Microbiological Assessment of Broiler Compound Feed Production as Part of the Food Chain—A Case Study in a Romanian Feed Mill
Abstract
1. Introduction
2. Materials and Methods
2.1. Feed Samples
2.2. Yeasts and Molds Analysis
2.3. Salmonella spp. Analysis
2.4. Escherichia coli Analysis
2.5. Clostridium perfringens Analysis
2.6. Statistical Analysis
3. Results
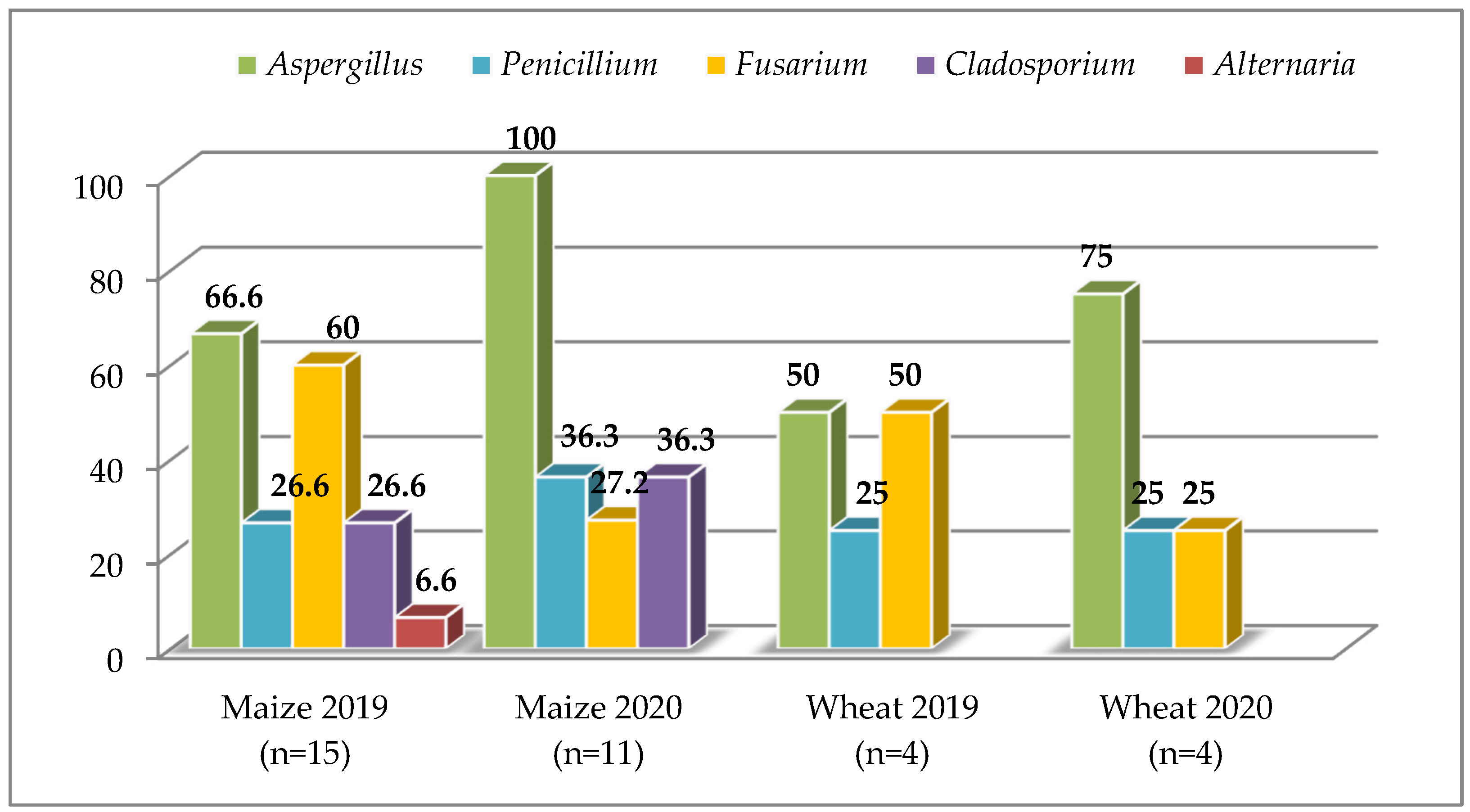
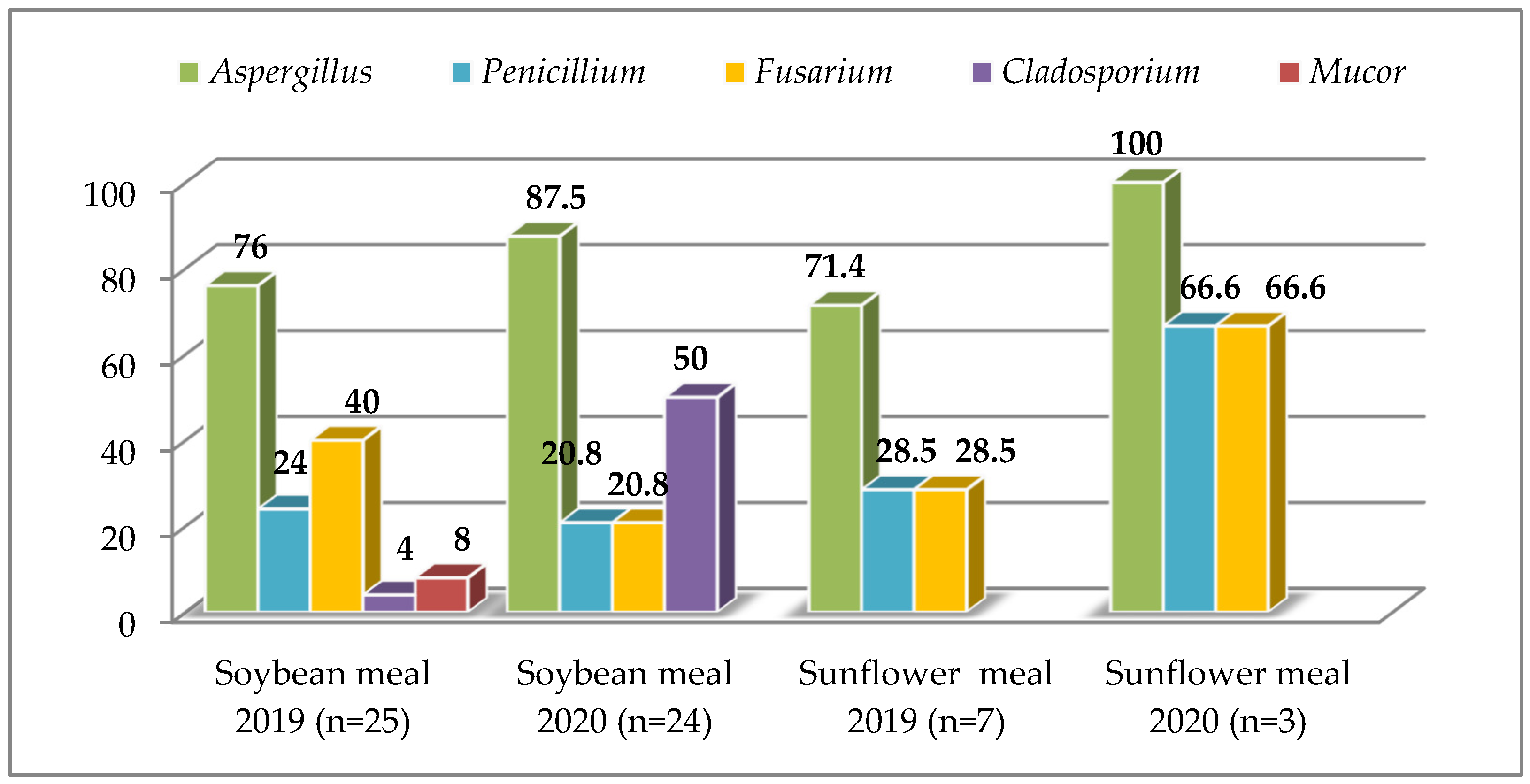
3.1. Raw Materials
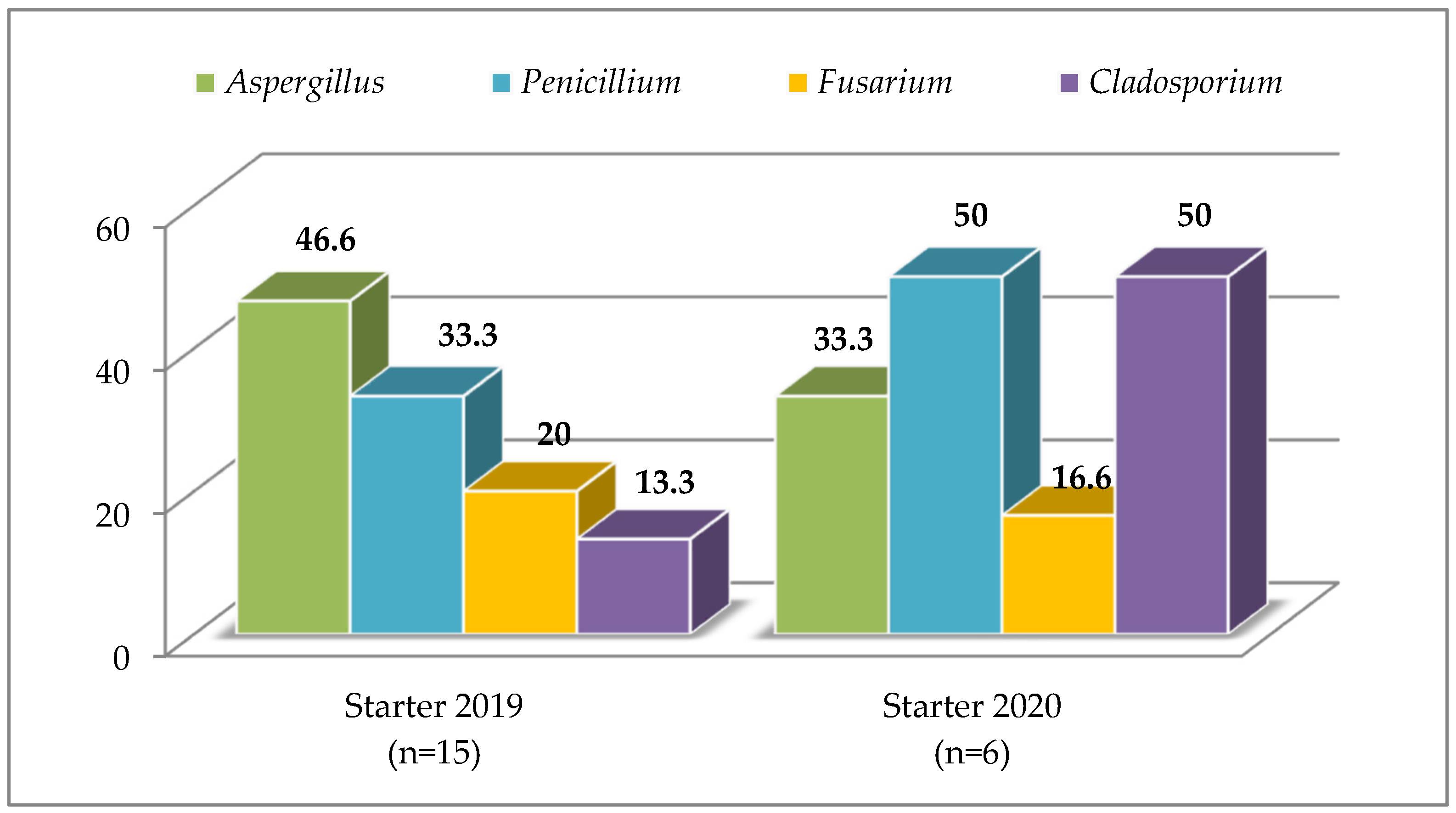
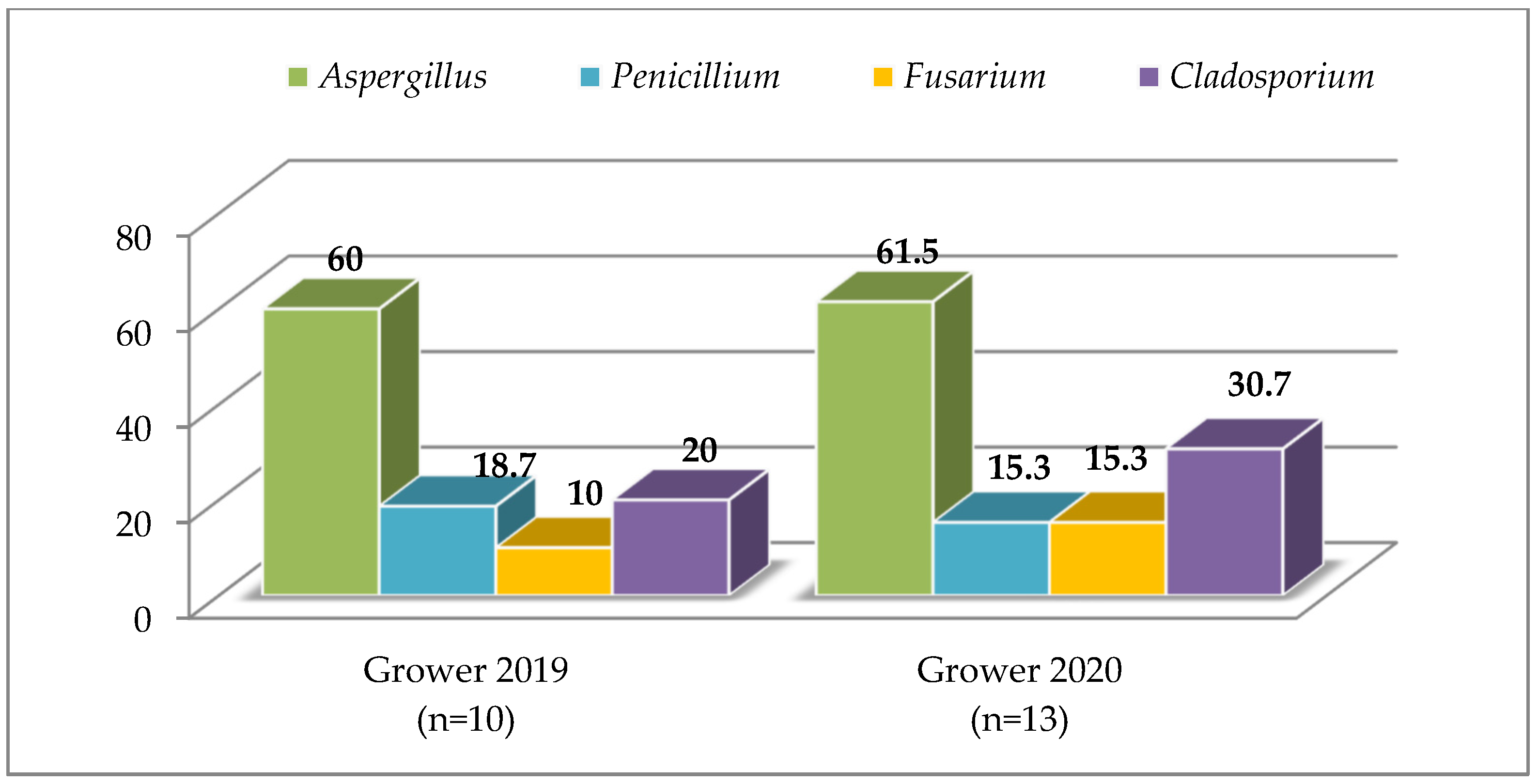
3.2. Compound Feed
4. Discussion
4.1. Raw Materials
4.2. Compound Feed
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Alali, W.Q.; Ricke, S.C. The ecology and control of bacterial pathogens in animal feed. In Animal Feed Contamination-Effects on Livestock and Food Safety, 1st ed.; Fink-Gremmels, J., Ed.; Woodhead Publishing: Cambridge, MA, USA, 2012; pp. 35–55. [Google Scholar] [CrossRef]
- Chatterjee, A.; Abraham, J. Microbial contamination, prevention and early detection in food industry. In Microbial Contamination and Food Degradation; Grumezescu, A.T., Holban, A.M., Eds.; Academic Press: Amsterdam, The Netherlands, 2018; pp. 21–47. [Google Scholar] [CrossRef]
- Wielogórska, E.; Mac Donald, S.; Elliott, C.T. A review of the efficacy of mycotoxin detoxifying agents used in feed in light of changing global environment and legislation. World Mycotoxin J. 2016, 9, 419–433. [Google Scholar] [CrossRef]
- Pinotti, L.; Ottoboni, M.; Giromini, C.; Dell’Orto, V.; Cheli, F. Mycotoxin contamination in the EU feed supply chain: A focus on cereal byproducts. J. Toxins 2016, 8, 45. [Google Scholar] [CrossRef]
- Atungulu, G.G.; Zhong, S.; Thote, A.; Okeyo, A.; Couch, A. Microbial Prevalence on Freshly-Harvested Long-Grain Pureline, Hybrid, and Medium-Grain Rice Cultivars. Appl. Eng. Agric. 2015, 31, 949–956. [Google Scholar] [CrossRef]
- Bento, L.F.; Caneppele, M.A.B.; Albuquerque, M.C.F.; Kobayasti, L.; Caneppele, C.; Andrade, P.J. Occurrence of fungi and aflatoxins in corn kernels. Rev. Do Inst. Adolfo Lutz 2012, 71, 44–49. [Google Scholar]
- Ruiz, M.J.; Macáková, P.; Juan-García, A.; Font, G. Cytotoxic effects of mycotoxin combinations in mammalian kidney cells. Food Chem. Toxicol. 2011, 49, 2718–2724. [Google Scholar] [CrossRef] [PubMed]
- Savi, G.D.; Piacentini, K.C.; Marchi, D.; Scussel, V.M. Fumonisins B1 and B2 in the corn-milling process and corn-based products, and evaluation of estimated daily intake. Food Addit. Contam. A 2016, 33, 339–345. [Google Scholar] [CrossRef]
- Abdollahi, M.R.; Ravindran, V.; Wester, T.J.; Ravindran, G.; Thomas, D.V. Influence of conditioning temperature on the performance, nutrient utilisation and digestive tract development of broilers fed on maize-and wheat-based diets. Brit. Poult. Sci. 2010, 51, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Briyones-Reyes, D.; Gómez-Martinez, L.; Cueva-Rolon, R. Zearalenone contamination in corn for human consumption in the state of Tlaxcala, Mexico. Food Chem. 2007, 100, 693–698. [Google Scholar] [CrossRef]
- Liu, M.T.; Ram, B.P.; Hart, L.P.; Pestka, J.J. Indirect Enzyme-Linked Immunosorbent Assay for the Mycotoxin Zearalenone. Appl. Environ. Microb. 1985, 50, 332–336. [Google Scholar] [CrossRef]
- Bhunia, A.K. Salmonella Enterica. In Foodborne Microbial Pathogens; Springer: New York, NY, USA, 2008; pp. 201–216. [Google Scholar]
- Pui, C.F.; Wong, W.C.; Chai, L.C.; Robin, T.; Ponniah, J.; Hidayah, M.S.; Anyi, U.; Mohamad Ghazali, F.; Cheah, Y.K.; Son, R. Review article Salmonella: A foodborne pathogen. Int. Food Res. J. 2011, 18, 465–473. [Google Scholar]
- Antunes, P.; Mourão, J.; Campos, J.; Peixe, L. Salmonellosis: The role of poultry meat. Clin. Microbiol. Infect. 2016, 22, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Gast, R.K. Serotype-specific and serotype-independent strategies for preharvest control of food-borne Salmonella in poultry. Avian Dis. 2007, 51, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, R.G.; Rosario, D.K.A.; Cunha-Neto, A.; Mano, S.B.; Figueiredo, E.E.S.; Conte-Junior, C.A. Worldwide Epidemiology of Salmonella serovars in animal-based foods: A meta-analysis. Appl. Environ. Microbiol. 2019, 85, e00591-19. [Google Scholar] [CrossRef] [PubMed]
- Popa, G.L.; Popa, M.I. Salmonella spp. Infection—A continuous threat worldwide. Germs 2021, 11, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Ruvalcaba-Gómez, J.M.; Villagrán, Z.; Valdez-Alarcón, J.J.; Martínez-Núñez, M.; Gomez- Godínez, L.J.; Ruesga-Gutiérrez, E.; Anaya-Esparza, L.M.; Arteaga- Garibay, R.I.; Villarruel-López, A. Non-Antibiotics Strategies to Control Salmonella Infection in Poultry. Animals 2022, 12, 102. [Google Scholar] [CrossRef]
- Desin, T.S.; Köster, W.; Potter, A.A. Salmonella vaccines in poultry: Past, present and future. Expert Rev. Vaccines 2013, 12, 87–96. [Google Scholar] [CrossRef]
- Shah, D.H.; Paul, N.C.; Sischo, W.C.; Crespo, R.; Guard, J. Population dynamics and antimicrobial resistance of the most prevalent poultry-associated Salmonella serotypes. Poult. Sci. 2017, 96, 687–702. [Google Scholar] [CrossRef]
- Crouch, C.F.; Pugh, C.; Patel, A.; Brink, H.; Wharmby, C.; Watts, A.; van Hulten, M.C.W.; de Vries, S.P.W. Reduction in intestinal colonization and invasion of internal organs after challenge by homologous and heterologous serovars of Salmonella Enterica following vaccination of chickens with a novel trivalent inactivated Salmonella vaccine. Avian Pathol. 2020, 49, 666–677. [Google Scholar] [CrossRef]
- Cadirci, O.; Gucukoglu, A.; Gulel, G.T.; Gunaydin, E.; Uyanik, T.; Kanat, S. Determination and antibiotic resistance profiles of Salmonella serotypes isolated from poultry meat. Fresenius Environ. Bull. 2021, 30, 4251–4261. [Google Scholar]
- Tauxe, R.V. Salmonella: A Postmodern Pathogen. J. Food Prot. 1991, 54, 563–568. [Google Scholar] [CrossRef]
- Bryan, F.L.; Doyle, M.P. Health Risks and Consequences of Salmonella and Campylobacter jejuni in Raw Poultry. J. Food Prot. 1995, 58, 326–344. [Google Scholar] [CrossRef]
- Jones, F.T.; Axtell, R.C.; Rives, D.V.; Scheideler, S.E.; Tarver, F.R.; Walker, R.L.; Wineland, M.J. A Survey of Salmonella Contamination in Modern Broiler Production. J. Food Prot. 1991, 54, 502–507. [Google Scholar] [CrossRef]
- Maciorowski, K.G.; Jones, F.T.; Pillai, S.D.; Ricke, S.C. Incidence, sources, and control of food-borne Salmonella spp. in poultry feeds. World Poult. Sci. J. 2004, 60, 446–457. [Google Scholar] [CrossRef]
- Cason, J.A.; Cox, N.A.; Bailey, J.S. Transmission of Salmonella typhimurium during Hatching of Broiler Chicks. Avian Dis. 1994, 38, 583–588. [Google Scholar] [CrossRef]
- Petkar, A.; Alali, W.Q.; Harrison, M.A.; Beuchart, L.R. Survival of Salmonella in organic and conventional broiler feeds as affected by temperature and water activity. Agric. Food Anal. Bacteriol. 2011, 1, 175–185. [Google Scholar]
- Blackburn, C.d.W.; Curtis, L.M.; Humpheson, L.; Billon, C.; McClure, P.J. Development of thermal inactivation models for Salmonella enteridis and Escherichia coli O157:H7 with temperature, pH and NaCl as controlling factors. Int. J. Food Microbiol. 1997, 38, 31–44. [Google Scholar] [CrossRef]
- Tenaillon, O.; Skurnik, D.; Picard, B.; Denamur, E. The population genetics of commensal Escherichia coli. Nat. Rev. Microbiol. 2010, 8, 207–217. [Google Scholar] [CrossRef]
- Ali, M.A.; Eldin, T.A.S.; El Moghazy, G.M.; Tork, I.M.; Omara, I.I. Detection of E. coli O157:H7 in feed samples using gold nanoparticles sensor. Int. J. Curr. Microbiol. App. Sci. 2014, 3, 697–708. [Google Scholar]
- Kabir, S.M.L. Avian colibacillosis and salmonellosis: A closer look at epidemiology, pathogenesis, diagnosis, control and public health concerns. Int. J. Environ. Res. Public Health 2010, 7, 89–114. [Google Scholar] [CrossRef]
- Markland, S.M.; LeStrange, K.J.; Sharma, M.; Knie, K.E. Old Friends in New Places: Exploring the Role of xtraintestinal E. coli in Intestinal Disease and Foodborne Illness. Zoonoses Public Health 2015, 62, 491–496. [Google Scholar] [CrossRef]
- Songer, J.G.; Meer, R.R. Genotyping of Clostridium perfringens by Polymerase Chain Reaction is a Useful Adjunct to Diagnosis of Clostridial Enteric Disease in Animals. Anaerobe 1996, 2, 197–203. [Google Scholar] [CrossRef]
- Petit, L.; Gibert, M.; Popoff, M.R. Clostridium perfringens: Toxinotype and genotype. Trends Microbiol. 1999, 7, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Craven, S.E.; Stern, N.J.; Bailey, J.S.; Cox, N.A. Incidence of Clostridium perfringens in broiler chickens and their environment during production and processing. Avian Dis. 2001, 45, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Stanley, D.; Bajagai, Y.S. Feed Safety and the Development of Poultry Intestinal Microbiota. Animals 2022, 12, 2890. [Google Scholar] [CrossRef]
- Schuring, M.; van Gils, B. Clostridium perfringens-a nutritional challenge? Feed Mix 2001, 9, 26–28. [Google Scholar]
- Van Immersel, F.; De Zutter, L.; Houf, K.; Pasmans, F.; Haesebrouck, F.; Ducatelle, R. Strategies to control Salmonella in the broiler production chain. World Poult. Sci. J. 2009, 65, 367–392. [Google Scholar] [CrossRef]
- Buzby, J.; Roberts, T. Economic costs and trade impacts of microbial foodborne ilness. World Health Statist. Quart. 1997, 50, 57–66. [Google Scholar]
- Pop, I.M. Food quality and safety management. In Managementul Calității și Siguranței Alimentelor; Tipo Moldova: Iași, Romania, 2022. [Google Scholar]
- Regulation (EC) No 2160/2003 of the European Parliament and of the Council of 17 November 2003 on the Control of Salmonella and Other Specified Food-Borne Zoonotic Agents. Available online: https://eur-lex.europa.eu/legal-content/en/ALL/?uri=CELEX:32003R2160 (accessed on 26 December 2022).
- Regulation (EC) No 183/2005 of the European Parliament and of the Council of 12 January 2005 Laying down Requirements for Feed Hygiene. Available online: https://leap.unep.org/countries/eu/national-legislation/regulation-ec-no-1832005-european-parliament-and-council-laying#:~:text=(EC)%20No.-,183%2F2005%20of%20the%20European%20Parliament%20and%20of%20the%20Council,down%20requirements%20for%20feed%20hygiene.&text=The%20present%20Regulation%20lays%20down,registration%20and%20approval%20of%20establishments (accessed on 26 December 2022).
- Pop, I.M.; Halga, P.; Avarvarei, T. Animal nutrition and feeding. In Nutriția și Alimentația Animalelor; Tipo Moldova: Iași, Romania, 2006. [Google Scholar]
- SR ISO 21527-2:2009; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Yeasts and Moulds—Part 2: Colony Count Technique in Products with Water Activity Less than or Equal to 0.95. Romanian Standards Association: Bucharest, Romania, 2009.
- SR EN ISO 6579-1:2017; Microbiology of the Food Chain—Horizontal Method for the Detection, Enumeration and Serotyping of Salmonella—Part 1: Detection of Salmonella spp. Romanian Standards Association: Bucharest, Romania, 2017.
- SR ISO 7251:2009; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Detection and Enumeration of Presumptive Escherichia coli—Most Probable Number Technique. Romanian Standards Association: Bucharest, Romania, 2009.
- SR EN ISO 7937:2005; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Clostridium perfringens—Colony-Count Technique. Romanian Standards Association: Bucharest, Romania, 2005.
- Rayat, C.S. Applications of Microsoft Excel in Statistical Methods. In Statistical Methods in Medical Research, 1st ed.; Rayat, C.S., Ed.; Springer: Singapore, 2018; pp. 139–146. [Google Scholar] [CrossRef]
- Creppy, E.E. Update of survey, regulation and toxic effects of mycotoxins in Europe. Toxicol. Lett. 2002, 127, 19–28. [Google Scholar] [CrossRef]
- Pacin, A.M.; González, H.H.L.; Etcheverry, M.; Resnik, S.L.; Vivas, L.; Espin, S. Fungi associated with food and feed commodities from Ecuador. Mycopathologia 2002, 156, 87–92. [Google Scholar] [CrossRef]
- Krnjaja, V.; Stanojković, A.; Stanković, S.Ž.; Lukić, M.; Bijelić, Z.; Mandić, V.; Mićić, N. Fungal contamination of maize grain samples with a special focus on toxigenic genera. Biotechnol. Anim. Husb. 2017, 33, 233–241. [Google Scholar] [CrossRef]
- Marin, S.; Ramos, A.J.; Cano-Sancho, G.; Sanchis, V. Mycotoxins: Occurrence, toxicology, and exposure assessment. Food Chem. Toxicol. 2013, 60, 218–237. [Google Scholar] [CrossRef]
- Völkel, I.; Schröer-Merker, E.; Czerny, C.P. The carry-over of mycotoxins in products of animal origin with special regards to its implications for the european food safety legislation. Food Nutr. Sci. 2011, 2, 852–867. [Google Scholar] [CrossRef]
- Khoshal, A.K.; Novak, B.; Martin, P.G.P.; Jenkins, T.; Neves, M.; Schatzmayr, G.; Oswald, I.P.; Pinton, P. Co-Occurrence of DON and emerging mycotoxins in worldwide finished pig feed and their combined toxicity in intestinal cells. Toxins 2019, 11, 727. [Google Scholar] [CrossRef] [PubMed]
- Jones, F.T.; Richardson, K.E. Salmonella in commercially manufactured feeds. Poult. Sci. 2004, 83, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Ge, B.; LaFon, P.C.; Carter, P.J.; McDermott, S.D.; Abbott, J.; Glenn, A.; Ayers, S.L.; Friedman, S.L.; Paige, J.C.; Wagner, D.D.; et al. Retrospective Analysis of Salmonella, Campylobacter, Escherichia coli, and Enterococcus in Animal Feed Ingredients. Foodborne Pathog. Dis. 2013, 10, 684–691. [Google Scholar] [CrossRef]
- Da Costa, P.M.; Oliveira, M.; Bica, A.; Vaz-Pires, P.; Bernardo, F. Antimicrobial resistance in Enterococcus spp. and Escherichia coli isolated from poultry feed and feed ingredients. Vet. Microbiol. 2007, 120, 122–131. [Google Scholar] [CrossRef]
- Casagrande, M.F.; Cardozo, M.V.; Beraldo-Massoli, M.C.; Boarini, L.; Longo, F.A.; Paulilo, A.C.; Schocken-Iturrino, R.P. Clostridium perfringens in ingredients of poultry feed and control of contamination by chemical treatments. J. Appl. Poultry Res. 2013, 22, 771–777. [Google Scholar] [CrossRef]
- Priό, P.; Gasol, R.; Soriano, R.C.; Pérez-Rigau, A. Effect of raw material microbial contamination over microbiological profile of ground and pelleted feeds. Cah. Opt. Méditerran. 2001, 54, 197–199. [Google Scholar]
- Udhayavel, S.; Thippichettypalayam Ramasamy, G.; Gowthaman, V.; Malmarugan, S.; Senthilvel, K. Occurrence of Clostridium perfringens contamination in poultry feed ingredients: Isolation, identification and its antibiotic sensitivity pattern. Anim. Nutr. 2017, 3, 309–312. [Google Scholar] [CrossRef]
- Order no. 249 of March 31, 2003 for the Approval of the Norms Regarding the Quality and Sanitation Parameters for the Production, Import, Quality Control, Marketing and Use of Simple, Compound Feeds, Feed Additives, Premixtures, Energy Substances, Mineral Substances and Special Feeds; Ministry of Agriculture, Food and Forest of Romania: București, Romania, 2003.
- Order no. 110 of June 16, 2020 for the Approval of the Veterinary Sanitary Norm Regarding the Maximum Limits Provided for Microorganisms in Certain Categories of Feed; National Veterinary Sanitary and Food Safety Authority: București, Romania, 2020.
- Dalcero, A.; Magnoli, C.; Luna, M.; Ancasi, G.; Reynoso, M.M.; Chiacchiera, S.; Miazzo, R.; Palacio, G. Mycoflora and naturally occurring mycotoxins in poultry feeds in Argentina. Mycopathologia 1998, 141, 37–43. [Google Scholar] [CrossRef]
- Rosa, C.A.R.; Ribeiro, J.M.M.; Fraga, M.J.; Gatti, M.; Cavaglieri, L.R.; Magnoli, C.E.; Dalcero, A.M.; Lopes, C.W.G. Mycoflora of poultry feeds and ochratoxin-producing ability of isolated Aspergillus and Penicillium species. Vet. Microbiol. 2006, 113, 89–96. [Google Scholar] [CrossRef]
- Shareef, A.M. Molds and mycotoxins in poultry feeds from farms of potential mycotoxicosis. Iraqui J. Vet. Sci. 2010, 24, 17–25. [Google Scholar] [CrossRef]
- Cegielska-Radziejewska, R.; Stuper, K.; Szablewski, T. Microflora and mycotoxin contamination in poultry feed mixtures from western Poland. Ann. Agric. Environ. Med. 2013, 20, 30–35. [Google Scholar]
- Greco, M.V.; Franchi, M.L.; Rico Golba, S.L.; Pardo, A.G.; Pose, G.N. Mycotoxins and Mycotoxigenic Fungi in Poultry Feed for Food-Producing Animals. Sci. World J. 2014, 2014, 968215. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.E. Salmonellas in poultry feeds—A worldwide review. Poult. Sci. J. 1981, 37, 97–105. [Google Scholar] [CrossRef]
- MacKenzie, M.A.; Bains, B.S. Dissemination of Salmonella serotypes from raw feed ingredients to chicken carcases. Poult. Sci. 1976, 55, 957–960. [Google Scholar] [CrossRef] [PubMed]
- Parker, E.M.; Edwards, L.J.; Mollenkopf, D.F.; Ballash, G.A.; Wittum, T.E.; Parker, A.J. Salmonella monitoring programs in Australian feed mills: A retrospective analysis. Aust. Vet. J. 2019, 97, 336–342. [Google Scholar] [CrossRef]
- Sokolovic, M.; Šimpraga, B.; Amšel-Zelenika, T.; Berendika, M.; Krstulovi’c, F. Prevalence and Characterization of Shiga Toxin Producing Escherichia coli Isolated from Animal Feed in Croatia. Microorganisms 2022, 10, 1839. [Google Scholar] [CrossRef]
- Ge, B.; Domesle, K.J.; Gaines, S.A.; Lam, C.; Bodeis Jones, S.M.; Yang, Q.; Ayers, S.L.; McDermott, P.F. Prevalence and Antimicrobial Susceptibility of Indicator Organisms Escherichia coli and Enterococcus spp. Isolated from U.S. Animal Food, 2005–2011. Microorganisms 2020, 8, 1048. [Google Scholar] [CrossRef]
- Schocken-Iturrino, R.P.; Vittori, J.; Beraldo-Massoli, M.C.; Delphino, T.P.C.; Damasceno, P.R. Clostridium perfringens search in water and ration used in the raising of broiler in sheds of São Paulo State–Brazil. Ciência Rural 2010, 40, 197–199. [Google Scholar] [CrossRef][Green Version]
- Tessari, E.N.C.; Cardoso, A.L.S.P.; Kanashiro, A.M.I.; Stoppa, G.F.Z.; Luciano, R.L.; de Castro, A.G.M. Analysis of the presence of Clostridium perfringens in feed and raw material used in poultry production. Food Nutr. Sci. 2014, 5, 614–617. [Google Scholar] [CrossRef]
| Specification | 2019 Year | 2020 Year | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Positive (%) | s | Min. | Max. | n | Positive (%) | s | Min. | Max. | ||||
| Yeasts and molds (cfu/g) | Maize | 17 | 88.2 | 1.3 × 103 | 12.4 × 102 | 6.0 × 102 | 4.3 × 103 | 13 | 84.6 | 1.5 × 103 | 1.6 × 103 | 4 × 102 | 4.9 × 103 |
| Wheat | 4 | 100 | 9.5 × 102 | 9.7 × 102 | 4.0 × 102 | 2.4 × 103 | 4 | 100 | 1.0 × 103 | 1.1 × 103 | 4 × 102 | 2.8 × 103 | |
| Soybean meal | 27 | 92.5 | 6.4 × 102 | 2.5 × 102 | 4.0 × 102 | 1.0 × 103 | 28 | 85.7 | 5.2 × 102 | 1.9 × 102 | 4 × 102 | 1.0 × 103 | |
| Sunflower meal | 7 | 100 | 7.4 × 102 | 2.5 × 102 | 4.0 × 102 | 1.0 × 103 | 3 | 100 | 7.1 × 102 | 3.0 × 102 | 4 × 102 | 1.0 × 103 | |
| Salmonella spp. /25 g | Maize | 8 | 0 | 0 | 0 | absent/25 g | 0 | - | - | - | - | - | |
| Wheat | 4 | 0 | 0 | 0 | absent/25 g | 2 | 0 | 0 | 0 | absent/25 g | |||
| Soybean meal | 32 | 0 | 0 | 0 | absent/25 g | 39 | 0 | 0 | 0 | absent/25 g | |||
| Sunflower meal | 10 | 0 | 0 | 0 | absent/25 g | 4 | 0 | 0 | 0 | absent/25 g | |||
| E.coli (cfu/g) | Maize | 7 | 0 | 0 | 0 | 0 | 0 | 0 | - | - | - | - | - |
| Wheat | 3 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | |
| Soybean meal | 31 | 0 | 0 | 0 | 0 | 0 | 39 | 0 | 0 | 0 | 0 | 0 | |
| Sunflower meal | 10 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | |
| Clostridium perfringens (cfu/g) | Maize | 5 | 0 | 0 | 0 | 0 | 0 | 0 | - | - | - | - | - |
| Wheat | 4 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | |
| Soybean meal | 17 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | |
| Sunflower meal | 5 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | |
| Specification | 2019 Year | 2020 Year | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Positive (%) | s | Min. | Max. | n | Positive (%) | s | Min. | Max. | ||||
| Yeasts and molds (cfu/g) | Starter | 30 | 50.0 | 5.9 × 102 | 6.4 × 102 | 4.0 × 102 | 2.9 × 103 | 14 | 42.8 | 5.3 × 102 | 1.9 × 102 | 4.0 × 102 | 9.0 × 102 |
| Grower | 26 | 38.4 | 4.2 × 102 | 6.3 × 101 | 4.0 × 102 | 6.0 × 102 | 29 | 44.8 | 6.5 × 102 | 6.4 × 102 | 4.0 × 102 | 26.5 × 102 | |
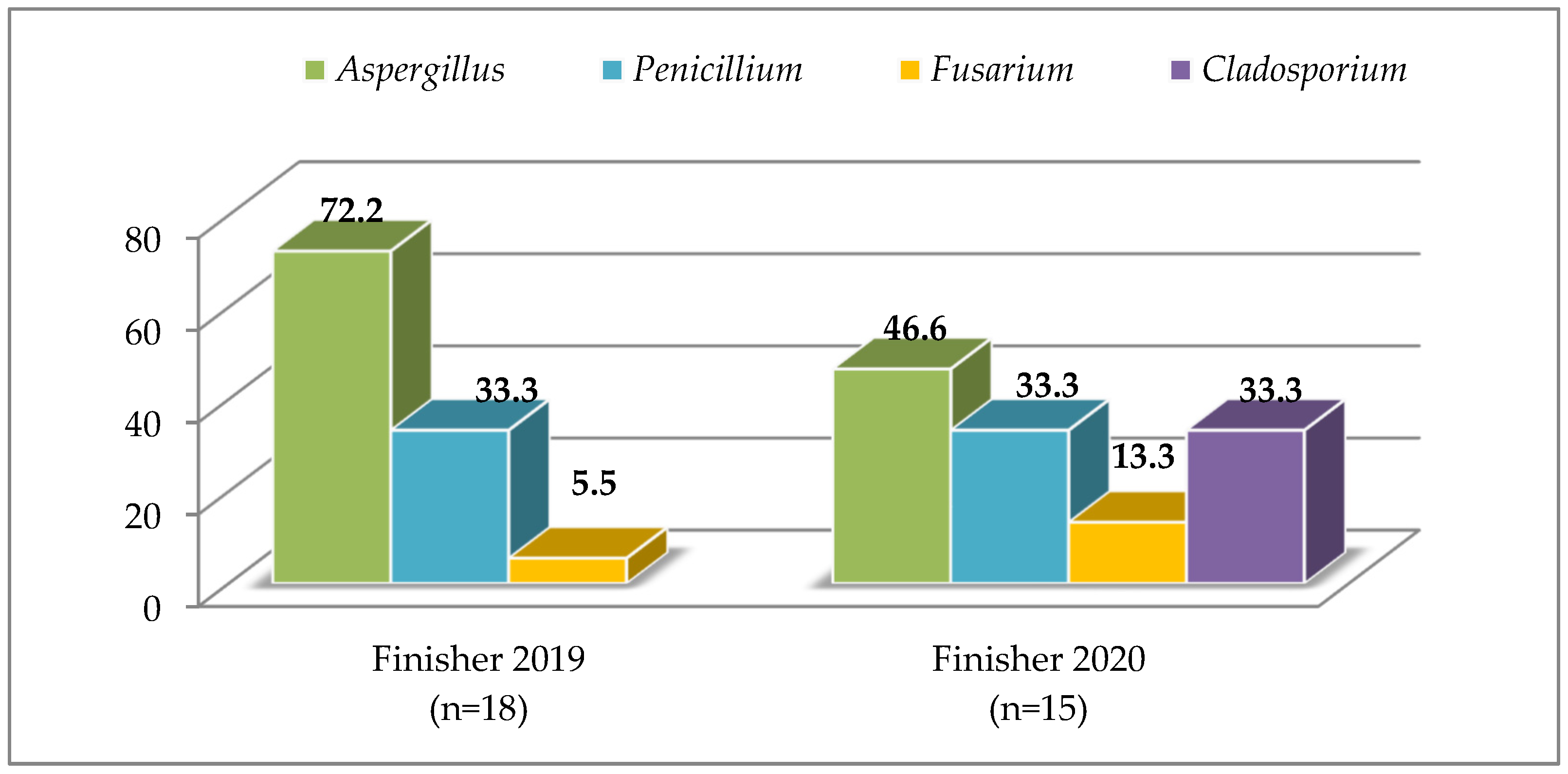
| Finisher | 48 | 37.5 | 4.2 × 102 | 3.3 × 101 | 4.0 × 102 | 7.0 × 102 | 37 | 40.5 | 5.8 × 102 | 5.1 × 102 | 4.0 × 102 | 23.5 × 102 | |
| Salmonella spp. /25 g | Starter | 31 | 0 | 0 | 0 | absent/25 g | 14 | 0 | 0 | 0 | absent/25 g | ||
| Grower | 26 | 0 | 0 | 0 | absent/25 g | 27 | 0 | 0 | 0 | absent/25 g | |||
| Finisher | 48 | 0 | 0 | 0 | absent/25 g | 37 | 0 | 0 | 0 | absent/25 g | |||
| E.coli (cfu/g) | Starter | 29 | 0 | 0 | 0 | 0 | 0 | 14 | 0 | 0 | 0 | 0 | 0 |
| Grower | 25 | 0 | 0 | 0 | 0 | 0 | 27 | 0 | 0 | 0 | 0 | 0 | |
| Finisher | 47 | 0 | 0 | 0 | 0 | 0 | 37 | 0 | 0 | 0 | 0 | 0 | |
| Clostridium perfringens (cfu/g) | Starter | 13 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 |
| Grower | 13 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | |
| Finisher | 24 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lăpușneanu, D.M.; Simeanu, D.; Radu-Rusu, C.-G.; Zaharia, R.; Pop, I.M. Microbiological Assessment of Broiler Compound Feed Production as Part of the Food Chain—A Case Study in a Romanian Feed Mill. Agriculture 2023, 13, 107. https://doi.org/10.3390/agriculture13010107
Lăpușneanu DM, Simeanu D, Radu-Rusu C-G, Zaharia R, Pop IM. Microbiological Assessment of Broiler Compound Feed Production as Part of the Food Chain—A Case Study in a Romanian Feed Mill. Agriculture. 2023; 13(1):107. https://doi.org/10.3390/agriculture13010107
Chicago/Turabian StyleLăpușneanu, Dragoș Mihai, Daniel Simeanu, Cristina-Gabriela Radu-Rusu, Roxana Zaharia, and Ioan Mircea Pop. 2023. "Microbiological Assessment of Broiler Compound Feed Production as Part of the Food Chain—A Case Study in a Romanian Feed Mill" Agriculture 13, no. 1: 107. https://doi.org/10.3390/agriculture13010107
APA StyleLăpușneanu, D. M., Simeanu, D., Radu-Rusu, C.-G., Zaharia, R., & Pop, I. M. (2023). Microbiological Assessment of Broiler Compound Feed Production as Part of the Food Chain—A Case Study in a Romanian Feed Mill. Agriculture, 13(1), 107. https://doi.org/10.3390/agriculture13010107







