Genome-Wide Identification, In Silico Analysis and Expression Profiling of SWEET Gene Family in Loquat (Eriobotrya japonica Lindl.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Identification and Characterization of EjSWEET Genes
2.2. Gene Structure, Conserved Motif, and Promoter Region Analyses of EjSWEET Genes
2.3. Chromosomal Mapping and Syntenic Analysis of SWEETs in Loquat
2.4. Ka and Ks Calculation
2.5. Multiple Sequence Alignment and Phylogenetic Analysis
2.6. Plant Sampling, RNA Isolation, and Quantitative RT-PCR Analysis
2.7. Statistical Analysis
3. Results
3.1. Identification and Characterization of EjSWEET Genes in Loquat
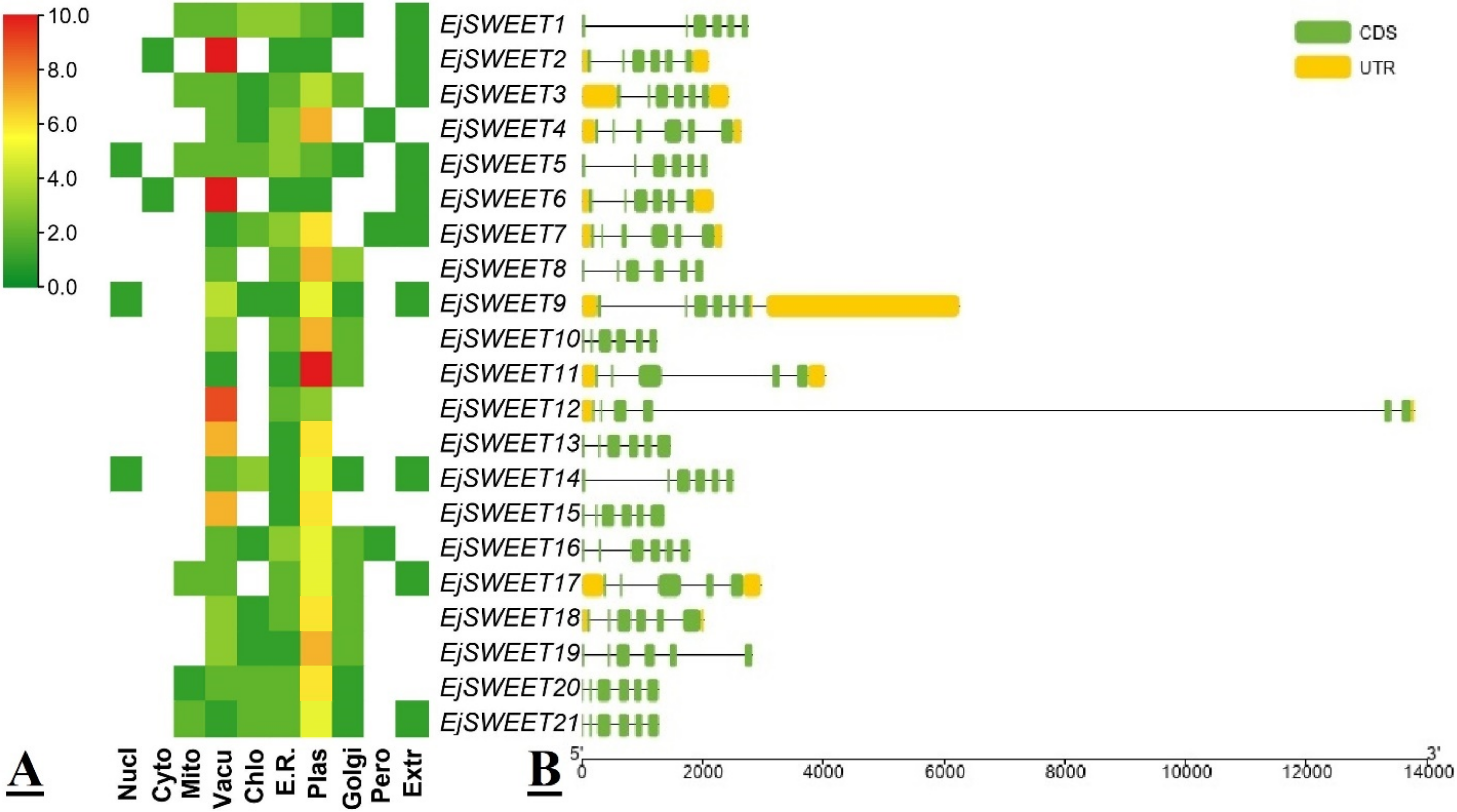
3.2. Protein Conserved Domain, Subcellular Localization, and Gene Structural Analysis of EjSWEET Genes
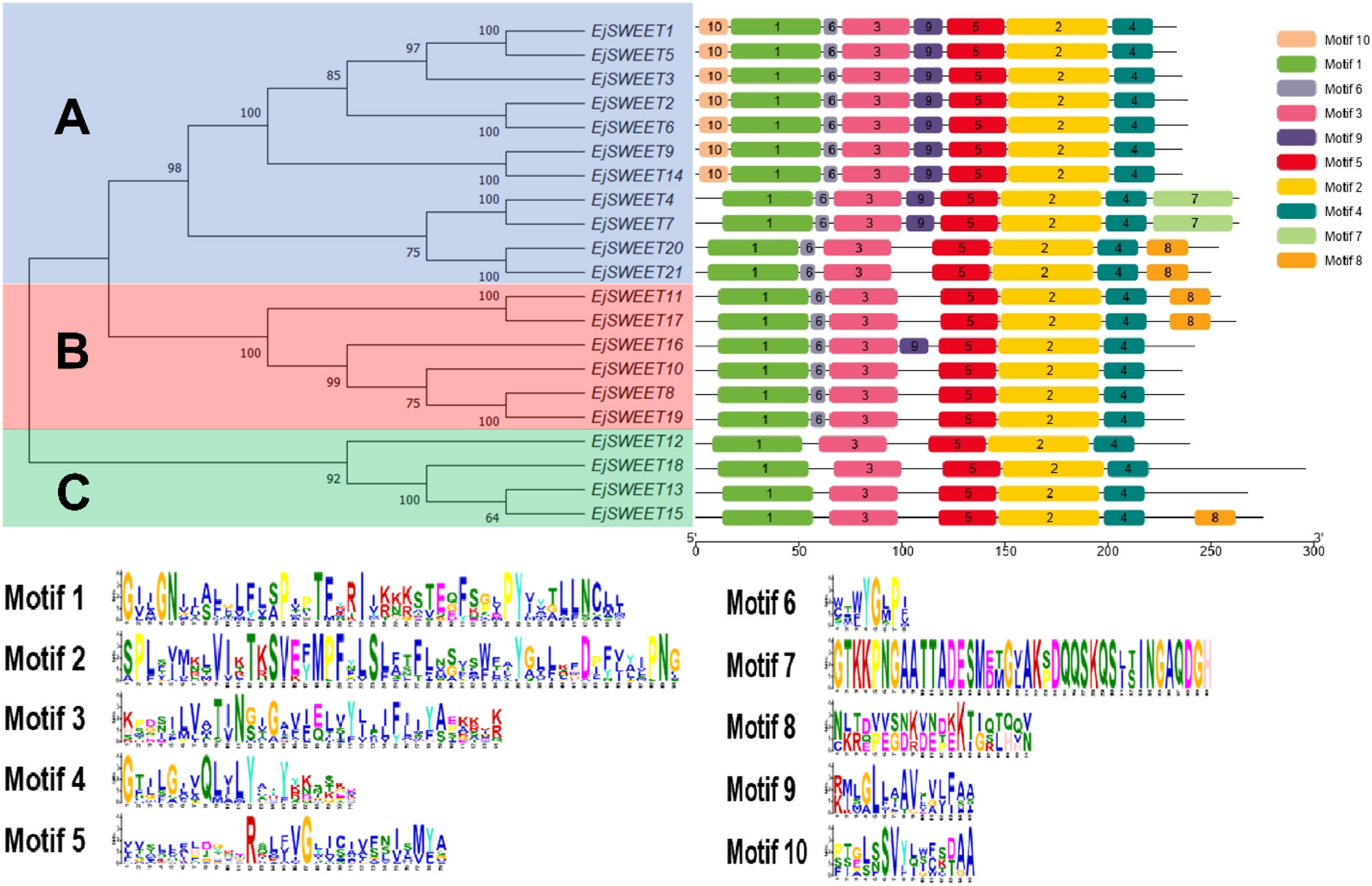
3.3. Phylogenetic and Conserved Motif Analysis of EjSWEET Genes
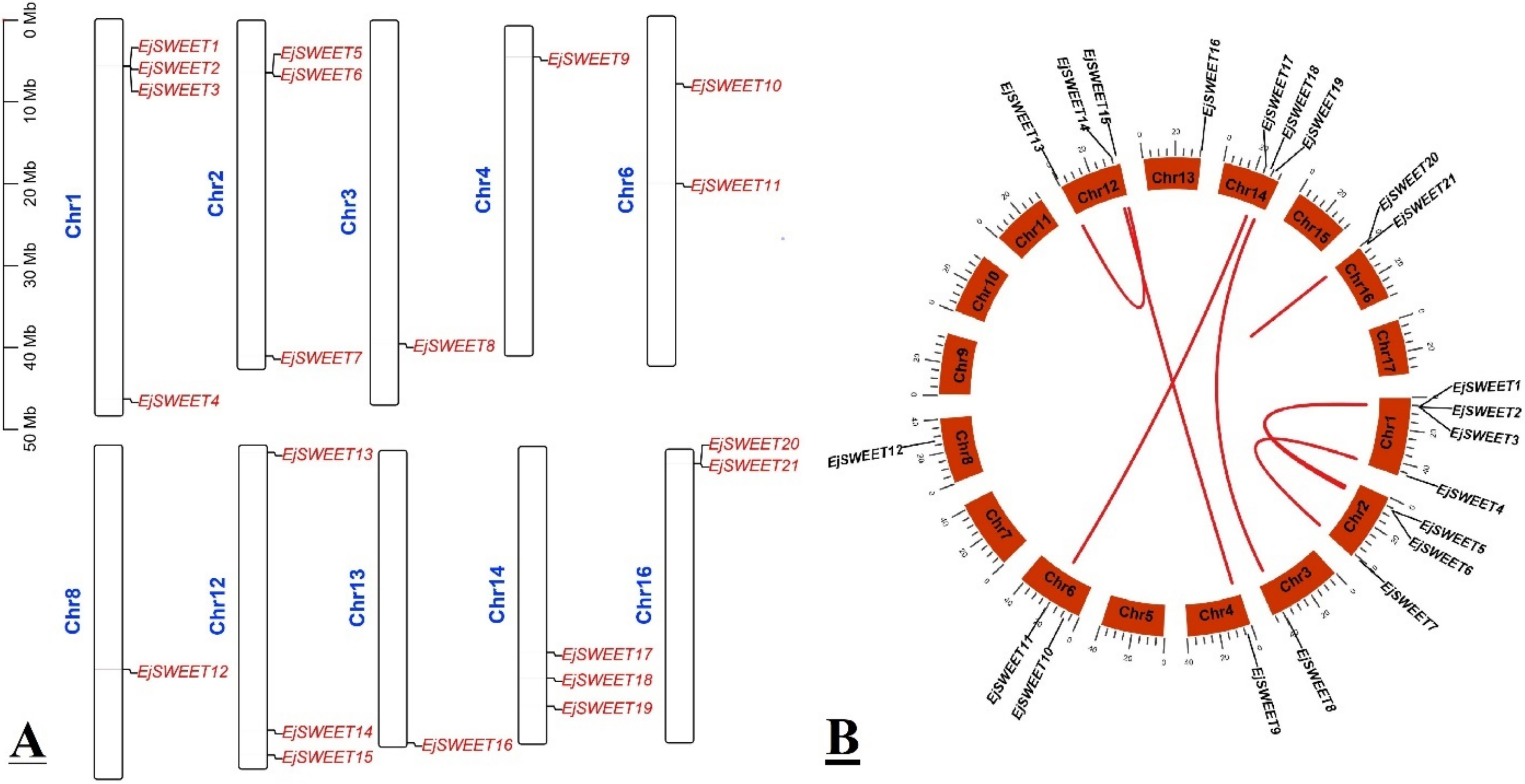
3.4. Chromosomal Mapping and Syntenic Analysis of EjSWEET Genes
3.5. Analysis of Cis-Acting Regulatory Elements in EjSWEET Promoter Region
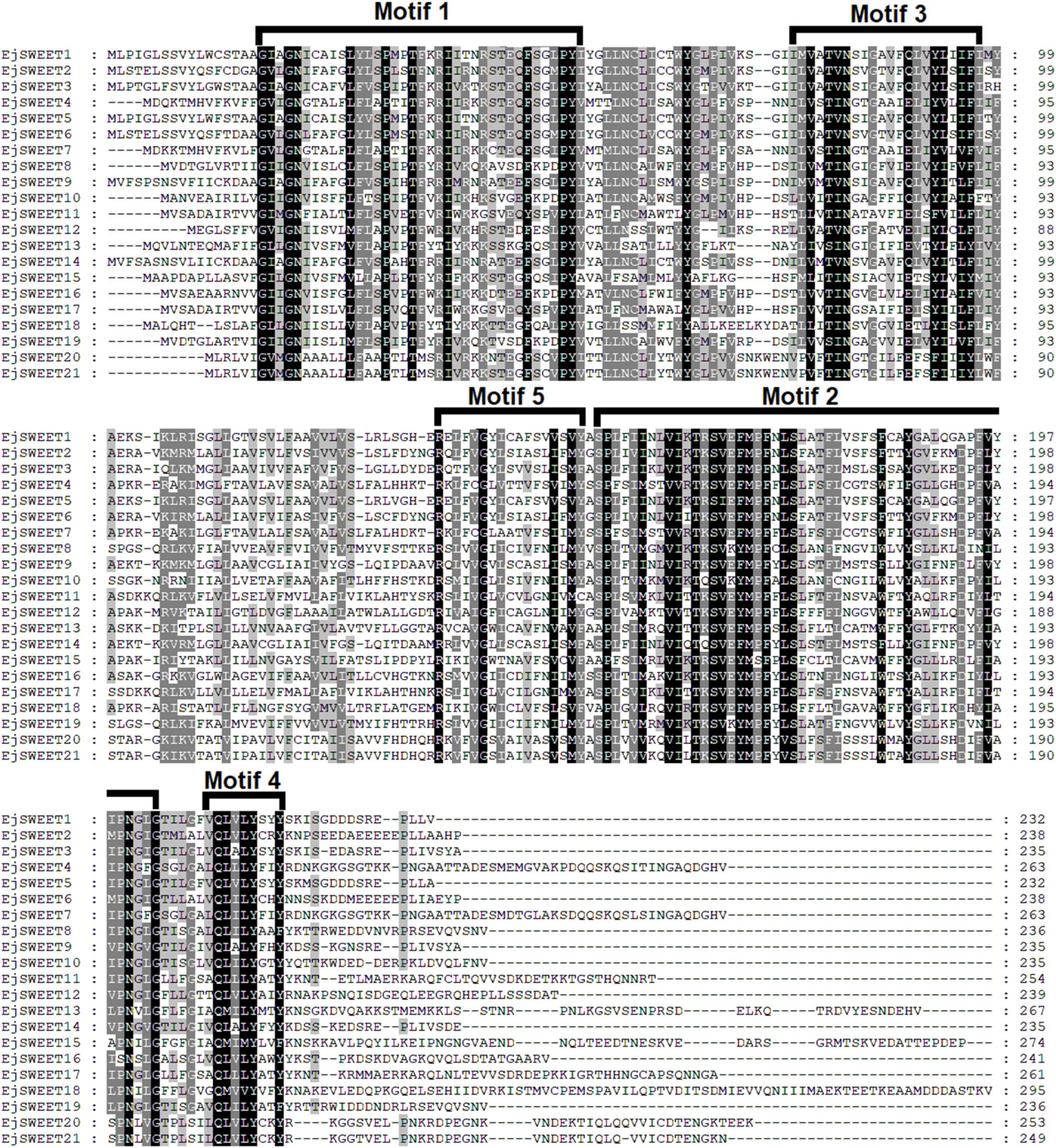
3.6. Multiple Sequence Alignment of EjSWEET Proteins
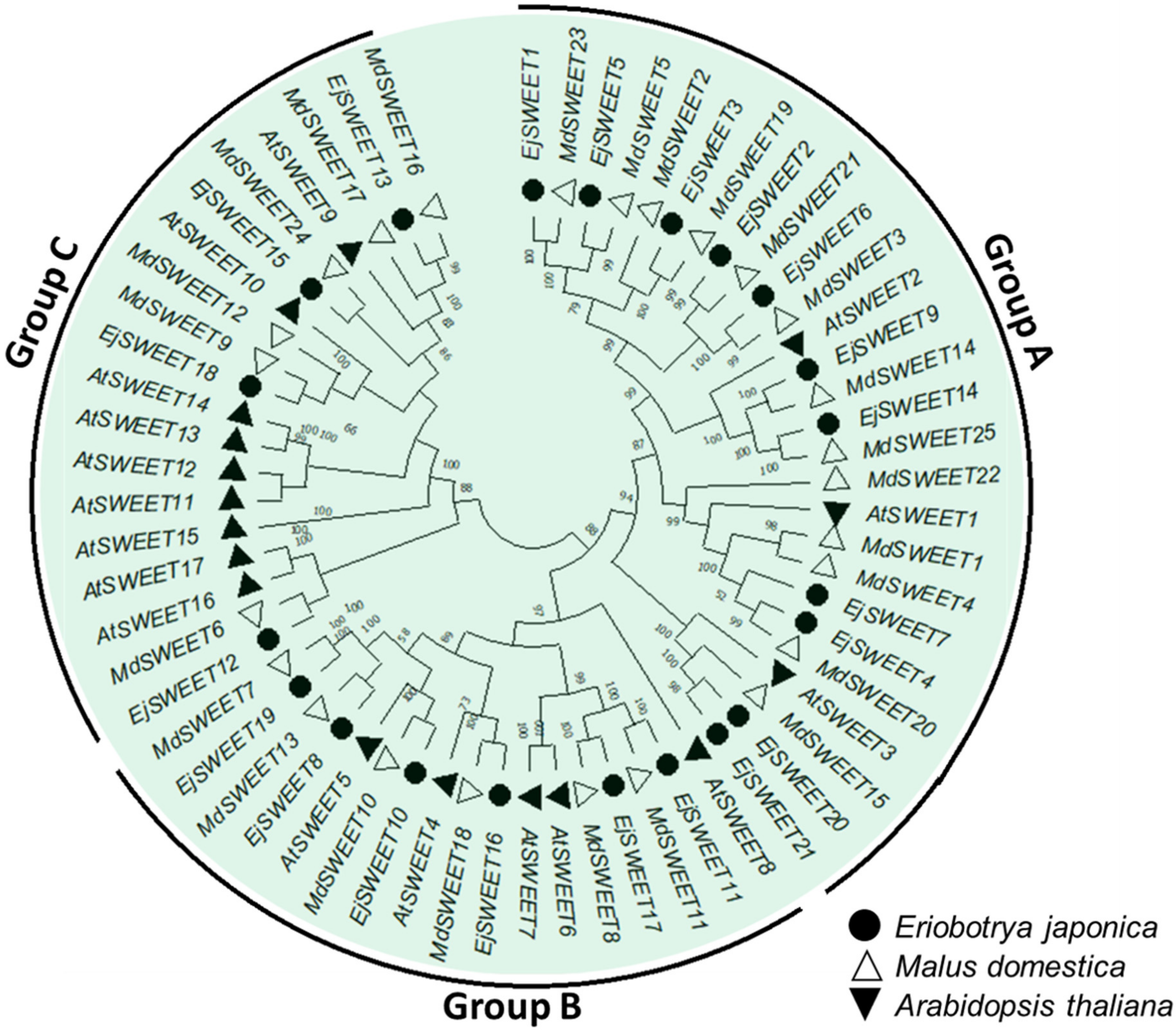
3.7. Phylogenetic Analysis of the EjSWEET Genes in Eriobotrya Japonica, Malus Domestica, and Arabidopsis Thaliana
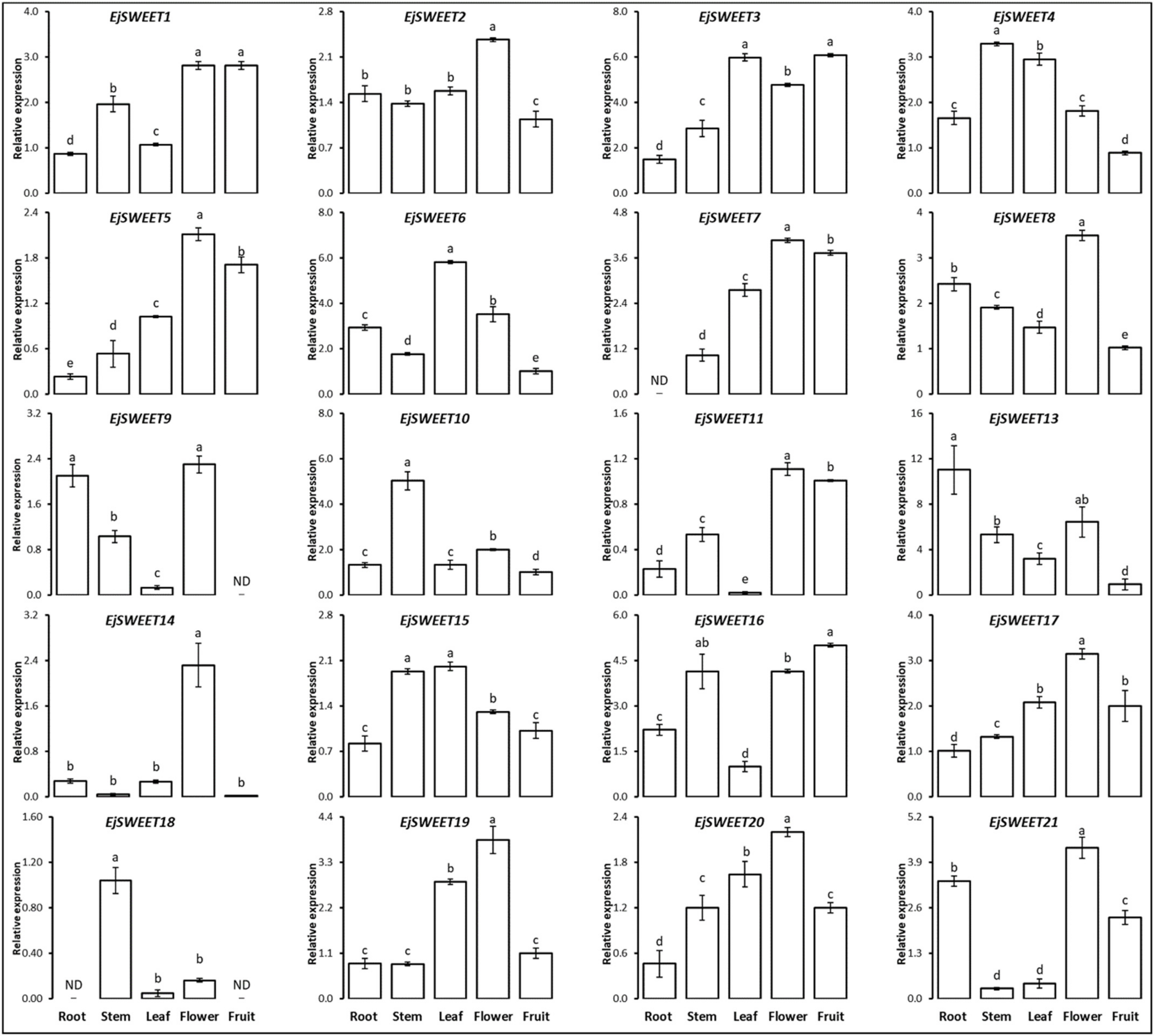
3.8. Expression Profiling of EjSWEET Genes in Different Tissues of Loquat
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, L.-Q.; Cheung, L.S.; Feng, L.; Tanner, W.; Frommer, W.B. Transport of Sugars. Annu. Rev. Biochem. 2015, 84, 865–894. [Google Scholar] [CrossRef] [PubMed]
- Sadat-Hosseini, M.; Bakhtiarizadeh, M.R.; Boroomand, N.; Tohidfar, M.; Vahdati, K. Combining independent de novo assemblies to optimize leaf transcriptome of Persian walnut. PLoS ONE 2020, 15, e0232005. [Google Scholar] [CrossRef] [PubMed]
- Chen, L. SWEET sugar transporters for phloem transport and pathogen nutrition. New Phytol. 2014, 201, 1150–1155. [Google Scholar] [CrossRef] [PubMed]
- Lalonde, S.; Wipf, D.; Frommer, W.B. Transport mechanisms for organic forms of carbon and nitrogen between source and sink. Annu. Rev. Plant Biol. 2004, 55, 341–372. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Ali, M.M.; Li, B.; Fang, T.; Chen, F. Transcriptome data-based identification of candidate genes involved in metabolism and accumulation of soluble sugars during fruit development in ‘Huangguan’ plum. J. Food Biochem. 2021, 45, e13878. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.; Ali, M.M.; Gong, J.; She, W.; Pan, D.; Guo, Z.; Yu, Y.; Chen, F. Fruit Physiology and Sugar-Acid Profile of 24 Pomelo (Citrus grandis (L.) Osbeck) Cultivars Grown in Subtropical Region of China. Agronomy 2021, 11, 2393. [Google Scholar] [CrossRef]
- Eom, J.-S.; Chen, L.-Q.; Sosso, D.; Julius, B.T.; Lin, I.; Qu, X.-Q.; Braun, D.M.; Frommer, W.B. SWEETs, transporters for intracellular and intercellular sugar translocation. Curr. Opin. Plant Biol. 2015, 25, 53–62. [Google Scholar] [CrossRef]
- Kühn, C.; Grof, C.P. Sucrose transporters of higher plants. Curr. Opin. Plant Biol. 2010, 13, 287–297. [Google Scholar] [CrossRef]
- Ayre, B.G. Membrane-Transport Systems for Sucrose in Relation to Whole-Plant Carbon Partitioning. Mol. Plant 2011, 4, 377–394. [Google Scholar] [CrossRef]
- Slewinski, T.L. Diverse Functional Roles of Monosaccharide Transporters and their Homologs in Vascular Plants: A Physiological Perspective. Mol. Plant 2011, 4, 641–662. [Google Scholar] [CrossRef]
- Chen, L.-Q.; Hou, B.-H.; Lalonde, S.; Takanaga, H.; Hartung, M.L.; Qu, X.-Q.; Guo, W.-J.; Kim, J.-G.; Underwood, W.; Chaudhuri, B.; et al. Sugar transporters for intercellular exchange and nutrition of pathogens. Nature 2010, 468, 527–532. [Google Scholar] [CrossRef]
- Xuan, Y.H.; Hu, Y.B.; Chen, L.-Q.; Sosso, D.; Ducat, D.C.; Hou, B.-H.; Frommer, W.B. Functional role of oligomerization for bacterial and plant SWEET sugar transporter family. Proc. Natl. Acad. Sci. USA 2013, 110, 3685–3694. [Google Scholar] [CrossRef]
- Yuan, M.; Wang, S. Rice MtN3/Saliva/SWEET Family Genes and Their Homologs in Cellular Organisms. Mol. Plant 2013, 6, 665–674. [Google Scholar] [CrossRef]
- Patil, G.; Valliyodan, B.; Deshmukh, R.; Prince, S.; Nicander, B.; Zhao, M.; Sonah, H.; Song, L.; Lin, L.; Chaudhary, J.; et al. Soybean (Glycine max) SWEET gene family: Insights through comparative genomics, transcriptome profiling and whole genome re-sequence analysis. BMC Genom. 2015, 16, 520. [Google Scholar] [CrossRef]
- Chandran, D. Co-option of developmentally regulated plant SWEET transporters for pathogen nutrition and abiotic stress tolerance. IUBMB Life 2015, 67, 461–471. [Google Scholar] [CrossRef]
- Chen, L.-Q.; Qu, X.-Q.; Hou, B.-H.; Sosso, D.; Osorio, S.; Fernie, A.R.; Frommer, W.B. Sucrose Efflux Mediated by SWEET Proteins as a Key Step for Phloem Transport. Science 2012, 335, 207–211. [Google Scholar] [CrossRef]
- Lin, I.W.; Sosso, D.; Chen, L.-Q.; Gase, K.; Kim, S.-G.; Kessler, D.; Klinkenberg, P.M.; Gorder, M.K.; Hou, B.-H.; Qu, X.-Q.; et al. Nectar secretion requires sucrose phosphate synthases and the sugar transporter SWEET9. Nature 2014, 508, 546–549. [Google Scholar] [CrossRef]
- Engel, M.L.; Holmes-Davis, R.; McCormick, S. Green Sperm. Identification of Male Gamete Promoters in Arabidopsis. Plant Physiol. 2005, 138, 2124–2133. [Google Scholar] [CrossRef]
- Guan, Y.-F.; Huang, X.-Y.; Zhu, J.; Gao, J.-F.; Zhang, H.-X.; Yang, Z.-N. RUPTURED POLLEN GRAIN1, a Member of the MtN3/saliva Gene Family, Is Crucial for Exine Pattern Formation and Cell Integrity of Microspores in Arabidopsis. Plant Physiol. 2008, 147, 852–863. [Google Scholar] [CrossRef]
- Sun, M.-X.; Huang, X.-Y.; Yang, J.; Guan, Y.-F.; Yang, Z.-N. Arabidopsis RPG1 is important for primexine deposition and functions redundantly with RPG2 for plant fertility at the late reproductive stage. Plant Reprod. 2013, 26, 83–91. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, L.; Huang, W.; Yuan, M.; Zhou, F.; Li, X.; Lin, Y. Overexpression of OsSWEET5 in Rice Causes Growth Retardation and Precocious Senescence. PLoS ONE 2014, 9, e94210. [Google Scholar] [CrossRef]
- Arab, M.M.; Brown, P.J.; Abdollahi-Arpanahi, R.; Sohrabi, S.S.; Askari, H.; Aliniaeifard, S.; Mokhtassi-Bidgoli, A.; Mesgaran, M.B.; Leslie, C.A.; Marrano, A.; et al. Genome-wide association analysis and pathway enrichment provide insights into the genetic basis of photosynthetic responses to drought stress in Persian walnut. Hortic. Res. 2022, 9, uhac124. [Google Scholar] [CrossRef]
- Arab, M.M.; Marrano, A.; Abdollahi-Arpanahi, R.; Leslie, C.A.; Askari, H.; Neale, D.B.; Vahdati, K. Genome-wide patterns of population structure and association mapping of nut-related traits in Persian walnut populations from Iran using the Axiom J. regia 700K SNP array. Sci. Rep. 2019, 9, 6376. [Google Scholar] [CrossRef]
- Chen, L.-Q.; Lin, I.W.; Qu, X.-Q.; Sosso, D.; McFarlane, H.E.; Londoño, A.; Samuels, A.L.; Frommer, W.B. A Cascade of Sequentially Expressed Sucrose Transporters in the Seed Coat and Endosperm Provides Nutrition for the Arabidopsis Embryo. Plant Cell 2015, 27, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Sosso, D.; Luo, D.; Li, Q.-B.; Sasse, J.; Yang, J.; Gendrot, G.; Suzuki, M.; Koch, K.E.; McCarty, D.R.; Chourey, P.S.; et al. Seed filling in domesticated maize and rice depends on SWEET-mediated hexose transport. Nat. Genet. 2015, 47, 1489–1493. [Google Scholar] [CrossRef]
- Yuan, M.; Chu, Z.; Li, X.; Xu, C.; Wang, S. Pathogen-Induced Expressional Loss of Function is the Key Factor in Race-Specific Bacterial Resistance Conferred by a Recessive R Gene xa13 in Rice. Plant Cell Physiol. 2009, 50, 947–955. [Google Scholar] [CrossRef]
- Streubel, J.; Pesce, C.; Hutin, M.; Koebnik, R.; Boch, J.; Szurek, B. Five phylogenetically close rice SWEET genes confer TAL effector-mediated susceptibility to Xanthomonas oryzae pv. oryzae. New Phytol. 2013, 200, 808–819. [Google Scholar] [CrossRef]
- Chong, J.; Piron, M.-C.; Meyer, S.; Merdinoglu, D.; Bertsch, C.; Mestre, P. The SWEET family of sugar transporters in grapevine: VvSWEET4 is involved in the interaction with Botrytis cinerea. J. Exp. Bot. 2014, 65, 6589–6601. [Google Scholar] [CrossRef] [PubMed]
- Cohn, M.; Bart, R.S.; Shybut, M.; Dahlbeck, D.; Gomez, M.; Morbitzer, R.; Hou, B.-H.; Frommer, W.B.; Lahaye, T.; Staskawicz, B.J. Xanthomonas axonopodis Virulence Is Promoted by a Transcription Activator-Like Effector–Mediated Induction of a SWEET Sugar Transporter in Cassava. Mol. Plant Microbe Interact. 2014, 27, 1186–1198. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhang, J.; Jia, H.; Sosso, D.; Li, T.; Frommer, W.B.; Yang, B.; White, F.F.; Wang, N.; Jones, J.B. Lateral organ boundaries 1 is a disease susceptibility gene for citrus bacterial canker disease. Proc. Natl. Acad. Sci. USA 2014, 111, E521–E529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, H.; Wang, D.; Qin, Y.; Ma, A.; Fu, J.; Qin, Y.; Hu, G.; Zhao, J. Genome-wide identification and expression analysis of SWEET gene family in Litchi chinensis reveal the involvement of LcSWEET2a/3b in early seed development. BMC Plant Biol. 2019, 19, 499. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.M.; Anwar, R.; Shafique, M.W.; Yousef, A.F.; Chen, F. Exogenous Application of Mg, Zn and B Influences Phyto-Nutritional Composition of Leaves and Fruits of Loquat (Eriobotrya japonica Lindl.). Agronomy 2021, 11, 224. [Google Scholar] [CrossRef]
- Ali, M.M.; Li, B.; Zhi, C.; Yousef, A.F.; Chen, F. Foliar-Supplied Molybdenum Improves Phyto-Nutritional Composition of Leaves and Fruits of Loquat (Eriobotrya japonica Lindl.). Agronomy 2021, 11, 892. [Google Scholar] [CrossRef]
- Ali, M.M.; Rizwan, H.M.; Yousef, A.F.; Zhi, C.; Chen, F. Analysis of toxic elements in leaves and fruits of loquat by inductively coupled plasma-mass spectrometry (ICP-MS). Acta Sci. Pol. Hortorum Cultus 2021, 20, 33–42. [Google Scholar] [CrossRef]
- Badenes, M.L.; Canyamas, T.; Romero, C.; Soriano, J.M.; Martínez, J.; Llácer, G. Genetic diversity in european collection of loquat (Eriobotrya japonica Lindl.). Acta Hortic. 2003, 620, 169–174. [Google Scholar] [CrossRef]
- Zhi, C.; Ali, M.M.; Zhang, J.; Shi, M.; Ma, S.; Chen, F. Effect of Paper and Aluminum Bagging on Fruit Quality of Loquat (Eriobotrya japonica Lindl.). Plants 2021, 10, 2704. [Google Scholar] [CrossRef]
- Jiang, S.; An, H.; Xu, F.; Zhang, X. Chromosome-level genome assembly and annotation of the loquat (Eriobotrya japonica) genome. GigaScience 2020, 9, giaa015. [Google Scholar] [CrossRef]
- Daccord, N.; Celton, J.-M.; Linsmith, G.; Becker, C.; Choisne, N.; Schijlen, E.; van de Geest, H.; Bianco, L.; Micheletti, D.; Velasco, R.; et al. High-quality de novo assembly of the apple genome and methylome dynamics of early fruit development. Nat. Genet. 2017, 49, 1099–1106. [Google Scholar] [CrossRef]
- Finn, R.D.; Mistry, J.; Tate, J.; Coggill, P.; Heger, A.; Pollington, J.E.; Gavin, O.L.; Gunasekaran, P.; Ceric, G.; Forslund, K.; et al. The Pfam protein families database. Nucleic Acids Res. 2010, 38, D211–D222. [Google Scholar] [CrossRef]
- Eddy, S.R. Accelerated Profile HMM Searches. PLoS Comput. Biol. 2011, 7, e1002195. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; DeBarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.-H.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef]
- Gan, X.; Jing, Y.; Shahid, M.Q.; He, Y.; Baloch, F.S.; Lin, S.; Yang, X. Identification, phylogenetic analysis, and expression patterns of the SAUR gene family in loquat (Eriobotrya japonica). Turk. J. Agric. For. 2020, 44, 15–23. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, Y.; Zhang, Z.; Zhu, J.; Yu, J. KaKs_Calculator 2.0: A Toolkit Incorporating Gamma-Series Methods and Sliding Window Strategies. Genom. Proteom. Bioinform. 2010, 8, 77–80. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Ali, M.M.; Alam, S.M.; Anwar, R.; Ali, S.; Shi, M.; Liang, D.; Lin, Z.; Chen, F. Genome-Wide Identification, Characterization and Expression Profiling of Aluminum-Activated Malate Transporters in Eriobotrya japonica Lindl. Horticulturae 2021, 7, 441. [Google Scholar] [CrossRef]
- Zhi, C.; Ali, M.M.; Alam, S.M.; Gull, S.; Ali, S.; Yousef, A.F.; Ahmed, M.A.A.; Ma, S.; Chen, F. Genome-Wide In Silico Analysis and Expression Profiling of Phosphoenolpyruvate Carboxylase Genes in Loquat, Apple, Peach, Strawberry and Pear. Agronomy 2021, 12, 25. [Google Scholar] [CrossRef]
- Akhunov, E.D.; Sehgal, S.; Liang, H.; Wang, S.; Akhunova, A.R.; Kaur, G.; Li, W.; Forrest, K.L.; See, D.; Šimková, H.; et al. Comparative Analysis of Syntenic Genes in Grass Genomes Reveals Accelerated Rates of Gene Structure and Coding Sequence Evolution in Polyploid Wheat. Plant Physiol. 2012, 161, 252–265. [Google Scholar] [CrossRef]
- Yuan, M.; Zhao, J.; Huang, R.; Li, X.; Xiao, J.; Wang, S. Rice MtN3/saliva/SWEET gene family: Evolution, expression profiling, and sugar transport. J. Integr. Plant Biol. 2014, 56, 559–570. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, F.; Song, S.; Tang, X.; Xu, H.; Liu, G.; Wang, Y.; He, H. Genome-wide identification, characterization, and expression analysis of the SWEET gene family in cucumber. J. Integr. Agric. 2017, 16, 1486–1501. [Google Scholar] [CrossRef] [Green Version]
- Feng, C.-Y.; Han, J.-X.; Han, X.-X.; Jiang, J. Genome-wide identification, phylogeny, and expression analysis of the SWEET gene family in tomato. Gene 2015, 573, 261–272. [Google Scholar] [CrossRef]
- Wei, X.; Liu, F.; Chen, C.; Ma, F.; Li, M. The Malus domestica sugar transporter gene family: Identifications based on genome and expression profiling related to the accumulation of fruit sugars. Front. Plant Sci. 2014, 5, 569. [Google Scholar] [CrossRef]
- Ardito, F.; Giuliani, M.; Perrone, D.; Troiano, G.; Lo Muzio, L. The crucial role of protein phosphorylation in cell signaling and its use as targeted therapy (Review). Int. J. Mol. Med. 2017, 40, 271–280. [Google Scholar] [CrossRef]
- Zhen, Q.; Fang, T.; Peng, Q.; Liao, L.; Zhao, L.; Owiti, A.; Han, Y. Developing gene-tagged molecular markers for evaluation of genetic association of apple SWEET genes with fruit sugar accumulation. Hortic. Res. 2018, 5, 14. [Google Scholar] [CrossRef]
- Lo, E.Y.Y.; Donoghue, M.J. Expanded phylogenetic and dating analyses of the apples and their relatives (Pyreae, Rosaceae). Mol. Phylogenet. Evol. 2012, 63, 230–243. [Google Scholar] [CrossRef]
- Chu, Z.; Yuan, M.; Yao, J.; Ge, X.; Yuan, B.; Xu, C.; Li, X.; Fu, B.; Li, Z.; Bennetzen, J.L.; et al. Promoter mutations of an essential gene for pollen development result in disease resistance in rice. Genes Dev. 2006, 20, 1250–1255. [Google Scholar] [CrossRef]
- Antony, G.; Zhou, J.; Huang, S.; Li, T.; Liu, B.; White, F.; Yang, B. Rice xa13 Recessive Resistance to Bacterial Blight Is Defeated by Induction of the Disease Susceptibility Gene Os- 11N3. Plant Cell 2010, 22, 3864–3876. [Google Scholar] [CrossRef]
- Rennie, E.A.; Turgeon, R. A comprehensive picture of phloem loading strategies. Proc. Natl. Acad. Sci. USA 2009, 106, 14162–14167. [Google Scholar] [CrossRef]
- Hu, L.-P.; Meng, F.-Z.; Wang, S.-H.; Sui, X.-L.; Li, W.; Wei, Y.-X.; Sun, J.-L.; Zhang, Z.-X. Changes in carbohydrate levels and their metabolic enzymes in leaves, phloem sap and mesocarp during cucumber (Cucumis sativus L.) fruit development. Sci. Hortic. 2009, 121, 131–137. [Google Scholar] [CrossRef]
- Bachmann, M.; Matile, P.; Keller, F. Metabolism of the Raffinose Family Oligosaccharides in Leaves of Ajuga reptans L. (Cold Acclimation, Translocation, and Sink to Source Transition: Discovery of Chain Elongation Enzyme). Plant Physiol. 1994, 105, 1335–1345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yadav, U.P.; Ayre, B.G.; Bush, D.R. Transgenic approaches to altering carbon and nitrogen partitioning in whole plants: Assessing the potential to improve crop yields and nutritional quality. Front. Plant Sci. 2015, 6, 275. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Sun, H.; Li, R.; Zhang, L.; Wang, S.; Sui, X.; Zhang, Z. Phloem unloading follows an extensive apoplasmic pathway in cucumber (Cucumis sativus L.) fruit from anthesis to marketable maturing stage. Plant. Cell Environ. 2011, 34, 1835–1848. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.A.; Davies, C.; Dry, I.B. Isolation, functional characterization, and expression analysis of grapevine (Vitis vinifera L.) hexose transporters: Differential roles in sink and source tissues. J. Exp. Bot. 2007, 58, 1985–1997. [Google Scholar] [CrossRef]
- Cheng, J.-T.; Li, X.; Yao, F.-Z.; Shan, N.; Li, Y.-H.; Zhang, Z.-X.; Sui, X.-L. Functional characterization and expression analysis of cucumber (Cucumis sativus L.) hexose transporters, involving carbohydrate partitioning and phloem unloading in sink tissues. Plant Sci. 2015, 237, 46–56. [Google Scholar] [CrossRef]
- Guo, W.-J.; Nagy, R.; Chen, H.-Y.; Pfrunder, S.; Yu, Y.-C.; Santelia, D.; Frommer, W.B.; Martinoia, E. SWEET17, a Facilitative Transporter, Mediates Fructose Transport across the Tonoplast of Arabidopsis Roots and Leaves. Plant Physiol. 2014, 164, 777–789. [Google Scholar] [CrossRef]
- Feng, L.; Frommer, W.B. Structure and function of SemiSWEET and SWEET sugar transporters. Trends Biochem. Sci. 2015, 40, 480–486. [Google Scholar] [CrossRef]
- Zheng, Q.-M.; Tang, Z.; Xu, Q.; Deng, X.-X. Isolation, phylogenetic relationship and expression profiling of sugar transporter genes in sweet orange (Citrus sinensis). Plant Cell Tissue Organ Cult. 2014, 119, 609–624. [Google Scholar] [CrossRef]

| Gene Name | Gene ID | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|---|
| EjSWEET1 | EVM0038444.1 | CCCCAATGCCAACATTTAAG | GGAAAACAGCTCCGATTGAA |
| EjSWEET2 | EVM0001232.1 | CCGAAAGAGCGGTTAAGATG | TGGCGAACCATACATGAAAA |
| EjSWEET3 | EVM0035130.1 | TCTTTCTCTCGCGACGTTTT | TGGCTCTCTTGAGGCATCTT |
| EjSWEET4 | EVM0038533.1 | CGCCTTTCTCGATTATGAGC | TCCCAAACCCATTTGGAATA |
| EjSWEET5 | EVM0008081.1 | TGTCACCAATGCCAACATTT | GAGCAATCCCGATATCCTCA |
| EjSWEET6 | EVM0039968.1 | TTGCGTCCATTGTCTTTGTC | GGCATGAACTCAACGCTTTT |
| EjSWEET7 | EVM0006793.1 | TTGTGTCCGCAAACAACATT | AGAACAGAGCAAGCACAGCA |
| EjSWEET8 | EVM0033338.1 | TCCCCAATCCCAACATTTTA | ACCTTCAAGCGTTGTGAACC |
| EjSWEET9 | EVM0009105.1 | TTGGTGCGGTGTTTCAATTA | CTTTGTCCGGATCACCAAGT |
| EjSWEET10 | EVM0029629.1 | ATGGAATTTTGTGGCTCGTC | TTTGGCCTTTCATCATCCTC |
| EjSWEET11 | EVM0019072.1 | ATGGGCTGGGCTTACTTTTT | CATGTCCGGTTGTTCTGATG |
| EjSWEET12 | EVM0043755.1 | TTTGCACACTGCTCAACTCC | CCACATCCAAGGTTCCAATC |
| EjSWEET13 | EVM0005220.1 | TCATCGAAAGGGTTCCAATC | CCGAAAGCGGCTACATTTAC |
| EjSWEET14 | EVM0003016.1 | CGTGGTATGGATCGCCTATT | AATCTGCAAGCTCCCAAAGA |
| EjSWEET15 | EVM0006392.1 | CATGTCCTTCCCTTTGTCGT | CATTTTCTGCAACCCCATTT |
| EjSWEET16 | EVM0043307.1 | CTCCTTCGGGCTTTTTCTCT | CAAGAGTGCTGTCAGGGTGA |
| EjSWEET17 | EVM0019370.1 | GCTCGTTTTCATGGCTCTTC | CATATGTGAACCAGGCAACG |
| EjSWEET18 | EVM0020014.1 | TGACTCGCTTTCTAGCCACA | TGATAACGGAAATGGCATGA |
| EjSWEET19 | EVM0044002.1 | CCGGTCTTTGGTAGTTGGAA | GACCAGCCAAACAACTCCAT |
| EjSWEET20 | EVM0026582.1 | AAGCAACAAATGGGAAAACG | GCTGAGATAATGGCGGTGAT |
| EjSWEET21 | EVM0012820.1 | TCTTCACCATTAACGGCACA | AACGACGGCTGAGATAATGG |
| EjAct | EVM0004523.1 | GGAGCGTGGATATTCCTTCA | GCTGCTTCCATTCCAATCAT |
| Gene Name | Gene ID | Chromosome | Start Site | End Site | Strand | CDS (bp) |
|---|---|---|---|---|---|---|
| EjSWEET1 | EVM0038444.1 | 1 | 5761008 | 5763757 | + | 699 |
| EjSWEET2 | EVM0001232.1 | 1 | 5771748 | 5773852 | + | 717 |
| EjSWEET3 | EVM0035130.1 | 1 | 5776406 | 5778843 | + | 708 |
| EjSWEET4 | EVM0038533.1 | 1 | 46469811 | 46472452 | + | 792 |
| EjSWEET5 | EVM0008081.1 | 2 | 6408991 | 6411071 | + | 699 |
| EjSWEET6 | EVM0039968.1 | 2 | 6430524 | 6432707 | + | 717 |
| EjSWEET7 | EVM0006793.1 | 2 | 41029948 | 41032274 | + | 792 |
| EjSWEET8 | EVM0033338.1 | 3 | 39566451 | 39568463 | − | 711 |
| EjSWEET9 | EVM0009105.1 | 4 | 3837836 | 3844089 | − | 708 |
| EjSWEET10 | EVM0029629.1 | 6 | 8278274 | 8279519 | − | 708 |
| EjSWEET11 | EVM0019072.1 | 6 | 20451938 | 20455969 | − | 765 |
| EjSWEET12 | EVM0043755.1 | 8 | 27415495 | 27429282 | − | 720 |
| EjSWEET13 | EVM0005220.1 | 12 | 889913 | 891387 | + | 804 |
| EjSWEET14 | EVM0003016.1 | 12 | 34849459 | 34851971 | + | 708 |
| EjSWEET15 | EVM0006392.1 | 12 | 37885082 | 37886461 | − | 825 |
| EjSWEET16 | EVM0043307.1 | 13 | 35717082 | 35718865 | + | 726 |
| EjSWEET17 | EVM0019370.1 | 14 | 25165282 | 25168251 | − | 786 |
| EjSWEET18 | EVM0020014.1 | 14 | 28293338 | 28295358 | + | 888 |
| EjSWEET19 | EVM0044002.1 | 14 | 31729522 | 31732348 | − | 711 |
| EjSWEET20 | EVM0026582.1 | 16 | 1749505 | 1750777 | − | 762 |
| EjSWEET21 | EVM0012820.1 | 16 | 1768563 | 1769839 | − | 750 |
| Gene Name | Protein Length (A.A.) | MW (kDa) | pI | Instability Index | Aliphatic Index | GRAVY |
|---|---|---|---|---|---|---|
| EjSWEET1 | 232 | 25.35819 | 8.71 | 34.89 | 128.1 | 0.936 |
| EjSWEET2 | 238 | 26.6245 | 5.42 | 43.89 | 115.42 | 0.758 |
| EjSWEET3 | 235 | 25.87286 | 8.91 | 32.61 | 126.89 | 0.926 |
| EjSWEET4 | 263 | 28.84401 | 9.77 | 36.02 | 98.97 | 0.502 |
| EjSWEET5 | 232 | 25.37417 | 8.55 | 38.09 | 127.28 | 0.929 |
| EjSWEET6 | 238 | 26.69948 | 4.93 | 45.91 | 114.62 | 0.777 |
| EjSWEET7 | 263 | 28.63981 | 9.57 | 30.76 | 102.36 | 0.521 |
| EjSWEET8 | 236 | 26.58405 | 9.03 | 39.64 | 132.33 | 0.992 |
| EjSWEET9 | 235 | 25.99204 | 9.03 | 45.23 | 121.53 | 0.871 |
| EjSWEET10 | 235 | 26.53263 | 8.99 | 34.12 | 121.53 | 0.716 |
| EjSWEET11 | 254 | 28.66118 | 9.41 | 30.9 | 117.76 | 0.63 |
| EjSWEET12 | 239 | 26.42614 | 5.67 | 43.5 | 118.28 | 0.714 |
| EjSWEET13 | 267 | 29.88251 | 9.34 | 24.63 | 111.69 | 0.59 |
| EjSWEET14 | 235 | 25.92469 | 6.55 | 45.96 | 123.19 | 0.857 |
| EjSWEET15 | 274 | 30.57331 | 8.24 | 41.78 | 111.79 | 0.596 |
| EjSWEET16 | 241 | 26.69083 | 9.22 | 31.14 | 121.29 | 0.687 |
| EjSWEET17 | 261 | 29.34506 | 9.71 | 38.69 | 124.33 | 0.628 |
| EjSWEET18 | 295 | 33.0874 | 5.97 | 48.15 | 120.24 | 0.664 |
| EjSWEET19 | 236 | 26.84237 | 9.57 | 41.76 | 135.64 | 0.911 |
| EjSWEET20 | 253 | 28.09724 | 9.15 | 40.88 | 115.06 | 0.532 |
| EjSWEET21 | 249 | 27.58569 | 9.36 | 39.2 | 117.31 | 0.584 |
| Gene 1 | Gene 2 | Ka | Ks | Ka/Ks | Duplication |
|---|---|---|---|---|---|
| EjSWEET1 | EjSWEET5 | 0.02442 | 0.1683363 | 0.1450679 | segmental |
| EjSWEET2 | EjSWEET6 | 0.048297 | 0.1254252 | 0.3850654 | segmental |
| EjSWEET4 | EjSWEET7 | 0.046486 | 0.2183978 | 0.2128524 | segmental |
| EjSWEET8 | EjSWEET19 | 0.096019 | 0.1580628 | 0.6074743 | segmental |
| EjSWEET9 | EjSWEET14 | 0.052542 | 0.0856763 | 0.6132632 | segmental |
| EjSWEET11 | EjSWEET17 | 0.086059 | 0.135099 | 0.6370108 | segmental |
| EjSWEET13 | EjSWEET15 | 0.452987 | 2.3006035 | 0.1968992 | tandem |
| EjSWEET20 | EjSWEET21 | 0.014411 | 0.032882 | 0.4382501 | tandem |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Ali, M.M.; Guo, T.; Alam, S.M.; Gull, S.; Iftikhar, J.; Yousef, A.F.; Mosa, W.F.A.; Chen, F. Genome-Wide Identification, In Silico Analysis and Expression Profiling of SWEET Gene Family in Loquat (Eriobotrya japonica Lindl.). Agriculture 2022, 12, 1312. https://doi.org/10.3390/agriculture12091312
Li B, Ali MM, Guo T, Alam SM, Gull S, Iftikhar J, Yousef AF, Mosa WFA, Chen F. Genome-Wide Identification, In Silico Analysis and Expression Profiling of SWEET Gene Family in Loquat (Eriobotrya japonica Lindl.). Agriculture. 2022; 12(9):1312. https://doi.org/10.3390/agriculture12091312
Chicago/Turabian StyleLi, Binqi, Muhammad Moaaz Ali, Tianxin Guo, Shariq Mahmood Alam, Shaista Gull, Junaid Iftikhar, Ahmed Fathy Yousef, Walid F. A. Mosa, and Faxing Chen. 2022. "Genome-Wide Identification, In Silico Analysis and Expression Profiling of SWEET Gene Family in Loquat (Eriobotrya japonica Lindl.)" Agriculture 12, no. 9: 1312. https://doi.org/10.3390/agriculture12091312
APA StyleLi, B., Ali, M. M., Guo, T., Alam, S. M., Gull, S., Iftikhar, J., Yousef, A. F., Mosa, W. F. A., & Chen, F. (2022). Genome-Wide Identification, In Silico Analysis and Expression Profiling of SWEET Gene Family in Loquat (Eriobotrya japonica Lindl.). Agriculture, 12(9), 1312. https://doi.org/10.3390/agriculture12091312









