Abstract
The impact of proline, methionine, and melatonin on cauliflower plants under drought stress is still unclear in the available publications. So, this research aims to study these biochemical compounds’ effects on cauliflower plants grown under well-irrigated and drought-stressed conditions. The obtained results showed that under drought-stressed conditions, foliar application of proline, methionine, and melatonin significantly (p ≤ 0.05) enhanced leaf area, leaf chlorophyll content, leaf relative water content (RWC), vitamin C, proline, total soluble sugar, reducing sugar, and non-reducing sugar compared to the untreated plants. These treatments also significantly increased curd height, curd diameter, curd freshness, and dry matter compared to untreated plants. Conversely, the phenolic-related enzymes including polyphenol oxidase (PPO), peroxidase (POD), and phenylalanine ammonia-lyase (PAL) were significantly reduced compared to the untreated plants. A similar trend was observed in glucosinolates, abscisic acid (ABA), malondialdehyde (MDA), and total phenols. Eventually, it can be concluded that the foliar application of proline, methionine, and melatonin can be considered a proper strategy for enhancing the growth performance and productivity of cauliflower grown under drought-stressed conditions.
1. Introduction
Cauliflower (Brassica oleracea var. botrytis L.) is one of the important vegetables belonging to Brassicaceae. It is nutritious healthy food rich in minerals (P, K, Mg, Mn), vitamins (C, B6), fiber, phenolic compounds, glucosinolates, carotenoids, and polyamines [1]. These compounds can elevate antioxidant activity and promote human health [2,3]. Cauliflower production could be negatively affected by water shortage. This shortage in water, particularly at critical stages of plant growth, enhances the metabolic process i.e., the accumulation of carbohydrates, amino acids, and other osmolyte compounds in order to increase plant tolerance against drought stresses [4].
Water deficit may trigger a wide series of reverse impacts, which can limit plant growth and development via the decline of cell division and enlargement [5], photosynthesis [6], plant growth regulators imbalance [7], and induce oxidative damages [8] that affected cell membrane integrity, leaf water state and finally decreasing yield [9]. It modifies stomata activities and restricts nutrient uptake resulting in decreased yield [10]. Water deficit excites the generation of reactive oxygen species (ROS) deactivating cellular redox regulatory assignment [11]. Additionally, stressed plants evolve drought tolerance strategies including promoting biosynthesis of compatible solutes and increasing enzymatic and non-enzymatic components of the antioxidant device [12]. To safeguard plant survival under water deficit, there are various ways to stimulate drought tolerance in plants, involving foliar applications of several activators such as proline, methionine, and melatonin.
Proline is one of the most efficient osmoprotectants that mitigate stress in plants [13]. It can induce protein stabilization and conserve membrane integrity through binding to hydrogen bonds [14], in addition, protect cells via enhancing water uptake potential and enzyme activation [15], and is considered a vigorous antioxidative defense molecule, ROS scavenger [16,17]. Foliar application with proline enhanced phenolic compounds, proline content, and antioxidant enzyme activity that decreased drought stress symptoms as enhanced ROS production, electrolyte leakage, and lipid peroxidation [18]. Additionally, proline is a proteinogenic secondary amino acid and it is classified as a safe compound when used in human feeding according to FDA (https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=582.5650; ITE: 21 CFR 582.5 650; Title 21, Volume 6, accessed on 22 August 2022).
Methionine is one of the fundamental amino acids, which collaborate in a diversity of physiological functions and is an effective regulator of plant growth and development under water deficit [19]. It regulates transpiration, protein synthesis, and photosynthetic rate, maintains membrane stability, relative water content, and reduces ROS production, H2O2, and MDA contents as well as enhances enzyme activities thus it protects plant cells from oxidative damage under water deficit conditions [20,21]. According to FDA (https://www.accessdata.fda.gov; CITE: 21CFR172.372; Title 21, Volume 3, accessed on 22 August 2022), methionine is one of nine essential amino acids in humans, and it could be safely added to food for except infant foods.
Melatonin plays a vital role in plant safeguarding against water deficit [22,23]. It protects the photosynthetic apparatus by enhancing the scavenging efficiency of ROS and decreases the oxidative damages induced by drought [24]. Melatonin can preserve the water status of plants under drought via regulating stomatal motion [25], maintaining cell membrane integrity [26], decreasing the cytotoxic biochemical cellular markers, such as H2O2, and MDA, enhance antioxidant enzymes activities [27]. Melatonin restricts the reverse impacts of drought stress via controlling the activity and production of core organic chemical compounds, especially chlorophyll, proteins, and nitrogen-related molecules [28]. Melatonin is a natural product found in plants and animals. It is considered to be safe as mentioned, as reported by FDA (https://www.fda.gov, accessed on 22 August 2022).
There are many studies on cauliflower under water deficit, but until now, there have been insufficient researches related to the impact of proline, methionine, and melatonin on cauliflower plants and the accumulation of glycosinolates as well antioxidant enzymes under water deficit conditions. Therefore, the purpose of this study is to investigate the impacts of proline, methionine, and melatonin on the agronomical and physiological characteristics of cauliflower plants under water deficit.
2. Material and Methods
2.1. Experimental Layout and Treatments
To investigate the effects of proline, methionine, and melatonin on cauliflower plants exposed to drought stress, 45 days old homogenous cauliflower (cv. Arasya) seedlings were transplanted into plastic pots (40 cm diameter) filled with 16 kg pre-washed sand (one seedling/pot). After transplantation, all plants were acclimated for a month by keeping them at 60–70% field capacity. Then, all pots (288) were divided into 3 groups (96 pots/group) and subjected to the following treatments: (I) control (well-watered), in which the plants were kept at 60–70% field capacity, (II) moderate drought stress, in which the plants were kept at 40–50% field capacity, and (III) severe drought stress where the plants were kept at 30–40% field capacity. To determine the field capacity of each level of irrigation, 12 calibrated tensiometers were distributed on the different treatments (4 devices/irrigation level). All pots were daily monitored and maintained at the investigated level of field capacity using specific volume of irrigation water according to the readings of different devices. Each group of irrigation treatments (96 pots) was divided into 4 subgroups to apply the foliar treatments. The first subgroup was sprayed with distilled water + 0.05% Tween-20 at (v/v) as a wetting agent, the second subgroup was sprayed with Pro (25 mg L−1) + 0.05% Tween-20 at (v/v), the third subgroup was sprayed with Met (25 mg L−1) + 0.05% Tween-20 at (v/v), and the fourth subgroup was sprayed with MT (25 mg L−1) + 0.05% Tween-20 at (v/v). All foliar treatments were performed 5 times at 30, 45, 60, 75, and 90 days after transplanting. Each plant sprayed with a hand-held sprayer at 120 mL on vegetative growth. All pots were left to grow for additional 15 days; then, gathered to determine the different studied variables. The experimental layout was a complete randomized design (CRD) including 3 levels of irrigation × 4 foliar treatments × 8 pots × 3 replicates.
2.2. Studied Parameters
Six samples were randomly chosen from each treatment after 105 days from transplanting to determine all the following parameters:
2.2.1. Vegetative Growth and Chlorophyll
Number of leaves was recorded for each selected plant and leaf area (cm2)/plant was calculated according to Iqbal and Hidayat [29]. Chlorophyll content was measured by using a SPAD meter (SPAD 502 Minolta Co., Osaka, Japan) as described by Abdelgawad et al. [30].
2.2.2. Relative Water Content (RWC)
RWC was estimated as described by Abd Elbar et al. [31]. Discs of leaves (2 cm diameter) from fully developed leaves were taken and weighted (FW) then placed in distilled water for 2 h at 25 °C subsequently weighted for turgid weights (TW). The dry weight (DW) was taken after dried samples in an oven for 24 h at 110 °C [32].
2.2.3. Determination of Glucosinolates Content
Glucosinolates (µmol g−1): It was determined as described by Slominski and Campbell [33]. Briefly, the leaf samples (50 mg) were added to 10 mL of distilled water after extraction for 30 min, then added 5 mL of 20% trichloroacetic acid. Thereafter, the mixtures of samples were centrifuged at 3000 rpm for 10 min and 3 mL aliquots of supernatant were mixed with 3 mL of 0.4M Fe (NO3)3 in 1N HNO3. The absorbance was read at 460 nm. The results are expressed as potassium thiocyanate equivalents in μg/5 mg of extract.
2.2.4. Determination of Vitamin C
Vitamin C (mg/g FW): It was estimated by titration methods [34], mixed 10 g of leaf samples with 90 mL of oxalic acid (6%), and filtered, then titration 25 mL of filtrated solution by 2,6–dichlorophenol indophenol.
2.2.5. Determination of Total Soluble Sugars
Total soluble sugars were determined in leaves of cauliflower according to the anthrone method stated by Yemm and Willis [35]. Reducing, non-reducing, and total sugars (%): Total sugars were determined by colorimetric method of anthrone and sulfuric acid [36] while reducing sugars were estimated by the method of 3, 5-Dinitrosalicylic acid according to Miller [37]. Non-reducing sugar content was calculated by the difference between them.
2.2.6. Determination of Total Soluble Phenols
Total soluble phenols (mg/100 g FW) were determined by the method of Folin–Ciocalteu’s reagent, according to Awad et al. [38]. Briefly, the leaf samples (2 g) were extracted with methanol for 30 min. Thereafter, a spectrophotometer at 765 nm was used to estimate phenolic compounds’ contents.
2.2.7. Determination of Proline
Proline (µg.g−1 F.W): was determined by ninhydrin method using a spectrophotometer at 520 nm according to Bates et al. [39], by extracted leaf samples of cauliflower with 3% sulfosalicylic acid, and diluted in an equal volume of glacial acetic acid and ninhydrin solutions, then placed in a water bath at 100 °C. Thereafter, cooling down the sample with ice, 5 mL of toluene was added.
2.2.8. Determination of Antioxidant Enzymes Activity
The fresh leaf samples (0.2 g) were ground in 4 mL of 0.1 M ice-cold sodium phosphate buffer (pH 7.0) containing 1% (w/v) polyvinylpyrrolidone (PVP) and 0.1 mM EDTA, centrifuged at 10,000× g at 4 °C for 20 min. The supernatant was used for determining total soluble protein and activity of different enzymes as described by Bradford [40]. Polyphenol oxidase activity (PPO) (Unit min−1 g−1) (EC 1.14.18.1) was determined at absorbance 420 nm at zero time and after 1 min as described by Oktay et al. [41]. The mixture of reaction consisted of 100 μL crude enzyme, 600 μL catechol, and 2.3 mL phosphate buffer (0.1 M, pH 6.5).
Peroxidase activity (POD) (unit mg−1 protein) (EC 1.11.1.7) was estimated as described by Tarchoune et al. [42]. Briefly, the fresh leaf samples were pulverized and extracted in 50 mM potassium phosphate (pH 7.0). It was estimated as guiacol-induced peroxidation of H2O2. Phenylalanine ammonia-lyase (PAL) (µmol g−1 h−1 F.W): It was estimated as Trans-cinnamic acid according to Lister et al. [43]. The PAL assay reaction contains 100 μL crude extract and 900 μL of 6 μmol phenylalanine in 500 mM tris-HCl buffer (pH 8.5). Then incubated mixture at 37 °C for 1 h and measured on spectrophotometer at 290 nm.
2.2.9. Determination of Lipid Peroxidation and Abscisic Acid
Lipid peroxidation (MDA) was assessed using the technique described by Horie et al. [44]. Approximately 100 mg of leaf samples of cauliflower plants were homogenized in 10 mL of trichloroacetic acid (TCA). Then homogenate was centrifuged at 15,000× g for 5 min. After that, the obtained mixture was heated at 95 °C for 30 min, rapidly cooled on ice bath, and then centrifuged again. The absorbance of the supernatant was performed at 450, 532, and 600 nm were determined using microplate reader (Infinite 200 PRO, Tecan Group Ltd., Männedorf, Switzerland). The values of MDA were reported as nmol g−1 F.W. Leaf abscisic acid (ABA) was determined using the method stated by Furniss [45]. The quantification of the ABA was performed using ati-Unicum gas–liquid chromatography, 610 Series, equipped with flame ionization detector according to the method stated by Bates [39].
2.2.10. Yield and Curd Parameters
Six curds were chosen randomly from each treatment to determine curd height and diameter (cm), curd fresh weight (g), and curd dry matter %. The curd height was measured using the gradual meter. Curd fresh weight was directly assessed after sampling using a digital balance, whereas curd dry matter was estimated by drying the samples in an air-forced ventilated oven at 105 °C until the weight was constant.
2.3. Statistical Analysis
The data of factorial experiments for two seasons 2021 and 2022 were analyzed using the SPSS software program (SPSS for Windows, SPSS Inc., Chicago, IL, USA). Combined analysis was performed over seasons to indicate normality distribution and homogeneity tests [46,47]. Two-Way analysis of variance (ANOVA) and means were compared at the 0.05 level according to the Tukey HSD test (p ≤ 0.05). All the data are shown as means values of the interaction between treatments and water levels with standard error (SE). Furthermore, Pearson’s correlations between the studied variables were performed using the XLSTAT program (Addinsoft Company, Paris, France, Version 2014).
3. Results
3.1. Plant Growth and Chlorophyll Content
Data in Table 1 shows the impact of proline, methionine, and melatonin on vegetative growth measurements, including leaf area, chlorophyll content, and number of leaves. All cauliflower plants exposed to drought stress showed a significant reduction in all the recorded plant growth parameters (p ≤ 0.05) in comparison to the well-irrigated plants (p ≤ 0.05). Under the control plants, leaf area decreased by 25.13% and 39.14% in moderate drought stress and severe drought stress, respectively, compared to well-watered plants. Furthermore, the reduction in the growth parameters was less pronounced in the cauliflower plants sprayed with proline, methionine, and melatonin than in untreated plants.

Table 1.
Impact of interaction between treatments (proline, methionine, and melatonin) and water levels on leaf area, leaf chlorophyll content, and number of leaves of cauliflower plants grown under drought. ±Value indicates standard error (SE) of the means (n = 6). Means followed by different letters point to significant differences between the treatments at according to Tukey’s multiple range test (p ≤ 0.05).
The higher values of leaf area and leaf chlorophyll content of cauliflower plants were recorded in the plants sprayed with proline, methionine, and melatonin compared to untreated plants, under water-stressed and non-stressed conditions. In this context, spraying with proline, methionine, and melatonin did not show any significant changes in the number of leaves under well-irrigated conditions compared to untreated plants.
3.2. Relative Water and Osmolytes Content
Moderate drought stress and severe drought stress significantly decreased relative water, reducing, non-reducing and total sugars (p ≤ 0.05) in comparison to the well-irrigated plants (Figure 1A,C–E), while proline content was increased with drought stress (Figure 1B).
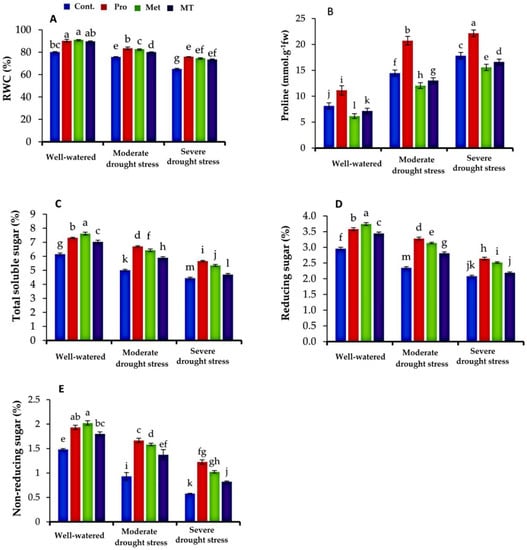
Figure 1.
Impact of interaction between treatments (proline, methionine, and melatonin) and water levels on relative water content (RWC, (A)), proline (B), total soluble sugar (C), reducing sugar (D), and non-reducing sugar (E) of cauliflower plants grown under different levels of irrigation water. Bars indicates standard error (SE) of the means (n = 6). Different letters indicate significant differences between the treatments at according to Tukey’s multiple range test (p ≤ 0.05).
Exogenous application of proline, methionine, and melatonin, as a foliar spray, displayed a significant (p ≤ 0.05) enhancement in relative water content (RWC) and proline compared to the untreated plants, under well-watered and moderate and severe drought stress conditions (Figure 1A,B). Compared to the untreated plants, the maximum values of leaf relative water content and proline content were achieved by proline treatment, followed by methionine and melatonin. Meanwhile, the leaf proline content increased with reducing the watering levels. Compared to the plants sprayed with tap water, the highest content of leaf proline was recorded in the plants treated with exogenous proline; conversely, lower values were observed in the plants treated with methionine, and melatonin, under different levels of irrigation water.
Furthermore, total soluble sugars, reducing and non-reducing sugar, were considerably affected by proline, methionine, and melatonin treatments under different levels of watering (Figure 1C–E). Under the well-watered condition, the application of proline, methionine, and melatonin improved the total soluble sugars by 16.27%, 19.78%, and 12.45%, respectively, compared to the untreated control (Figure 1C). Under drought stress conditions, the ratio of total sugar content was elevated due to proline, methionine, and melatonin treatments by 25.50%, 22.48%, and 15.41% at moderate drought stress and 21.1%, 16.44%, and 5.51% at severe drought stress, respectively, as compared to the untreated plants.
Observing the plants sprayed with tap water (untreated plants), drought stress significantly decreased the content of leaf-reducing and non-reducing sugar (p ≤ 0.05), as shown in Figure 1E. In contrast, the treatments with proline, methionine, and melatonin enhanced the concentration of leaf-reducing and non-reducing sugar. The improvement ratio caused by proline, methionine, and melatonin in the sugar reached 28.46%, 25.19%, and 16.49% at moderate drought stress and 52.86%, 43.65%, and 29.67% at severe drought stress for non-reducing sugar, respectively, compared to the untreated plants.
3.3. Lipid Peroxidation and Abscisic Acid Content
In this study, changes in the concentration of leaf lipid peroxidation (MDA) and abscisic acid (ABA) of cauliflower leaves as a result of the applied chemicals are shown in Figure 2A,B. The findings of the current study showed that MDA and ABA levels were lower in cauliflower plants under well-watered conditions compared with moderate and severe drought-stressed conditions (Figure 2). Foliar application of proline, methionine, and melatonin significantly reduced the levels of MDA and ABA compared to untreated plants. Furthermore, significant differences were observed between treated plants, under water-stressed conditions. The lowest values of MDA and ABA were found in cauliflower plants treated with proline, followed by methionine, and melatonin, while the highest value was observed in the untreated plants (tap water, control).
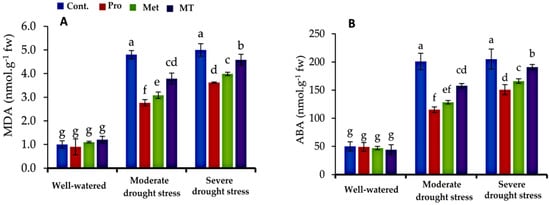
Figure 2.
Impact of interaction between treatments (proline, methionine, and melatonin) and water levels on malondialdehyde (MDA, (A)) and abscisic acid (ABA, (B)) of cauliflower plants grown under different levels of irrigation water. Bars indicates standard error (SE) of the means (n = 6). Different letters indicate significant differences between the treatments at according to Tukey’s multiple range test (p ≤ 0.05).
3.4. Phenolic-Related Enzymes and Secondary Metabolites
Concerning the phenolic-related enzymes (Figure 3A–C), it can be noted that the activities of polyphenol oxidase (PPO), peroxidase (POD), and phenylalanine ammonia-lyase (PAL), under water-stressed conditions, are significantly upraised in cauliflower plants compared to those of well-watered conditions. In this concern, moderate drought stress recorded the highest activity of antioxidant enzymes, followed by severe drought stress. Moderate drought stress increased PPO, POD, and PAL by 15.92%, 37.78%, and 23.33%, respectively, compared to well-watered plants, while severe drought stress increased PPO, POD, and PAL by 23.0%, 57.78%, and 36.67%, respectively, compared to well-watered plants. In general, the maximum activity of PPO, POD, and PAL was recorded in the leaves of untreated cauliflower plants, followed by melatonin and methionine, but the lowest value was observed in the leaves of plants treated with exogenous proline (Figure 3A–C).
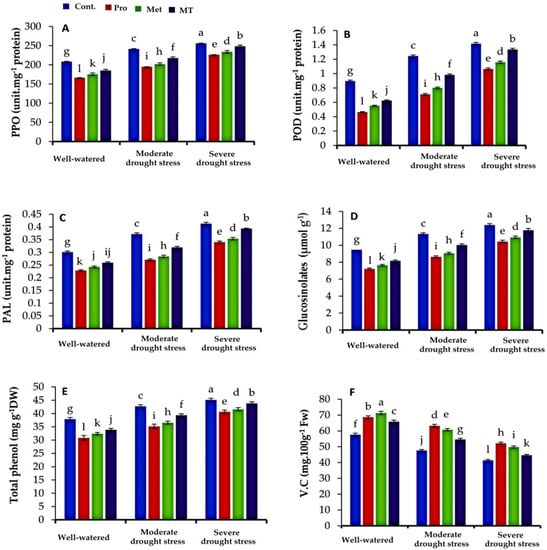
Figure 3.
Impact of interaction between treatments (proline, methionine, and melatonin) and water levels on polyphenol oxidase (PPO, (A)), peroxidase (POD, (B)), and phenylalanine ammonia-lyase (PAL, (C)), glucosinolates (D), total phenols (E) and vitamin C (F) of cauliflower plants grown under different levels of irrigation water. Bars indicates standard error (SE) of the means (n = 6). Different letters indicate significant differences between the treatments at according to Tukey’s multiple range test (p ≤ 0.05).
A similar significance was observed in the leaf total phenols and glucosinolates content (Figure 3D,E). Generally, the concentration of total phenols and glucosinolates in leaves increased with decreasing the irrigation water level. In this concern, moderate drought stress recorded the maximum values of total phenols and glucosinolates, followed by severe drought stress. Under the control plants, leaf total phenols increased by 12.77% and 19.10% in moderate drought stress and severe drought stress, respectively, compared to well-watered plants, as well as glucosinolates increased by 19.64% and 30.94% in moderate drought stress and severe drought stress, respectively, compared to well-watered plants. The obtained findings in Figure 3D,E exhibited that foliar application of proline, methionine, and melatonin extensively reduced the concentration of leaf total phenols and glucosinolates compared to the untreated plants, under both stressed and non-stressed conditions. Compared to all the treatments, the highest significant findings were recorded in the untreated plants exposed to severe drought stress in this respect. While the lowest content of total phenols and glucosinolates were registered in the leaves of cauliflower plants under well-watered conditions.
In contrast, the obtained results of this study showed that the content of vitamin C was higher in the leaves of cauliflower plants under well-watered conditions compared to moderate and severe drought stress conditions (Figure 3F). In this concern, the lowest contents of vitamin C were noticed in plants under severe drought stress Moreover, foliar spraying with proline, methionine, and melatonin elevated vitamin C content in the leaves of cauliflower plants compared to untreated plants under different levels of irrigation water. Under well-watered conditions, the proline, methionine, and melatonin treatments increased the leaf vitamin C content by 15.95%, 18.99%, and 12.23%, respectively. Under the water-stressed conditions, the aforementioned treatments improved the vitamin C in the leaves of cauliflower plants exposed to moderate drought stress by 24.65%, 21.63%, and 12.69%, and by 20.73%, 16.98%, and 7.18% in the leaves of cauliflower plants exposed to severe drought stress. While the lowest value of vitamin C was obtained in untreated plants, either under well-watered or water-stressed conditions.
3.5. Yield Parameters
Data in Table 2 shows significant differences in the curd height, curd fresh weight (plant yield) and curd dry matter of treated and untreated cauliflower plants, under stress and non-stress conditions. Cauliflower plants exposed to drought stress showed a significant reduction in yield parameters (p ≤ 0.05) in comparison to the well-irrigated plants (p ≤ 0.05). Under the control plants, plant yield decreased by 22.18% and 34.15% in moderate drought stress and severe drought stress, respectively, compared to well-watered plants. Generally, the higher values of curd height, curd fresh, and dry matter of cauliflower plants were recorded in the plants sprayed with proline, methionine, and melatonin compared to untreated plants, under water-stressed and non-stressed conditions (Table 2). In this context, spraying with proline, methionine, and melatonin did not show any significant changes in the cured diameter of the cauliflower plants under well-irrigated conditions compared to untreated plants. But the changes in the cured diameter were more pronounced under moderate and severe irrigation conditions. Compared to the untreated plants, the maximum values of cured diameter were recorded in the cauliflower plants treated with methionine, followed by melatonin and proline.

Table 2.
Impact of interaction between treatments (proline, methionine, and melatonin) and water levels on curd height, curd diameter, curd fresh weight, curd dry matter, and total yield of cauliflower plants grown under drought. ±Value indicates standard error (SE) of the means (n = 6). Means followed by different letters point to significant differences between the treatments at according to Tukey’s multiple range test (p ≤ 0.05).
3.6. Correlation Study
Pearson’s correlation coefficient is used to detect the positive and negative relationship among the agronomical and biochemical parameters of cauliflower plants sprayed with proline, methionine, and melatonin, under well-watered or water-stressed conditions. The significant relationship (bold numbers) and insignificant correlation (nonbolded numbers) are shown in Table 3. The Pearson’s correlation analysis exhibited that leaf chlorophyll content was significantly and positively correlated with the vegetative growth characters (leaf area, and RWC). A similar correlation was found between leaf chlorophyll content and curd height, curd diameter, curd fresh weight, and curd dry matter. Furthermore, leaf chlorophyll content is linked significantly and negatively with MDA and ABA content. A similar negative association was observed between ABA and yield parameters (curd fresh and dry matter). On the other hand, the leaf chlorophyll content also correlated significantly and positively with vitamin C, total soluble sugar, reducing, and non-reducing sugar. These results imply that foliar spraying with proline, methionine, and melatonin were effective compounds for mitigating drought stress in cauliflower plants by modulating osmolytes, sugar biosynthesis, and antioxidant compounds.

Table 3.
Pearson’s correlation analysis among agro-physiological characteristics of the cauliflower plant sprayed with melatonin either under well-watered or water-stressed conditions. Values in bold are different from 0 with a significance level alpha = 0.05. RWC = relative water content, V.C = Vitamin C, TSS = total soluble sugar, Rsu = reducing sugar, TP = total phenol, PPO = polyphenol oxidase, POD = peroxidase, PAL = phenylalanine ammonia-lyase, GLC = glucosinolates, and Chl. = chlorophyll.
4. Discussion
Drought is considered one of the most important environmental stresses that has serious limitations on economic crop productivity worldwide [48,49,50]. Approximately 45% of the globe’s cultivated area is affected by frequent and continuous water shortages. This stands threatening the food security of about 38% of the world population that lives in drought-prone areas [51]. Drought-susceptible vegetables such as cauliflower plants can be extremely influenced by water scarcity. Water shortages can cause a reduction in the photosynthesis parameters, biochemical compounds, nutrient and water absorption, growth characteristics, and yield production of sensitive vegetable crops [52,53]. In this study, plants exposed to drought stress (moderate drought stress and severe drought stress) exhibited significant decreases in leaf area, leaf chlorophyll content, and the number of leaves compared to well-watered plants (Table 1). These declines might be due to the result of confusion in the photosynthetic process by pigment retraction, stomatal conductance restrictions, and reduction in the photochemical quantum yield [9,11]. Many strategies have been suggested to mitigate the adverse impacts of water shortages and improve crop productivity. These strategies include the utilization of grafting, biochar, and biofertilizer application and spraying with some chemicals or/and nanoparticles of some nutrients. In this respect, foliar spraying with some chemical compounds such as salicylic acid and jasmonic acid has been widely studied to induce drought tolerance in different economic crops [54,55,56,57].
Likewise, biochemical application treatments such as proline, methionine, and melatonin have been suggested to alleviate the adverse impacts of abiotic stresses, and they affect the plant in the same way [58,59,60]. Furthermore, several reports have shown that exogenous proline, methionine, and melatonin can certainly influence the growth performance of different crops [13,58,59,60,61]. In the present study, the roles of exogenous proline, methionine, and melatonin in mitigating water stress in cauliflower plants were examined. The current study confirmed that all the aforementioned treatments (proline, methionine, and melatonin) significantly improved the leaf chlorophyll content in cauliflower plants under water-stressed conditions. This effect could be related to the ability of proline, methionine, and melatonin to protect cauliflower plants from drought-induced oxidative damage [58,61,62]. These results were in harmony with several investigators who stated that the application of proline, methionine, and melatonin considerably improved the concentration of leaf chlorophyll and reduced the oxidative damages that occurred by ROS molecules [58,60,61,62,63]. On the other hand, the significant reduction in the studied vegetative growth of untreated cauliflower plants, under water-stressed conditions, might be associated with the reduction of water and nutrient uptake from the soil and consequently diminish cell division and elongation as well as plant growth and development [48,51,53,64].
In addition, drought stress significantly disturbs the physiological processes in the leaves of plants, particularly photosynthetic rate, respiration, stomatal conductance, and transpiration rate. Those processes are regulated by the leaf stomata through opening and closing, which are impacted by water balance [48,65]. Relative water content (RWC) is considered a vital physiological parameter for the water status and supports the plants’ ability to survive under water-stressed conditions.
In the present research, moderate drought stress and severe drought stress significantly decreased relative water and osmolytes content except for proline in comparison to the well-irrigated plants (Figure 1). Moderate drought stress and severe drought stress decreased RWC and this decline could be explained by the negative effects on the plants’ conductivity, availability, and water absorption [11]. Water deficiency considerably decreased the RWC, proline, reducing sugar, non-reducing sugar, and total soluble sugar in untreated cauliflower plants. Conversely, the exogenous proline, methionine, and melatonin, as foliar spraying, increased the RWC, proline, reducing sugar, non-reducing sugar, and total soluble sugar of treated cauliflower plants (Figure 1). The leaf proline content increased with reducing the watering levels. Compared to the plants sprayed with tap water, the highest content of leaf proline was recorded in the plants treated with exogenous proline; conversely, lower values were observed in the plants treated with methionine, and melatonin under different levels of irrigation water. These findings imply that the high accumulation of endogenous proline in plant leaves under different levels of irrigation water may be related to the additional concentration of proline that plants acquire after spraying the cauliflower plants with exogenous proline. This finding suggests that the previous treatments enhance the function of leaf stomata by inducing the plant to reopen its stomata [66,67,68], improving photosynthesis, increasing the soluble carbohydrates, and permitting the RWC to rise under water-stressed conditions. Besides, in this study, there is a positive correlation between RWC and reducing sugar, non-reducing sugar, and total soluble sugar (Table 3). These results indicate that foliar application with proline, methionine, and melatonin, under water stress conditions, may stimulate the sucrose biosynthesis (non-reducing sugar) that is considered the main form of soluble sugars. Similar results were observed by several investigators who stated that under osmatic stresses, osmolyte substances, i.e., free amino and total soluble sugar content, were significantly improved by exogenous proline and melatonin treatment, which may lead to osmotic adjustment [11,58,69]. Moreover, El-Yazied et al. [58] found that a high positive correlation between the increased concentration of soluble carbohydrates and the ability of the plant to survive under water-stressed conditions. Many reports declared that leaf stomata and pore numbers were reduced in plants under water-stressed conditions compared to untreated and treated plants. Moreover, the closure of stomata decreases the water loss from the plant leaves by transpiration, which could be related to abscisic acid accumulation [57,59]. Moreover, the closure of stomata decreases the water loss from the plant leaves by transpiration, which could be related to abscisic acid accumulation [57,59]. Our study confirmed that the ABA significantly increased in the untreated cauliflower plants and was reduced in the treated plants under water-stressed conditions (Figure 2B). In addition, a negative correlation was found between ABA level and reducing sugar, non-reducing sugar, and total soluble sugar content (Table 3). This finding indicated that a higher level of ABA, under water-stressed conditions, could be related to reducing the photosynthesis process and consequently decreasing the accumulation of osmolyte substances in plants, which play a vital role in mitigating environmental stress tolerance by minimizing water loss and protecting the cell membrane [14,58].
The biotic and abiotic stresses cause serious changes in the production of, lipid peroxidation (MDA), and phytohormones, which lead to great alterations in plant growth and crop production [48,49]. Malondialdehyde (MDA), a constituent produced by membrane lipids in response to reactive oxygen species (ROS), can be used as an indicator to assess the degree of plasma membrane damage and the ability of plants to drought stress tolerance (moderate and severe drought-stress) [70]. In the present study, MDA levels were lowered in cauliflower plants under well-watered conditions compared with moderate and severe drought-stressed conditions (Figure 2). The plants could respond to drought stress (moderate and severe drought-stress) by producing fewer leaves, stomata, and decreasing leaf area, in addition to enhancing stress hormone concentrations such as ABA (Figure 2B). In the present research, the level of MDA was higher in untreated plants while its level was low in the treated plants with proline, methionine, and melatonin (Figure 2A). Similar findings were observed by several researchers who found that the application of proline, methionine, and melatonin significantly reduced the MDA level in different plants [58,71].
Numerous plant species have multifaceted antioxidant systems consisting of enzymatic and non-enzymatic processes involved in detoxifying, removing, and neutralizing ROS overproduction to preserve cellular redox stability [48,58,60,72]. Antioxidant enzymes are essential protective enzymes associated with the enzymatic defense system, efficiently removing the toxicity of ROS, and reducing H2O2 levels in plant cells [11,73]. Under osmotic stresses such as water shortages, plants change the mechanisms of tolerance to reduce the adverse effect of abiotic stress through the activation of many antioxidant enzymes (PAL, PPO, CAT, and POD) to protect the plant cells from oxidative injury [48,49,51]. Moreover, secondary metabolites, i.e., non-enzymatic antioxidants (vitamin C, phenols), proline, ABA, free amino acids, and glucosinolates, play an important role in improving the tolerance of plants against drought stress conditions [58,74,75].
In the current research, phenolic-related enzymes (Figure 3A–C) significantly upraised in moderate and severe drought stress of cauliflower plants compared to those of well-watered conditions. The levels of phenolic-related enzymes (PAL, POD, and PPO) and secondary metabolites, i.e., total phenols and glucosinolates content, significantly increased in untreated plants than in the treated plants with proline, methionine, and melatonin, except for vitamin C; this result could be associated with the toxic effect of ROS under drought stress conditions [58,75]. These findings suggest that proline, methionine, and melatonin may play a vital role in the elevation of glucosinolates, enzymatic, and non-enzymatic antioxidants (total phenols and vitamin C) to protect the cells of the cauliflower plants from injury under water-stressed conditions. Similarly, the application of proline, methionine, and melatonin increased the activity of enzymatic antioxidants (PPO, SOD, and CAT) and non-enzymatic antioxidants in treated plant plants under environmental stress [13,20,58]. In the current study reflected an increase in the level of vitamin C in response to water shortages (moderate and severe drought stress) of cauliflower plants compared to those of well-watered conditions. Compared to the untreated plants, the maximum vitamin C was noted in treated plants with proline, methionine, and melatonin, under both well-watered and water-stressed conditions. Vitamin C, as a non-enzymatic antioxidant, also plays an important role in removing or detoxifying the reactive oxygen species (ROS) and protects the plant cell from oxidative damage [76,77].
Regarding the yield of cauliflower, drought stress had an adverse effect and reduced the yield measurements of cauliflower (curd height, fresh and dry matter of curd). Cauliflower plants exposed to drought stress (moderate and severe drought stress) showed a significant reduction in yield parameters (p ≤ 0.05) in comparison to the well-irrigated plants (Table 2). The results of this study cleared that the curd height, fresh and dry matter of curd, and total yield improved due to foliar application of proline, methionine, and melatonin under drought stress conditions (Table 2). Moreover, yield was correlated positively with chlorophyll content, non-reducing sugar, curd fresh weight, and curd dry matter and negatively with ABA and MDA levels (Table 3). These findings imply that proline, methionine, and melatonin have an important role in the improvement of yield and its measurements under water stress by regulating leaf water status and ABA level, non-reducing sugar biosynthesis, as well as the formation of enzymatic and non-enzymatic antioxidants. Consistent with the current study’s findings, many researchers found that foliar spraying with proline, methionine, and melatonin increased the yield of potato, corn, and tomato plants under drought conditions [11,58,69]. Therefore, this study suggests that applying either of the biochemical treatments (proline, methionine, and melatonin) induces the curd formation and development under water-stressed conditions in the same way by improving leaf chlorophyll content in cauliflower plants under water-stressed conditions, which consequently leads to increasing the photosynthetic rate and improving the accumulation of osmolytes, and carbohydrates in addition to reducing ABA and MDA in plant cells [13,20,58,78,79,80,81,82].
5. Conclusions
The results of the current study mentioned the importance of foliar applications of proline, methionine, and melatonin to mitigate drought stress. Furthermore, all treatments increased the accumulation of total soluble sugar, reducing and non-reducing sugar, which consequently leads to improving the plant growth and productivity of cauliflower plants under drought stress conditions (Figure 4). Thus, our treatments could be used as an agricultural treatment to reduce the harmful effects of drought. Moreover, these compounds are safe for use.
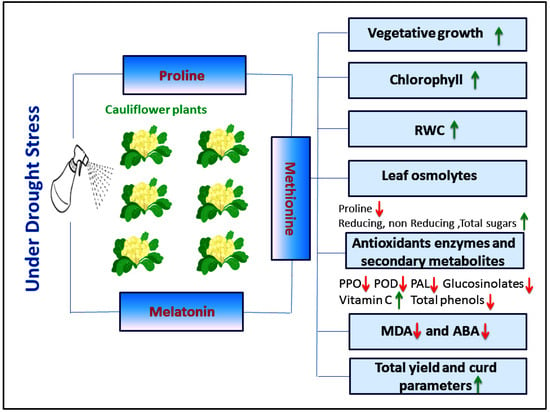
Figure 4.
Simplified conclusion for the suggested effect of proline, methionine, and melatonin application as a foliar spray on the growth and bioactive compounds of cauliflower plants grown under drought stress.
Author Contributions
Conceptualization, H.A.E.-B., M.A.M.A.E.-H., M.A.B. and S.M.A.D.; methodology, H.A.E.-B., E.A.A., I.A.I. and D.B.E.D.; software, M.M.E.-M., E.A.A., N.A.A.-H. and E.S.D.; validation, M.S.A., M.M.E.-M., E.S.D. and D.B.E.D.; formal analysis, H.A.E.-B., E.A.A., M.A.B. and S.M.A.-Q.; investigation, E.A.A., M.A.M.A.E.-H., N.A.A.-H. and F.M.A.; resources, M.A.M.A.E.-H., A.A., S.M.A.D. and I.A.I.; data curation, H.A.E.-B., E.A.A., E.S.D. and S.M.A.D.; writing—original draft preparation, H.A.E.-B., E.A.A., M.A.M.A.E.-H., A.A. and S.M.A.D.; writing—review and editing, D.B.E.D., M.S.A., F.M.A., M.M.E.-M. and E.S.D.; visualization, E.A.A., M.S.A., M.A.B. and S.M.A.-Q., supervision, H.A.E.-B. and S.M.A.D.; project administration, H.A.E.-B., N.A.A.-H. and A.A.; funding acquisition, I.A.I., F.M.A., E.A.A., S.M.A.-Q., M.A.M.A.E.-H. and S.M.A.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Mansoura University, Faculty of Agriculture, Mansoura, Egypt. The funding number is NA.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors extend their appreciation to Taif University for funding current work by Taif University Researchers Supporting Project number (TURSP-2020/120), Taif University, Taif, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kapusta-Duch, J.; Szelag-Sikora, A.; Sikora, J.; Niemiec, M.; Grodek-Szostak, Z.; Kubon, M.; Leszczynska, T.; Borczak, B. Health-Promoting Properties of Fresh and Processed Purple Cauliflower. Sustainability 2019, 11, 4008. [Google Scholar] [CrossRef]
- Munoz-Esparza, N.C.; Latorre-Moratalla, M.L.; Comas-Baste, O.; Toro-Funes, N.; Veciana-Nogues, M.T.; Vidal-Carou, M.C. Polyamines in Food. Front. Nutr. 2019, 6, 00108. [Google Scholar] [CrossRef] [PubMed]
- Picchi, V.; Fibiani, M.; Scalzo, R.L. Cauliflower. In Nutritional Composition and Antioxidant Properties of Fruits and Vegetables; Academic Press: Cambrige, MA, USA, 2020; Volume 1, pp. 19–32. [Google Scholar]
- Sohail; Khan, N.; Ullah, Z.; Ahmad, J.; Khan, A.; Nawaz, F.; Khan, R. Effect of deficit irrigation and nitrogen levels on growth and yield of cauliflower under drip irrigation. Pure Appl. Biol. 2018, 7, 910–921. [Google Scholar]
- Hashim, A.; Alharbi, B.; Abdulmajeed, A.; Elkelish, A.; Hozzein, W.; Hassan, H. Oxidative Stress Responses of Some Endemic Plants to High Altitudes by Intensifying Antioxidants and Secondary Metabolites Content. Plants 2020, 9, 869. [Google Scholar] [CrossRef] [PubMed]
- Elkelish, A.; Ibrahim, M.F.M.; Ashour, H.; Bondok, A.; Mukherjee, S.; Aftab, T.; Hikal, M.; El-Yazied, A.A.; Azab, E.; Gobouri, A.A.; et al. Exogenous Application of Nitric Oxide Mitigates Water Stress and Reduces Natural Viral Disease Incidence of Tomato Plants Subjected to Deficit Irrigation. Agronomy 2021, 11, 87. [Google Scholar] [CrossRef]
- Saied, E.M.; Arenz, C. Small Molecule Inhibitors of Ceramidases. Cell. Physiol. Biochem. 2014, 34, 197–212. [Google Scholar] [CrossRef]
- Ahmad, P.; Jamsheed, S.; Hameed, A.; Rasool, S.; Sharma, I.; Azooz, M.; Hasanuzzaman, M. Drought Stress Induced Oxidative Damage and Antioxidants in Plants. In Oxidative Damage to Plants; Elsevier: Amsterdam, The Netherlands, 2014; pp. 345–367. [Google Scholar] [CrossRef]
- Elkelish, A.; El-Mogy, M.M.; Niedbała, G.; Piekutowska, M.; Atia, M.A.M.; Hamada, M.M.A.; Shahin, M.; Mukherjee, S.; El-Yazied, A.A.; Shebl, M.; et al. Roles of Exogenous α-Lipoic Acid and Cysteine in Mitigation of Drought Stress and Restoration of Grain Quality in Wheat. Plants 2021, 10, 2318. [Google Scholar] [CrossRef]
- Kumawat, K.R.; Sharma, N. Effect of drought stress on plants growth. Pop. Kheti 2018, 6, 239–241. [Google Scholar]
- Ibrahim, M.F.M.; Abd Elbar, O.H.; Farag, R.; Hikal, M.; El-Kelish, A.; El-Yazied, A.A.; Alkahtani, J.; Abd El-Gawad, H.G. Melatonin Counteracts Drought Induced Oxidative Damage and Stimulates Growth, Productivity and Fruit Quality Properties of Tomato Plants. Plants 2020, 9, 1276. [Google Scholar] [CrossRef]
- Ibrahim, M.F.M.; El-Samad, G.A.; Ashour, H.; El-Sawy, A.M.; Hikal, M.; Elkelish, A.; El-Gawad, H.A.; El-Yazied, A.A.; Hozzein, W.N.; Farag, R. Regulation of Agronomic Traits, Nutrient Uptake, Osmolytes and Antioxidants of Maize as Influenced by Exogenous Potassium Silicate under Deficit Irrigation and Semiarid Conditions. Agronomy 2020, 10, 1212. [Google Scholar] [CrossRef]
- Hosseinifard, M.; Stefaniak, S.; Javid, M.G.; Soltani, E.; Wojtyla, L.; Garnczarska, M. Contribution of Exogenous Proline to Abiotic Stresses Tolerance in Plants: A Review. Int. J. Mol. Sci. 2022, 23, 5186. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Kumar, V.; Burritt, D.J.; Fujita, M.; Mäkelä, P. Osmoprotectant-Mediated Abiotic Stress Tolerance in Plants. In Proline Metabolism and Its Functions in Development and Stress Tolerance; Springer Nature: Cham, Switzerland, 2019; pp. 41–72. [Google Scholar]
- Burritt, D.J. Proline and the cryopreservation of plant tissues: Functions and practical applications. Curr. Front. Cryopreserv. 2012, 20, 415–426. [Google Scholar]
- Dar, M.I.; Naikoo, M.I.; Rehman, F.; Naushin, F.; Khan, F.A. Proline Accumulation in Plants: Roles in Stress Tolerance and Plant Development. In Osmolytes and Plants Acclimation to Changing Environment: Emerging Omics Technologies; Springer: Berlin/Heidelberg, Germany, 2016; pp. 155–166. [Google Scholar]
- Adejumo, S.A.; Oniosun, B.; Akpoilih, O.A.; Adeseko, A.; Arowo, D.O. Anatomical changes, osmolytes accumulation and distribution in the native plants growing on Pb-contaminated sites. Environ. Geochem. Health 2021, 43, 1537–1549. [Google Scholar] [CrossRef] [PubMed]
- AlKahtani, M.D.F.; Hafez, Y.M.; Attia, K.; Rashwan, E.; Husnain, L.A.; AlGwaiz, H.I.M.; Abdelaal, K.A.A. Evaluation of Silicon and Proline Application on the Oxidative Machinery in Drought-Stressed Sugar Beet. Antioxidants 2021, 10, 398. [Google Scholar] [CrossRef]
- You, J.; Zhang, Y.; Liu, A.; Li, D.; Wang, X.; Dossa, K.; Zhou, R.; Yu, J.; Zhang, Y.; Wang, L.; et al. Transcriptomic and metabolomic profiling of drought-tolerant and susceptible sesame genotypes in response to drought stress. BMC Plant Biol. 2019, 19, 267. [Google Scholar] [CrossRef]
- Mehak, G.; Akram, N.A.; Ashraf, M.; Kaushik, P.; El-Sheikh, M.A.; Ahmad, P. Methionine-induced regulation of growth, secondary metabolites and oxidative defense system in sunflower (Helianthus annuus L.) plants subjected to water deficit stress. PLoS ONE 2021, 16, e0259585. [Google Scholar] [CrossRef]
- Merwad, A.-R.M.A.; Desoky, E.-S.M.; Rady, M.M. Response of water deficit-stressed Vigna unguiculata performances to silicon, proline or methionine foliar application. Sci. Hortic. 2018, 228, 132–144. [Google Scholar] [CrossRef]
- Wang, J.; Chen, J.; Sharma, A.; Tao, S.; Zheng, B.; Landi, M.; Yuan, H.; Yan, D. Melatonin Stimulates Activities and Expression Level of Antioxidant Enzymes and Preserves Functionality of Photosynthetic Apparatus in Hickory Plants (Carya cathayensis Sarg.) under PEG-Promoted Drought. Agronomy 2019, 9, 702. [Google Scholar] [CrossRef]
- Ahmad, S.; Muhammad, I.; Wang, G.Y.; Zeeshan, M.; Yang, L.; Ali, I.; Zhou, X.B. Ameliorative effect of melatonin improves drought tolerance by regulating growth, photosynthetic traits and leaf ultrastructure of maize seedlings. BMC Plant Biol. 2021, 21, 368. [Google Scholar] [CrossRef]
- Sharma, A.; Zheng, B. Melatonin Mediated Regulation of Drought Stress: Physiological and Molecular Aspects. Plants 2019, 8, 190. [Google Scholar] [CrossRef]
- Wei, J.; Li, D.-X.; Zhang, J.-R.; Shan, C.; Rengel, Z.; Song, Z.-B.; Chen, Q. Phytomelatonin receptor PMTR1-mediated signaling regulates stomatal closure in Arabidopsis thaliana. J. Pineal Res. 2018, 65, e12500. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Lian, H.; Mou, X. Effect of foliar spraying exogenous melatonin on physiological and biochemical characteristics of Dendranthema morifolium ‘Chuju’ seedlings under drought stress. Acta Bot. Boreali Occident. Sin. 2016, 36, 2241–2246. [Google Scholar]
- Khan, A.; Numan, M.; Khan, A.; Lee, L.; Imran, M.; Asaf, S.; Al-Harrasi, A. Melatonin: Awakening the Defense Mechanisms during Plant Oxidative Stress. Plants 2020, 9, 407. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Su, T.; Huo, L.; Wei, H.; Jiang, Y.; Xu, L.; Ma, F. Unveiling the mechanism of melatonin impacts on maize seedling growth: Sugar metabolism as a case. J. Pineal Res. 2015, 59, 255–266. [Google Scholar] [CrossRef]
- Iqbal, A.; Hidayat, Z. Potassium Management for Improving Growth and Grain Yield of Maize (Zea mays L.) under Moisture Stress Condition. Sci. Rep. 2016, 6, 34627. [Google Scholar] [CrossRef]
- Abdelgawad, K.F.; El-Mogy, M.M.; Mohamed, M.I.A.; Garchery, C.; Stevens, R.G. Increasing Ascorbic Acid Content and Salinity Tolerance of Cherry Tomato Plants by Suppressed Expression of the Ascorbate Oxidase Gene. Agronomy 2019, 9, 51. [Google Scholar] [CrossRef]
- Abd Elbar, O.H.; Elkelish, A.; Niedbała, G.; Farag, R.; Wojciechowski, T.; Mukherjee, S.; Abou-Hadid, A.F.; El-Hennawy, H.M.; Abou El-Yazied, A.; Abd El-Gawad, H.G. Protective Effect of γ-Aminobutyric Acid Against Chilling Stress during Reproductive Stage in Tomato Plants Through Modulation of Sugar Metabolism, Chloroplast Integrity, and Antioxidative Defense Systems. Front. Plant Sci. 2021, 12, 663750. [Google Scholar] [CrossRef]
- Perdones, Á.; Escriche, I.; Chiralt, A.; Vargas, M. Effect of chitosan–Lemon essential oil coatings on volatile profile of strawberries during storage. Food Chem. 2016, 197, 979–986. [Google Scholar] [CrossRef]
- Slominski, B.A.; Campbell, L.D. Indoleacetonitriles—thermal degradation products of indole glucosinolates in commercial rapeseed (Brassica napus) meal. J. Sci. Food Agric. 1989, 47, 75–84. [Google Scholar] [CrossRef]
- Shehata, S.A.; Elmogy, M.; Mohamed, H.F.Y. Postharvest quality and nutrient contents of long sweet pepper enhanced by supplementary potassium foliar application. Int. J. Veg. Sci. 2019, 25, 196–209. [Google Scholar] [CrossRef]
- Yemm, E.W.; Willis, A.J. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508–514. [Google Scholar] [CrossRef]
- Helrich, K. Official Methods of Analysis, 15th ed.; Association of Official Agricultural Chemist: Arlington, VA, USA, 1990; Volume 1, p. 673. [Google Scholar]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Awad, A.; Parmar, A.; Ali, M.; El-Mogy, M.; Abdelgawad, K. Extending the Shelf-Life of Fresh-Cut Green Bean Pods by Ethanol, Ascorbic Acid, and Essential Oils. Foods 2021, 10, 1103. [Google Scholar] [CrossRef] [PubMed]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Oktay, M.; Küfreviolu, I.; Kocaçali¸skan, I.; ¸Saklrolu, H. Polyphenoloxidase from Amasya apple. J. Food Sci. 1995, 60, 494–496. [Google Scholar] [CrossRef]
- Tarchoune, I.; Sgherri, C.; Izzo, R.; Lachaâl, M.; Navari-Izzo, F.; Ouerghi, Z. Changes in the antioxidative systems of Ocimum basilicum L. (cv. Fine) under different sodium salts. Acta Physiol. Plant. 2012, 34, 1873–1881. [Google Scholar] [CrossRef]
- Lister, C.; Lancaster, J.E.; Walker, J.R. Phenylalanine Ammonia-lyase (PAL) Activity and its Relationship to Anthocyanin and Flavonoid Levels in New Zealand-grown Apple Cultivars. J. Am. Soc. Hortic. Sci. 1996, 121, 281–285. [Google Scholar] [CrossRef]
- Sunarpi; Horie, T.; Motoda, J.; Kubo, M.; Yang, H.; Yoda, K.; Horie, R.; Chan, W.-Y.; Leung, H.-Y.; Hattori, K.; et al. Enhanced salt tolerance mediated by AtHKT1 transporter-induced Na+ unloading from xylem vessels to xylem parenchyma cells. Plant J. 2005, 44, 928–938. [Google Scholar] [CrossRef] [PubMed]
- Furniss, B.S. Vogel’s Textbook of Practical Organic Chemistry; Pearson Education: Delhi, India, 1989. [Google Scholar]
- Shapiro, S.S.; Wilk, M.B. Analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Hartley, H. The maximum F-ratio as a short cut test for homogeneity of variance. Biometrika 1950, 37, 308–312. [Google Scholar] [PubMed]
- Abdelaziz, M.E.; Atia, M.A.M.; Abdelsattar, M.; Abdelaziz, S.M.; Ibrahim, T.A.A.; Abdeldaym, E.A. Unravelling the Role of Piriformospora indica in Combating Water Deficiency by Modulating Physiological Performance and Chlorophyll Metabolism-Related Genes in Cucumis sativus. Horticulturae 2021, 7, 399. [Google Scholar] [CrossRef]
- Mahmoud, A.W.M.; Samy, M.M.; Sany, H.; Eid, R.R.; Rashad, H.M.; Abdeldaym, E.A. Nanopotassium, Nanosilicon, and Biochar Applications Improve Potato Salt Tolerance by Modulating Photosynthesis, Water Status, and Biochemical Constituents. Sustainability 2022, 14, 723. [Google Scholar] [CrossRef]
- Zali, A.G.; Ehsanzadeh, P. Exogenous proline improves osmoregulation, physiological functions, essential oil, and seed yield of fennel. Ind. Crop. Prod. 2018, 111, 133–140. [Google Scholar] [CrossRef]
- El-Mogy, M.M.; Atia, M.A.M.; Dhawi, F.; Fouad, A.S.; Bendary, E.S.A.; Khojah, E.; Samra, B.N.; Abdelgawad, K.F.; Ibrahim, M.F.M.; Abdeldaym, E.A. Towards Better Grafting: SCoT and CDDP Analyses for Prediction of the Tomato Rootstocks Performance under Drought Stress. Agronomy 2022, 12, 153. [Google Scholar] [CrossRef]
- Abdallah, I.S.; Atia, M.A.M.; Nasrallah, A.K.; El-Beltagi, H.S.; Kabil, F.F.; El-Mogy, M.M.; Abdeldaym, E.A. Effect of New Pre-Emergence Herbicides on Quality and Yield of Potato and Its Associated Weeds. Sustainability 2021, 13, 9796. [Google Scholar] [CrossRef]
- Abdeldym, E.A.; El-Mogy, M.M.; Abdellateaf, H.R.L.; Atia, M.A.M. Genetic Characterization, Agro-Morphological and Physiological Evaluation of Grafted Tomato under Salinity Stress Conditions. Agronomy 2020, 10, 1948. [Google Scholar] [CrossRef]
- Zhu, Y.; Gong, H. Beneficial effects of silicon on salt and drought tolerance in plants. Agron. Sustain. Dev. 2014, 34, 455–472. [Google Scholar] [CrossRef]
- Ilyas, N.; Gull, R.; Mazhar, R.; Saeed, M.; Kanwal, S.; Shabir, S.; Bibi, F. Influence of Salicylic Acid and Jasmonic Acid on Wheat Under Drought Stress. Commun. Soil Sci. Plant Anal. 2017, 48, 2715–2723. [Google Scholar] [CrossRef]
- Kareem, F.; Rihan, H.; Fuller, M.P. The Effect of Exogenous Applications of Salicylic Acid on Drought Tolerance and Up-Regulation of the Drought Response Regulon of Iraqi Wheat. J. Crop Sci. Biotechnol. 2019, 22, 37–45. [Google Scholar] [CrossRef]
- Yong, B.; Xie, H.; Li, Z.; Li, Y.-P.; Zhang, Y.; Nie, G.; Zhang, X.-Q.; Ma, X.; Huang, L.-K.; Yan, Y.-H.; et al. Exogenous Application of GABA Improves PEG-Induced Drought Tolerance Positively Associated with GABA-Shunt, Polyamines, and Proline Metabolism in White Clover. Front. Physiol. 2017, 8, 1107. [Google Scholar] [CrossRef] [PubMed]
- El-Yazied, A.A.; Ibrahim, M.F.M.; Ibrahim, M.A.R.; Nasef, I.N.; Al-Qahtani, S.M.; Al-Harbi, N.A.; Alzuaibr, F.M.; Alaklabi, A.; Dessoky, E.S.; Alabdallah, N.M.; et al. Melatonin Mitigates Drought Induced Oxidative Stress in Potato Plants through Modulation of Osmolytes, Sugar Metabolism, ABA Homeostasis and Antioxidant Enzymes. Plants 2022, 11, 1151. [Google Scholar] [CrossRef] [PubMed]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akram, N.A.; Ashraf, M.; Ashraf, M.; Sadiq, M. Exogenous application of L-methionine mitigates the drought-induced oddities in biochemical and anatomical responses of bitter gourd (Momordica charantia L.). Sci. Hortic. 2020, 267, 109333. [Google Scholar] [CrossRef]
- Zhang, N.; Sun, Q.; Zhang, H.; Cao, Y.; Weeda, S.; Ren, S.; Guo, Y.-D. Roles of melatonin in abiotic stress resistance in plants. J. Exp. Bot. 2014, 66, 647–656. [Google Scholar] [CrossRef]
- Elhady, S.A.A.; El-Gawad, H.; Ibrahim, M.; Mukherjee, S.; Elkelish, A.; Azab, E.; Gobouri, A.; Farag, R.; Ibrahim, H.; El-Azm, N. Hydrogen Peroxide Supplementation in Irrigation Water Alleviates Drought Stress and Boosts Growth and Productivity of Potato Plants. Sustainability 2021, 13, 899. [Google Scholar] [CrossRef]
- Li, H.; Guo, Y.; Cui, Q.; Zhang, Z.; Yan, X.; Ahammed, G.J.; Yang, X.; Yang, J.; Wei, C.; Zhang, X. Alkanes (C29 and C31)-Mediated Intracuticular Wax Accumulation Contributes to Melatonin- and ABA-Induced Drought Tolerance in Watermelon. J. Plant Growth Regul. 2020, 39, 1441–1450. [Google Scholar] [CrossRef]
- Braga, L.N.; Silva, L.M.; Miranda, F.R.; Silva, E.O.; Canuto, K.M.; Miranda, M.R.; de Brito, E.S.; Zocolo, G.J. Physiological changes for drought resistance in different species of Phyllanthus. Sci. Rep. 2018, 8, 15141. [Google Scholar] [CrossRef]
- Ibrahim, M.; Ibrahim, H.A. Assessment of selenium role in promoting or inhibiting potato plants under water stress. J. Hortic. Sci. Ornam. Plants 2016, 8, 125–139. [Google Scholar]
- Sharma, A.; Wang, J.; Xu, D.; Tao, S.; Chong, S.; Yan, D.; Li, Z.; Yuan, H.; Zheng, B. Melatonin regulates the functional components of photosynthesis, antioxidant system, gene expression, and metabolic pathways to induce drought resistance in grafted Carya cathayensis plants. Sci. Total Environ. 2020, 713, 136675. [Google Scholar] [CrossRef]
- Zhao, C.; Guo, H.; Wang, J.; Wang, Y.; Zhang, R. Melatonin Enhances Drought Tolerance by Regulating Leaf Stomatal Behavior, Carbon and Nitrogen Metabolism, and Related Gene Expression in Maize Plants. Front. Plant Sci. 2021, 12, 779382. [Google Scholar] [CrossRef] [PubMed]
- Abdelaal, K.A.A.; Attia, K.A.; Alamery, S.F.; El-Afry, M.M.; Ghazy, A.I.; Tantawy, D.S.; Al-Doss, A.A.; El-Shawy, E.-S.E.; Abu-Elsaoud, A.M.; Hafez, Y.M. Exogenous Application of Proline and Salicylic Acid can Mitigate the Injurious Impacts of Drought Stress on Barley Plants Associated with Physiological and Histological Characters. Sustainability 2020, 12, 1736. [Google Scholar] [CrossRef]
- Anjum, S.A.; Ashraf, U.; Tanveer, M.; Khan, I.; Hussain, S.; Shahzad, B.; Zohaib, A.; Abbas, F.; Saleem, M.F.; Ali, I.; et al. Drought Induced Changes in Growth, Osmolyte Accumulation and Antioxidant Metabolism of Three Maize Hybrids. Front. Plant Sci. 2017, 8, 69. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Luan, Q.; Jiang, J.; Li, Y. Prediction and Utilization of Malondialdehyde in Exotic Pine Under Drought Stress Using Near-Infrared Spectroscopy. Front. Plant Sci. 2021, 12, 735275. [Google Scholar] [CrossRef]
- Ghafoor, R.; Akram, N.A.; Rashid, M.; Ashraf, M.; Iqbal, M.; Lixin, Z. Exogenously applied proline induced changes in key anatomical features and physio-biochemical attributes in water stressed oat (Avena sativa L.) plants. Physiol. Mol. Biol. Plants 2019, 25, 1121–1135. [Google Scholar] [CrossRef]
- Hoque, T.S.; Hossain, M.A.; Mostofa, M.G.; Burritt, D.J.; Fujita, M.; Tran, L.-S.P. Methylglyoxal: An Emerging Signaling Molecule in Plant Abiotic Stress Responses and Tolerance. Front. Plant Sci. 2016, 7, 1341. [Google Scholar] [CrossRef]
- Youssef, M.H.; Raafat, A.; El-Yazied, A.A.; Selim, S.; Azab, E.; Khojah, E.; El Nahhas, N.; Ibrahim, M.F. Exogenous Application of Alpha-Lipoic AcidMitigates Salt-Induced Oxidative Damage in Sorghum Plants through Regulation Growth, Leaf Pigments, Ionic Homeostasis, Antioxidant Enzymes, and Expression of Salt Stress Responsive Genes. Plants 2021, 10, 2519. [Google Scholar] [CrossRef]
- Del Carmen Martinez-Ballesta, M.; Moreno, D.A.; Carvajal, M. The Physiological Importance of Glucosinolates on Plant Response to Abiotic Stress in Brassica. Int. J. Mol. Sci. 2013, 14, 11607–11625. [Google Scholar] [CrossRef]
- Li, Z.; Su, X.; Chen, Y.; Fan, X.; He, L.; Guo, J.; Wang, Y.; Yang, Q. Melatonin Improves Drought Resistance in Maize Seedlings by Enhancing the Antioxidant System and Regulating Abscisic Acid Metabolism to Maintain Stomatal Opening Under PEG-Induced Drought. J. Plant Biol. 2021, 64, 299–312. [Google Scholar] [CrossRef]
- Shehata, S.A.; Abdelrahman, S.Z.; Megahed, M.M.A.; Abdeldaym, E.A.; El-Mogy, M.M.; Abdelgawad, K.F. Extending Shelf Life and Maintaining Quality of Tomato Fruit by Calcium Chloride, Hydrogen Peroxide, Chitosan, and Ozonated Water. Horticulturae 2021, 7, 309. [Google Scholar] [CrossRef]
- Vwioko, E.D.; El-Esawi, M.A.; Imoni, M.E.; Al-Ghamdi, A.A.; Ali, H.M.; El-Sheekh, M.M.; Abdeldaym, E.A.; Al-Dosary, M.A. Sodium Azide Priming Enhances Waterlogging Stress Tolerance in Okra (Abelmoschus esculentus L.). Agronomy 2019, 9, 679. [Google Scholar] [CrossRef]
- Agami, R.A. Applications of ascorbic acid or proline increase resistance to salt stress in barley seedlings. Biol. Plant. 2014, 58, 341–347. [Google Scholar] [CrossRef]
- El-Mogy, M.M.; Salama, A.M.; Mohamed, H.F.Y.; Abdelgawad, K.; Abdeldaym, E.A. Responding of Long Green Pepper Plants to Different Sources of Foliar Potassium Fertiliser; Sciendo: Warsaw, Poland, 2019; Volume 65, pp. 59–76. [Google Scholar] [CrossRef]
- Abuarab, M.E.; Hafez, S.M.; Shahein, M.M.; Hassan, A.M.; El-Sawy, M.B.; El-Mogy, M.M.; Abdeldaym, E.A. Irrigation scheduling for green beans grown in clay loam soil under a drip irrigation system. Water SA 2020, 46, 573–582. [Google Scholar] [CrossRef]
- Liu, W.; Liu, K.; Chen, D.; Zhang, Z.; Li, B.; El-Mogy, M.M.; Tian, S.; Chen, T. Solanum lycopersicum, a Model Plant for the Studies in Developmental Biology, Stress Biology and Food Science. Foods 2022, 11, 2402. [Google Scholar] [CrossRef]
- Shehata, S.A.; Omar, H.S.; Elfaidy, A.G.; EL-Sayed, S.S.; Abuarab, M.E.; Abdeldaym, E.A. Grafting enhances drought stress tolerance by regulating stress-responsive gene expression and antioxidant enzyme activities in cucumbers. BMC Plant Biol. 2022, 22, 408. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).