Construction of a Chlorophyll Content Prediction Model for Predicting Chlorophyll Content in the Pericarp of Korla Fragrant Pears during the Storage Period
Abstract
:1. Introduction
2. Materials and Methods
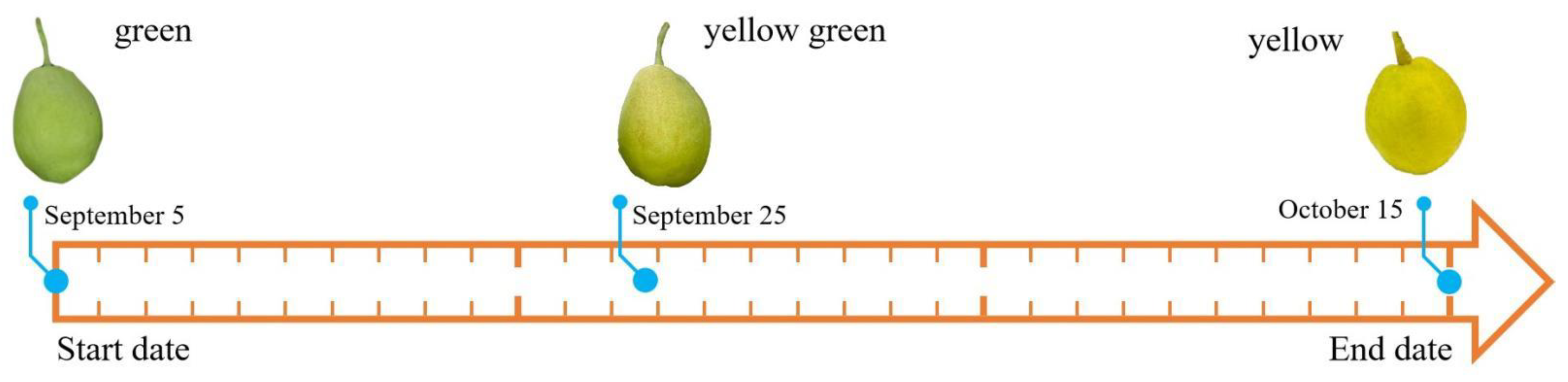
2.1. Sample Preparation
2.2. Measurement of Chlorophyll Content in the Pericarp
2.3. Modeling Methods
2.3.1. BPNN Model
2.3.2. GRNN Model
2.3.3. ANFIS Model
2.3.4. Determination of the Optimal Prediction Model
3. Results and Analysis
3.1. Variation Laws of Chlorophyll Content in The Pericarp of Fragrant Pears during the Storage Period
3.2. Prediction Model for Chlorophyll Content in the Pericarp of Fragrant Pears
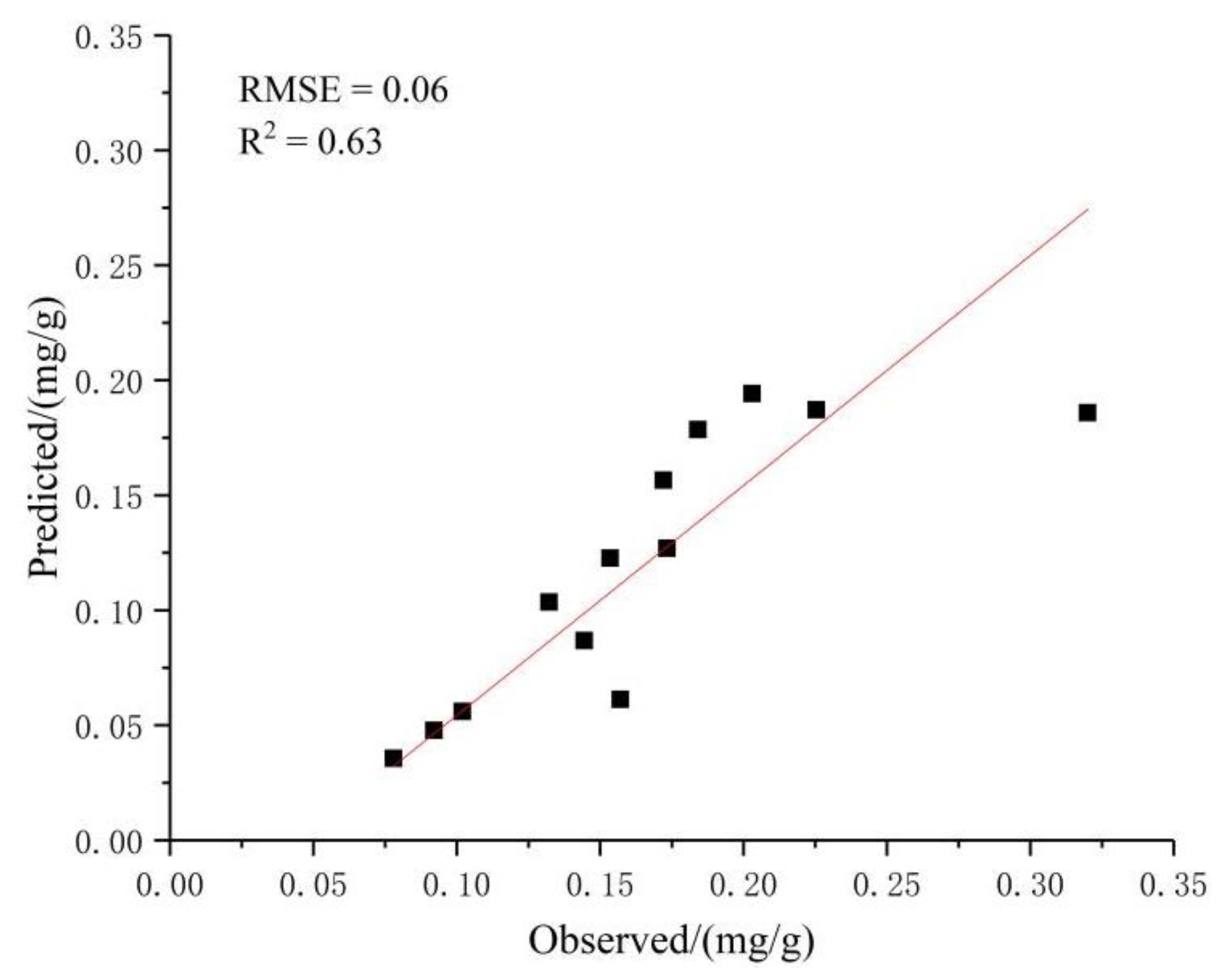
3.2.1. Prediction of Chlorophyll Content in the Pericarp Based on the BPNN Model
3.2.2. Prediction of Chlorophyll Content in the Pericarp Based on the GRNN Model
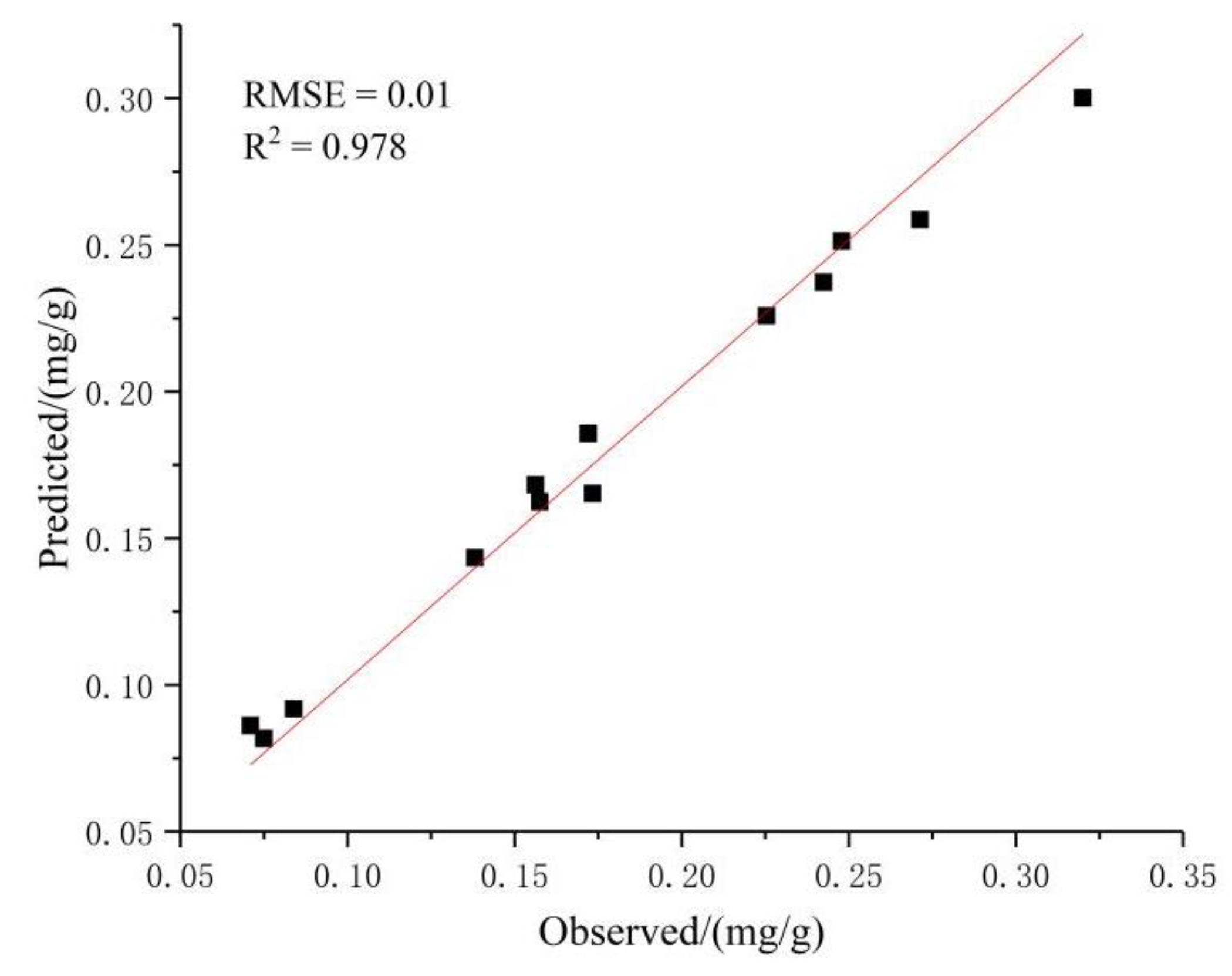
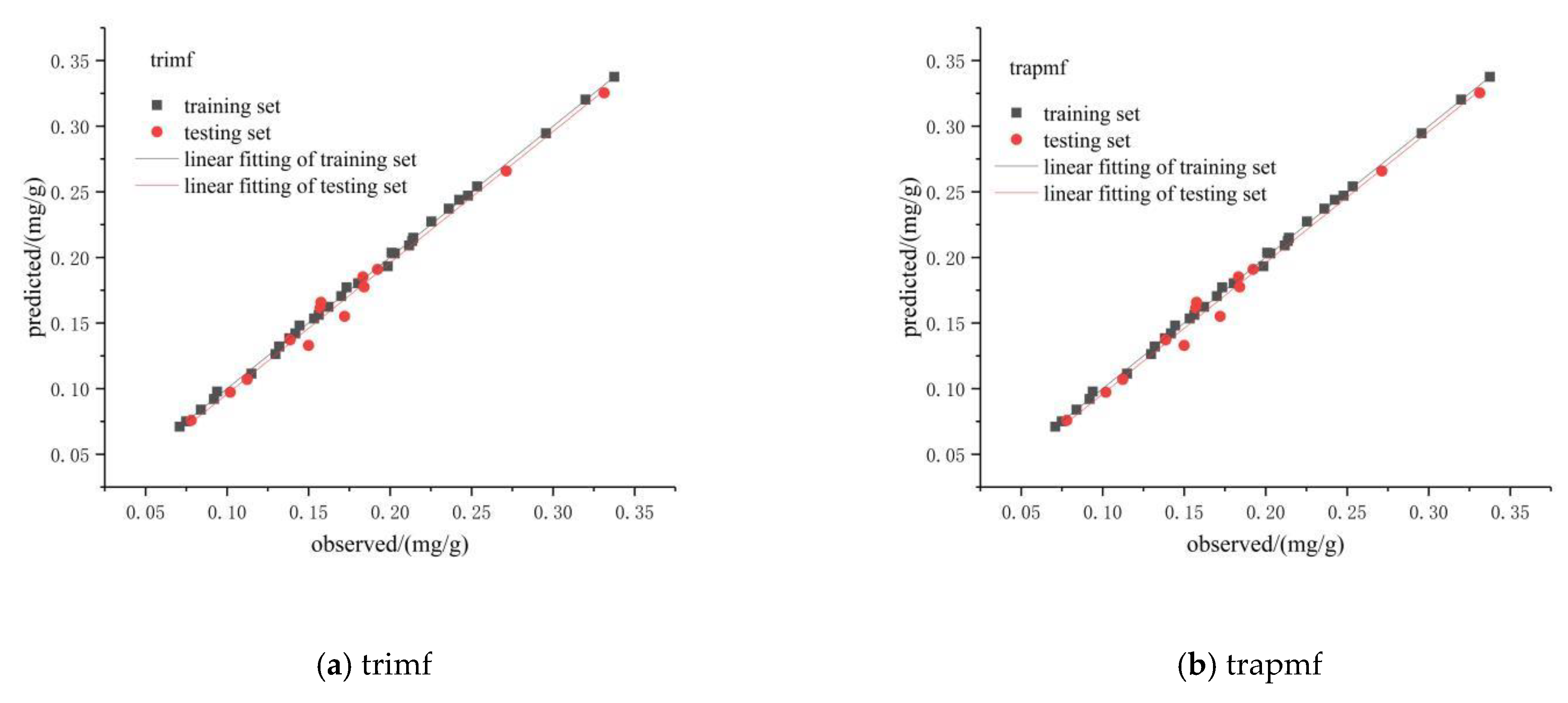
3.2.3. Prediction of Chlorophyll Content in the Pericarp Based on the ANFIS Model
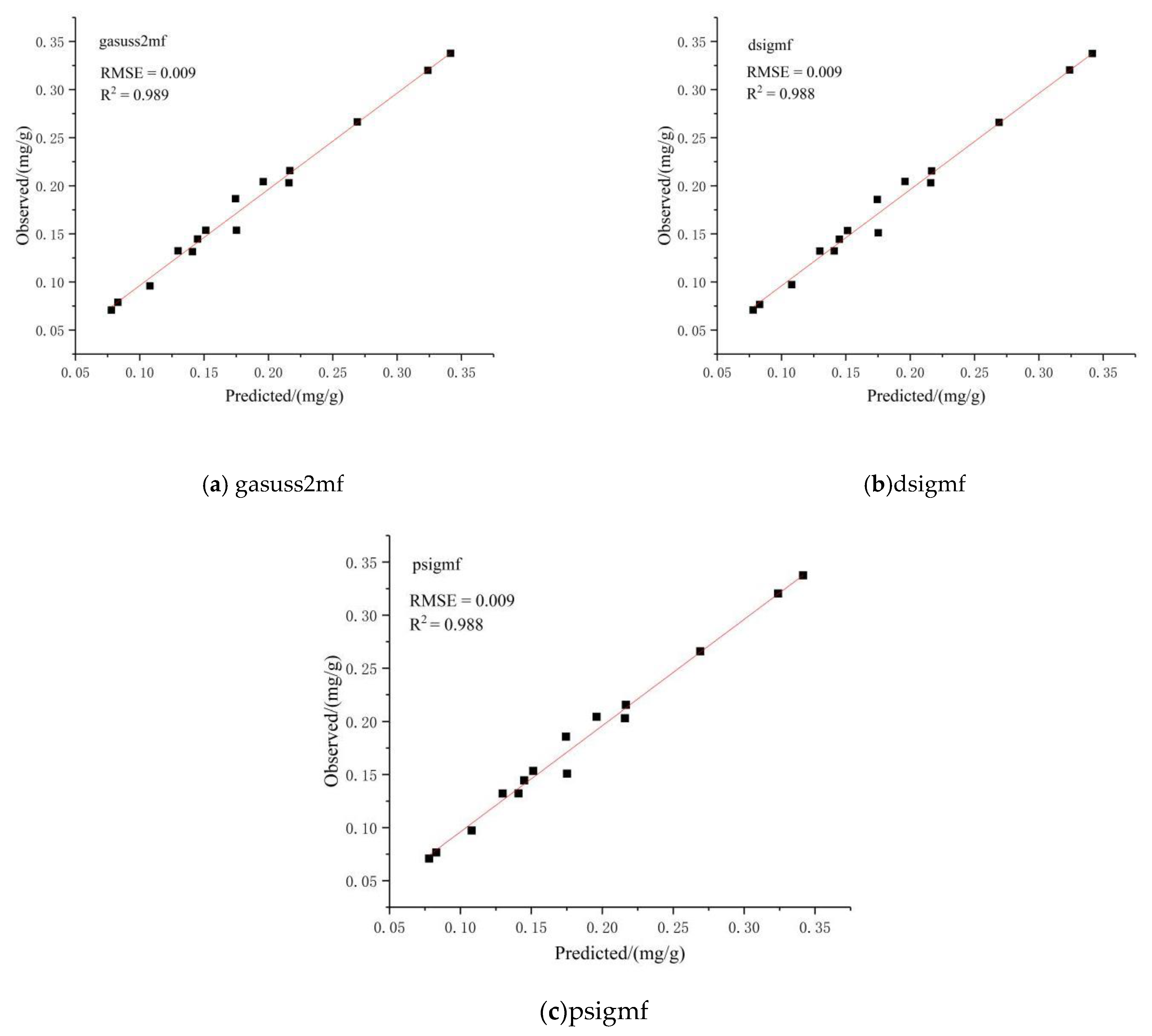
3.2.4. Determining the Optimal Prediction Model
3.2.5. Model Verification
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Y.; Wang, T.; Su, R.; Hu, C.; Chen, F.; Cheng, J. Quantitative evaluation of color, firmness, and soluble solid content of Korla fragrant pears via IRIV and LS-SVM. Agriculture 2021, 11, 731. [Google Scholar] [CrossRef]
- Wu, J.; Guo, K.Q. Dynamic viscoelastic behaviour and microstructural changes of Korla pear (Pyrus bretschneideri rehd) under varying turgor levels. Biosyst. Eng. 2010, 106, 485–492. [Google Scholar] [CrossRef]
- Wang, B.-H.; Sun, X.-X.; Dong, F.-Y.; Zhang, F.; Niu, J.-X. Cloning and expression analysis of an MYB gene associated with calyx persistence in Korla fragrant pear. Plant Cell Rep. 2014, 33, 1333–1341. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, X.; Li, Z.; Zhang, X.; Jemric, T.; Wang, X. Quality monitoring and analysis of Xinjiang ‘Korla’ fragrant pear in cold chain logistics and home storage with multi-sensor technology. Appl. Sci. 2019, 9, 3895. [Google Scholar] [CrossRef]
- Li, X.; Zhang, H.; Han, J.; Teng, K. Changes in postharvest physiology and quality of fragrant pear fruit with different maturities at harvest. Xinjiang Agric. Sci. 2007, 44, 264–267. [Google Scholar]
- Kan, C.; Liu, S.; Chen, M.; Chen, C.; Gao, Y.; Chen, J. Effects of different harvest time on fruit color and texture of Cuiguan pear during shelf life. Acta Agric. Univ. Jiangxiensis 2018, 40, 49–55. [Google Scholar]
- Lan, H.; Tang, Y.; Zhang, H.; An, J.; Liu, W.; Li, F. A Experimental Study on the Variation of Physical-Chemical Indicators for Korla Fragrant Pear in the Maturation Stage. In Proceedings of the 2014 Fifth International Conference on Intelligent Systems Design and Engineering Applications, Zhangjiajie, China, 15–16 June 2014. [Google Scholar]
- Rizzolo, A.; Grassi, M.; Vanoli, M. Influence of storage (time, temperature, atmosphere) on ripening, ethylene production and texture of 1-MCP treated ‘Abbé Fétel’ pears. Postharvest Biol. Technol. 2015, 109, 20–29. [Google Scholar] [CrossRef]
- Choi, J.-H.; Yim, S.-H.; Kim, S.-J.; Lee, H.-C.; Kwon, Y.-H.; Park, Y.-S.; Jung, S.-K.; Choi, H.-S. Effect of harvest date on fruit quality and core breakdown of ‘Wonhwang’ Pears. Korean J. Org. Agric. 2015, 23, 103–112. [Google Scholar] [CrossRef]
- Saquet, A.A. Storage of pears. Sci. Hortic. 2019, 246, 1009–1016. [Google Scholar] [CrossRef]
- Lan, H.; Jia, F.; Tang, Y.; Zhang, Q.; Han, Y.; Liu, Y. Quantity evaluation method of maturity for Korla fragrant pear. Trans. CSAE 2015, 31, 325–330. [Google Scholar]
- Niu, H.; Liu, Y.; Wang, Z.; Zhang, H.; Zhang, Y.; Lan, H. Effects of harvest maturity and storage time on storage quality of Korla fragrant pear based on GRNN and ANFIS models: Part I Firmness Study. Food Sci. Technol. Res. 2020, 26, 363–372. [Google Scholar] [CrossRef]
- Wang, Z.; Tang, Y.; Liu, Y.; Zhang, H.; Zhang, Y.; Lan, H. Inhibitory effect of CaCl2 and carboxymethyl chitosan coating on the after-ripening of Korla fragrant pears in cold storage. Int. J. Food Sci. Technol. 2021, 56, 6777–6790. [Google Scholar] [CrossRef]
- Jia, X.-H.; Wang, W.-H.; Du, Y.-M.; Tong, W.; Wang, Z.-H.; Gul, H. Optimal storage temperature and 1-MCP treatment combinations for different marketing times of Korla Xiang pears. J. Integr. Agric. 2018, 17, 693–703. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Y.; Zhou, Y.; Zhang, H.; Niu, H.; Lan, H. Prediction method of changes in storage quality of Korla fragrant pear based on kinetic modeling. Int. Agric. Eng. J. 2020, 29, 245–254. [Google Scholar]
- Yu, S.; Lan, H.; Li, X.; Zhang, H.; Zeng, Y.; Niu, H.; Niu, X.; Xiao, A.; Liu, Y. Prediction method of shelf life of damaged Korla fragrant pears. J. Food Process Eng. 2021, 44, e13902. [Google Scholar] [CrossRef]
- Mohammadi Torkashvand, A.; Ahmadi, A.; Gómez, P.A.; Maghoumi, M. Using artificial neural network in determining postharvest LIFE of kiwifruit. J. Sci. Food Agric. 2019, 99, 5918–5925. [Google Scholar] [CrossRef]
- Lan, H.; Wang, Z.; Niu, H.; Zhang, H.; Zhang, Y.; Tang, Y.; Liu, Y. A nondestructive testing method for soluble solid content in Korla fragrant pears based on electrical properties and artificial neural network. Food Sci. Nutr. 2020, 8, 5172–5181. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Q.; Niu, H.; Zhang, H.; Lan, H.; Zeng, Y.; Jia, F. Prediction method for nutritional quality of Korla pear during storage. Int. J. Agric. Biol. Eng. 2021, 14, 247–254. [Google Scholar] [CrossRef]
- Guo, W.; Fang, L.; Liu, D.; Wang, Z. Determination of soluble solids content and firmness of pears during ripening by using dielectric spectroscopy. Comput. Electron. Agric. 2015, 117, 226–233. [Google Scholar] [CrossRef]
- T/XLXH001-2019, Korla Pear Group Standard. Korla Fragrant Pear Association of Bayingolin Mongolian Autonomous Prefecture. 2019, p. 29. Available online: www.ttbz.org.cn/Home/Show/8587?tdsourcetag=s_pcqq_aiomsg (accessed on 1 August 2019).
- Gao, J. Experimental Guidance for Plant Physiology, 1st ed.; Higher Education Press: Beijing, China, 2006; pp. 74–76. [Google Scholar]
- Wei, Y. Studies on the Different Mechanism of Pigment Development in ‘Nanguo’ Pear and ‘Nanhong’ Pear. Master’s Thesis, Shenyang Agricultural University, Shenyang, China, 2016. [Google Scholar]
- Ma, W.; Tian, Y.; Zhao, T.; Zhang, Q. Changes of weight loss rate, chlorophyll and wax content in different pear varieties (lines) during storage. North. Fruits 2019, 4, 5–12. [Google Scholar]
- Sun, G.-D.; Qin, L.-A.; Hou, Z.-H.; Jing, X.; He, F.; Tan, F.-F.; Zhang, S.-L.; Zhang, S.-C. Feasibility analysis for acquiring visibility based on lidar signal using genetic algorithm-optimized back propagation algorithm. Chin. Phys. B 2019, 28, 024213. [Google Scholar] [CrossRef]
- Heddam, S. Generalized regression neural network based approach as a new tool for predicting total dissolved gas (TDG) downstream of spillways of dams: A case study of columbia river basin dams, USA. Environ. Processes 2017, 4, 235–253. [Google Scholar] [CrossRef]
- Taghinezhad, E.; Kaveh, M.; Szumny, A. Optimization and prediction of the drying and quality of turnip slices by convective-infrared dryer under various pretreatments by RSM and ANFIS Methods. Foods 2021, 10, 284. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Zheng, H.; Mantri, N.; Qi, Z.; Zhang, X.; Hou, Z.; Chang, J.; Liu, H.; Liang, Z. Prediction of relationship between surface area, temperature, storage time and ascorbic acid retention of fresh-cut pineapple using adaptive neuro-fuzzy inference system (ANFIS). Postharvest Biol. Technol. 2016, 113, 1–7. [Google Scholar] [CrossRef]
- Lan, H.; Zhang, Q.; Tang, Y.; Liu, Y.; Zhang, H.; Jia, F. Research of the maturity law and the evaluation method for the ripeness of the Korla fragrant pear based on the effective accumulated temperature. Int. Agric. Eng. J. 2016, 25, 10–19. [Google Scholar]
- Cheng, Y.; Dong, Y.; Yan, H.; Ge, W.; Shen, C.; Guan, J.; Liu, L.; Zhang, Y. Effects of 1-MCP on chlorophyll degradation pathway-associated genes expression and chloroplast ultrastructure during the peel yellowing of Chinese pear fruits in storage. Food Chem. 2012, 135, 415–422. [Google Scholar] [CrossRef]
- Bernhard, K. Chlorophyll breakdown and chlorophyll catabolites in leaves and fruit. Photochem. Photobiol. Sci. 2008, 7, 1114–1120. [Google Scholar]
- Dong, Y.; Zhao, Q.; Guan, J.; Gao, M.; Li, Y. Effect of 1-MCP on the postharvest fruit softening, chlorophyll content and peel structure in Zaokui pear. J. Agric. Univ. Hebei 2013, 36, 33–37. [Google Scholar]

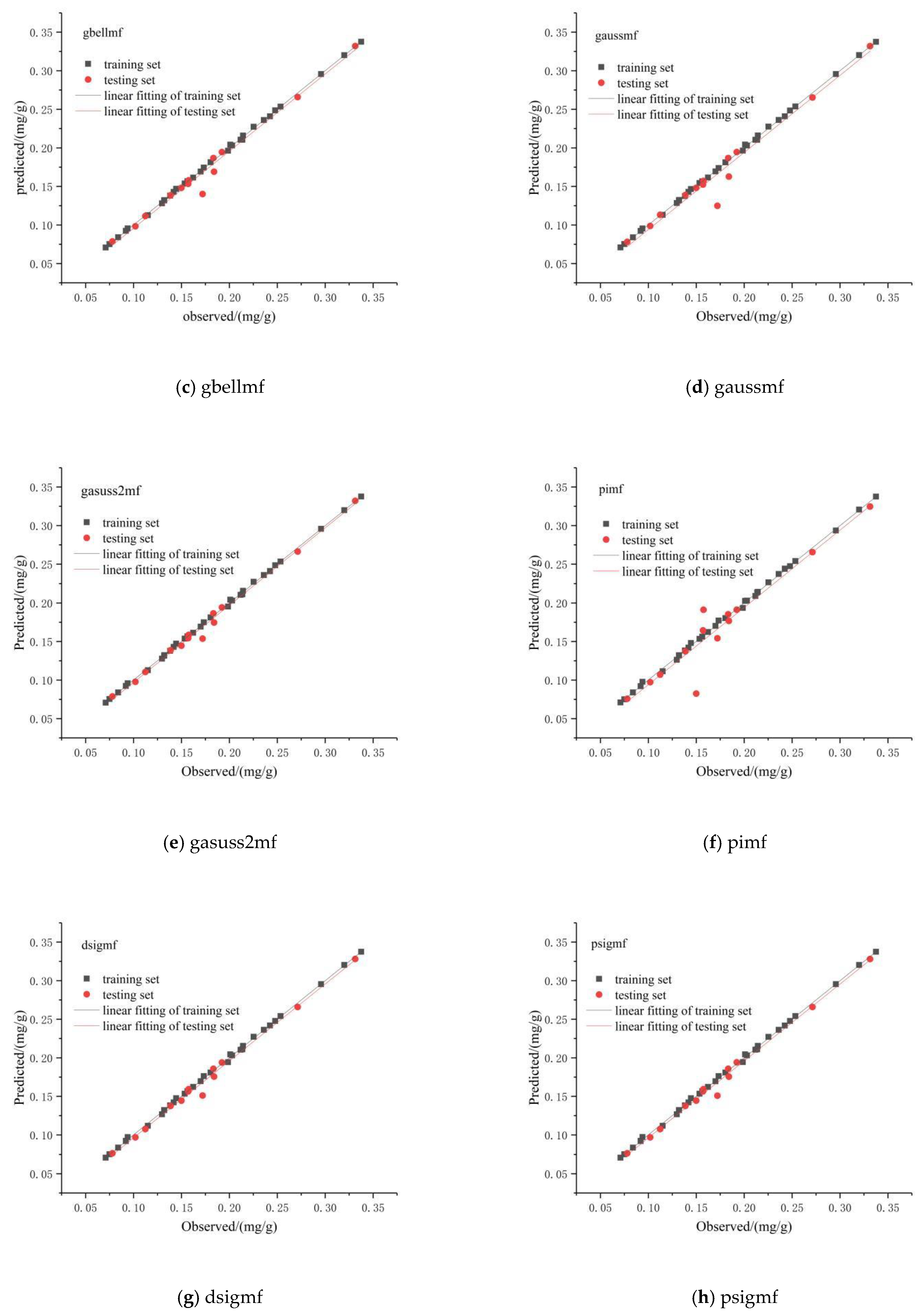
| Membership Function | Training Stage | Prediction Stage | ||
|---|---|---|---|---|
| RMSE | R2 | RMSE | R2 | |
| trimf | 0.001 | 0.999 | 0.018 | 0.989 |
| trapmf | 0.002 | 0.999 | 0.008 | 0.989 |
| gbellmf | 0.001 | 0.999 | 0.010 | 0.980 |
| gaussmf | 0.001 | 0.999 | 0.015 | 0.959 |
| gasuss2mf | 0.001 | 0.999 | 0.006 | 0.993 |
| pimf | 0.002 | 0.999 | 0.022 | 0.905 |
| dsigmf | 0.002 | 0.999 | 0.007 | 0.992 |
| psigmf | 0.002 | 0.999 | 0.007 | 0.992 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Zhao, J.; Tang, Y.; Jiang, X.; Liao, J. Construction of a Chlorophyll Content Prediction Model for Predicting Chlorophyll Content in the Pericarp of Korla Fragrant Pears during the Storage Period. Agriculture 2022, 12, 1348. https://doi.org/10.3390/agriculture12091348
Liu Y, Zhao J, Tang Y, Jiang X, Liao J. Construction of a Chlorophyll Content Prediction Model for Predicting Chlorophyll Content in the Pericarp of Korla Fragrant Pears during the Storage Period. Agriculture. 2022; 12(9):1348. https://doi.org/10.3390/agriculture12091348
Chicago/Turabian StyleLiu, Yang, Jinfei Zhao, Yurong Tang, Xin Jiang, and Jiean Liao. 2022. "Construction of a Chlorophyll Content Prediction Model for Predicting Chlorophyll Content in the Pericarp of Korla Fragrant Pears during the Storage Period" Agriculture 12, no. 9: 1348. https://doi.org/10.3390/agriculture12091348
APA StyleLiu, Y., Zhao, J., Tang, Y., Jiang, X., & Liao, J. (2022). Construction of a Chlorophyll Content Prediction Model for Predicting Chlorophyll Content in the Pericarp of Korla Fragrant Pears during the Storage Period. Agriculture, 12(9), 1348. https://doi.org/10.3390/agriculture12091348









