High-Vigor Maize Seeds Resist Fusarium graminearum Infection through Stronger Ca2+ Signaling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Measurements and Methods
2.2.1. Acquisition of Homozygous Transgenic Seeds
2.2.2. Subcellular Localization Assays
2.2.3. Determination of Free Ca2+ Concentration in Embryo Cells
2.2.4. Aging Treatment in Maize Seeds
2.2.5. Treatment of Maize Seeds with Ca2+ Channel Inhibitors
2.2.6. Treatments of Maize Seeds with Exogenous Ca2+
2.2.7. Standard Germination Test of Maize Seeds
2.2.8. Inoculation of Maize Seeds with F. graminearum
2.2.9. Determination of Enzyme Activity
2.2.10. Statistical Analysis
3. Results
3.1. High-Vigor Seeds Had Higher Resistance to F. graminearum
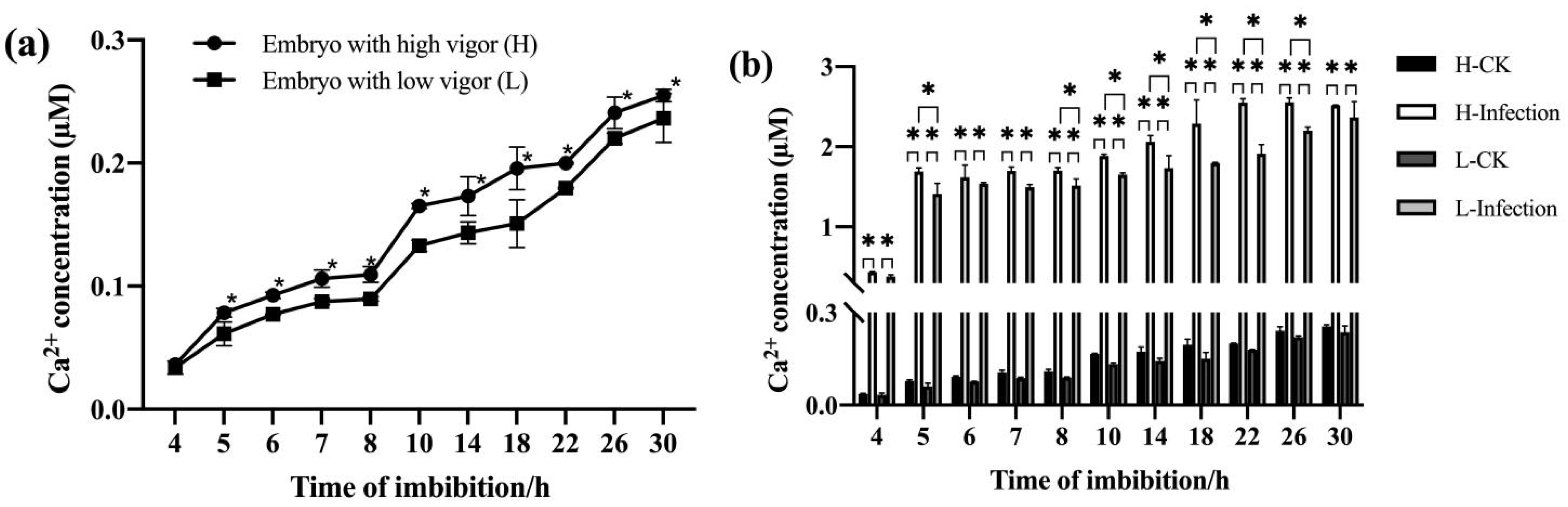
3.2. F. graminearum-Infected High-Vigor Maize Seeds Had Stronger Ca2+ Signals during Imbibition
3.3. Effects of the Inhibition of Ca2+ Entry into the Cytoplasm and Exogenous Ca2+ Treatments on the Resistance of Maize Seeds to F. graminearum
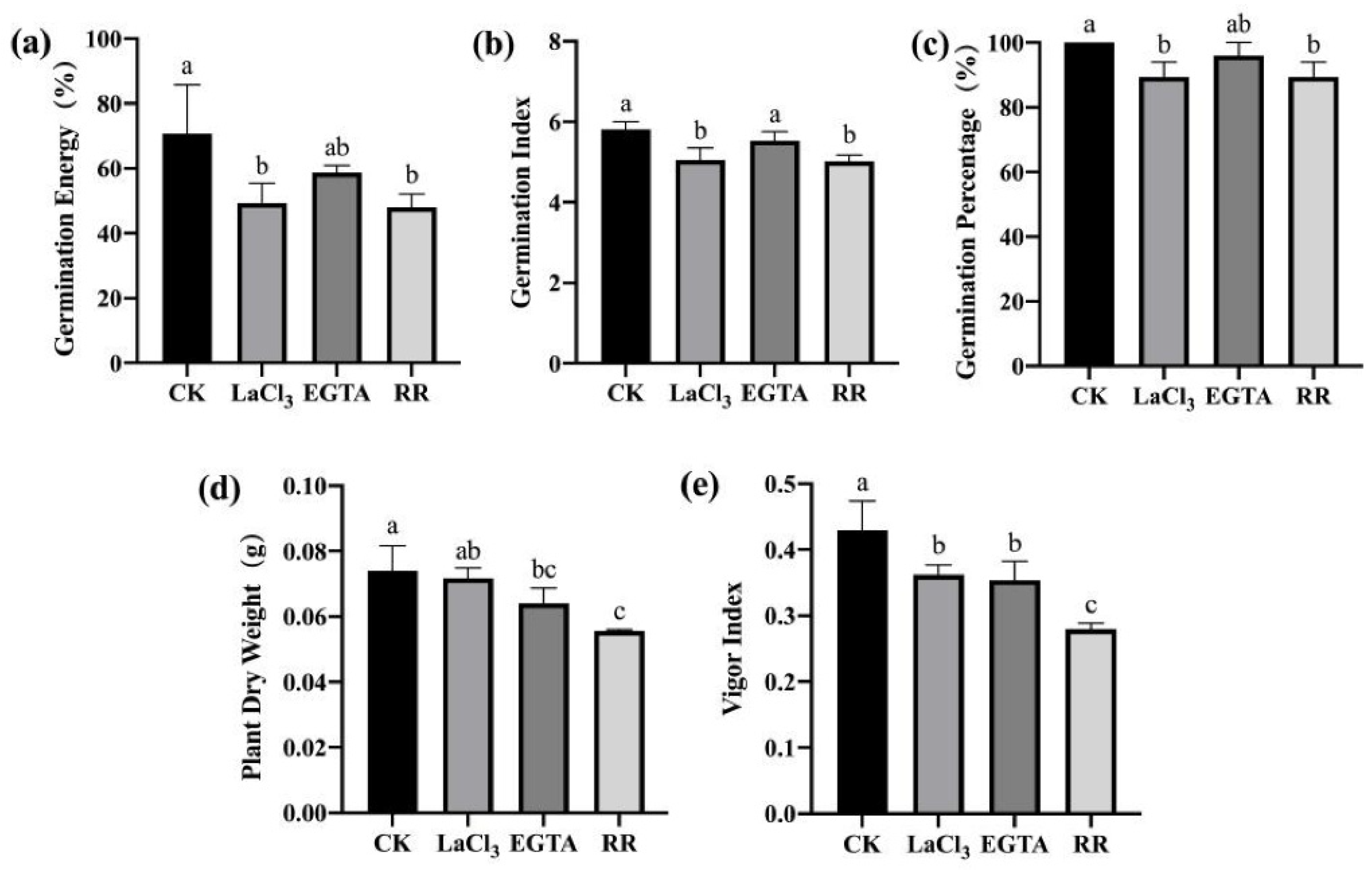
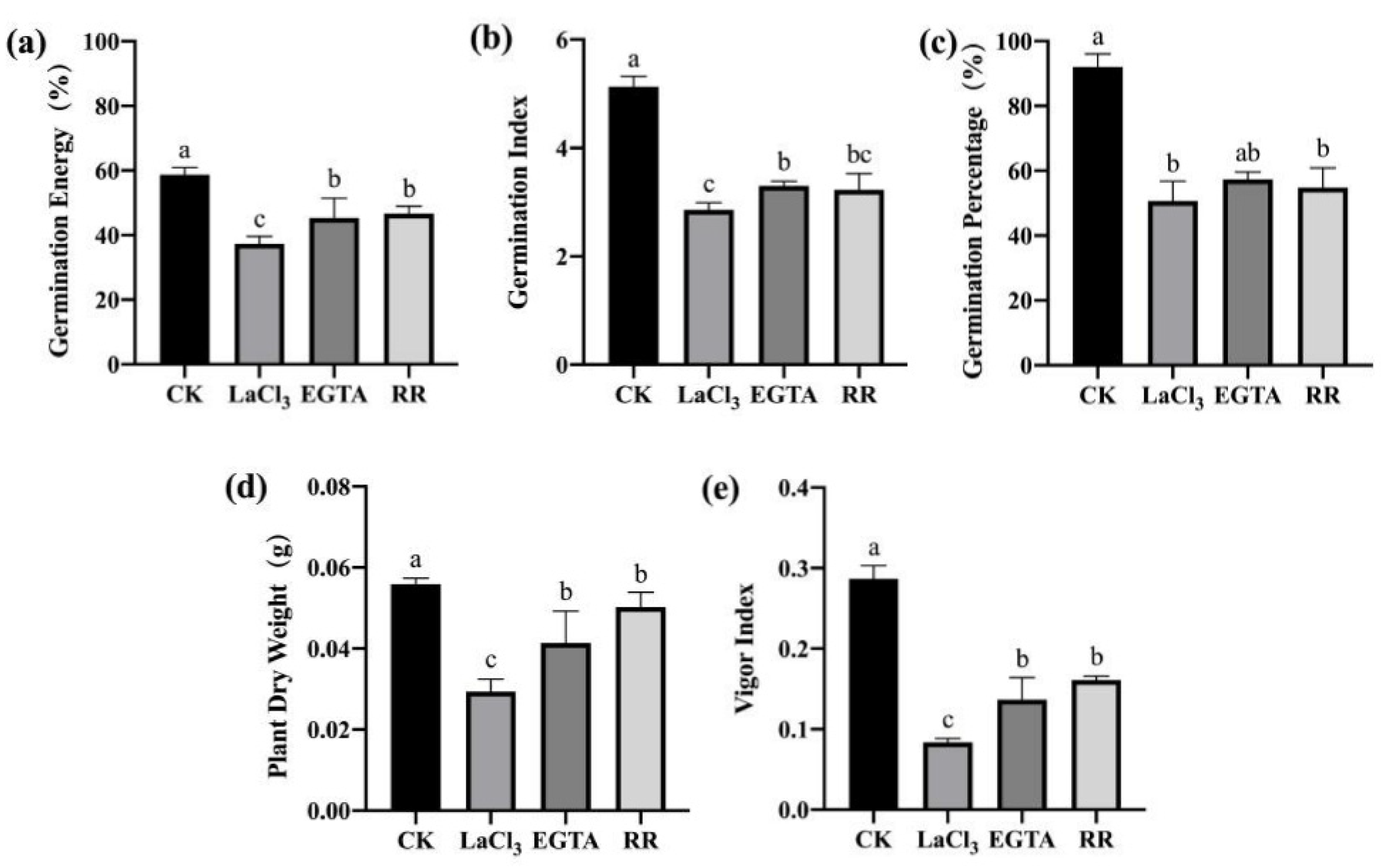
3.3.1. Inhibiting the Entry of Ca2+ into the Cytoplasm Reduced the Resistance of Maize Seeds to F. graminearum
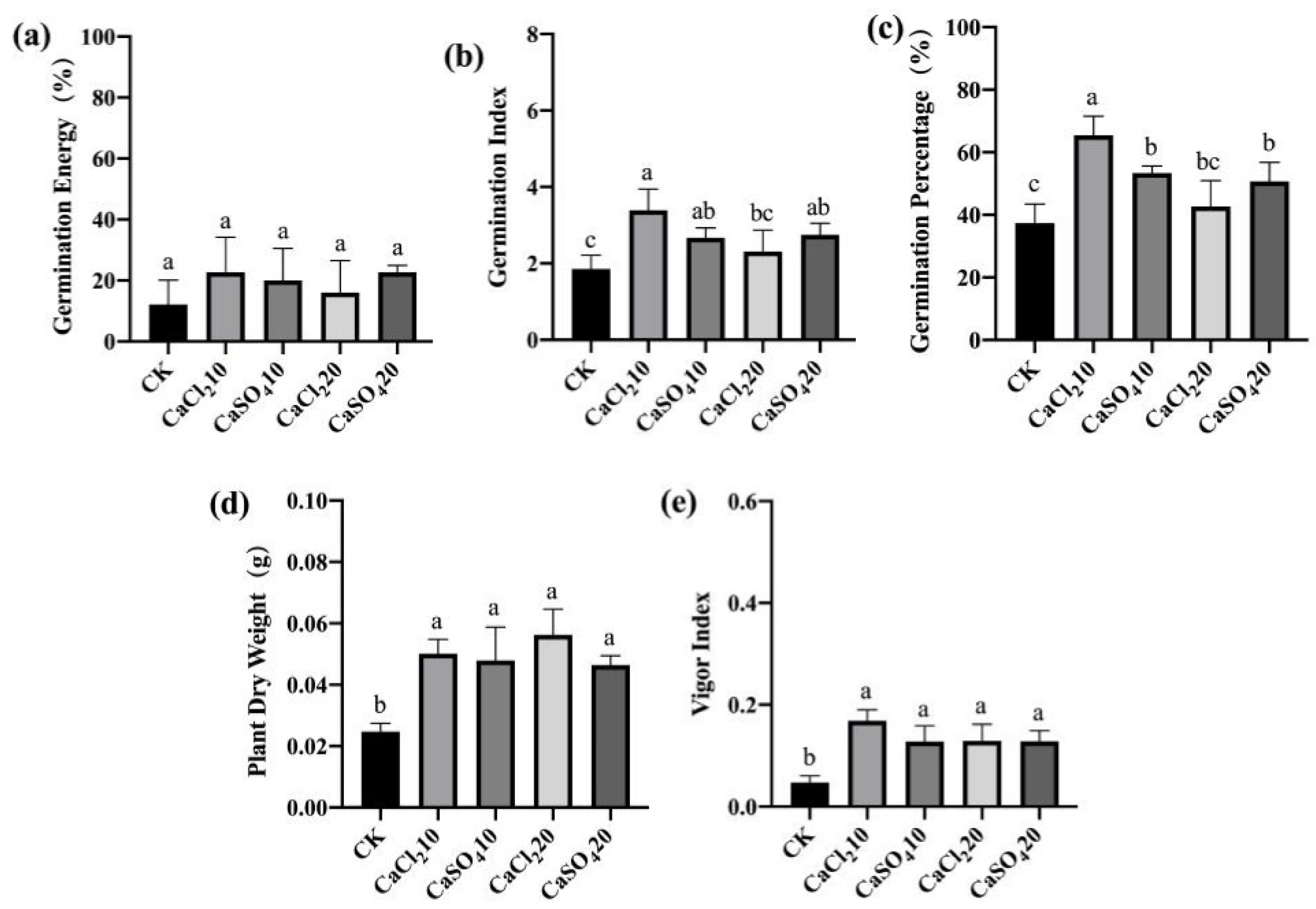
3.3.2. Exogenous Ca2+ Treatments Enhanced the Resistance of Maize Seeds to F. graminearum
3.4. Effects of Inhibiting Ca2+ Entry into the Cytoplasm and Exogenous Ca2+ Treatments on the Activity of Defensive Enzymes in Maize Seeds
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tian, W.; Wang, C.; Gao, Q.; Li, L.; Luan, S. Calcium spikes, waves and oscillations in plant development and biotic interactions. Nat. Plants 2020, 6, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Wallrad, L.; Almutairi, B.O.; Kudla, J. Ca2+ signaling in plant responses to abiotic stresses. J. Integr. Plant Biol. 2022, 64, 287–300. [Google Scholar]
- Kudla, J.; Becker, D.; Grill, E.; Hedrich, R.; Hippler, M.; Kummer, U.; Parniske, M.; Romeis, T.; Schumacher, K. Advances and current challenges in calcium signaling. New Phytol. 2018, 218, 414–431. [Google Scholar] [CrossRef]
- Kong, D.; Ju, C.; Parihar, A.; Kim, S.; Cho, D.; Kwak, J.M. Arabidopsis glutamate receptor homolog3.5 modulates cytosolic Ca2+ level to counteract effect of abscisic acid in seed germination. Plant Physiol. 2015, 167, 1630–1642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fallah, S.; Malekzadeh, S.; Pessarakli, M. Seed priming improves seedling emergence and reduces oxidative stress in Nigella sativa under soil moisture stress. J. Plant Nutr. 2018, 41, 29–40. [Google Scholar] [CrossRef]
- Singh, K.L.; Mukherjee, A.; Kar, R.K. Early axis growth during seed germination is gravitropic and mediated by ROS and calcium. J. Plant Physiol. 2017, 216, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Basra, S.; Hafeez, K. Seed invigoration by osmohardening in coarse and fine rice. Seed Sci. Technol. 2006, 34, 181–187. [Google Scholar] [CrossRef]
- Guan, Y.; Li, Z.; He, F.; Huang, Y.; Song, W.; Hu, J. “On-Off” thermoresponsive coating agent containing salicylic acid applied to maize seeds for chilling tolerance. PLoS ONE 2015, 10, e0120695. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Pan, B.; Wang, Y.; Xu, W.; Zhang, S. Exogenous calcium improved resistance to Botryosphaeria dothidea by increasing autophagy activity and salicylic acid level in pear. Mol. Plant Microbe Interact. 2020, 33, 1150–1160. [Google Scholar] [CrossRef]
- Tan, W.; Meng, Q.W.; Brestic, M.; Olsovska, K.; Yang, X. Photosynthesis is improved by exogenous calcium in heat-stressed tobacco plants. J. Plant Physiol. 2011, 168, 2063–2071. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Ye, T.; Zhong, B.; Liu, X.; Chan, Z. Comparative proteomic and metabolomic analyses reveal mechanisms of improved cold stress tolerance in bermudagrass (Cynodon dactylon (L.) Pers.) by exogenous calcium. J. Integr. Plant Biol. 2014, 56, 1064–1079. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Yin, H.; Peng, S.; Khan, F.A.; Khan, F.; Sameeullah, M.; Hussain, H.A.; Huang, J.; Cui, K.; Nie, L. Comparative transcriptional profiling of primed and non-primed rice seedlings under submergence stress. Front. Plant Sci. 2016, 7, 1125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valivand, M.; Amooaghaie, R.; Ahadi, A. Seed priming with H2S and Ca2+ trigger signal memory that induces cross-adaptation against nickel stress in zucchini seedlings. Plant Physiol. Biochem. 2019, 143, 286–298. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xi, M.; Li, Y.; Cheng, Z.; Wang, S.; Kong, F. Improvement in salt tolerance of Iris pseudacorus L. in constructed wetland by exogenous application of salicylic acid and calcium chloride. J. Environ. Manag. 2021, 300, 113703. [Google Scholar] [CrossRef] [PubMed]
- Finch-Savage, W.E.; Bassel, G.W. Seed vigour and crop establishment: Extending performance beyond adaptation. J. Exp. Bot. 2016, 67, 567–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, K.; Zhang, Y.; Sun, J.; Meng, J.; Tao, J. Deterioration of orthodox seeds during ageing: Influencing factors, physiological alterations and the role of reactive oxygen species. Plant Physiol. Biochem. 2021, 158, 475–485. [Google Scholar] [CrossRef]
- Han, C.; Zhen, S.; Zhu, G.; Bian, Y.; Yan, Y. Comparative metabolome analysis of wheat embryo and endosperm reveals the dynamic changes of metabolites during seed germination. Plant Physiol. Biochem. 2017, 115, 320–327. [Google Scholar] [CrossRef]
- Sych, T.; Mély, Y.; Römer, W. Lipid self-assembly and lectin-induced reorganization of the plasma membrane. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018, 373, 20170117. [Google Scholar] [CrossRef]
- Tilden, R.L.; West, S.H. Reversal of the effects of aging in soybean seeds. Plant Physiol. 1985, 77, 584–586. [Google Scholar] [CrossRef]
- Jones, J.D.G.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [Green Version]
- Kadota, Y.; Shirasu, K.; Zipfel, C. Regulation of the NADPH oxidase RBOHD during plant immunity. Plant Cell Physiol. 2015, 56, 1472–1480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Guo, Y.; Ma, C.; Zhang, D.; Wang, C.; Yang, Q. Transcriptome analysis of maize resistance to Fusarium graminearum. BMC Genom. 2016, 17, 477. [Google Scholar]
- Nomura, H.; Komori, T.; Uemura, S.; Kanda, Y.; Shimotani, K.; Nakai, K.; Furuichi, T.; Takebayashi, K.; Sugimoto, T.; Sano, S.; et al. Chloroplast-mediated activation of plant immune signalling in Arabidopsis. Nat. Commun. 2012, 3, 926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Figueroa, M.; Hammond-Kosack, K.E.; Solomon, P.S. A review of wheat diseases-a field perspective. Mol. Plant Pathol. 2018, 19, 1523–1536. [Google Scholar] [CrossRef]
- Mehlmer, N.; Parvin, N.; Hurst, C.H.; Knight, M.R.; Teige, M.; Vothknecht, U.C. A toolset of aequorin expression vectors for in planta studies of subcellular calcium concentrations in Arabidopsis thaliana. J. Exp. Bot. 2012, 63, 1751–1761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tu, X.; Mejía-Guerra, M.K.; Valdes Franco, J.A.; Tzeng, D.; Chu, P.Y.; Shen, W.; Wei, Y.; Dai, X.; Li, P.; Buckler, E.S.; et al. Reconstructing the maize leaf regulatory network using ChIP-seq data of 104 transcription factors. Nat. Commun. 2020, 11, 5089. [Google Scholar] [CrossRef] [PubMed]
- Mittal, D.; Mishra, S.; Prajapati, R.; Vadassery, J. Forward genetic screen using transgenic calcium reporter aequorin to identify novel targets in calcium signaling. J. Vis. Exp. 2020, 162, e61259. [Google Scholar] [CrossRef]
- Wang, M.; Qu, H.; Zhang, H.; Liu, S.; Li, Y.; Zhang, C. Hormone and RNA-seq analyses reveal the mechanisms underlying differences in seed vigour at different maize ear positions. Plant Mol. Biol. 2019, 99, 461–476. [Google Scholar] [CrossRef]
- Zhu, P.; Song, X.; Mao, Y.; Li, Y.; Zhang, C. The flux rate of Ca2+ into embryo can be used to evaluate the vigour level of maize seeds. Qual. Assur. Saf. Crop. Foods 2020, 12, 81–88. [Google Scholar] [CrossRef]
- Kaneko, M.; Itoh, H.; Ueguchi-Tanaka, M.; Ashikari, M.; Matsuoka, M. The α-amylase induction in endosperm during rice seed germination is caused by gibberellin synthesized in epithelium. Plant Physiol. 2002, 128, 1264–1270. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.; Verma, G.; Sharma, S. A novel Ca2+-activated protease from germinating Vigna radiata seeds and its role in storage protein mobilization. J. Plant Physiol. 2010, 167, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Zehra, A.; Gul, B.; Ansari, R.; Khan, M.A. Role of calcium in alleviating effect of salinity on germination of Phragmites karka seeds. S. Afr. J. Bot. 2012, 78, 122–128. [Google Scholar] [CrossRef] [Green Version]
- Salahshoor, F.; Kazemi, F. Effect of calcium on reducing salt stress in seed germination and early growth stage of Festuca ovina L. Plant Soil Environ. 2016, 62, 460–466. [Google Scholar] [CrossRef] [Green Version]
- Verma, G.; Khan, S.; Agarwal, S.K.; Sharma, S. Role of apoplastic calcium during germination and initial stages of seedling establishment in Vigna radiata seeds. J. Plant Physiol. 2019, 236, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Pirayesh, N.; Giridhar, M.; Khedher, A.B.; Vothknecht, U.C.; Chigri, F. Organellar calcium signaling in plants: An update. BBA-Mol. Cell Res. 2021, 1868, 118948. [Google Scholar] [CrossRef]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, B.; Liu, X.; Song, X.; Guo, Q.; Yin, Y.; Zhang, C.; Li, Y. High-Vigor Maize Seeds Resist Fusarium graminearum Infection through Stronger Ca2+ Signaling. Agriculture 2022, 12, 992. https://doi.org/10.3390/agriculture12070992
Xu B, Liu X, Song X, Guo Q, Yin Y, Zhang C, Li Y. High-Vigor Maize Seeds Resist Fusarium graminearum Infection through Stronger Ca2+ Signaling. Agriculture. 2022; 12(7):992. https://doi.org/10.3390/agriculture12070992
Chicago/Turabian StyleXu, Baokuan, Xiyan Liu, Xuejiao Song, Qifang Guo, Yongqi Yin, Chunqing Zhang, and Yan Li. 2022. "High-Vigor Maize Seeds Resist Fusarium graminearum Infection through Stronger Ca2+ Signaling" Agriculture 12, no. 7: 992. https://doi.org/10.3390/agriculture12070992
APA StyleXu, B., Liu, X., Song, X., Guo, Q., Yin, Y., Zhang, C., & Li, Y. (2022). High-Vigor Maize Seeds Resist Fusarium graminearum Infection through Stronger Ca2+ Signaling. Agriculture, 12(7), 992. https://doi.org/10.3390/agriculture12070992





