Abstract
The bioenergy crop switchgrass (Panicum virgatum L.) has been recognized as friendly to the soil of cultivated land depending on the previous land use types and management practices. However, the effects of switchgrass establishment on soil properties at a broader depth when it is harvested annually without any fertilization in northern China largely remain unknown. To explore the impacts of unfertilized switchgrass on soil physical and chemical properties, 0–100 cm soil samples were collected from 7-year cropland-to-switchgrass conversion and the bare land (control). The results showed that switchgrass establishment increased soil total and capillary porosity, CFU numbers of the microbial communities (fungi, bacteria, and actinomycetes), contents of microbial biomass (carbon, nitrogen, and phosphorus), and water-soluble organic carbon, and decreased soil bulk density, mostly at 0–60 cm depths, compared to the control values. Notably, the annual harvest of switchgrass insignificantly increased soil total and available nitrogen contents and slightly reduced available phosphorus and potassium contents. In conclusion, long-term cropland conversion to unfertilized switchgrass could ameliorate soil properties and does not cause soil depletion. The output of this study could inspire governments and farmers to make large-scale use of switchgrass in the ecological restoration of abandoned cropland in north China.
1. Introduction
The pursuit of crop production in the last 60 years has forced vast areas of land that were unsuitable for agricultural production, including 8 × 106 hm2 of grassland, to be reclaimed for farming in China [1,2]. Many severe ecological problems, such as soil degradation and acidification, soil erosion by wind and water, and agricultural non-point source pollution have already emerged and severely affected the safety of the ecological environment in these areas [1,3]. Conversion of cropland to grassland or forests in these areas has been evidenced as a profitable strategy for such environmental problems that has been implemented in many other countries worldwide and adopted by Chinese governments recently [4,5]. Meanwhile, the conversion of cropland, especially the marginal cropland in these areas, provides enormous opportunity for the large-scale use of switchgrass due to its great potential in bioenergy and multiple ecological services.
Switchgrass (Panicum virgatum L.) is a C4, warm season, deeply rooted perennial grass native to North America [6]. It has a wide range of ecological adaptations due to its high tolerance to various stresses and high nutrient- and water-use efficiencies [7,8]. Switchgrass, as one of the most photosynthetically efficient crops, can generate a large quantity of lignocellulosic biomass with adequate nutrient and high-quality fibers, and therefore has been proposed as a model feedstock crop to generate both forage and non-food bioethanol [9]. Moreover, switchgrass is of great interest due to its multiple ecological services such as soil erosion control, wildlife habitats, ornamental purposes, and nitrogen leakage prevention [10]. Recently switchgrass has been successfully introduced in northern China and shows good ecological adaptability [11]. Thus, switchgrass has been considered a good candidate for environmental restoration of marginal cropland, which is a promising solution to address the increasing demand for bioenergy feedstock, as well as to mitigate the environmental problems there, such as by its amelioration effects on degraded soil.
In addition to the biomass yield potential of switchgrass, the effects of its long-term cultivation on the soil micro-environment are also significant concerns. Several publications have revealed that soil physical properties were distinctively ameliorated after switchgrass establishment, e.g., decreased soil bulk density (BD) and increased soil porosity and aggregates [12,13,14], but this was mostly confined to a shallow soil layer (0–40 cm). For soil chemical properties, a plethora of papers have reported carbon sequestration effects after the cultivation of switchgrass independent of the establishment years, soil types, climate characteristics, and management practices. However, the quantity of the sequestrated carbon differs under those different conditions [15,16,17,18,19,20]. In contrast, Kasanke et al. (2020) reported that the long-term carbon accrual in soils under switchgrass production depends mainly on the initial site selection [21]. Soil nutrient levels after switchgrass establishment is another research focus worldwide. The annual harvest of switchgrass biomass indicates the long-term export of element nutrients from the cultivated land, which implies soil depletion risk if there is not any fertilization, primarily when harvested at a high frequency [22] and at an earlier time [23,24]. However, the removal rate of switchgrass was much lower than the annual crops [25]. The positive effects of fertilization on switchgrass yield have been proved. However, soil heath status after the annual harvest of switchgrass without fertilization compensation is greatly ignored. The uses of fertilization for switchgrass in many field experiments complicates soil nutrient variations, impeding people’s understanding of this question. Hence, the long-term nutrient removal by biomass harvest in unfertilized switchgrass land greatly challenges soil health [14,22,23,24,26]. To date, the effects of switchgrass establishment on soil properties in the absence of any fertilization have not been addressed, which remarkably prevents the large-scale uses of switchgrass in northern China.
Soil microbial activity usually reflects microbiological processes of soil microorganisms and is a potential indicator of soil quality, as plants rely on soil microorganisms to mineralize organic nutrient for growth and development [27]. Soil microbial communities play a critical role in the cycling of soil nutrient materials, which is usually affected by crop cover, management practices, soil types, and soil depths. Switchgrass establishment has been evidenced to increase soil bacteria and fungi communities and their activities [28,29,30], while lacking exploration at a deeper soil depth. It’s worth noting that switchgrass establishment might promote soil microbial nitrogen fixation, especially under low nitrogen levels, which accounts for a nonnegligible amount of nitrogen for the growth and development of switchgrass [31,32,33]. Hence, it is attractive to detect soil nitrogen levels after switchgrass establishment, especially when fertilization is not applied. It is necessary to uncover the microbial alterations after the conversion of cropland to unfertilized switchgrass.
We hypothesized that long-term switchgrass cultivation could dramatically ameliorate soil properties, but may generate some adverse effects on soil nutrient levels due to the annual export of element materials from the field. The objectives of this study were to (1) evaluate the effects of 7-year switchgrass establishment on soil physical and chemical properties; (2) to illustrate changes in soil nutrient levels after switchgrass cultivation compared to the bare land; and (3) to reveal the alterations in soil microbial communities including the quantity of soil bacteria, fungi, and actinomycetes as well as the contents of microbial carbon, nitrogen, and phosphorus.
2. Materials and Methods
2.1. Study Site Description and Management
The experiment site was located at the experimental base of the Institute of Grassland, Flowers and Ecology, Beijing Academy of Agricultural and Forestry Sciences (40°23′ N, 116°28′ E) at Town in Beijing, China (Figure S1). The soil type, climate characteristics, and the initial soil properties were well described in our previously published paper [34]. The experiment was based on a long-term yield evaluation test platform for switchgrass, which was built in 2006. There were two types of land in this study, switchgrass-cultivated land (switchgrass plot) and the bare land (bare land plot). We set five 4 × 5 m subplots for each type of land; these five subplots were not adjacent and were distributed randomly in the experiment field. We considered the five subplots of each plot to be five replicates in this study.
Before switchgrass plantation, rotary tillage was conducted at around 30 cm depth, and then about 150 kg/ha compound fertilizer (N:P:K = 20:12.5:10) was spread evenly over the whole experiment area. In mid-April 2006, we planted switchgrass seedlings (four leaf stage) with 80 cm plant spacing. Then, about 200 m3/ha irrigation was provided by sprinkler irrigation after plantation to ensure the expected growth of switchgrass according to our previous experience. Beyond that, there was just natural rainfall during the whole experiment period. The weeds in the switchgrass and bare land were manually prevented. In early November of each year, the above-ground biomass, including all the litter, was harvested, leaving around 10 cm stubble height. Beyond that, there were not any other management practices in this experiment. The amount of rainfall ranged from 318.00 mm to 733.20 mm, and the average temperature was 13.29 during the 2006 to 2012 period in this area.
2.2. Soil Sampling and Properties Determination
Soil samples were collected in mid-November 2012 from the two plots. Before sampling, the apparent litter and small rocks were carefully removed. We dug a quadrat pit layer by layer (20 cm for each layer to 100 cm) in each subplot of the two plots. The exact location of the quadrat is displayed in Figure S2. Soil from the layers 0–20, 20–40, 40–60, 60–80, and 80–100 cm was thoroughly mixed using the soil cone method, and then ten random subsamples were acquired from the cone to form one composed replicate. Hence, there were five soil replicates for each layer of the two plots. Undisturbed soil cores at different soil layers of each quadrate were taken by a cylindrical steel ring of 100 cm3 volume with five replicates for soil physical property analysis. The visible rocks were removed from soil samples and all soil samples were passed through a 2 mm sieve. Each soil replicate was divided into two parts; one part was transported back to the laboratory in a 4 °C incubator and then stored in a 4 °C refrigerator for the determination of microbial indicators. The other part was naturally air-dried and crushed by a micro-mill (Tianjin TEST Instrument Co., LTD, Tianjin, China) for the determination of soil organic carbon (SOC), total nitrogen (TN), total phosphorous (TP), total potassium (TK), available nitrogen (AN), available phosphorus (AP), available potassium (AK), and soil pH.
Soil capillary porosity (CP) was determined by the core method [35]. Soil total porosity (TPO) was calculated as the difference between the saturated soil weight and the dry soil weight divided by the sample volume [35]. Soil bulk density (BD) and soil water contents were determined based on the oven-dried cutting ring-acquired soil samples. Soil pH was measured using a 1:2.5 ratio of 0.01 M CaCl2 solution/soil suspension according to the professional standard of China (LY/T 1239-1999) by a Nahita pH meter, model ST 5000 (Ohaus International Trading (Shanghai) Co., LTD, Shanghai, China). A Vario Macro CHNS instrument (Elementar Analys ensysteme GmbH, Hanau, Germany) was used to determine soil TN [34]. We determined soil AN, AP, and AK contents using the alkaline KMnO4 oxidation method, Olsen’s extract method, and the NH4OAc extract method, respectively [36]. About 10 g of fresh soil samples were used to determine microbial C and N contents using the chloroform fumigation-K2SO4 extraction and potassium persulfate digestion methods, respectively [37]. Soil microbial phosphorus (MP) contents were determined by chloroform fumigation method coupled with phosphorus detection by molybdate colorimetry [38]. The values were corrected for phosphate sorption to the soil during the extraction and for microbial phosphorus not recovered by fumigation [38]. The potassium dichromate oxidation method coupled with ferrous sulfate titrimetry was used to determine SOC and water-soluble organic carbon (WSOC) contents. Digestion and colorimetric methods were used to determine soil TP content [39]. The atomic absorption method was used to determine soil total K (TK) contents [40]. Soil actinomycete is the third most abundant community and plays crucial roles in the mineralization process of soil organic materials, e.g., the degradation of cellulosic materials, and hence participates primarily in soil element cycling and soil amelioration. Hence, we herein analyzed these three dominant microbial communities. We used the solid medium made from beef extract, Martin’s medium [41], and Gause I Medium [42] to culture soil bacteria, fungi, and actinomycetes, respectively. The medium was invertedly cultured at 2 °C in a constant temperature incubator for one week. The CFU numbers were calculated according to the following formula,
where CFU indicates the CFU numbers of soil bacteria, fungi, and actinomycetes. C, n, and m represented the average community number, dilution ratio, and soil dry weights, respectively.
2.3. Statistical Analysis
The experiment data were organized and prepared using Microsoft Office Excel 2010. Shapiro–Wilk and Levene tests were performed to check the data distribution and homoscedasticity before the analysis, respectively. A Wilcoxon rank-sum test corrected by the “Bonferroni” method was carried out to verify the significant differences in soil indexes of the paired groups at p < 0.05 and 0.01. The correlation analysis based on Pearson’s method at p < 0.05 (*) and 0.01 (**) levels in this work were carried out using the “psych” package in R software (3.5.2). The figures were created using Origin (2019b, OriginLab Corporation, Northampton, MA, USA) and R software (Bell Laboratories, Inc., Windsor, ON, Canada).
3. Results
3.1. Soil Physical Properties of the Bare Land and Switchgrass Land
Figure 1 showed the great changes in soil physical properties after switchgrass establishment compared to the bare land. The switchgrass establishment significantly (p < 0.05, 0.01, or 0.001) increased 0–60 cm soil CP by 40.51%, 16.92%, and 14.33% sequentially from the top to the bottom (Figure 1a). Soil CP from 60–100 cm exhibited a slight (p > 0.05) decrease by 5.89% and 10.92% sequentially compared to the bare land (Figure 1a). Soil TP at 0–100 cm depth of switchgrass land was significantly (p < 0.05, 0.01, or 0.001) higher by 30.98%, 24.05%, 7.90%, 18.34, and 11.96% sequentially compared to the bare land (Figure 1b). For soil BD, 0–60 cm soil of switchgrass land significantly (p < 0.05 or 0.01) decreased by 6.37%, 4.58%, and 2.17% sequentially compared to the bare land (Figure 1c), while BD of 60–100 cm soil of the two land types seemed to be similar (Figure 1c). The water contents of 0–100 cm soil all showed an increased trend compared to the bare land, and the increase percentages reached 38.26%, 16.08%, 9.63%, 10.09%, and 5.13%, sequentially, though the difference at 80–100 cm was not statistically significant (p > 0.05, Figure 1d).
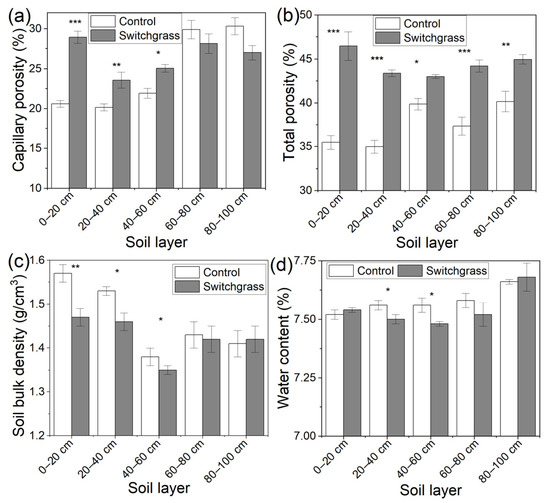
Figure 1.
Soil (a) capillary porosity, (b) total porosity, (c) bulk density, and (d) water contents in the bare land (control) and switchgrass-cultivated land. Error bars (standard deviation) were correctly added at the top of the mean columns. A Wilcoxon rank-sum test was used to analyze the significant differences in each soil index between control and switchgrass land at p < 0.05 (*), 0.01 (**), and 0.001 (***) levels.
As is shown in Figure 2, no significant difference in SOC was observed at 0–100 cm soil between the two types of land. The WSOC of switchgrass land soil at 0–40 cm showed significant (p < 0.05) increases by 86.79% and 23.97%, respectively, compared to the bare land (Figure 2b). Soil WSOC at 40–100 cm depths of switchgrass cultivated land all exhibited an increasing trend compared to the bare land, but the differences were not significant (Figure 2b).

Figure 2.
Soil (a) organic carbon and (b) water-soluble carbon content in bare land (control) and switchgrass-cultivated land. Error bars (standard deviation) were correctly added at the top of the mean columns. A Wilcoxon rank-sum test was used to analyze the significant differences between the bare land and switchgrass cultivated land at p < 0.05 (*).
3.2. Soil Chemical Properties of the Bare Land and Switchgrass Land
Soil nutrient levels after switchgrass establishment showed some differences compared to the bare land (Figure 3). Soil TN, TP, and AN content all showed a gradual decreasing trend with soil depths, while TP and AK showed the opposite trend (Figure 3). We found significant (p < 0.05 or 0.01) increases in soil TN at 0–40 and 60–80 cm by 7.92%, 30.67%, and 20.34%, sequentially, compared to the bare land (Figure 3a). In contrast, no significant difference was observed in soil TN between switchgrass and bare land (Figure 3a). The decreasing trend is presented in Figure 3b in soil TP at 0–100 cm (except for 20–40 cm) of switchgrass land compared to the bare land, though the differences were insignificant in some layers. For 0–20 cm and 80–100 cm soil, the decreasing percentages reached 2.78% and 9.68%, respectively, whereas the increasing percentage in 20–40 cm was 3.03% (Figure 3b). For soil TK, 0–40 cm and 80–100 cm soil of switchgrass land exhibited significant (p < 0.01) increases by 3.80%, 4.30%, and 5.69%, sequentially, compared to the bare land, and 40–80 cm soil showed a similar value (Figure 3c). Soil AN of 0–80 cm in switchgrass land was significantly (p < 0.01 or 0.001) higher by 27.97%, 47.19%, 21.68%, and 59.16%, sequentially, than the values in the bare land (Figure 3d). The 80–100 cm soil exhibited no significant difference (Figure 3d). For AP, we did not observe a significant difference in 0–20 cm soil between the two types of land (Figure 3e), and a significant (p < 0.05) increase of 7.51% was found in 20–40 cm soil (Figure 3e). On the contrary, AP of 40–100 cm soil in switchgrass land increased significantly (p < 0.05) by 5.70%, 13.00%, and 9.50%, sequentially, compared to the bare land (Figure 3e). Soil AK contents of the two types of land showed different changes in different soil depths (Figure 3f). A significant (p < 0.05) increase by 11.73% at 0–20 cm and significant decreases (p < 0.05) by 13.31% and 13.56% at 40–80 cm, respectively, were observed in switchgrass land compared to the bare land (Figure 3f). No significant differences were found in other soil depths (Figure 3f).

Figure 3.
Soil total (a) nitrogen, (b) phosphorus, (c) potassium, and available (d) nitrogen, (e) phosphorus, (f) potassium in the bare land and switchgrass-cultivated land. Error bars (standard deviation) were correctly added at the top of the mean columns. A Wilcoxon rank-sum test was used to analyze the significant differences between the bare land and switchgrass cultivated land at p < 0.05 (*), 0.01 (**), and 0.001 (***) levels.
3.3. Soil Microbial Indicators and Correlations with Soil Other Properties
Soil microbial communities are primarily distributed in 0–40 cm soil (Figure 4). The large differences in the CFU number of fungi, bacteria, and actinomycetes occurred mainly in 0–40 cm soil depths between the two types of land (Figure 4). At 0–20 cm soil depth, the CFU numbers of fungi, bacteria, and actinomycetes in switchgrass land were significantly (p < 0.05, 0.01 or 0.001) higher by 149.27%, 37.60%, and 56.40% than the bare land (Figure 4a–c), and, in 20–40 cm soil, the increase percentages reached 775.37%, 70.80%, and 338%, respectively (Figure 4a–c). In 40–60 cm soil, only the CFU number of actinomycetes in switchgrass land increased significantly (p < 0.001), by 152% compared with the bare land (Figure 4c). Few differences were observed at other soil depths in the CFU number of the three microbial communities, but they mostly exhibited an increase (Figure 4).

Figure 4.
CFU numbers of (a) fungi, (b) bacteria, and (c) actinomycetes in the bare land and switchgrass-cultivated land. Error bars (standard deviation) were correctly added at the top of the mean columns. A Wilcoxon rank-sum test was used to analyze the significant differences between the bare land and switchgrass cultivated land at p < 0.05 (*), 0.01 (**), and 0.001 (***) levels.
Soil MC, MN, and MP at all five soil depths showed an increase in switchgrass land compared to the bare land (Figure 5). The significant (p < 0.001) increase percentages of soil MC in 0–40 cm soil in switchgrass land were large, reaching 117.42% and 57.58%, respectively, and at 40–100 cm soil depths, they reached 18.30%, 11.34%, and 37.87%, sequentially, when compared to the bare land (Figure 5a). For soil MN, the increase percentages reached 20.48% (p > 0.05), 41.01% (p < 0.05), 12.45% (p > 0.05), 42.20% (p < 0.05), and 11.45% (p > 0.05), sequentially, in switchgrass land (Figure 5b). The significant (p < 0.05 or 0.01) increases in soil MP mainly happened at 60–100 cm depths, and the increase percentages were 67.57% and 103.03%, sequentially; at the other soil depths, slight increases were observed (Figure 5c).
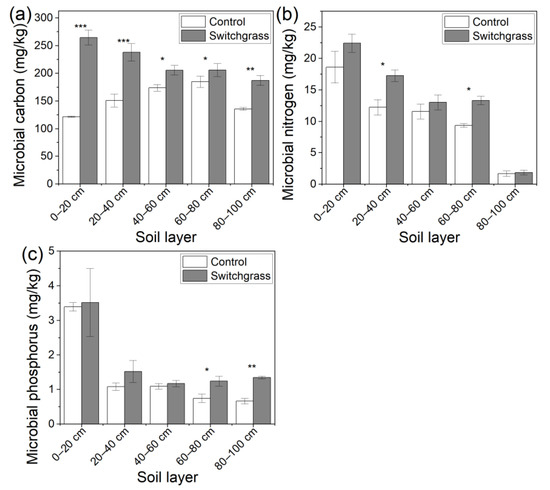
Figure 5.
Soil microbial (a) carbon, (b) nitrogen, and (c) phosphorus contents in bare land and switchgrass-cultivated land. Error bars (standard deviation) were correctly added at the top of the mean columns. A Wilcoxon rank-sum test was used to analyze the significant differences between the bare land and switchgrass cultivated land at p < 0.05 (*), 0.01 (**), and 0.001 (***) levels.
According to the heatmap results, the microbial indicators, including the CFU numbers of the bacteria, fungi, and actinomycetes as well as MC, MN, and MP contents, were all positively correlated with WSOC, TP, SOC, TN, and AN (Figure 6). The microbial indicators (excluding MC) were negatively correlated with soil pH, TK, and AK. Soil AK showed significantly (p < 0.05 or 0.01) negative correlations with the CFU numbers of three microbial communities and MN (Figure 6). Soil pH, as an important soil indicator, was also included into the correlation analysis although few differences were observed in this study (the data was not shown). Soil MN exhibited significantly (p < 0.01) negative correlations with soil pH, TK, and AK (Figure 6). Soil MC only had significant (p < 0.01) positive correlations with soil TPO (Figure 6).
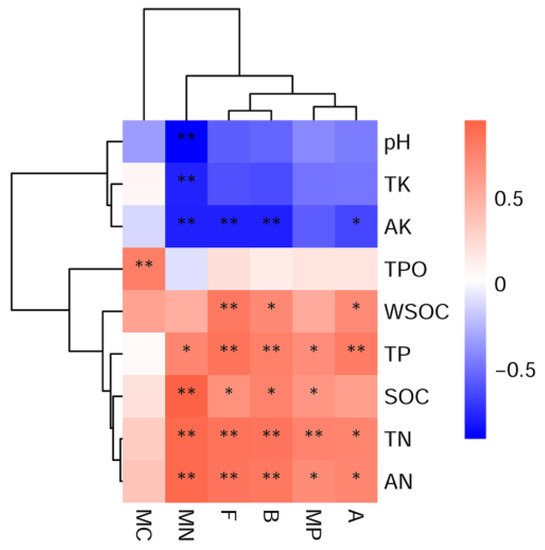
Figure 6.
Pearson correlation analysis between soil microbial communities and properties. A, B, and F represent the actinomycetes, bacteria, and fungi. MN, MP, and MC indicate microbial nitrogen, phosphorus, and carbon. TN, TP, TK, TPO, AK, AN, SOC, and WSOC indicate soil total nitrogen, total phosphorus, total potassium, total porosity, and water-soluble organic carbon, respectively. The symbol * and ** indicate significant correlations at p < 0.05 and 0.01, respectively. The colored bar from white to red and blue shows the gradual increasing R2 value of the positive and negative correlations, respectively.
4. Discussion
Switchgrass shows excellent potential as bioenergy raw material. It provides multiple ecological services, but the effects of its establishment without any fertilization on soil properties in abandoned cropland are unclear, which proposes the necessity of this work. To address these questions, we determined the main soil physical and chemical properties and CFU numbers of microbial communities, as well as the microbial biomass based on 7-year cropland to switchgrass conversion.
4.1. Effects of 7-Year Switchgrass Establishment on Soil Physical Properties
Potential improvements in soil physical properties are significant should switchgrass be cultivated on abandoned cropland, as it has a very developed root system. The increased soil porosity can promote soil aeration and water entry, which is an important indicator of soil quality [43]. Several studies have evidenced the increased soil CP and TPO in marginal cropland after switchgrass cultivation, which was mainly due to switchgrass developing root systems in deep soil and stalk residues on the land surface [14,44,45]. However, the effects of long-term (7-year) production of unfertilized switchgrass on soil physical properties in cropland in northern China are still ambiguous. In this study, the significantly (p < 0.05, 0.01, or 0.001) increased soil TPO (Figure 1b) indicates the dramatical amelioration effects of switchgrass on soil physical structure. However, the increases of soil CP seemed more pronounced at 0–60 cm depths than at 60–100 cm (Figure 1a), which implies that the amelioration effects primarily exist within a limited range of soil depth. Due to the changes in soil CP, the capacity of soil to absorb water was dramatically enhanced, which led to a significantly (p < 0.05, 0.01, or 0.001) higher water content in 0–80 cm soil of switchgrass land (Figure 1d). We speculate that root residues contributed significantly to the improved soil porosity at deeper soil depths. The stubble residue after annual harvest was the predominant factor influencing the surface soil porosity [44,45].
Soil BD, which is also a critical soil property and indicates soil quality, represents the mass of dry soil per unit volume and essentially measures soil porosity, as a high number of soil pores results in low bulk density values [43]. A plethora of papers have reported decreased soil BD after switchgrass establishment, and the surface soil usually shows a more extensive range of variation than soil at deeper depths [14,43,46,47,48]. Consistent with the conclusion of the increased soil porosity in switchgrass land, the 0–60 cm soil BD was significantly (p < 0.05 or 0.01) decreased in the range of 0.03 to 0.1 g/cm3 compared with the cropland (Figure 1c), which was consistent with most of the previous studies [14,43,46,47,48]. The minor sensitivity of soil BD at 60–100 cm was probably attributable to the predominant influences of clay content rather than switchgrass establishment [48]. In addition, the relatively rare root distribution in deeper soil was also another factor that should be considered. As we hypothesized, all these results evidenced the distinct amelioration effects of switchgrass establishment on soil physical structures, though there was no fertilization applied over a 7-year biomass harvest.
4.2. Effects of 7-Year Unfertilized Switchgrass on Soil Nutrient Levels
SOC, which is another important indicator of soil quality [49], has a large pool size and plays a vital role in global carbon cycling because of its slower turnover rate than atmospheric carbon [50]. The carbon sequestration potential of switchgrass establishment has been a great concern worldwide, and its carbon accrual effect has been widely evidenced [15,16,17,18,19,20]. However, there have been some particular case indicating experiment-site-dependent conclusions [21]. Unlike most studies, we herein observed similar SOC contents in 0–100 cm soil in switchgrass land compared to the bare land (Figure 2a). It is hard for us to provide reasonable explanations, but this was probably due to soil carbon priming effects, which should be explored further over a continuous time scale. Soil WSOC usually indicates carbon resources that could be directly used by the soil microbial communities. In this work, the significantly increased WSOC contents in switchgrass land imply relatively abundant carbon resources, which could benefit the enrichment of soil microbial communities, especially at 0–40 cm depths (Figure 2b).
Besides carbon accrual after switchgrass cultivation, the primary nutrients levels, such as those of nitrogen, phosphorus, and potassium, are also significant concerns for most people, especially in China. It is unrealistic for Chinese farmers to input any fertilization during switchgrass production due to the uncertain economic benefits that could be derived from the plantation system. Hence, there is a significant doubt about whether the soil quality was sustainable in the absence of any fertilization over a long-term period. However, the net element exports of switchgrass were much lower than those of cereal crops [25]. In this study, it is interesting that soil TN contents exhibited a significant increase instead of sharp decreases (Figure 3a). Several papers have evidenced the existence of non-symbiotic nitrogen-fixing bacteria and remarkable contributions to nitrogen requirements during the growth and development of switchgrass [31,32,33,51,52], which was the critical reason for the higher TN and AN (Figure 3a,d) in the switchgrass land. Moreover, the prevention of soil nitrogen leaching by the developed switchgrass root system [53] and decreased nitrogen emissions [54] are two factors that should not be ignored for nitrogen accumulation in switchgrass land.
The decreased soil TP and AP contents were reported in both 5-year and 9-year unfertilized switchgrass field experiments [14,17]. In this work, the significantly reduced soil TP and AP contents in deeper soil depths (40–100 cm, Figure 3b,e) imply soil phosphorus consumption through annual biomass harvest, which requires appropriate phosphorus compensation. The degradation of the stubble residues after annual harvest of switchgrass might contribute to the similar AP levels between the switchgrass and control group, but it cannot change the decreasing trend of soil TP in surface soil (0–20 cm). Switchgrass roots were mainly distributed within 0–40 cm soil and were more dispersive in 20–40 cm soil. We speculated that the activation effects by the more abundant capillary roots on soil phosphorus, together with more root turnover, induced the significant increase in soil TP and AP contents in switchgrass land. The accrual of soil potassium caused by the degradation of switchgrass stubbles after biomass harvest accounted mainly for the significantly increased soil TK and AK levels in surface soil (0–40 cm), while it hardly changed the results of the significantly reduced AK contents in deeper soils, although the TK contents were similar.
4.3. Effects of 7-Year Unfertilized Switchgrass on Soil Microbial Indicators
The soil microbial community performs many pivotal ecological functions in soil nutrient and energy cycles [55]. Land-use changes have been widely evidenced to affect soil physical and chemical properties drastically and thus reshape soil microbial communities [56]. Several studies have reported increased numbers of soil bacteria, fungi, and actinomycetes, as well as microbial biomass [28,29,30,37]. However, a large knowledge gap exists in the current understanding of changes in the soil microbial communities and N cycling after the long-term cropland conversion to switchgrass in a temperate continental monsoon climate. In this work, consistent with the previous conclusions, significantly increased numbers of soil bacteria, fungi, and actinomycetes were observed predominantly in 0–40 cm soil after switchgrass establishment, which led to significantly increased microbial carbon, nitrogen, and phosphorus contents. These results are consistent with the significantly increased WSOC (Figure 2b) contents in this study. The increased microbial C/N (data not shown) usually indicate the shift of soil bacterial communities to fungi communities [57], which was also evidenced by the increased fungal and bacterial CFU ratio in this study (data not shown here).
5. Conclusions
In conclusion, we showed that switchgrass establishment can ameliorate soil physical properties, and the nutrient levels did not seem to be depleted except for the slight decrease of soil TP level, even when the biomass was annually harvested without any fertilization. The alterations of soil physical properties, such as increased soil porosity and decreased BD, and chemical properties, such as increased WSOC, TN, and AN, drove the shift of soil bacteria, fungi, and actinomycetes from the bare land pattern to the switchgrass land pattern. Outputs of this study could firmly dispel anxiety that soil nutrients might be exhausted under such a model over a long-term period, which could inspire the government and farmers to large-scale use of switchgrass on abandoned cropland in northern China. Based on our findings, it is exciting and of great significance to explore the mechanisms of the increased TN levels over the long-term switchgrass production without any nitrogen compensation through high-throughput sequencing and metagenomic strategies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agriculture12081138/s1. Figure S1: Schematic diagram of the experiment site location. Figure S2: Schematic diagram of the location of the plot pit.
Author Contributions
Conceptualization, C.Z., X.F. and X.H.; methodology, C.Z., Q.G. and Y.Y.; software, C.Z., Y.Y., X.H. and Y.C.; validation, C.Z., J.W., Q.G. and X.H.; formal analysis, C.Z., X.H., Z.W. and Y.Y.; investigation, C.Z., X.F. and X.H.; resources, X.F. and J.W.; data curation, C.Z., X.F., X.H. and Z.W.; writing—original draft preparation, C.Z., Y.C., Z.W., Q.W. and C.L., writing—review and editing, C.Z., X.H., Q.G., Y.Y., J.W., Q.W. and C.L., visualization, C.Z., Y.C., Q.W., C.L. and Z.W., supervision, X.F., X.H. and Y.Y.; project administration, X.F. and J.W.; funding acquisition, X.F. and J.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Special Project for Capacity of the Scientific and Technological Innovation, grant numbers KJCX20210419 and KJCX20200210, and The National Natural Science Foundation of China (31971752). This study was also supported by the Lush Mountains Project Special Fund of China Environmental Protection Foundation ‘Carbon sequestration and ecological restoration potential of switchgrass and its demonstration in saline-alkali land of the Yellow River Delta’, grant number CEPFQS202169-16.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
We thank researcher Wenjun Teng and others who contributed significantly to the establishment and maintenance of the experimental site. This research was funded by the Special Project for Capacity of the Scientific and Technological Innovation, grant numbers KJCX20210419 and KJCX20200210, and The National Natural Science Foundation of China (31971752). This study was also supported by the Lush Mountains Project Special Fund of China Environmental Protection Foundation, ‘Carbon sequestration and ecological restoration potential of switchgrass and its demonstration in saline-alkali land of the Yellow River Delta’, grant number CEPFQS202169-16.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Xu, Z.; Li, Y. Effects of grassland degradation on soil and water loss. Arid. Land Res. Manag. 2003, 17, 65–68. [Google Scholar]
- Li, B.; Tang, H.; Wu, L.; Li, Q.; Zhou, C. Relationships between the soil organic carbon density of surface soils and the influencing factors in differing land uses in Inner Mongolia. Environ. Earth Sci. 2012, 65, 195–202. [Google Scholar] [CrossRef]
- Ongley, E.D.; Xiaolan, Z.; Tao, Y. Current status of agricultural and rural non-point source pollution assessment in China. Environ. Pollut. 2010, 158, 1159–1168. [Google Scholar] [CrossRef]
- Bullock, A.; King, B. Evaluating China’s Slope Land Conversion Program as sustainable management in Tianquan and Wuqi Counties. J. Environ. Manag. 2011, 92, 1916–1922. [Google Scholar] [CrossRef]
- Wei, H.; Zhang, K.; Zhang, J.; Li, D.; Zhang, Y.; Xiang, H. Grass cultivation alters soil organic carbon fractions in a subtropical orchard of southern China. Soil Tillage Res. 2018, 181, 110–116. [Google Scholar] [CrossRef]
- Slessarev, E.W.; Nuccio, E.E.; McFarlane, K.J.; Ramon, C.E.; Saha, M.; Firestone, M.K.; Pett-Ridge, J. Quantifying the effects of switchgrass (Panicum virgatum) on deep organic C stocks using natural abundance 14C in three marginal soils. GCB Bioenergy 2020, 12, 834–847. [Google Scholar] [CrossRef]
- Berti, M.T.; Johnson, B.L. Switchgrass establishment as affected by seeding depth and soil type. Ind. Crop. Prod. 2013, 41, 289–293. [Google Scholar] [CrossRef]
- McLaughlin, S.B.; Kszos, L.A. Development of switchgrass (Panicum virgatum) as a bioenergy feedstock in the United States. Biomass-Bioenergy 2005, 28, 515–535. [Google Scholar] [CrossRef]
- Guretzky, J.A.; Biermacher, J.T.; Cook, B.J.; Kering, M.K.; Mosali, J. Switchgrass for forage and bioenergy: Harvest and nitrogen rate effects on biomass yields and nutrient composition. Plant Soil 2011, 339, 69–81. [Google Scholar] [CrossRef] [Green Version]
- Phouthavong-Murphy, J.C.; Merrill, A.K.; Zamule, S.; Giacherio, D.; Brown, B.; Roote, C.; Das, P. Phytoremediation potential of switchgrass (Panicum virgatum), two United States native varieties, to remove bisphenol-A (BPA) from aqueous media. Sci. Rep. 2020, 10, 835. [Google Scholar] [CrossRef]
- Yue, Y.; Hou, X.; Fan, X.; Zhu, Y.; Zhao, C.; Wu, J. Biomass yield components for 12 switchgrass cultivars grown in Northern China. Biomass-Bioenergy 2017, 102, 44–51. [Google Scholar] [CrossRef]
- He, H.; Wu, N.; Liu, J.; Chen, J.; Liu, X.; Chang, W. Effects of planting years of Panicum virgatum on soil physical and chemical properties. Ecol. Environ. Sci. 2020, 29, 285–292. (In Chinese) [Google Scholar]
- He, H.; Wu, N.; Liu, J.; Xu, X. Effects of different fertilization treatments on soil chemical properties and bacterial diversity in switchgrass field. Soil Fertil. Sci. China 2022, 3, 164–172. (In Chinese) [Google Scholar]
- Stewart, C.E.; Follett, R.F.; Pruessner, E.G.; Varvel, G.E.; Vogel, K.P.; Mitchell, R.B. Nitrogen and harvest effects on soil properties under rainfed switchgrass and no-till corn over 9 years: Implications for soil quality. GCB Bioenergy 2015, 7, 288–301. [Google Scholar] [CrossRef]
- Lemus, R.; Lal, R. Bioenergy Crops and Carbon Sequestration. Crit. Rev. Plant Sci. 2005, 24, 1–21. [Google Scholar] [CrossRef]
- Liebig, M.; Johnson, H.; Hanson, J.; Frank, A. Soil carbon under switchgrass stands and cultivated cropland. Biomass-Bioenergy 2005, 28, 347–354. [Google Scholar] [CrossRef]
- Lai, L.; Kumar, S.; Osborne, S.; Owens, V.N. Switchgrass impact on selected soil parameters, including soil organic carbon, within six years of establishment. Catena 2018, 163, 288–296. [Google Scholar] [CrossRef]
- Chatterjee, A.; Long, D.S.; Pierce, F.J. Switchgrass influences on soil biogeochemical processes in the dryland region of the Pacific Northwest. Commun. Soil Sci. Plant Anal. 2013, 44, 2314–2326. [Google Scholar] [CrossRef]
- Martinez-Feria, R.; Basso, B. Predicting soil carbon changes in switchgrass grown on marginal lands under climate change and adaptation strategies. GCB Bioenergy 2020, 12, 742–755. [Google Scholar] [CrossRef]
- Ledo, A.; Smith, P.; Zerihun, A.; Whitaker, J.; Vicente-Vicente, J.L.; Qin, Z.; McNamara, N.P.; Zinn, Y.L.; Llorente, M.; Liebig, M.; et al. Changes in soil organic carbon under perennial crops. Glob. Chang. Biol. 2020, 26, 4158–4168. [Google Scholar] [CrossRef]
- Kasanke, C.P.; Zhao, Q.; Bell, S.; Thompson, A.M.; Hofmockel, K.S. Can switchgrass increase carbon accrual in marginal soils? The importance of site selection. GCB Bioenergy 2021, 13, 320–335. [Google Scholar] [CrossRef]
- Zhao, F.; Yang, W.; Zeng, Z.; Li, H.; Yang, X.; He, Z.; Gu, B.; Rafiq, M.T.; Peng, H. Nutrient removal efficiency and biomass production of different bioenergy plants in hypereutrophic water. Biomass-Bioenergy 2012, 42, 212–218. [Google Scholar] [CrossRef]
- Kering, M.K.; Guretzky, J.A.; Interrante, S.M.; Butler, T.J.; Biermacher, J.T.; Mosali, J. Harvest timing affects switchgrass production, forage nutritive value, and nutrient removal. Crop Sci. 2013, 53, 1809–1817. [Google Scholar] [CrossRef] [Green Version]
- Kimura, E.; Collins, H.P.; Fransen, S. Biomass production and nutrient removal by switchgrass under irrigation. Agron. J. 2015, 107, 204–210. [Google Scholar] [CrossRef]
- Propheter, J.L.; Staggenborg, S. Performance of annual and perennial biofuel crops: Nutrient removal during the first two years. Agron. J. 2010, 102, 798–805. [Google Scholar] [CrossRef]
- Kumar, P.; Lai, L.; Battaglia, M.L.; Kumar, S.; Owens, V.; Fike, J.; Galbraith, J.; Hong, C.O.; Farris, R.; Crawford, R.; et al. Impacts of nitrogen fertilization rate and landscape position on select soil properties in switchgrass field at four sites in the USA. Catena 2019, 180, 183–193. [Google Scholar] [CrossRef]
- Chen, G.; Zhu, H.; Zhang, Y. Soil microbial activities and carbon and nitrogen fixation. Res. Microbiol. 2003, 154, 393–398. [Google Scholar] [CrossRef]
- Liang, C.; Jesus, E.D.C.; Duncan, D.; Jackson, R.D.; Tiedje, J.M.; Balser, T.C. Soil microbial communities under model biofuel cropping systems in southern Wisconsin, USA: Impact of crop species and soil properties. Appl. Soil Ecol. 2012, 54, 24–31. [Google Scholar] [CrossRef]
- Mafa-Attoye, T.G.; Thevathasan, N.V.; Dunfield, K.E. Indications of shifting microbial communities associated with growing biomass crops on marginal lands in Southern Ontario. Agrofor. Syst. 2019, 94, 735–746. [Google Scholar] [CrossRef] [Green Version]
- Sekaran, U.; McCoy, C.; Kumar, S.; Subramanian, S. Soil microbial community structure and enzymatic activity responses to nitrogen management and landscape positions in switchgrass (Panicum virgatum L.). GCB Bioenergy 2019, 11, 836–851. [Google Scholar] [CrossRef] [Green Version]
- Roley, S.S.; Ulbrich, T.C.; Robertson, G.P. Nitrogen fixation and resorption efficiency differences among twelve upland and lowland switchgrass cultivars. Phytobiomes J. 2021, 5, 97–107. [Google Scholar] [CrossRef]
- Wewalwela, J.J.; Tian, Y.; Donaldson, J.R.; Baldwin, B.S.; Varco, J.J.; Rushing, B.; Lu, H.; Williams, M.A. Associative nitrogen fixation linked with three perennial bioenergy grasses in field and greenhouse experiments. GCB Bioenergy 2020, 12, 1104–1117. [Google Scholar] [CrossRef]
- Roley, S.S.; Xue, C.; Hamilton, S.K.; Tiedje, J.M.; Robertson, G.P. Isotopic evidence for episodic nitrogen fixation in switchgrass (Panicum virgatum L.). Soil Biol. Biochem. 2019, 129, 90–98. [Google Scholar] [CrossRef]
- Zhao, C.; Fan, X.; Li, X.; Hou, X.; Zhang, W.; Yue, Y.; Zhu, Y.; Wang, C.; Zuo, Y.; Wu, J. Miscanthus sacchriflorus exhibits sustainable yields and ameliorates soil properties but potassium stocks without any input over a 12-year period in China. GCB Bioenergy 2020, 12, 556–570. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, S.L.; Feng, Z.W.; Wang, H.; Huang, H. Comparative study of selected soil properties following introduction of broad-leaf trees into clear-felled Chinese fir forest. Commun. Soil Sci. Plant Anal. 2005, 36, 1385–1403. [Google Scholar] [CrossRef]
- Dhillon, N.; Dev, G. Changes in available nitrogen, phosphorus and potassium in soils of different fertility status as affected by groundnut-wheat rotation. J. Indian Soc. Soil Sci. 1979, 27, 138–141. [Google Scholar]
- Li, J.; Guo, C.; Jian, S.; Deng, Q.; Yu, C.-L.; Dzantor, K.E.; Hui, D. Nitrogen fertilization elevated spatial heterogeneity of soil microbial biomass carbon and nitrogen in switchgrass and gamagrass croplands. Sci. Rep. 2018, 8, 1734. [Google Scholar] [CrossRef] [Green Version]
- Brookes, P.C.; Powlson, D.S.; Jenkinson, D.S. Measurement of microbial biomass phosphorus in soil. Soil Biol. Biochem. 1982, 14, 319–329. [Google Scholar] [CrossRef]
- Bertheux, M.H. A modified procedure for the fractionation and determination of soil phosphorus. J. Sci. Food Agric. 1958, 9, 177–181. [Google Scholar] [CrossRef]
- Khreish, E.A.; Boltz, D.F. Indirect spectrophotometric and atomic absorption spectrometric methods for the determination of potassium. Mikrochim. Acta 1970, 58, 1174–1180. [Google Scholar] [CrossRef]
- Martin, J.P. Use of acid, rose-bengal and streptomycin in the plate method for estimating soil fungi. Soil Sci. 1950, 69, 215–232. [Google Scholar] [CrossRef]
- Atlas, R.M.; Park, L.C. Handbook of Microbiological Media; CRC Press, Inc.: Boca Raton, FL, USA, 2000. [Google Scholar]
- Murphy, C.A.; Foster, B.L.; Ramspott, M.E.; Price, K.P. Grassland management effects on soil bulk density. Trans. Kans. Acad. Sci. 2004, 107, 45–54. [Google Scholar] [CrossRef]
- Singh, N.; Dhaliwal, J.K.; Sekaran, U.; Kumar, S. Soil hydrological properties as influenced by long-term nitrogen application and landscape positions under switchgrass seeded to a marginal cropland. GCB Bioenergy 2019, 11, 1026–1040. [Google Scholar] [CrossRef] [Green Version]
- Blanco-Canqui, H.; Gilley, J.E.; Eisenhauer, D.E.; Jasa, P.J.; Boldt, A. Soil carbon accumulation under switchgrass barriers. Agron. J. 2014, 106, 2185–2192. [Google Scholar] [CrossRef] [Green Version]
- Schmer, M.R.; Liebig, M.A.; Vogel, K.P.; Mitchell, R.B. Field-scale soil property changes under switchgrass managed for bioenergy. GCB Bioenergy 2011, 3, 439–448. [Google Scholar] [CrossRef] [Green Version]
- Burylo, M.; Hudek, C.; Rey, F. Soil reinforcement by the roots of six dominant species on eroded mountainous marly slopes (Southern Alps, France). Catena 2011, 84, 70–78. [Google Scholar] [CrossRef]
- Mudgal, A.; Anderson, S.H.; Baffaut, C.; Kitchen, N.R.; Sadler, E. Effects of long-term soil and crop management on soil hydraulic properties for claypan soils. J. Soil Water Conserv. 2010, 65, 393–403. [Google Scholar] [CrossRef] [Green Version]
- Andrews, S.S.; Karlen, D.L.; Cambardella, C.A. The soil management assessment framework: A quantitative soil quality evaluation method. Soil Sci. Soc. Am. J. 2004, 68, 1945–1962. [Google Scholar] [CrossRef]
- Dou, F.; Hons, F.; Ocumpaugh, W.; Read, J.; Hussey, M.; Muir, J. Soil organic carbon pools under switchgrass grown as a bioenergy crop compared to other conventional crops. Pedosphere 2013, 23, 409–416. [Google Scholar] [CrossRef]
- Bahulikar, R.A.; Chaluvadi, S.R.; Torres-Jerez, I.; Mosali, J.; Bennetzen, J.L.; Udvardi, M. Nitrogen fertilization reduces nitrogen fixation activity of diverse diazotrophs in switchgrass roots. Phytobiomes J. 2021, 5, 80–87. [Google Scholar] [CrossRef] [Green Version]
- Roley, S.S.; Duncan, D.S.; Liang, D.; Garoutte, A.; Jackson, R.D.; Tiedje, J.M.; Robertson, G.P. Associative nitrogen fixation (ANF) in switchgrass (Panicum virgatum) across a nitrogen input gradient. PLoS ONE 2018, 13, e0197320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vadas, P.A.; Barnett, K.H.; Undersander, D.J. Economics and energy of ethanol production from alfalfa, corn, and switchgrass in the Upper Midwest, USA. BioEnergy Res. 2008, 1, 44–55. [Google Scholar] [CrossRef]
- Monti, A.; Barbanti, L.; Zatta, A.; Zegada-Lizarazu, W. The contribution of switchgrass in reducing GHG emissions. GCB Bioenergy 2012, 4, 420–434. [Google Scholar] [CrossRef] [Green Version]
- Philippot, L.; Raaijmakers, J.M.; Lemanceau, P.; van der Putten, W.H. Going back to the roots: The microbial ecology of the rhizosphere. Nat. Rev. Microbiol. 2013, 11, 789–799. [Google Scholar] [CrossRef]
- van Delden, L.; Rowlings, D.W.; Scheer, C.; Grace, P.R. Urbanisation-related land use change from forest and pasture into turf grass modifies soil nitrogen cycling and increases N2O emissions. Biogeosciences 2016, 13, 6095–6106. [Google Scholar] [CrossRef] [Green Version]
- Marschner, P.; Kandeler, E.; Marschner, B. Structure and function of the soil microbial community in a long-term fertilizer experiment. Soil Biol. Biochem. 2003, 35, 453–461. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).