Abstract
Resistant crop varieties can usually decrease the population density of insect pests; however, they can also easily cause the occurrence of highly virulent pest populations when repeatedly grown. Whether herbivorous insects feeding intermittently on a susceptible variety affects their subsequent virulence has rarely been investigated. In this paper, we examined the variations in the virulence of the brown planthopper (BPH), Nilaparvata lugens (Stål) (Hemiptera: Delphacidae), by alternately rearing three resistant rice varieties, Mudgo, ASD7, and Rathu Heenati, with a susceptible rice variety (TN1) in indoor experiments. The results showed that, while the susceptible rice variety was used in alternate rearing for several generations, the BPHs exhibited a higher intrinsic rate of increase (rm) and were identified as less virulent to all three resistant varieties. Such virulence reduction by experience with a susceptible variety could delay the progression of resistance-breaking toward resistant varieties. The results suggested that careful alternation with susceptible varieties in fields is a potential method for pest variety-resistance management.
1. Introduction
Rice (Oryza sativa L.) is an important economic crop in China, and its average yield is one of the largest in Asia. As the global population grows, the rice demand will rise by 30% by 2050; people are becoming increasingly concerned about the reduction in rice production []. The brown planthopper (Nilaparvata lugens (Stål)) is a rice pest that can migrate over long distances and has strong adaptability to the environment []. The BPH is mainly distributed in tropical regions and invades seasonally from elsewhere in the next year. BPH causes more than one billion dollars in economic losses in food production worldwide each year. For traditional agriculture, farmers’ use of pesticides to kill insects leads to negative effects, such as drug resistance in the brown planthopper and environmental pollution. Rice production faces huge challenges [].
Insect pests have become successively virulent to resistant varieties, and such virulent populations of pests have come to be known as the so-called ‘biotype’. The existence of resistance-breaking ‘biotypes’ has been reported in many crop pests [,]. For example, ‘biotypes’ of the whitebacked planthopper (WBPH) Sogatella furcifera (Horvath) and the brown planthopper (BPH) Nilaparvata lugens (Stål) were observed after resistant varieties of rice were cultivated for a few years []. ‘Biotypes’ were also found in the green leafhopper Nephotettix virescens [], the Asian rice gall midge Orseolia oryzae [], the Hessian fly Mayetiola destructor [], etc. Resistance breakdown due to the emergence of ‘biotypes’ has been a serious challenge in breeding programs for resistance variety.
Among the cases mentioned above, resistant varieties and ‘biotypes’ of BPH are the most extensively studied. Resistant varieties carrying quantitative trait loci (QTLs) or major genes associated with resistance are used to control insect pests [,]. The virulence evolution of BPH is currently the main obstacle to the breeding of resistant rice [,]. For example, the International Rice Research Institute (IRRI) developed the first resistant rice, IR26 (Bph1), which was deployed in fields in the Philippines in 1973. However, BPH adapted to the resistant rice after only two years []. Laboratory experiments also showed that BPH genotypes with virulence to different rice varieties. The field population of BPH can complete the selection of resistance genes in 5–10 generations; therefore, the virulence evolution of BPH is hereditary [].
Variation in virulence is the basis for the formation of a new virulent BPH ‘biotype’. Usually, some individuals within a population can overcome resistance in the early stages, and the proportion of highly virulent individuals gradually becomes higher. Finally, a new virulent ‘biotype’ is formed that is adapted to resistant rice varieties harboring one or several major resistance genes [,]. While BPHs present high levels of virulence, it is hard to reduce their virulence []. Therefore, strategies for delaying the occurrence of more highly virulent types are becoming important for BPH control in paddy fields. Previous studies have shown that a mixture of resistant rice lines was effective in delaying the development of a varietal-resistant BPH population for several generations []. However, whether a mixture of resistant and susceptible rice varieties can delay the development of BPH virulence is currently unknown. In this study, the susceptible rice variety Taichung native 1 (TN1, carrying no BPH-resistance genes) was mixed with the resistant rice varieties Mudgo (Bph1), ASD7 (bph2), and Rathu Heenati (Bph3), and fed to BPH for five generations. The aim was to determine whether feeding alternately with resistant and susceptible varieties can delay the occurrence of more virulent insect pests.
2. Materials and Methods
2.1. Plant Materials
The rice varieties TN1 (susceptible, carrying no resistance genes), Mudgo (carrying Bph1 resistance genes), ASD7 (bph2), and Rathu Heenati (Bph3) were used in this study. Seeds were germinated on filter paper placed in a Petri dish (90 mm diameter) and then transferred to a pot (12 cm diameter) containing multipurpose compost. The plant was maintained in a growth room at 24 ± 2 °C and 76 ± 5% relative humidity (r.h.), with a 12:12 h dark: light (DL) photoperiod. Plants aged 30–40 days were used for the experiment.
2.2. BPH Colony
The brown planthopper colony was provided by the Plant Protection Research Institute, Guangdong Agricultural Academy of Sciences, Guangzhou, China. BPHs were transferred to tillering-stage TN1 and kept in a net cage with the same conditions as described above. The rice plant was replaced weekly. Newly hatched nymphs were selected for the experiments.
2.3. Multi-Generation Rearing Experiments
Three resistant varieties, Mudgo, ASD7, and Rathu Heenati, carrying the resistance genes Bph1, bph2, and Bph3, respectively, were chosen for the multi-generation rearing experiments (Table S1). Experiments were carried out following the protocols of Sogawa [] and Khush [], with some modifications. Approximately 10 rice seeds of each variety (including the resistant varieties Mudgo, ASD7, and Rathu Heenati and the susceptible variety TN1) were sown in plastic pots (12 cm diameter and 5 cm height) containing moist soil. After 40 days, rice seedlings were covered with cylindrical vinyl chloride cages (11 cm in diameter, 50 cm high) containing two Teforon gauze windows for multi-generation feeding experiments. In the alternate rearing experiments, four days was a suitable interval time, because large individuals’ deaths would occur when BPHs stayed on the resistant rice cultivar Rathu Heenati continuously for more than 4 days (Figure S1). Firstly, 20 newly hatched BPH nymphs were introduced into the cages with Mudgo, ASD7, or Rathu Heenati seedlings, and each treatment was repeated five times. After 4 days (Figure S1), BPHs were transferred to TN1 plants, and then returned to the resistant varieties after another 4 days. We chose the susceptible line to susceptible line and resistant line to resistant rice line as the control treatments in this study. The interval was also four days (Figure S1). While the BPH nymphs developed into adults, eight pairs of emerged males and females were picked out individually, and the egg production per female was checked. Mortality and the emergence of offspring were counted daily until the adults died. One hundred newly hatched nymphs were picked out and put into five individual pots per treatment for the next-generation rearing experiment. These procedures continued for five generations. A randomized complete block design was employed, with Mudgo/TN1, ASD7/TN1, and Rathu Heenati/TN1 as the experimental group and TN1/TN1, Mudgo/Mudgo, ASD7/ASD7, and Rathu Heenati/Rathu Heenati as the control group; each treatment was replicated five times.
2.4. Evaluation of Virulence of BPH
The virulence of each BPH line selected for five generations was evaluated by using the standard seedbox screening test (SSST) to clarify the differences in virulence on the various resistant rice varieties and between single-variety rearing and alternate-variety rearing. The SSST has been described in detail in several publications and widely used in rice breeding programs throughout Asia [,,]. To ensure that the seedling was at the same growth stage for BPH infestation, seeds of TN1, Mudgo, ADS7, and Rathu Heenati were sown in plastic pots. The distance between the seedlings was 2.5 cm. The pots were placed in a 70 × 45 × 15 cm plastic seed box filled with water to a depth of about 2 cm until the test was completed. A total of 25 pots (four pots for every treatment and one pot for control variety TN1) were randomly arranged. Sowing after seven days, the seedlings were thinned to 20 plants per pot. Seedlings were infested with BPH at the age of 1st to 2nd, each seedling having a density of five individuals.
When the susceptible rice plant was completely wilted due to BPH feeding, the experiment was stopped and the condition of the seedling was graded using the standard evaluation system (SES) where 0 = no damage, 1 = slight damage to a few plants within a row, 3 = first leaf and second leaf of each plant partially yellowing, 5 = pronounced yellowing or stunting of the plant or between 10% and 25% of plants wilted within a row, 7 = more than 50% of the plants severely stunted or dying and the remaining plants dead or wilted, and 9 = plants dead or wilted. The SSST of each population was replicated three times.
2.5. Data Analysis
Life tables were constructed using the age-specific fecundity (mx) and survival rates (lx) for each age interval (x) per day [], and the intrinsic rate of increase (rm) was assessed by the equation:
where rm was calculated for the original data (rall). The significance of the differences in the rm values was tested by estimating variance using the jackknife method []. The jackknife pseudo-value rj was computed for n samples using the following equation:
rj = n × rall − (n − 1) × ri.
According to the simulation output, the smoothed allocation pattern of the estimates of rm by delete-1 jackknife adapted the corresponding regular density. The mean (n − 1) jack-knife pseudo-values for each treatment were subjected to Z-test criterion (two-level) or analysis of variance (ANOVA), and the differences between the mean jackknife pseudo-values were analyzed by Tukey’s test (multilevel).
The mean virulence levels of the BPH reared on resistant/resistant and resistant/susceptible plants after five generations of selection in the bulk seedling test were compared using the nonparametric Mann–Whiney U test.
IBM SPSS Statistics version 25.0 was used to perform the statistical tests (https://www.ibm.com/analytics/spss-statistics-software (accessed on 1 October 2017)).
3. Results
3.1. Survival and Fecundity
Alternately rearing BPH with susceptible rice varieties (TN1), resistant varieties, and resistant/susceptible varieties found that TN1 was susceptible to BPHs, with the highest nymphal survival rate (71.33%) and highest number of offspring (86.03) (p < 0.05). Rathu Heenati was highly resistant to BPH, with the lowest nymphal survival rate (5.83%) and lowest number of offspring (1.31) (p < 0.05). The highest values of rm (intrinsic rate of increase) were 0.2749, 0.2648, and 0.2549 for TN1/TN1, ASD7/TN1, and ADS7/ADS7 rearing, respectively. The rm values were 0.2749, 0.2442, and 0.2385 for TN1/TN1, Mudgo/TN1, and Mudgo/Mudgo rearing, respectively. The rm values were 0.2749, 0.0139, and 0.0019 for TN1/TN1, Rathu Heenati/TN1, and Rathu Heenati/Rathu Heenati rearing, respectively (Table 1, Figure 1). The rm values differed significantly among the BPH reared for just one generation on TN1/TN1, or Rathu Heenati/Rathu Heenati and Rathu Heenati/TN1 (p < 0.05) (Table 1).

Table 1.
Effect of different rearing treatments on the development of first-generation BPH.
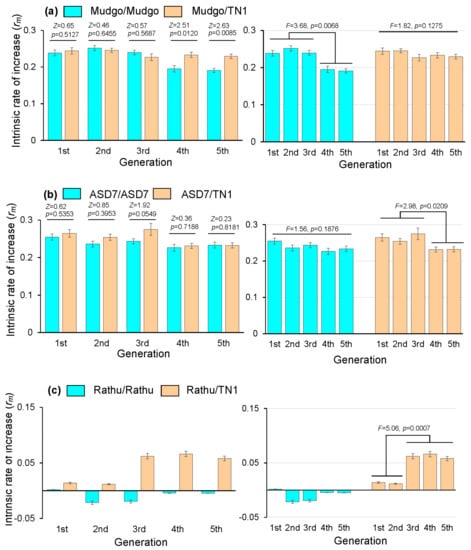
Figure 1.
Effects of rice varieties on the intrinsic rate of increase (rm) of Nilaparvata lugens over multiple generations under resistant/resistant and resistant/susceptible alternate rearing. (a) Mudgo/Mudgo and Mudgo/TN1 alternate rearing; (b) ASD7/ASD7 and ASD7/TN1 alternate rearing; (c) Rathu Heenati/Rathu Heenati and Rathu Heenati/TN1 alternate rearing. Data are the mean jackknife pseudo-values ± SEM (n = 40). Statistics and p-values were calculated using a two-tailed Z-test (two-level) or ANOVA (multilevel).
The effects of rice varieties on the rm of BPHs over multiple generations under single-variety and alternate-variety rearing were also investigated. The laboratory results showed that the rm of BPH increased after alternate-host rearing for five generations with the resistant rice varieties Mudgo, ASD7, and Rathu Heenati, and the susceptible rice variety TN1, compared with those of BPHs reared with single resistant rice varieties (Figure 1). In particular, for the BPHs reared only on the resistant variety Rathu Heenati, the rm was negative from the second to fifth generations. However, the rm of the second to fifth-generation BPHs became positive when reared alternately on Rathu Heenati and TN1 (Figure 1c).
3.2. Evaluation of BPH Virulence
In the seedling test, the virulence of BPHs between single-variety and alternate-variety rearing was evaluated based on the BPH resistance level after five generations of selection (Figure 2) []. The mean resistance levels of Mudgo, ADS7, and Rathu Heenati after five generations of BPH selection were 7.23, 6.95, and 5.68, respectively. When TN1 was added to the rotation, the mean resistance level of Mudgo, ADS7, and Rathu Heenati to BPHs after five generations of selection decreased to 5.30, 5.10, and 3.60, respectively (Table 2). It seemed that BPH was less virulent on all three resistant varieties after TN1 was added to the rotation at 4-day intervals for five generations of selection.
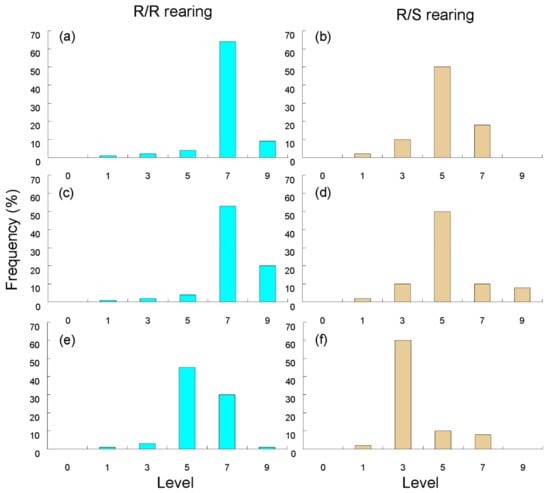
Figure 2.
Frequency distribution of the resistance level of BPH acclimated to resistant/resistant and resistant/susceptible alternate rearing for 5 generations in the seedling bulk test. (a) Virulent to ASD7 in ASD7/ASD7 rearing; (b) Virulent to ASD7 in ASD7/TN1 alternately rearing; (c) Virulent to Mudgo in Mudgo/Mudgo rearing; (d) Virulent to Mudgo in Mudgo/TN1 alternate rearing; (e) Virulent to Rathu Heenati in Rathu Heenati/Rathu Heenati rearing; (f) Virulent to Rathu Heenati in Rathu Heenati/TN1 alternate rearing.

Table 2.
Mean virulence levels of different BPH populations after five generations of selection, evaluated using the standard seedbox screening test (SSST).
4. Discussion
Resistant varieties of many crops have been bred and utilized for insect pest control. The use of the resistant crop varieties has advantages in that farmers need not pay additional costs in terms of pesticides and labor to control the pests, and the use of such crops has minimal deleterious effects on the environment []. However, the fact that some populations of insect pests have adapted and become virulent to previously resistant varieties presents a serious problem. Such virulence has evolved in many crop pests []. Our research group has continuously monitored the virulence of BPHs against resistant rice varieties in Guangdong’s rice planting regions since 1976 (Table S2); the field BPH populations have changed to ‘biotype 2′ since 1992, leading to the breakdown of the resistance of rice variety IR26 []. Such changes were also observed in Japan and northern Vietnam during the same period [,]. Varieties harboring the bph2 gene were subsequently released, but the BPH adapted quickly to these resistant varieties again []. At present, it appears from several reports that the BPH population throughout much of South and Southeast Asia has now adapted to the resistance conferred by both the Bph1 and bph2 genes [,,]. Recently, BPH populations in paddy fields have been reported to be present in the meta-population form [], consistent with the results of this study. The BPH colony used in our experiments was collected from the rice fields of Guangzhou, Guangdong province, China, and continuously reared with the susceptible rice variety TN1. Life table analysis results showed that Mudgo and ASD7 were susceptible to this BPH colony (Figure 3b,c) and were affected little by feeding alternately with resistant and susceptible hosts (Figure 1 and Figure 3e,f). The BPH colony in our study was a meta-population and retained strong virulence against rice varieties ASD7 and Mudgo. The occurrence of highly virulent BPHs would shorten the utilization period of resistant rice varieties. Strategies to prevent BPH adaptation to resistant rice varieties and the adaptation mechanism of highly virulent BPH populations to resistant rice varieties have been discussed in previous studies [,,]. The virulence of BPH continues to bring new challenges to rice breeding for BPH resistance.
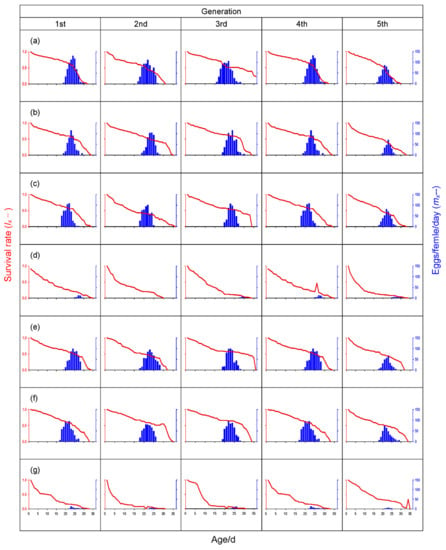
Figure 3.
Fecundity (mx━) and survival rate (lx━) of Nilaparvata lugens on different rice varieties in different rearing arrangements at 28 °C. (a) TN1/TN1; (b) Mudgo/Mudgo; (c) ADS7/ASD7; (d) Rathu Heenati/Rathu Heenati; (e) Mudgo/TN1; (f) ADS7/TN1; (g) Rathu Heenati/TN1.
The “high-dose/refuge (HDR)” strategy has been widely adopted to delay the evolution of pest virulence to Bt crops—as long as there are non-Bt crops as “refuges”, the validity period of Bt crops will be extended [,]. In the present study, our results of alternating resistant varieties (Mudgo, ASD7, and Rathu Heenati) with the susceptible variety TN1 to rear multiple generations of a BPH colony showed that feeding resistant and susceptible rice varieties alternately can delay the development of highly virulent BPH populations compared with feeding a single resistant rice variety. Meanwhile, the rm of the BPH population fed a mixture of varieties (Mudgo/TN1, ASD7/TN1, and Rathu Heenati/TN1) for just one generation was higher than that of insects fed only a resistant variety—Mudgo, ASD7, or Rathu Heenati. Moreover, if the resistant varieties retain greater resistance against the BPH population, the effect of delaying the development of BPH virulence will be stronger when incorporating susceptible varieties. The rm values significantly increased when the BPH was reared with alternating varieties (Rathu Heenati and the susceptible variety TN1) compared with feeding only the resistant variety Rathu Heenati (Table 1 and Figure 1). Kobayashi [] reviewed the interaction between BPH virulence and rice resistance. He proposed that the wild BPH population includes a certain level of genetic diversity, and each population is a collection of individual genotypes capable of living on rice varieties carrying different resistance genes. Similar to the strategy of susceptible rice with rice-blast-resistant rice, different resistant rice types increased the distance between the ‘biotype’ and diluted the inoculum of a given pathogenic race as it was dispersed between compossible host plant varieties, decreasing the outbreak rate of rice blast by 94% []. The major difference between a virulent ‘biotype’ and an avirulent ‘biotype’ results from the frequency of virulence in the BPH population. The highly virulent ‘biotype’ BPH consists of a high proportion of virulent genotype individuals and a very low proportion of avirulent genotype individuals. The opposite is true for an avirulent ‘biotype’. According to previous studies using the HDR strategy adopted to delay the adaptation of cotton bollworms to feed on Bt cotton [,], Huang confirmed that the HDR strategy successfully delayed the development of field cotton bollworm resistance to Bt crops [], a similar finding was also confirmed by our results. The avirulent genotype individuals in the BPH population which could not overcome the resistance genes carried by the different resistant rice varieties would have been seriously decreased while the population was reared with the resistant varieties (such as Rathu Heenati) continuously. However, in the BPH fed alternately with resistant and susceptible rice varieties, the death rate of the virulent genotype individuals was reduced because the selection pressure imposed by the host was weakened. Therefore, the virulence frequency distribution of BPH populations fed alternately with resistant and susceptible rice varieties was reduced (Figure 2), and the timing of the BPH becoming a highly virulent ‘biotype’ was delayed.
Here, we summarized the progress towards understanding virulence in BPH and resistance in rice through our experimental results and previous studies (Figure 4). Laboratory experiments showed that the BPH genotypes that were virulent to rice varieties carrying resistant genes remained virulent to them when reared on susceptible rice varieties, regardless of whether short- or long-term rearing was conducted []. This indicated that virulence had little fitness cost [,]. However, an avirulent ‘biotype’ easily changes to a virulent ‘biotype’ when reared on highly resistant rice varieties continuously, as indicated by many previous studies [,,,,,,]. Furthermore, our laboratory results suggested that feeding alternately with resistant and susceptible rice varieties might delay the development of virulence in BPH (Table 1 and Figure 1). This is consistent with the effect of mixing different Bt cotton types for rearing bollworm [,]. High host selection pressure (rearing on highly resistant rice varieties) caused virulence to increase quickly for the BPH population, no host selection pressure (rearing on susceptible varieties) had no effect on virulence, and weakened host selection pressure delayed the development of virulence in the BPH population (Figure 4). The present study provides a novel understanding of virulence control, and our results demonstrate that a simple, convenient, and ecological approach to pest control can be used effectively in fields to attain environmentally sound pest control. We provide growers with a green and controllable strategy—plant areas of a susceptible crop in the field while growing resistant crops. These areas have a refuge effect to protect resistant crops.
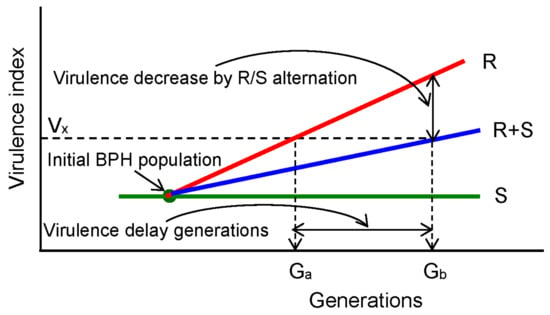
Figure 4.
Summary of the variation in BPH virulence while rearing with highly resistant rice varieties ®, susceptible rice varieties (S), and a mixture of resistant and susceptible rice varieties (R + S). Because the virulence of the BPH increases if it is reared with highly resistant rice varieties, Vx shows any value of virulence index higher than that of the initial BPH population. Ga indicates the number of generations required by the initial BPH population fed on a single highly resistant rice variety for its virulence index to reach Vx. Gb indicates the number of generations needed by the initial BPH population reared alternately with highly resistant rice varieties and a susceptible rice variety for its virulence index to reach Vx. Virulence delay generations indicates the generations’ Gb minus Ga. The virulence decrease by the H/S mixture indicates that the virulence index increased after the susceptible rice variety was included as an alternative highly resistant rice variety.
5. Conclusions
In our study, we examined the effects of alternately rearing different resistant rice varieties on the virulence, growth, and development of N. lugens. After five generations of selection, we confirmed that alternately rearing with a susceptible variety decreased the survival rate, fecundity, and virulence of the brown planthopper. Alternately rearing with a susceptible variety delayed the virulence development of insect pests to resistant varieties. On this basis, alternately rearing as an effective green control strategy has important guiding significance for field planting.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agriculture12070991/s1, Figure S1: The experimental arrangements in this study; Table S1: Variety lines used for multi-generation rearing experiments; Table S2: BPH virulence monitoring results in Guangdong Province, China.
Author Contributions
Data curation, Y.-D.Z.; Investigation, Y.-D.Z.; Methodology, Y.-D.Z.; Resources, Z.-F.Z. and W.-J.W.; Software, Y.-D.Z.; Writing—original draft, G.G.; Writing—review and editing, Z.-F.Z. and W.-J.W. All authors have read and agreed to the published version of the manuscript.
Funding
We are grateful to Yang Zhang from the Guangdong Academy of Agricultural Sciences for his valuable assistance with the standard seedbox screening test. This work was supported by the National Key Research and Development Program of China (2021 YFD1401100) and the National Natural Science Foundation of China (Grant No. 32172507).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated for this study are accessible upon request from the authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cheng, K.; Yan, M.; Nayak, D.; Pan, G.; Zheng, J. Carbon footprint of crop production in china: An analysis of national statistics data. J. Agric. Sci. 2015, 153, 422–431. [Google Scholar] [CrossRef]
- Sogawa, K. Biotypes of phytophagous insects: Intraspecific variations in host affinity and infestivity (1). Plant. Prot. 1983, 37, 7–10. [Google Scholar]
- Hereward, J.P.; Cai, X.; Matias, A.; Walter, G.H.; Xu, C.; Wang, Y. Migration dynamics of an important rice pest: The brown planthopper (Nilaparvata lugens) across Asia-Insights from population genomics. Evol. Appl. 2020, 13, 2449–2459. [Google Scholar] [CrossRef] [PubMed]
- Sogawa, K. Biotypes of phytophagous insects: Intraspecific cvariations in iost affinity and infestivity (2). Plant. Prot. 1983, 37, 63–68. [Google Scholar]
- Heinrichs, E.A. Perspectives and directions for the continued development of insect resistant rice varieties. Agric. Ecosyst. Environ. 1986, 18, 9–36. [Google Scholar] [CrossRef]
- Takita, T.; Habibuddin, H. Relationship between laboratory-developed biotypes of green leafhopper and resistant varieties of rice in malaysia. JARQ-Jpn. Agric. Res. Q. 1985, 19, 219–223. [Google Scholar]
- Atray, I.; Bentur, J.S.; Nair, S. The Asian rice gall midge (Orseolia oryzae) mitogenome has evolved novel gene boundaries and tandem repeats that distinguish its biotypes. PLoS ONE 2015, 10, e0134625. [Google Scholar] [CrossRef][Green Version]
- Li, C.; Chen, M.S.; Chao, S.; Yu, J.; Bai, G. Identification of a novel gene, H34, in wheat using recombinant inbred lines and single nucleotide polymorphism markers. Theor. Appl. Genet. 2013, 126, 2065–2071. [Google Scholar] [CrossRef] [PubMed]
- Jena, K.K.; Kim, S.M. Currect status of brown planthopper (BPH) resistance and genetics. Rice 2010, 3, 161–171. [Google Scholar] [CrossRef]
- Fujita, D.; Kohli, A.; Horgan, F.G. Rice resistance to planthoppers and leafhoppers. Crit. Rev. Plant. Sci. 2013, 32, 162–191. [Google Scholar] [CrossRef]
- Sogawa, K. Planthopper Outbreaks in Different Paddy Ecosystems in Asia: Man-Made Hopper Plagues that Threatened the Green Revolution in Rice; Springer: Dordrecht, The Netherlands, 2015; pp. 33–63. [Google Scholar]
- Kobayashi, T. Evolving ideas about genetics underlying insect virulence to plant resistance in rice-brown planthopper interactions. J. Insect Physiol. 2016, 84, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Fauer, R. Biotype 2 brown planthopper in the philippines. Int. Rice Res. Newsl. 1976, 1, 15. [Google Scholar]
- Claridge, M.F.; Den, H.J. Virulence to rice cultivars and selection for virulence in populations of the brown planthopper Nilaparvata lugens. Entomol. Exp. Appl. 1982, 32, 213–221. [Google Scholar] [CrossRef]
- Gallagher, K.D.; Kenmore, P.E.; Sogawa, K. Judicial Use of Insecticide Deter Planthopper Outbreaks and Extend the Life of Resistant Varieties in Southeast Asian Rice; Chapman & Hall: London, UK, 1994; pp. 599–614. [Google Scholar]
- Ketipearachchi, Y.; Kaneda, C.; Nakamura, C. Adaptation of the brown planthopper (BPH), Nilaparvata lugens (Stål) (Homoptera: Delphacidae) to BPH resistant rice cultivars carrying bph8 or Bph. Appl. Entomol. Zool. 1998, 33, 497–505. [Google Scholar] [CrossRef]
- Myint, K.K.; Yasui, H.; Takagi, M.; Matsumura, M. Virulence of long-Term laboratory populations of the brown planthopper, Nilaparvata lugens (Stål), and whitebacked planthopper, Sogatella furcifera (Horváth) (Homoptera: Delphacidae), on rice differential varieties. Appl. Entomol. Zool. 2009, 44, 149–153. [Google Scholar] [CrossRef]
- Nemoto, H.; Yokoo, M. Experimental selection of a brown planthopper population on mixtures of resistant rice lines. Breeding Sci. 1994, 44, 133–136. [Google Scholar] [CrossRef]
- Sogawa, K. A Change in biotype property of brown planthopper populations immigrating into Japan and their probable source areas. Kyushu Plant. Prot. Res. 1992, 38, 63–68. [Google Scholar] [CrossRef][Green Version]
- Khush, G.S. Multiple disease and insect resistance for increased yield stability in rice. In Progress in Irrigated Rice Research; International Rice Research Institute: Manila, Philippines, 1989; pp. 79–92. [Google Scholar]
- Velusamy, R.; Heinrichs, E.A.; Medrano, F.G. Greenhouse techniques to identify field resistance to the brown planthopper, Nilaparvata lugens (Stål) (Homoptera: Delphacidae), in rice cultivars. Crop. Prot. 1986, 5, 328–333. [Google Scholar] [CrossRef]
- Horgan, F.G. Mechanisms of Resistance: A Major Gap in Understanding Planthopper-Rice Interactions; International Rice Research Institute: Los Baños, Philippines, 1960; pp. 281–302. [Google Scholar]
- Horgan, F.G.; Ramal, A.F.; Bentur, J.S.; Kumar, R.; Bhanu, K.V. Virulence of brown planthopper (Nilaparvata lugens) populations from South and South East against resistant rice varieties. Crop. Prot. 2015, 78, 222–231. [Google Scholar] [CrossRef]
- Andrewartha, H.G.; Birch, L.C. Distribution and Abundance of Animal Populations; University of Chicago Press: Chicago, IL, USA, 1954. [Google Scholar]
- Meyer, J.S.; Ingersoll, C.G.; McDonald, L.L.; Boyce, M.S. Estimating uncertainty in population growth rates: Jack knife vs. bootstrap techniques. Ecology 1986, 67, 1156–1166. [Google Scholar] [CrossRef]
- Shangguan, X.; Zhang, J.; Liu, B.; Zhao, Y.; Wang, H.; Wang, Z.; Guo, J.; Rao, W.; Jing, S.; Guan, W.; et al. A Mucin-Like Protein of Planthopper Is Required for Feeding and Induces Immunity Response in Plants. Plant. Physiol. 2018, 176, 552–565. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Matsumura, M. Development of virulence to resistant rice varieties in the brown planthopper, Nilaparvata lugens (Homoptera: Delphacidae), immigrating into Japan. Appl. Entomol. Zool. 2000, 35, 529–533. [Google Scholar] [CrossRef][Green Version]
- Diehl, S.R.; Bush, G.L. An evolutionaly and applied perspective of insect biotypes. Annu. Rev. Entomol. 1984, 29, 471–504. [Google Scholar] [CrossRef]
- Tan, Y.J.; Zhang, Y.; Huang, B.C. Monitoring the variation dynamic of brown planthopper, Nilaparvata lugens (Stål) biotypes and recommending the resistant rice cultivars and resources. Acta Enomol. Sin. 1997, 40, 32–39. [Google Scholar]
- Thuat, N.C.; Huong, N.T.; Binh, D.T.; Chien, H.V.; Chau, N.L. Virulence of brown planthopper (BPH) in Vietnam. Int. Rice Res. Newsl. 1992, 25, 11. [Google Scholar]
- Ali, M.P.; Alghamdi, S.S.; Begum, M.A.; Uddin, A.B.M.A.; Alam, M.Z. Screening of rice genotypes for resistance to the brown planthopper, Nilaparvata lugens Stål. Cereal Res. Commun. 2012, 40, 502–508. [Google Scholar] [CrossRef]
- Ferrater, J.B.; Jong, P.W.; Dicke, M.; Chen, Y.H.; Horgan, F.G. Symbiont-mediated adaptation by planthoppers and leafhoppers to resistant rice varieties. Arthropod-Plant Interact. 2013, 7, 591–605. [Google Scholar] [CrossRef]
- Ferrater, J.B.; Naredo, A.I.; Almazan, M.L.P.; Jong, P.W.; Dicke, M.; Horgan, F. Varied responses by yeast-like symbionts during virulence adaptation in a monophagous phloem-feeding insect. Arthropod-Plant Interact. 2015, 9, 215–224. [Google Scholar] [CrossRef]
- Horgan, F.G.; Srinivasan, T.S.; Bentur, J.S.; Kumar, R.; Bhanu, K.V. Geographic and research center origins of rice resistance to Asian planthoppers and Leafhoppers: Implications for rice breeding and gene deployment. Agronomy 2017, 7, 62. [Google Scholar] [CrossRef]
- Bates, S.L.; Zhao, J.Z.; Roush, R.T.; Shelton, A.M. Insect resistance management in GM crops: Past, present and future. Nat. Biotechnol. 2005, 23, 57–62. [Google Scholar] [CrossRef]
- Tabashnik, B.E.; Gassmann, A.J.; Crowder, D.W.; Carriére, Y. Insect resistance to Bt crops: Evidence versus theory. Nat. Biotechnol. 2008, 26, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Chen, H.; Fan, J.; Wang, Y.; Li, Y.; Chen, J.; Fan, J.; Yang, S.; Hu, L.; Leung, H.; et al. Genetic diversity and disease control in rice. Nature 2000, 406, 718–722. [Google Scholar] [CrossRef] [PubMed]
- Vacher, C.; Bourguet, D.; Rousset, F.; Chevillon, C.; Hochberg, M.E. Modelling the spatial configuration of refuges for a sustainable control of pests: A case study of Bt cotton. J. Evol. Biol. 2003, 16, 378–387. [Google Scholar] [CrossRef]
- Huang, F.; Andow, A.A.; Buschman, L.L. Success of the high-dose/refuge resistance management strategy after 15 years of Btcrop use in North America. Entomol. Exp. Appl. 2011, 140, 1–16. [Google Scholar] [CrossRef]
- Pathak, P.K.; Heinrichs, E.A. Selection of biotype populations 2 and 3 of Nilaparvata lugens by exposure to resistant rice varieties. Environ. Entomol. 1982, 11, 85–90. [Google Scholar] [CrossRef]
- Sogawa, K.; Kilin, D. Biotype shift in a brown planthopper population (BPH) on IR42. Int. Rice Res. Newsl. 1987, 12, 40. [Google Scholar]
- Cohen, M.B.; Alam, S.N.; Medina, E.B.; Bernal, C.C. Brown planthopper, Nilaparvata lugens, resistance in rice cultivar IR64: Mechanism and role in successful N. lugens management in Central Luzon, Philippines. Entomol. Exp. Appl. 1997, 85, 221–229. [Google Scholar] [CrossRef]
- Tanaka, K. Quantitative genetic analysis of biotypes of the brown planthopper Nilaparvata lugens: Heritability of virulence to resistant rice varieties. Entomol. Exp. Appl. 1999, 90, 279–287. [Google Scholar] [CrossRef]
- Tanaka, K. Recent status in virulence to resistant rice varieties of brown planthopper Nilaparvata lugens immigration into Japan. Ann. Rep. Kanto Plant Prot. Soc. 1999, 46, 85–88. [Google Scholar]
- Li, J.; Ke, S.S.; Liu, J.; Jiang, T.R.; Hu, D.B. Multi-generational effects of rice harboring Bph15 on brown planthopper, Nilaparvata lugens. Pest. Manag. Sci. 2014, 70, 310–317. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).