649 nm (chlorophyll b) and 665 nm (chlorophyll a). In the extract, the concentrations of each photosynthetic pigment (carotenoid, chlorophyll b and chlorophyll a) were calculated according to Wellburn equations [42].
2.5. Assay of Apple Fruits Quality Characteristics
From each treated replicate, 10 apple fruits were sampled and analyzed for quality characteristics. The dry matter content was determined by using gravimetry. Samples weighing about 5 g were dried at 105 °C until reaching constant weight. The total soluble solids in the fruits were analyzed by using refractometry. Briefly, 100 g of grated apple pulp was homogenized in a blender with 50 mL of milli-Q water. The suspension was centrifuged at 2500× g (Universal 320 R centrifuge, Hettich, Tuttlingen, Germany) and the total soluble solids were determined using a digital refractometer (RX 5000α, Atago, Tokyo, Japan). The juice’s acidity was titrated with a solution of 0.1 N NaOH until reaching pH 8.1 (Titrino 848 Plus, Metrohm, Herisau, Switzerland) and expressed as % malic acid. L-ascorbic acid was determined using an enzymatic test kit (R-Biopharm, Darmstadt, Germany) according to the manufacturer’s instructions. The total phenolic content of the apple fruits was determined with Folin-Ciocâlteu reagent [43], with some modifications [44]. Briefly, 4 mL of 15% Na2CO3 and distilled water were added to 150 µL of the sample and 750 µL of Folin–Ciocâlteu reagent, until a final volume of 15 mL was achieved. The absorbance at λ = 756 nm was measured after a 2 h incubation at room temperature. The total phenolic compounds were expressed as gallic acid (GA) equivalents, based on a calibration curve with known concentrations of gallic acid. All of the determinations were performed in triplicate. All of the reagents used were analytical-grade reagents purchased from Sigma–Aldrich (Merck Group, Darmstadt, Germany).
2.6. Statistical Analysis
The standard uncertainty for the analysis of the foliar fertilizers and the two-in-one products, SNNMs foliar fertilizers, was calculated as the standard deviation by assuming a rectangular distribution. The data from the apple orchard experiment were statistically analyzed by analysis of variance (ANOVA), using the SPSS V.21.0 software package (IBM, Armonk, NY, USA). Two-way ANOVA (treatment; year) with analysis of the interactions was also used for the yield and the fruit quality parameters. The effects of the treatments were studied using the analysis of variance (Fischer method) and Fisher’s Least Significant Difference (LSD) test.
3. Results
3.1. Analysis of the Foliar Fertilizer
We prepared a new type of foliar fertilizer, which is suitable for apple foliar fertilization and the correspondent foliar fertilizers with SNNMs as nanoporous carriers (and particle film-forming material). The product containing natural zeolites was called NanoFert Z and the product containing diatomaceous earth NanoFert D.
As already discussed, the prepared foliar fertilizers were NPK 5:25:3 with micro-elements. This foliar fertilizer composition was confirmed to be optimal for apple fruit trees. The estimated and determined values of the fertilizer composition in the mineral nutrients are presented in Table 2, Table 3 and Table 4.

Table 2.
Composition of the prepared foliar fertilizer F1, estimated value (according to receipt) and determined value.

Table 3.
Composition of the prepared foliar fertilizer, NanoFert Z, estimated value (according to receipt) and determined value.

Table 4.
Composition of the prepared foliar fertilizer, NanoFert D, estimated value (according to receipt) and determined value.
The SNNMs carrier did not significantly influence the distribution and homogenization of the raw materials in the final products. The uncertainty is similar for the prepared foliar fertilizers that are mixed with film-forming materials, as for the foliar fertilizers preparation. These results demonstrate the mixing compatibility between the chosen raw materials for foliar fertilizer and the efficiency of the preparation method developed by our team.
3.2. Effects on Apple Tree Yield
The influence of applying foliar fertilizers and foliar fertilizers embedded with the particle-forming materials in the experiment organized in 2021 and 2022 is presented in Table 5.

Table 5.
Productive efficiency (kg/ha) after foliar fertilization with fertilizers and fertilizers combined with film-forming material applied to apple (Idared variety) in an intensive, non-irrigated orchard compared to the control—2021 and 2022.
In 2021, the effects of applying the two-in-one products, foliar fertilizer embedded within the porous structure of the particle film forming SNNMs, were more significant than in 2022. In 2021, the precipitation level was lower (254.2 mm), with a decrease of 83.3 mm compared to normal (337.5 mm) and the average monthly temperatures (+17.6 °C) varied slightly around the thermal norm (+17.3 °C). Under moderate water deficit in the soil, the particle film-forming SNNMs have an additional positive effect on the yield with respect to Fert NPK alone, which is significant in the case of DE (NanoFert D) and at the limit of statistical significance in the case of the product with zeolites (NanoFert Z).
The climatic conditions in 2022 were characterized by a deficit in precipitation (75.0 mm compared to the period’s average) and a significant temperature increase- +4.8 °C. Under such conditions, with a combined pedological and atmospheric drought, the protective effects of the particle film-forming SNNMs were less significant. The difference between the effects of foliar fertilizer treatment and the application of the two-in-one product, foliar fertilizer and particle film-forming materials is limited and without statistical significance. The two-way ANOVA (treatment, year) with an analysis of the interactions (Table 6, Tests of within contrasts) demonstrates the major influence of the agroclimatic conditions (years) vs. the treatment. The effect of the foliar treatments on the increased yield is statistically significant. However, the two-in-one products do not perform better than foliar fertilizer alone, as indicated by the results from 2022.

Table 6.
Tests of within-subjects contrasts, two-way ANOVA (treatment, year) with analysis of interactions.
3.3. Effects on Photosynthetic Pigments and Fruit Quality
The effects of the experimental treatments using foliar fertilizers and foliar fertilizers with particle film-forming SNNMs on the photosynthetic pigments of the leaves from the apple trees are presented in Figure 2 for 2021 and in Figure 3 for 2022.
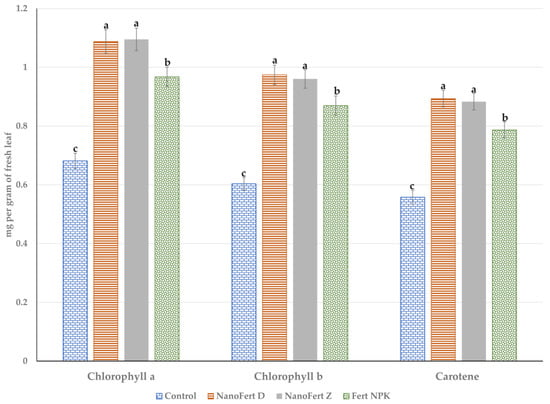
Figure 2.
The effects of the experimental treatment with foliar fertilizers and foliar fertilizers with particle film-forming SNNMs on the photosynthetic pigments of the leaves from the apple trees. Orchard of “Vasili Adamachi” experimental farm, Faculty of Horticulture, “Ion Ionescu de la Brad” Iasi University of Life Sciences, 2021. Values followed by the same letter do not differ significantly at p < 0.05.
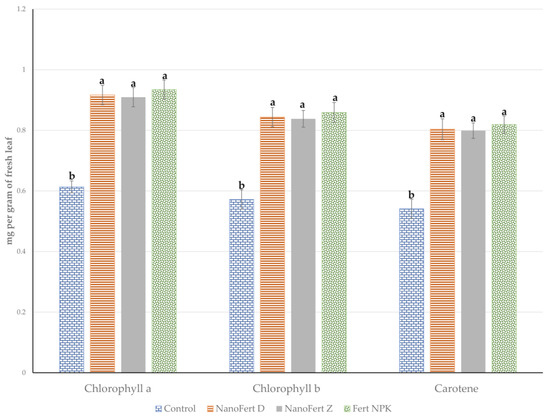
Figure 3.
The effects of the experimental treatment with foliar fertilizers and foliar fertilizers with particle film-forming SNNMs on the photosynthetic pigments of the leaves from the apple trees. Orchard of “Vasili Adamachi” experimental farm, Faculty of Horticulture, “Ion Ionescu de la Brad” Iasi University of Life Sciences, 2022. Values followed by the same letter do not differ significantly at p < 0.05.
The pattern for these two years of experimentation is similar to that observed in the case of the effect on the yield. In 2021, applying the two-in-one products, the foliar fertilizer embedded within the particle film’s porous structure forming SNNMs, promotes the accumulation of photosynthesis pigments. The effect of particle film-forming SNNMs was added to the effect of foliar fertilization. The protection against photobleaching due to high solar radiation and high temperatures was 61.41%, 59.13% and 44.04% for chlorophyll b for NanoFert D, NanoFert Z and Fert NPK, respectively. Almost similar values were registered for chlorophyll a: 59.61%, 60.70% and 41.99%. For total carotenoids, the results are as follows: 60.21%, 58.33% and 41.03% for NanoFert D, NanoFert Z and Fert NPK, respectively. Foliar fertilizers support the primary and secondary metabolism of plants [45,46], stimulate the synthesis of assimilatory pigments [47,48,49] and support photoprotective mechanisms against reactive oxygen species formed during the harvesting of light energy [50,51,52]. The effects of the two-in-one product, particles film-forming material and foliar fertilizers, are similar to that of foliar fertilizers.
The effects of the applied treatments on fruit quality are presented in Table 7.

Table 7.
The effect on apple fruit quality characteristics of the experimental treatment with foliar fertilizers and foliar fertilizers with particle film-forming SNNMs. Orchard of “Vasili Adamachi” experimental farm, Faculty of Horticulture, “Ion Ionescu de la Brad”, Iasi University of Life Sciences.
The results demonstrate similar patterns for the two experimental years. In 2021, applying SNNMs with foliar fertilizer significantly improved several quality characteristics of apple fruits, especially in the case of NanoFert D, a product made with diatomaceous earth (DE). In 2022, the effects on fruit quality were lower, with some effects still being present on the apple fruits harvested from the trees treated with NanoFert D.
The two-way ANOVA (treatment, year), with analysis of the interactions, revealed the limitation of the plant bio-stimulant-like effect on the crop quality of the two-in-one products, NanoFert D and NanoFert Z.
4. Discussion
Particle film technology aims to protect plants against biotic and abiotic stress. Due to its greater benefits, the film-forming technology was mainly applied to horticultural crops. In the years since its introduction, the application of particle film-forming materials to leaves demonstrated effects that are also related to improved fruit and vegetable quality [53,54,55,56].
Protection against abiotic stress and improved crop quality traits are agricultural functions that characterize plant bio-stimulants [1,2,57]. Plant bio-stimulants are a class of agricultural inputs that have been developed by the agrochemicals industry in the last 50 years and were introduced into the academic community 10 years ago during the 1st Bio-stimulant World Congress, which represented a milestone in plant bio-stimulant acceptance in the scientific community, held in 2012, in Strasbourg [58]. Another agricultural function of plant bio-stimulants is enhancing nutrient uptake and nutrient use efficiency [59,60]. This agricultural function still needed to be systematically investigated for the particle film-forming materials. Therefore, the beneficial effect of particle film-forming material on the performance of the treated plant should also involve enhanced nutrient uptake.
The ideal film-forming materials are porous [17]. Porous nanomaterial was described recently as the “main vein of agricultural nanotechnology” [52]. Despite their porosity and use as particle film-forming materials, siliceous natural nanomaterials (SNNMs), e.g., zeolites and diatomaceous earth, were not used as slow-release matrices/carriers for foliar fertilizer. Our approach was to use SNNMs as film-forming materials and as a slow-release matrix for foliar fertilizers to demonstrate the possibility of particle film-forming materials enhancing nutrient uptake [34]. Before mixing them with foliar fertilizers, we activated the SNNMs. Using the activation treatment, we enhanced the cation exchange capacity of natural zeolites and diatomaceous earth (DE). After activation, the zeolite exchange capacity (CEC) exceeded 2.5 meq/g and the CEC of diatomaceous earth reached almost 0.75 meg/g [34]. Kaolin, one of the main materials used in particle film technology, is less suitable as a carrier for foliar fertilizer. Kaolinite, the main clay mineral constituent of kaolin, has a relatively low CEC—0.38 meq/g [61]. Other particle film forming materials, for example, chabazite zeolite (CHA), have even higher CEC [62]. Formulations based on copper and chabazite zeolites were used to control plant diseases, e.g., grape downy mildew [63] and grapevine gray mold and sour rot [54], and not in combination with foliar fertilizers, for the foliar nutrition of the treated plants.
Foliar fertilizers emphasize the plant bio-stimulant effects of particle film-forming materials. The resulting two-in-one products, foliar fertilizers embedded in SNNMs, combine particle film-forming technology with foliar fertilization and complement plant protection effects against abiotic stress and plant nutrition. Due to their association with foliar fertilizers, the effects of particle film-forming materials became similar to those of plant bio-stimulants.
In our previous test on stone fruits during 2020 and 2021, we demonstrated the plant bio-stimulant effects for the two-in-one products, foliar fertilizers embedded in SNNMs. The anti-transpirant effect of the particle film enhanced water use efficiency by up to 30%. The nutritional effects of the foliar fertilizers on yield were amplified by the film-forming (nano)porous material by up to 8.1%. Peach and apricot fruit quality increased after applying the combined film-forming particle materials and foliar fertilizers [34].
The similarities between the particle film-forming materials and plant bio-stimulants are not only related to agricultural functions. These similar final effects on the treated plants also result from the similar response at the plant cellular level. High temperatures decrease the efficiency of photosynthetic machinery [64]. In apples, heat events significantly reduce the efficiency of photosystem II and increase non-photochemical quenching [65]. Non-photochemical quenching generates reactive oxygen species [66]. Excessive reactive oxygen species are quenched by antioxidant systems at a non-destructive level under normal conditions [67]. However, high temperatures and high light intensity produce ROS levels that have adverse effects on plants [68]. Increases in the leaf temperature inactivated Rubisco (D-ribulose 1, 5-bisphosphate carboxylase-oxygenase), the first enzyme of the Calvin cycle, reducing CO2 assimilation [63,69]. Heat stress also determines other adverse effects on chloroplasts, such as impairment of protein translation, chlorophyll breakdown and inactivation of PSII [19]. The drought stress amplifies the effects exerted by the heat stress on the photosynthetic pigments of fruit trees. Drought stress determined the accumulation of reactive oxygen species, leading to photosynthetic pigment peroxidation and photoinhibition [70].
Plant bio-stimulant applications reduce reactive oxygen species (ROS) formation in the treated plants, maintaining the ROS pool at a physiological level [71]. The foliar application of microbial plant bio-stimulants based on a Trichoderma consortium protects Passiflora caerulae (a shadow plant) from the damage induced by high light intensity [72]. The foliar application of plant bio-stimulants based on plant extracts rich in phytohormones, brassinosteroids, amino acids, or nitrophenolates (NP) resulted in a lower accumulation of ROS-stress-related markers (malondialdehyde and proline) in rice under heat stress [73]. The application of glycine-betaine on the sweet cherry tree (Prunus avium) improved water status, increased antioxidant activity and the polyphenol content and protected the photosynthetic pigments from photodegradation [74]. A plant bio-stimulant based on a magnesium–polyphenolic compound stimulated the accumulation of the pigments involved in heat dissipation by the xanthophyll cycle [75].
Particles film-forming material also reduce reactive oxygen species formation in leaves [76]. Kaolin application regulates secondary metabolism in grapes, enhancing accumulation of polyphenol compounds and boosting antioxidant capacity [77]. Phenylpropanoid and flavonoid pathways are stimulated in grapes by kaolin application [78]. Particle film determine a fine-tuning of the heat dissipation by xanthophyll cycle [79].
The main difference between plant bio-stimulants and particle film-forming materials is related to the mode of action. One of the main modes of action of particle film-forming material is reducing the leaf temperature by modifying the light reflection characteristics, especially IR light [80,81]. Our combined treatment reduces the leaf temperature of stone fruit trees, apricot and peach by up to 4.5 °C [34]. The two-in-one products, NanoFertD and NanoFertZ, shield the photosynthesis pigments from degradation due to excessive (UV and IR) solar radiation. Similar shielding effects were reported for other particle film-forming products [79,82]. In 2022, higher temperatures resulted from more intense solar radiations, combined with the decrease in precipitation, limiting the protective effects of the particle film-forming material on photosynthesis pigments. A decrease in the leaf temperature has several beneficial physiological effects. The mode of action of plant bio-stimulants is chemical/molecular priming, i.e., the preparedness/pre-activation of the metabolic pathways related to plant defense [83,84]. Molecular priming was demonstrated to be an efficient approach to controlling plant abiotic stress [85]. However, despite the different modes of action, the agricultural functions of plant bio-stimulants and particle film-forming materials are very similar. Particle film-forming materials, especially in combination with foliar fertilizers, have plant bio-stimulant-like effects on treated plants.
The results of our experiments using two-in-one products, foliar fertilizers embedded in SNNMs, on non-irrigated apple fruit trees were collected across two years under different forms of stress. 2021 was characterized by a moderate water/precipitation deficit during the apple vegetation period, with a temperature in the normal range. Applying the SNNMs combined with foliar fertilizers benefits nutrient uptake (higher yield), increases the protection of the photosynthetic pigments against inactivation and increases fruit quality. The year 2022 was characterized by a higher temperature (+4.8 °C) during the vegetation period, combined with a precipitation deficit. The average temperature was higher than the multiyear average by +8.6 °C, +10.0 °C and +7.2 °C in June, July and August 2022, respectively. Under such agrometeorological conditions, SNNM applications with foliar fertilizer could no longer exert the whole range of effects similar to those of plant bio-stimulants. The climatic conditions (higher temperatures/higher solar radiation and water deficit) limited the influence of the particle film-forming material on phytonutrients accumulation in apple fruits. The only exception is the accumulation of polyphenols in the case of NanoFert D. In this case, it could be speculated that this effect is the result of soluble silicon species slowly being released from the diatomaceous earth [20]. Additionally, the DE porosity, more complex than on zeolites, could be related to the enhanced effects of the DE observed in some situations.Under normal temperature conditions, particle-forming materials combined with foliar fertilizers exert similar effects with plant bio-stimulant: enhanced nutrient use efficiency, increased protection against abiotic stress and improved fruit quality. High-temperature conditions limit plant bio-stimulant-like effects of the particle-forming materials combined with foliar fertilizers. More investigations are necessary to find solutions for such high-temperature conditions.
5. Conclusions
The application of particle film-forming materials on crops can demonstrate plant bio-stimulant-like effects, such as protecting the treated plants against abiotic stress and increasing crop-quality traits. The agricultural functions of particle film-forming materials were more similar to those that define plant bio-stimulants when such particle film-forming materials are combined with foliar fertilizers. This two-in-one combination evidences the function related to enhanced nutrient uptake and nutrient use efficiency.
The effects of particle film-forming materials and plant bio-stimulants are also similar at the physiological, cellular and biochemical levels. However, the mode of action is different. Particle film-forming materials reduce the leaf temperature and plant bio-stimulants determine a molecular priming of the plant metabolic pathways related to defense.
Our present study reveals that high temperatures limit the plant bio-stimulant-like effects of the two-in-one combination, particle film-forming materials and foliar fertilizers. One potential solution for such high-temperature conditions are complementary/compatible combinations with plant bio-stimulants.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agriculture13010178/s1, Table S1. The agrometeorological data for 2020–2021, Iasi agrometeorological station; Table S2. The agrometeorological data for 2021–2022. Iasi agrometeorological station.
Author Contributions
Conceptualization, F.O. and T.M.C.; methodology, C.E.S., D.C.-A. and M.D.-A.; validation, T.M.C., C.E.S. and F.O.; formal analysis, C.E.S.; investigation, C.E.S., M.D.-A. and D.C.-A.; resources, F.O.; writing—original draft preparation, C.E.S.; writing—review and editing, F.O. and D.C.-A.; visualization, D.C.-A.; supervision, F.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Romanian Ministry of Agriculture and Rural Development, project Research on the biological activity of some nanomaterial-based products on major pest and pathogens of fruit trees and assessment of the ecotoxicological impact of these on useful entomofauna—ADER 7.3.9. and by the Romanian Ministry of Research, Innovation and Digitalization, project PN.19.23.01.01 Integrated platform for smart valorization of the biomass- Smart-Bi, Nucleu Programme ChemEmergent. The APC was funded by project ADER 7.3.9.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Authors thanks to Industriile de Diatomit, Pătârlagele, Buzău, Romania and Zeolites Production, Rupea, Brașov, Romania for the donation of natural zeolites and diatomaceous earth used for experiments. Additionally, the authors thank Bogdan Trică for his support with the statistical analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Li, J.; Van Gerrewey, T.; Geelen, D. A Meta-Analysis of Biostimulant Yield Effectiveness in Field Trials. Front. Plant Sci. 2022, 13, 836702. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Toward a Sustainable Agriculture Through Plant Biostimulants: From Experimental Data to Practical Applications. Agronomy 2020, 10, 1461. [Google Scholar] [CrossRef]
- Canellas, L.P.; Olivares, F.L.; Aguiar, N.O.; Jones, D.L.; Nebbioso, A.; Mazzei, P.; Piccolo, A. Humic and fulvic acids as biostimulants in horticulture. Sci. Hortic. 2015, 196, 15–27. [Google Scholar] [CrossRef]
- Jindo, K.; Olivares, F.L.; Malcher, D.J.d.P.; Sánchez-Monedero, M.A.; Kempenaar, C.; Canellas, L.P. From lab to field: Role of humic substances under open-field and greenhouse conditions as biostimulant and biocontrol agent. Front. Plant Sci. 2020, 11, 426. [Google Scholar] [CrossRef]
- EL Boukhari, M.E.M.; Barakate, M.; Bouhia, Y.; Lyamlouli, K. Trends in Seaweed Extract Based Biostimulants: Manufacturing Process and Beneficial Effect on Soil-Plant Systems. Plants 2020, 9, 359. [Google Scholar] [CrossRef]
- Battacharyya, D.; Babgohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed extracts as biostimulants in horticulture. Sci. Hortic. 2015, 196, 39–48. [Google Scholar] [CrossRef]
- Deolu-Ajayi, A.O.; van der Meer, I.M.; van der Werf, A.; Karlova, R. The power of seaweeds as plant biostimulants to boost crop production under abiotic stress. Plant Cell Environ. 2022, 45, 2537–2553. [Google Scholar] [CrossRef]
- Colla, G.; Nardi, S.; Cardarelli, M.; Ertani, A.; Lucini, L.; Canaguier, R.; Rouphael, Y. Protein hydrolysates as biostimulants in horticulture. Sci. Hortic. 2015, 196, 28–38. [Google Scholar] [CrossRef]
- Colla, G.; Hoagland, L.; Ruzzi, M.; Cardarelli, M.; Bonini, P.; Canaguier, R.; Rouphael, Y. Biostimulant Action of Protein Hydrolysates: Unraveling Their Effects on Plant Physiology and Microbiome. Front. Plant Sci. 2017, 8, 2202. [Google Scholar] [CrossRef]
- Santini, G.; Biondi, N.; Rodolfi, L.; Tredici, M.R. Plant Biostimulants from Cyanobacteria: An Emerging Strategy to Improve Yields and Sustainability in Agriculture. Plants 2021, 10, 643. [Google Scholar] [CrossRef] [PubMed]
- Colla, G.; Rouphael, Y. Microalgae: New Source of Plant Biostimulants. Agronomy 2020, 10, 1240. [Google Scholar] [CrossRef]
- Kapoore, R.V.; Wood, E.E.; Llewellyn, C.A. Algae biostimulants: A critical look at microalgal biostimulants for sustainable agricultural practices. Biotechnol. Adv. 2021, 49, 107754. [Google Scholar] [CrossRef] [PubMed]
- Bello, A.S.; Saadaoui, I.; Ben-Hamadou, R. “Beyond the Source of Bioenergy”: Microalgae in Modern Agriculture as a Biostimulant, Biofertilizer, and Anti-Abiotic Stress. Agronomy 2021, 11, 1610. [Google Scholar] [CrossRef]
- Savvas, D.; Ntatsi, G. Biostimulant activity of silicon in horticulture. Sci. Hortic. 2015, 196, 66–81. [Google Scholar] [CrossRef]
- Bishnoi, A.; Jangir, P.; Shekhawat, P.K.; Ram, H.; Soni, P. Silicon Supplementation as a Promising Approach to Induce Thermotolerance in Plants: Current Understanding and Future Perspectives. J. Soil Sci. Plant Nutr. 2022. [Google Scholar] [CrossRef]
- Sharma, R.R.; Vijay Rakesh Reddy, S.; Datta, S.C. Particle films and their applications in horticultural crops. Appl. Clay Sci. 2015, 116–117, 54–68. [Google Scholar] [CrossRef]
- Brito, C.; Dinis, L.T.; Moutinho-Pereira, J.; Correia, C. Kaolin, an emerging tool to alleviate the effects of abiotic stresses on crop performance. Sci. Hortic. 2019, 250, 310–316. [Google Scholar] [CrossRef]
- De Smedt, C.; Someus, E.; Spanoghe, P. Potential and actual uses of zeolites in crop protection. Pest Manag. Sci. 2015, 71, 1355–1367. [Google Scholar] [CrossRef] [PubMed]
- Constantinescu-Aruxandei, D.; Lupu, C.; Oancea, F. Siliceous Natural Nanomaterials as Biorationals—Plant Protectants and Plant Health Strengtheners. Agronomy 2020, 10, 1791. [Google Scholar] [CrossRef]
- De Smedt, C.; Steppe, K.; Spanoghe, P. Beneficial effects of zeolites on plant photosynthesis. Adv. Mater. Sci. 2017, 2, 1–11. [Google Scholar] [CrossRef]
- Bernardo, S.; Dinis, L.T.; Machado, N.; Barros, A.; Pitarch-Bielsa, M.; Malheiro, A.C.; Gómez-Cadenas, A.; Moutinho-Pereira, J. Uncovering the effects of kaolin on balancing berry phytohormones and quality attributes of Vitis vinifera grown in warm-temperate climate regions. J. Sci. Food Agric. 2022, 102, 782–793. [Google Scholar] [CrossRef]
- D’Aquino, S.; Palma, A.; Chessa, I.; Satta, D.; De Pau, L.; Inglese, P.; Ochoa, J.M.; Glenn, D.M. Effect of surround WP (a Kaolin-based particle film) on Ceratitis capitata infestation, quality and postharvest behavior of cactus pear fruit cv Gialla. Sci. Hortic. 2021, 289, 110484. [Google Scholar] [CrossRef]
- Maghsoodi, M.R.; Najafi, N.; Reyhanitabar, A.; Oustan, S. Hydroxyapatite nanorods, hydrochar, biochar, and zeolite for controlled-release urea fertilizers. Geoderma 2020, 379, 114644. [Google Scholar] [CrossRef]
- Sharma, S.; Sahu, B.K.; Cao, L.D.; Bindra, P.; Kaur, K.; Chandel, M.; Koratkar, N.; Huang, Q.L.; Shanmugam, V. Porous nanomaterials: Main vein of agricultural nanotechnology. Prog. Mater. Sci. 2021, 121, 100812. [Google Scholar] [CrossRef]
- Dubey, A.; Mailapalli, D.R. Zeolite coated urea fertilizer using different binders: Fabrication, material properties and nitrogen release studies. Environ. Technol. Innov. 2019, 16, 100452. [Google Scholar] [CrossRef]
- Nakhli, S.A.A.; Delkash, M.; Bakhshayesh, B.E.; Kazemian, H. Application of Zeolites for Sustainable Agriculture: A Review on Water and Nutrient Retention. Water Air Soil Pollut. 2017, 228, 464. [Google Scholar] [CrossRef]
- Cataldo, E.; Salvi, L.; Paoli, F.; Fucile, M.; Masciandaro, G.; Manzi, D.; Masini, C.M.; Mattii, G.B. Application of Zeolites in Agriculture and Other Potential Uses: A Review. Agronomy 2021, 11, 1547. [Google Scholar] [CrossRef]
- Jarosz, R.; Szerement, J.; Gondek, K.; Mierzwa-Hersztek, M. The use of zeolites as an addition to fertilisers—A review. Catena 2022, 213, 106125. [Google Scholar] [CrossRef]
- Mondal, M.; Biswas, B.; Garai, S.; Sarkar, S.; Banerjee, H.; Brahmachari, K.; Bandyopadhyay, P.K.; Maitra, S.; Brestic, M.; Skalicky, M. Zeolites enhance soil health, crop productivity and environmental safety. Agronomy 2021, 11, 448. [Google Scholar] [CrossRef]
- Babu, S.; Singh, R.; Yadav, D.; Rathore, S.S.; Raj, R.; Avasthe, R.; Yadav, S.K.; Das, A.; Yadav, V.; Yadav, B.; et al. Nanofertilizers for agricultural and environmental sustainability. Chemosphere 2022, 292, 133451. [Google Scholar] [CrossRef] [PubMed]
- Lateef, A.; Nazir, R.; Jamil, N.; Alam, S.; Shah, R.; Khan, M.N.; Saleem, M. Synthesis and characterization of zeolite based nano–composite: An environment friendly slow release fertilizer. Microporous Mesoporous Mater. 2016, 232, 174–183. [Google Scholar] [CrossRef]
- Vakal, S.; Vakal, V.; Artyukhov, A.; Shkola, V.; Yanovska, A. Granulated organo-mineral fertilizers: The process of formation and investigation of porous phosphate-diatomite shell. Appl. Nanosci. 2022. [Google Scholar] [CrossRef]
- Moale, C.; Ghiurea, M.; Sîrbu, C.E.; Somoghi, R.; Cioroianu, T.M.; Faraon, V.A.; Lupu, C.; Trică, B.; Constantinescu-Aruxandei, D.; Oancea, F. Effects of Siliceous Natural Nanomaterials Applied in Combination with Foliar Fertilizers on Physiology, Yield and Fruit Quality of the Apricot and Peach Trees. Plants 2021, 10, 2395. [Google Scholar] [CrossRef]
- Rotondi, A.; Morrone, L.; Facini, O.; Faccini, B.; Ferretti, G.; Coltorti, M. Distinct Particle Films Impacts on Olive Leaf Optical Properties and Plant Physiology. Foods 2021, 10, 1291. [Google Scholar] [CrossRef]
- Brito, C.; Dinis, L.-T.; Ferreira, H.; Rocha, L.; Pavia, I.; Moutinho-Pereira, J.; Correia, C.M. Kaolin particle film modulates morphological, physiological and biochemical olive tree responses to drought and rewatering. Plant Physiol. Biochem. 2018, 133, 29–39. [Google Scholar] [CrossRef]
- Neag, E.; Török, A.I.; Tanaselia, C.; Aschilean, I.; Senila, M. Kinetics and Equilibrium Studies for the Removal of Mn and Fe from Binary Metal Solution Systems Using a Romanian Thermally Activated Natural Zeolite. Water 2020, 12, 1614. [Google Scholar] [CrossRef]
- Tulan, E.; Reinhard, F.S.; Tari, G.; Witkowski, J.; Tămaș, D.M.; Horvat, A.; Tămaș, A. Hydrocarbon source rock potential and paleoenvironment of lower Miocene diatomites in the Eastern Carpathians Bend Zone (Sibiciu de Sus, Romania). Geol. Carpathica. 2020, 71, 424–443. [Google Scholar] [CrossRef]
- Lupu, C.; Delian, E.; Chira, L.; Chira, A. New insights into the multiple protective functions of diatomaceous earth during storage of agricultural products. Sci. Papers. Ser. A. Agron. 2018, 61, 487–496. [Google Scholar]
- Meier, U. Phenological growth stages. In Phenology: An Integrative Environmental Science; Springer: Berlin/Heidelberg, Germany, 2003; pp. 269–283. [Google Scholar]
- Prsa, I.; Stampar, F.; Vodnik, D.; Veberic, R. Influence of nitrogen on leaf chlorophyll content and photosynthesis of ‘Golden Delicious’ apple. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2007, 57, 283–289. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Craciunescu, O.; Constantin, D.; Gaspar, A.; Toma, L.; Utoiu, E.; Moldovan, L. Evaluation of antioxidant and cytoprotective activities of Arnica montana L. and Artemisia absinthium L. ethanolic extracts. Chem. Cent. J. 2012, 6, 97. [Google Scholar] [CrossRef]
- Niu, J.; Liu, C.; Huang, M.; Liu, K.; Yan, D. Effects of Foliar Fertilization: A Review of Current Status and Future Perspectives. J. Soil Sci. Plant Nutr. 2021, 21, 104–118. [Google Scholar] [CrossRef]
- Fernandez, V.; Brown, P.H. From plant surface to plant metabolism: The uncertain fate of foliar-applied nutrients. Front. Plant Sci. 2013, 4, 289. [Google Scholar] [CrossRef]
- Jarecki, W.; Buczek, J.; Bobrecka-Jamro, D. The response of winter oilseed rape to diverse foliar fertilization. Plant Soil Environ. 2019, 65, 125–130. [Google Scholar] [CrossRef]
- El-Mogy, M.M.; Mahmoud, A.W.M.; El-Sawy, M.B.; Parmar, A. Pre-harvest foliar application of mineral nutrients to retard chlorophyll degradation and preserve bio-active compounds in broccoli. Agronomy 2019, 9, 711. [Google Scholar] [CrossRef]
- Dima, S.-O.; Neamțu, C.; Desliu-Avram, M.; Ghiurea, M.; Capra, L.; Radu, E.; Stoica, R.; Faraon, V.-A.; Zamfiropol-Cristea, V.; Constantinescu-Aruxandei, D. Plant Biostimulant Effects of Baker’s Yeast Vinasse and Selenium on Tomatoes through Foliar Fertilization. Agronomy 2020, 10, 133. [Google Scholar] [CrossRef]
- Pavia, I.; Roque, J.; Rocha, L.; Ferreira, H.; Castro, C.; Carvalho, A.; Silva, E.; Brito, C.; Goncalves, A.; Lima-Brito, J. Zinc priming and foliar application enhances photoprotection mechanisms in drought-stressed wheat plants during anthesis. Plant Physiol. Biochem. 2019, 140, 27–42. [Google Scholar] [CrossRef]
- Kumari, V.V.; Banerjee, P.; Verma, V.C.; Sukumaran, S.; Chandran, M.A.S.; Gopinath, K.A.; Venkatesh, G.; Yadav, S.K.; Singh, V.K.; Awasthi, N.K. Plant nutrition: An effective way to alleviate abiotic stress in agricultural crops. Int. J. Mol. Sci. 2022, 23, 8519. [Google Scholar] [CrossRef]
- Erel, R.; Yermiyahu, U.; Ben-Gal, A.; Dag, A.; Shapira, O.; Schwartz, A. Modification of non-stomatal limitation and photoprotection due to K and Na nutrition of olive trees. J. Plant Physiol. 2015, 177, 1–10. [Google Scholar] [CrossRef]
- Rosati, A.; Metcalf, S.G.; Buchner, R.P.; Fulton, A.E.; Lampinen, B.D. Physiological effects of kaolin applications in well-irrigated and water-stressed walnut and almond trees. Ann. Bot. 2006, 98, 267–275. [Google Scholar] [CrossRef]
- Cantore, V.; Pace, B.; Albrizio, R. Kaolin-based particle film technology affects tomato physiology, yield and quality. Environ. Exp. Bot. 2009, 66, 279–288. [Google Scholar] [CrossRef]
- Glenn, D.M.; Puterka, G.J.; Drake, S.R.; Unruh, T.R.; Knight, A.L.; Baherle, P.; Prado, E.; Baugher, T.A. Particle Film Application Influences Apple Leaf Physiology, Fruit Yield, and Fruit Quality. J. Am. Soc. Hortic. Sci. Jashs 2001, 126, 175–181. [Google Scholar] [CrossRef]
- Schupp, J.R.; Fallahi, E.; Chun, I.-J. Effect of particle film on fruit sunburn, maturity and quality of Fuji’ and Honeycrisp’ apples. Horttechnology 2002, 12, 87–90. [Google Scholar] [CrossRef]
- Drobek, M.; Frąc, M.; Cybulska, J. Plant biostimulants: Importance of the quality and yield of horticultural crops and the improvement of plant tolerance to abiotic stress—A review. Agronomy 2019, 9, 335. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- De Pascale, S.; Rouphael, Y.; Colla, G. Plant biostimulants: Innovative tool for enhancing plant nutrition in organic farming. Eur. J. Hortic. Sci 2017, 82, 277–285. [Google Scholar] [CrossRef]
- Halpern, M.; Bar-Tal, A.; Ofek, M.; Minz, D.; Muller, T.; Yermiyahu, U. The use of biostimulants for enhancing nutrient uptake. Adv. Agron. 2015, 130, 141–174. [Google Scholar]
- Kahr, G.; Madsen, F.T. Determination of the cation exchange capacity and the surface area of bentonite, illite and kaolinite by methylene blue adsorption. Appl. Clay Sci. 1995, 9, 327–336. [Google Scholar] [CrossRef]
- Gualtieri, A.F.; Passaglia, E. Rietveld structure refinement of NH4-exchanged natural chabazite. Eur. J. Mineral. 2006, 18, 351–359. [Google Scholar] [CrossRef]
- Salvucci, M.E.; Crafts-Brandner, S.J. Inhibition of photosynthesis by heat stress: The activation state of Rubisco as a limiting factor in photosynthesis. Physiol. Plant. 2004, 120, 179–186. [Google Scholar] [CrossRef]
- Mathur, S.; Agrawal, D.; Jajoo, A. Photosynthesis: Response to high temperature stress. J. Photochem. Photobiol. B Biol. 2014, 137, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Greer, D.H. Temperature-dependent responses of the photosynthetic and chlorophyll fluorescence attributes of apple (Malus domestica) leaves during a sustained high temperature event. Plant Physiol. Biochem. 2015, 97, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Czarnocka, W.; Karpiński, S. Friend or foe? Reactive oxygen species production, scavenging and signaling in plant response to environmental stresses. Free Radic. Biol. Med. 2018, 122, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Krieger-Liszkay, A.; Shimakawa, G. Regulation of the generation of reactive oxygen species during photosynthetic electron transport. Biochem. Soc. Trans. 2022, 50, 1025–1034. [Google Scholar] [CrossRef] [PubMed]
- Dumanović, J.; Nepovimova, E.; Natić, M.; Kuča, K.; Jaćević, V. The Significance of Reactive Oxygen Species and Antioxidant Defense System in Plants: A Concise Overview. Front. Plant Sci. 2021, 11, 552969. [Google Scholar] [CrossRef]
- Sage, R.F.; Kubien, D.S. The temperature response of C3 and C4 photosynthesis. Plant Cell Environ. 2007, 30, 1086–1106. [Google Scholar] [CrossRef]
- Mihaljević, I.; Viljevac Vuletić, M.; Šimić, D.; Tomaš, V.; Horvat, D.; Josipović, M.; Zdunić, Z.; Dugalić, K.; Vuković, D. Comparative Study of Drought Stress Effects on Traditional and Modern Apple Cultivars. Plants 2021, 10, 561. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Parvin, K.; Bardhan, K.; Nahar, K.; Anee, T.I.; Masud, A.A.C.; Fotopoulos, V. Biostimulants for the regulation of reactive oxygen species metabolism in plants under abiotic stress. Cells 2021, 10, 2537. [Google Scholar] [CrossRef]
- Şesan, T.E.; Oancea, A.O.; Ştefan, L.M.; Mănoiu, V.S.; Ghiurea, M.; Răut, I.; Constantinescu-Aruxandei, D.; Toma, A.; Savin, S.; Bira, A.F. Effects of foliar treatment with a Trichoderma plant biostimulant consortium on Passiflora caerulea L. yield and quality. Microorganisms 2020, 8, 123. [Google Scholar] [CrossRef] [PubMed]
- Quintero-Calderon, E.H.; Sanchez-Reinoso, A.D.; Chavez-Arias, C.C.; Garces-Varon, G.; Restrepo-Diaz, H. Rice seedlings showed a higher heat tolerance through the foliar application of biostimulants. Not. Bot. Horti Agrobot. Cluj-Napoca 2021, 49, 12120. [Google Scholar] [CrossRef]
- Serapicos, M.; Afonso, S.; Gonçalves, B.; Silva, A.P. Exogenous Application of Glycine Betaine on Sweet Cherry Tree (Prunus avium L.): Effects on Tree Physiology and Leaf Properties. Plants 2022, 11, 3470. [Google Scholar] [CrossRef]
- Hamedeh, H.; Antoni, S.; Cocciaglia, L.; Ciccolini, V. Molecular and Physiological Effects of Magnesium–Polyphenolic Compound as Biostimulant in Drought Stress Mitigation in Tomato. Plants 2022, 11, 586. [Google Scholar] [PubMed]
- Bernardo, S.; Dinis, L.-T.; Luzio, A.; Pinto, G.; Meijón, M.; Valledor, L.; Conde, A.; Gerós, H.; Correia, C.; Moutinho-Pereira, J. Kaolin particle film application lowers oxidative damage and DNA methylation on grapevine (Vitis vinifera L.). Environ. Exp. Bot. 2017, 139, 39–47. [Google Scholar] [CrossRef]
- Dinis, L.T.; Bernardo, S.; Conde, A.; Pimentel, D.; Ferreira, H.; Félix, L.; Gerós, H.; Correia, C.M.; Moutinho-Pereira, J. Kaolin exogenous application boosts antioxidant capacity and phenolic content in berries and leaves of grapevine under summer stress. J. Plant Physiol. 2016, 191, 45–53. [Google Scholar] [CrossRef]
- Conde, A.; Pimentel, D.; Neves, A.; Dinis, L.-T.; Bernardo, S.; Correia, C.M.; Gerós, H.; Moutinho-Pereira, J. Kaolin Foliar Application Has a Stimulatory Effect on Phenylpropanoid and Flavonoid Pathways in Grape Berries. Front. Plant Sci. 2016, 7, 1150. [Google Scholar] [CrossRef]
- Bernardo, S.; Rodrigo, M.J.; Vives-Peris, V.; Gómez-Cadenas, A.; Zacarías, L.; Machado, N.; Moutinho-Pereira, J.; Dinis, L.-T. Fine-tuning of grapevine xanthophyll-cycle and energy dissipation under Mediterranean conditions by kaolin particle-film. Sci. Hortic. 2022, 291, 110584. [Google Scholar] [CrossRef]
- Glenn, D.M.; Puterka, G.J. Particle films: A new technology for agriculture. Hortic. Rev. 2005, 31, 1–44. [Google Scholar]
- Glenn, D.M.; Cooley, N.; Walker, R.; Clingeleffer, P.; Shellie, K. Impact of Kaolin Particle Film and Water Deficit on Wine Grape Water Use Efficiency and Plant Water Relations. Hortscience 2010, 45, 1178–1187. [Google Scholar] [CrossRef]
- Hamdy, A.E.; Abdel-Aziz, H.F.; El-khamissi, H.; AlJwaizea, N.I.; El-Yazied, A.A.; Selim, S.; Tawfik, M.M.; AlHarbi, K.; Ali, M.S.M.; Elkelish, A. Kaolin Improves Photosynthetic Pigments, and Antioxidant Content, and Decreases Sunburn of Mangoes: Field Study. Agronomy 2022, 12, 1535. [Google Scholar] [CrossRef]
- Canellas, L.P.; Canellas, N.O.; da S Irineu, L.E.S.; Olivares, F.L.; Piccolo, A. Plant chemical priming by humic acids. Chem. Biol. Technol. Agric. 2020, 7, 12. [Google Scholar] [CrossRef]
- Lephatsi, M.; Nephali, L.; Meyer, V.; Piater, L.A.; Buthelezi, N.; Dubery, I.A.; Opperman, H.; Brand, M.; Huyser, J.; Tugizimana, F. Molecular mechanisms associated with microbial biostimulant-mediated growth enhancement, priming and drought stress tolerance in maize plants. Sci. Rep. 2022, 12, 10450. [Google Scholar] [CrossRef] [PubMed]
- Kerchev, P.; van der Meer, T.; Sujeeth, N.; Verlee, A.; Stevens, C.V.; Van Breusegem, F.; Gechev, T. Molecular priming as an approach to induce tolerance against abiotic and oxidative stresses in crop plants. Biotechnol. Adv. 2020, 40, 107503. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).