Abstract
Seeds with high vigor have strong resistance to various adverse environmental conditions. However, little is known about how seed vigor affects the resistance of seeds to biotic stress. In this study, newly harvested seeds that had high vigor and seeds with low vigor, achieved via an artificially accelerated aging treatment, were used in the germination test after inoculation with Fusarium graminearum for 24 h. The results showed that high-vigor seed-related germination and seedling growth were not significantly affected by F. graminearum infection, while those related to low-vigor seeds were significantly inhibited. Analysis of transgenic maize seeds expressing the luminescent Ca2+ probe encoded by aequorin indicated that the concentration of free Ca2+ in the cytoplasm and nucleus of the embryo cells of high-vigor seeds was significantly higher than that of the low-vigor seeds. Through an experiment with Ca2+ inhibitor treatment and exogenous Ca2+ application, we further confirmed that Ca2+ played an important role in seed germination and seedling growth. Interestingly, in the presence of F. graminearum, the Ca2+ required for seed germination and seedling growth mainly came from the vacuolar calcium pool, while in the absence of F. graminearum, the required Ca2+ mainly came from the apoplastic calcium store. This study helps understand how high-vigor seeds resist disease and provides theoretical support for the wide application of high-vigor seeds in agricultural production.
1. Introduction
Ca2+ is a key signaling molecule in eukaryotes. It regulates the response of plants to various environmental signals and controls developmental processes in plants [,]. Ca2+ is rapidly released from the intracellular and apoplastic calcium pools to form pulsed Ca2+ signals in cytoplasm and some organelles including nuclei, mitochondria and chloroplasts, in response to various biotic and abiotic stimuli []. After Ca2+ signals are perceived by the sensor proteins of Ca2+, the downstream genes are regulated to respond to the stimuli.
Higher levels of cytoplasmic Ca2+ promote seed germination []. Ca2+ is involved in the upregulation of gibberellin to promote seed germination of Nigella sativa [] and participates in regulating the early growth of the plumular axis during the germination of mung bean seeds []. Treatment with exogenous calcium significantly increased the levels of auxin, gibberellin, and cytokinin during seed germination, thereby promoting seed germination [,]. In addition, appropriate concentrations of exogenous Ca2+ improve resistance to Botryosphaeria dothidea in pear [] and alleviates the damage resulting from abiotic stress caused by high temperature, drought, freezing, salinity, and heavy metal [,,,,].
Seed vigor is an important indicator for evaluating seed quality. High-vigor seeds generate evenly emerging and fast-growing seedlings that are robust and resistant to biotic and abiotic stress []. The level of seed vigor is gradually formed during seed development, reaches a peak at physiological maturity, and then decreases as the seed enters an irreversible aging process []. Oxidative damage caused by reactive oxygen species (ROS) is considered the main cause of seed aging []. During the long-term storage of seeds, as one of the byproducts of aerobic respiration, gradually accumulated ROS results in DNA/RNA damage, protein carbonylation, and lipid peroxidation []. ROS degrade long-chain fatty acids by attacking polyunsaturated fatty acids in membrane phospholipids, leading to the loss of membrane integrity [] and the compromised function of transmembrane proteins, such as transporters and signaling receptors [,]. During seed imbibition, phospholipids in cell membranes were rebuilt, and the function of transmembrane proteins was restored []. A higher degree of seed aging causes a higher degree of cell membrane damage, and as a result, it is more difficult to repair the membranes and restore the function of the transmembrane proteins [].
Living in complex environments, plants have evolved a sophisticated innate immune system that senses and responds to pathogenic infection. This system includes two aspects: pathogen-associated molecular patterns (PAMP)/microbe-associated molecular patterns (MAMP)-induced immune response (PTI) and effector-induced immune response (ETI) []. Initially, plant pattern-recognition receptors (PRRs) on cell membranes recognize microbial conserved PAMPs/MAMPs or plant-originated damage-associated molecular patterns (DAMPs) and activate PTI []. Plants trigger a series of cellular and physiological changes, including the rapid increase of cytoplasmic Ca2+ content, high levels of extracellular ROS, and the increase of mitogen-activated protein kinase (MAPK) activity, and then start the synthesis of plant hormones, such as salicylic acid, jasmonic acid, and ethylene [,]. About 30 s to 2 min after perceiving MAMPs/DAMPs, extracellular Ca2+ begins to flow into the cytoplasm, and about 4 to 6 min later, cytoplasmic Ca2+ content reaches its peak [].
Fusarium graminearum is a pathogenic fungus that is highly harmful to agricultural production and causes head scab in wheat and barley, and ear and stalk rot in maize, resulting in serious losses due to yield and quality reduction as well as to the contamination of trichothecene mycotoxins, which threaten the health of humans and animals []. The main aim of this present study was to determine how vigor level of seeds affected their resistance to F. graminearum. In this study, we found that high-vigor seeds were more resistant to F. graminearum infection. Using the luminescent Ca2+ probe aequorin transgenic maize seeds as materials, the study found that the concentration of free Ca2+ in cytoplasm and nuclei of the embryo cells of the high-vigor seeds was significantly higher than that of the low-vigor seeds. Furthermore, in the presence of F. graminearum, the Ca2+ required for seed germination and seedling growth mainly came from the vacuole, while when there was no F. graminearum infection, the required Ca2+ was mainly from the apoplastic calcium store.
2. Materials and Methods
2.1. Plant Materials
Maize hybrid “Xianyu 335” and transgenic maize expressing the luminescent Ca2+ probe aequorin were used in this study. The vector pBIN-YA (B) was used to express the 35S promoter-driven YFP-aequorin and was obtained from The Arabidopsis Information Resource (TAIR, www.arabidopsis.org, accessed on 2 November 2018). Transgenic maize seeds expressing the luminescent probe were used to detect free Ca2+ in the cytoplasm and nuclei []. The transformation of maize was conducted by the Center for Life Science and Technology of China National Seed Group (Wuhan, China). The seeds used in this study were generated through strict manual pollination.
2.2. Measurements and Methods
2.2.1. Acquisition of Homozygous Transgenic Seeds
Seeds of the T1 generation were sown, and DNA was extracted from leaves at the seedling stage. Primers pBIN-YA-F (5′-ATGACGCACAATCCCACTATCC-3′) and pBIN-YA-R (5′-AGTTCACCTTGATGCCGTTC-3′) were designed for PCR amplification according to the 35S promoter and YFP sequence of pBIN-YA (B) vector []. The plants without amplification bands were removed and the remaining plants were self-pollinated. Twenty seeds were randomly selected from each harvested T2 generation ear and germinated on a paper bed at 25 °C. Four days later, the germinated seeds were irradiated with LUYOR-3260 fluorescent protein excitation light source (Shanghai Luyang Instrument, Shanghai, China). If all the seeds emitted fluorescence, this indicated that all the seeds on the ear were homozygous. Homozygous seeds were used for further related assays.
2.2.2. Subcellular Localization Assays
The protoplast isolation and transient expression were performed as described by Tu et al. []. Mesophyll protoplasts were isolated using the 11 d old seedling leaves under dark conditions. The pBIN-YA (B) vector was transfected into protoplasts using the PEG-calcium solution (0.4 g/mL PEG4000, 0.8 M mannitol, and 0.1 M CaCl2). After being washed and resuspended with W5 solution (154 mM NaCl, 125 mM CaCl2, 5 mM KCl, and 2 mM MES), mesophyll protoplasts were incubated under dark conditions for 12–18 h. The YFP fluorescence was examined and imaged with a Leica TCS SP8 confocal microscope (Leica Microsystems, Mannheim, Germany).
2.2.3. Determination of Free Ca2+ Concentration in Embryo Cells
Undamaged transgenic maize seeds were randomly selected and placed in an embryo-upward position on five layers of germination paper saturated with deionized water in a petri dish. Subsequently, the seeds were covered with three sheets of water-saturated germination paper. The seeds were allowed to imbibe water for different durations at 25 °C. Excessive water on the seeds was then removed, and the embryos were carefully isolated with a scalpel blade and forceps. Fifteen embryos were prepared from each imbibition duration treatment, and the embryos were used to determine the free Ca2+ concentration.
The seed embryos were individually placed in the wells of a black opaque microplate, and each well included one embryo. After the addition of 200 μL coelenterazine solution (10 mmol/L) to each well, the microplate was covered with a lid to completely block out light and incubated on an 80 rpm shaker at 25 °C for 8 h.
After incubation, the coelenterazine solution in each well was removed and replaced with 200 μL F. graminearum suspension. Then, the fluorescence intensity (Lstimuli) was immediately measured with a microplate reader. After the measurement, the fungal suspension in each well was removed and replaced with 200 μL discharge buffer (2 M CaCl2 in 20% ethanol). The fluorescence intensity (Lrest) was measured immediately.
Lmax = Lstimuli + Lrest
k = Lstimuli/Lmax
pCa = 0.332588 (−logk) + 5.5593
The pCa value was multiplied by 106 to obtain the concentration of free Ca2+ in μM [].
2.2.4. Aging Treatment in Maize Seeds
For the seed aging treatment, mesh bags and the inside of a seed aging chamber were sterilized using 75% ethanol. Randomly selected undamaged maize seeds were placed in a mesh bag and then placed on a plate in a compartment of the seed aging chamber. The seeds were evenly spread out without overlapping. The seeds were treated continuously for 4 d under the conditions of 95% relative humidity and 45 °C.
2.2.5. Treatment of Maize Seeds with Ca2+ Channel Inhibitors
Maize seeds were placed in ethylene glycol-bis(beta-aminoethyl ether)-N-tetraacetic acid (EGTA, 5 mmol/L) solution to chelate extracellular free Ca2+, LaCl3 solution (3 mmol/L) to block the cell membrane Ca2+ channel, RR solution (Ruthenium Red, 0.5 mmol/L) to inhibit the release of vacuolar Ca2+, and distilled water (control) for 12 h at 25 °C.
2.2.6. Treatments of Maize Seeds with Exogenous Ca2+
Maize seeds were soaked in CaCl2 solution (10 and 20 mmol/L), CaSO4 solution (10 and 20 mmol/L), and distilled water (control) for 12 h at 25 °C.
2.2.7. Standard Germination Test of Maize Seeds
One kilogram of quartz sand (about 0.35 mm in diameter) was mixed well with 170 mL of deionized water. In a germination box (17 cm × 12 cm × 7 cm) with 800 g sand, scraped flat, 50 seeds were then placed in the sand with the embryo in an upward position. Subsequently, more sand was added to cover the seeds until a total of 1400 g sand was in the germination box. Each treatment included four replicates. The performance during seed germination and the calculation of related indices were carried out according to the previous description [].
2.2.8. Inoculation of Maize Seeds with F. graminearum
In an ultra-clean laminar flow hood, a F. graminearum spore suspension (1.5–2 × 105 spores/mL) was prepared. Four layers of germination paper saturated with the spore suspension were placed in a sterile petri dish. Maize seeds were evenly placed without overlapping on the germination paper with the embryos in an upward position, and then covered with 3 sheets of germination paper saturated with the spore suspension. The petri dish was placed in an incubator at 25 °C for 24 h.
2.2.9. Determination of Enzyme Activity
Maize seeds with high and low vigor were treated with a Ca2+ inhibitors and exogenous Ca2+, respectively, for 12 h and then co-cultured with F. graminearum for 24 h. Subsequently, the seeds were sampled at 0, 24, and 48 h after the end of the inoculation period. Enzyme assay kits produced by Cominbio (Suzhou, China) were used to determine the activity of chitinase, superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT). Each treatment included three biological replicates, and each sample had three technical replicates. The operation followed the instructions provided by the kit producer.
2.2.10. Statistical Analysis
The results are expressed as means ± SD. Data were tested for significance using one-way ANOVA with the IBM SPSS Statistics 19.0 software (SPSS, Chicago, IL, USA).
3. Results
3.1. High-Vigor Seeds Had Higher Resistance to F. graminearum
To clarify the resistance of seeds differing in vigor to F. graminearum, we used the newly harvested high-vigor seeds of maize hybrid “Xianyu 335” and low-vigor seeds obtained through an artificially accelerated aging treatment to perform germination tests after they were co-cultured with F. graminearum. The results showed that F. graminearum infection inhibited seed germination and seedling growth. The germination energy, germination index, germination percentage, and vigor index of the low-vigor seeds inoculated with F. graminearum were significantly decreased (Figure 1). High-vigor seeds related to germination and seedling growth were inhibited to a certain extent, but the inhibition effect was not significant (Figure 1), indicating that high-vigor seeds had higher resistance to F. graminearum at the germination stage and during seedling growth.
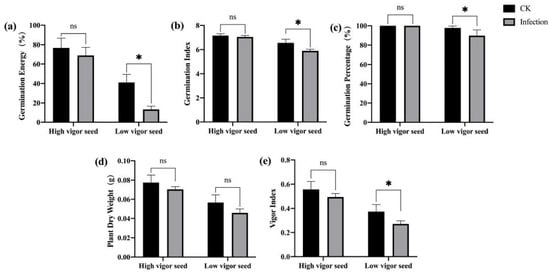
Figure 1.
Effects of F. graminearum infection on seed germination and seedling growth of maize with different vigor levels. (a) germination energy (%), (b) germination index, (c) germination percentage (%), (d) dry weight of single plant (g), (e) vigor index. * indicates p < 0.05 (Student’s t-test), and ns represents not significant.
3.2. F. graminearum-Infected High-Vigor Maize Seeds Had Stronger Ca2+ Signals during Imbibition
In a previous study, the flow rate of Ca2+ flowing from the extracellular matrix to the embryo cells showed a significant positive correlation with maize seed vigor during imbibition []. To determine how the vigor of maize seeds affected their resistance to F. graminearum, we transformed maize with the expression vector pBIN-YA (B) and obtained transgenic plants expressing the YFP-aequorin gene []. Homozygous transgenic seeds were obtained for subsequent analysis (Figure 2). The aequorin protein was used as a Ca2+ probe to detect the Ca2+ concentration in cytoplasmic and nuclear (Figure 3) prior to and after F. graminearum infection during seed imbibition.
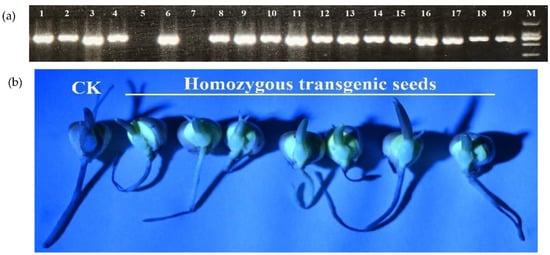
Figure 2.
Acquisition of homozygous transgenic seeds by PCR and fluorescence detection. (a) PCR products of T1 generation plants (lane 1–18; lane 5 and 7 are of negative plants), and pBIN-YA (B) plasmid (lane 19). M: DL 2000 marker; (b) fluorescence detection.
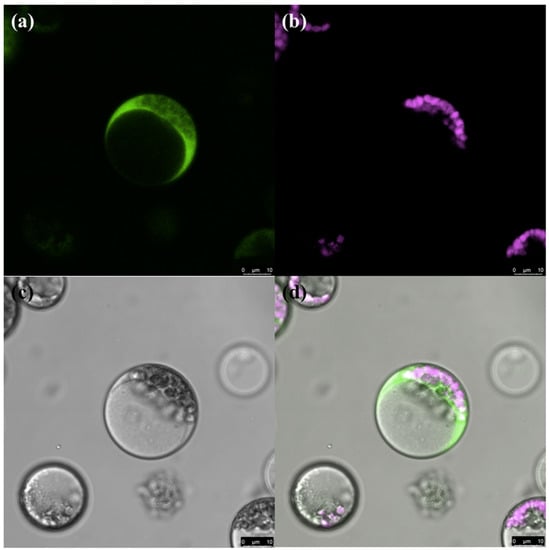
Figure 3.
Subcellular localization of YFP-aequorin in maize protoplast. (a) YFP-aequorin, (b) chlorophyll, (c) bright field, (d) merged image.
The embryos that were isolated from the seeds with different vigor levels and imbibed for different durations were used to determine the concentration of free Ca2+ in the cytoplasm and nuclei. The free Ca2+ in the embryo cells of high-vigor seeds was significantly higher than that in low-vigor seeds (Figure 4a). Exposure of the embryos to F. graminearum resulted in a rapid increase in the free Ca2+ concentration in the embryo cells of seeds with high and low vigor. The free Ca2+ concentration in the embryo cells of the high-vigor seeds was higher than that of the low-vigor seeds, and the differences reached a significant level for most imbibition durations (Figure 4b).
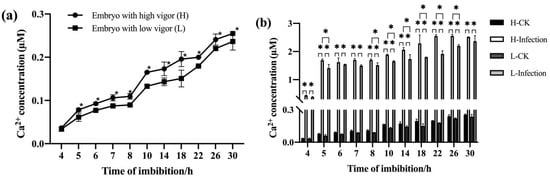
Figure 4.
Ca2+ concentration in the cytoplasm and nucleus of embryo cells during imbibition of maize seeds with different vigor levels. (a) Ca2+ concentration without F. graminearum infection, (b) Ca2+ concentration before and after F. graminearum infection. * indicates p < 0.05 (Student’s t-test), and ns represents not significant.
3.3. Effects of the Inhibition of Ca2+ Entry into the Cytoplasm and Exogenous Ca2+ Treatments on the Resistance of Maize Seeds to F. graminearum
3.3.1. Inhibiting the Entry of Ca2+ into the Cytoplasm Reduced the Resistance of Maize Seeds to F. graminearum
To clarify the effects of inhibiting the entry of Ca2+ into the cytoplasm on the disease resistance of maize seeds, high- and low-vigor maize seeds were treated with cell membrane Ca2+ channel inhibitor LaCl3, extracellular Ca2+ chelator EGTA, and vacuolar Ca2+ channel inhibitor RR. After F. graminearum infection, a standard germination test was used to estimate indices related to seed germination and seedling growth. For high-vigor seeds, Ca2+ channel inhibitor/chelator treatments inhibited germination and seedling growth. LaCl3 treatment significantly reduced germination energy, germination index, germination percentage, and vigor index. EGTA treatment significantly reduced the vigor index and average dry weight of a single seedling. RR treatment significantly reduced the germination energy, germination index, germination percentage, average dry weight of a single seedling, and vigor index. Among the treatments, the RR treatment had the largest effect (Figure 5). Similar results were obtained for low-vigor seeds after treatments with Ca2+ channel inhibitors/chelators (Figure 6).
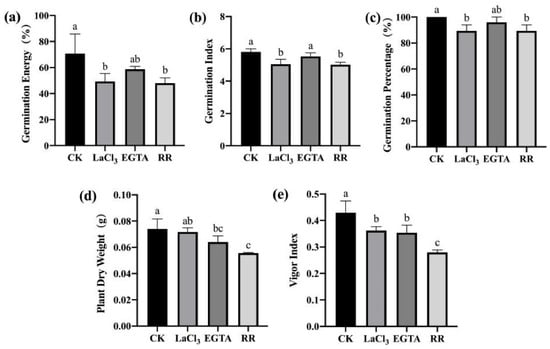
Figure 5.
Ca2+ chelating agent/channel inhibitor treatments reduced F. graminearum resistance in high-vigor maize seeds. (a) germination energy (%), (b) germination index, (c) germination percentage (%), (d) dry weight of single plant (g), (e) vigor index. EGTA, ethylene glycol-bis(beta-aminoethyl ether)-N-tetraacetic acid; RR, Ruthenium Red; CK, control. Different letters indicate significant differences among different lines (Tukey’s test).
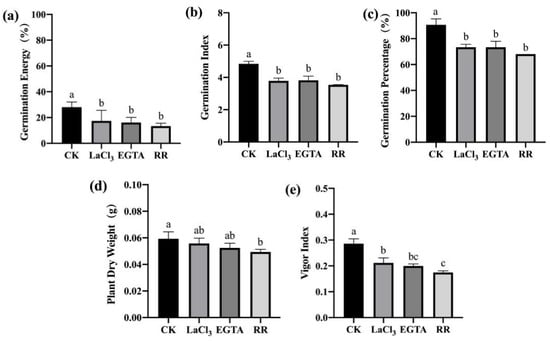
Figure 6.
Ca2+ chelating agent/channel inhibitor treatments reduced F. graminearum resistance in low-vigor maize seeds. (a) germination energy (%), (b) germination index, (c) germination percentage (%), (d) dry weight of single plant (g), (e) vigor index. EGTA, ethylene glycol-bis(beta-aminoethyl ether)-N-tetraacetic acid; RR, Ruthenium Red; CK, control. Different letters indicate significant differences among different lines (Tukey’s test).
To further examine the effects of LaCl3, EGTA, and RR treatments on the germination and seedling growth of maize seeds with different vigor levels, a standard germination test was conducted on maize seeds treated with three inhibitors but not inoculated with F. graminearum. The three inhibitors also significantly inhibited seed germination and seedling growth (Figure 7 and Figure 8). However, after inoculation with F. graminearum, RR treatment had the highest effect in the case of F. graminearum infection (Figure 5 and Figure 6), while LaCl3 had the highest effect in the absence of F. graminearum infection (Figure 7). This indicates that the Ca2+ required for germination was mainly from the vacuolar calcium pool when the seeds were treated with the inhibitors and exposed to F. graminearum, while the Ca2+ required for germination was mainly from the apoplastic calcium store when the seeds were treated only with the inhibitors.
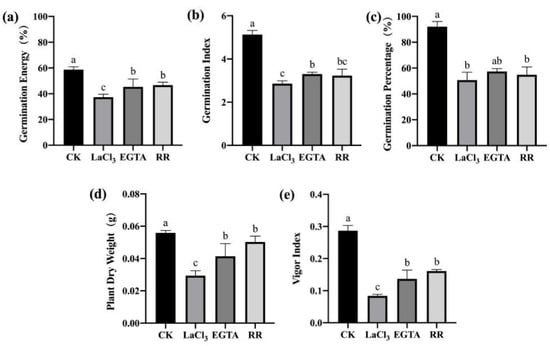
Figure 7.
Ca2+ chelating agent/channel inhibitor treatments inhibited seed germination and seedling growth of high-vigor maize seeds. (a) germination energy (%), (b) germination index, (c) germination percentage (%), (d) dry weight of single plant (g), (e) vigor index. EGTA, ethylene glycol-bis(beta-aminoethyl ether)-N-tetraacetic acid; RR, Ruthenium Red; CK, control. Different letters indicate significant differences among different lines (Tukey’s test).
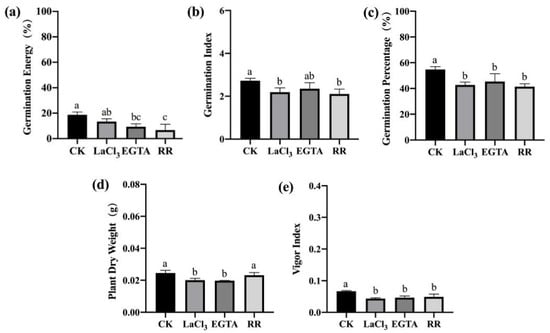
Figure 8.
Ca2+ chelating agent/channel inhibitor treatments inhibited seed germination and seedling growth of low-vigor maize seeds. (a) germination energy (%), (b) germination index, (c) germination percentage (%), (d) dry weight of single plant (g), (e) vigor index. EGTA, ethylene glycol-bis(beta-aminoethyl ether)-N-tetraacetic acid; RR, Ruthenium Red; CK, control. Different letters indicate significant differences among different lines (Tukey’s test).
3.3.2. Exogenous Ca2+ Treatments Enhanced the Resistance of Maize Seeds to F. graminearum
To clarify the effect of exogenous Ca2+ application on the disease resistance of maize seeds, we used CaCl2 solution (10 and 20 mmol/L) and CaSO4 solution (10 and 20 mmol/L) to treat maize seeds. After F. graminearum infection, a standard germination test was performed to estimate the indices related to seed germination and seedling growth. Meanwhile, non-inoculated maize seeds treated with exogenous Ca2+ were also germinated. For both high- and low-vigor seeds, exogenous Ca2+ treatment promoted germination and seedling growth; the germination index, germination percentage, and vigor index of the seeds and the dry weight of seedlings were significantly higher than those of the control (Figure 9 and Figure 10). The results indicate that exogenous Ca2+ treatment significantly improves the resistance of maize seeds to F. graminearum.
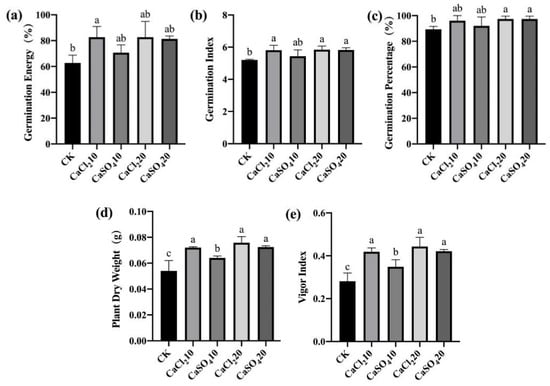
Figure 9.
Exogenous Ca2+ enhanced the resistance of high-vigor maize seeds to F. graminearum. (a) germination energy (%), (b) germination index, (c) germination percentage (%), (d) dry weight of single plant (g), (e) vigor index. CaCl210/20, 10/20 mmol/L CaCl2 solution; CaSO410/20, 10/20 mmol/L CaSO4 solution; CK, control. Different letters indicate significant differences among different lines (Tukey’s test).
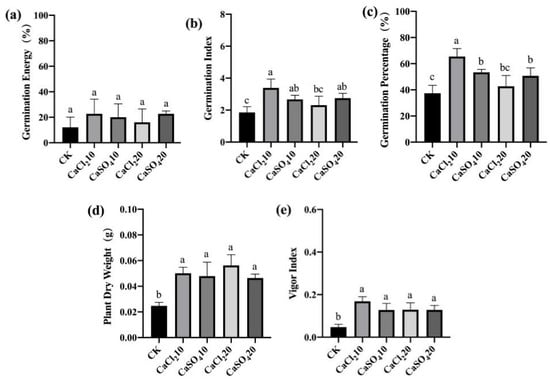
Figure 10.
Exogenous Ca2+ enhanced the resistance of low-vigor maize seeds to F. graminearum. (a) germination energy (%), (b) germination index, (c) germination percentage (%), (d) dry weight of single plant (g), (e) vigor index. CaCl210/20, 10/20 mmol/L CaCl2 solution; CaSO410/20, 10/20 mmol/L CaSO4 solution; CK, control. Different letters indicate significant differences among different lines (Tukey’s test).
3.4. Effects of Inhibiting Ca2+ Entry into the Cytoplasm and Exogenous Ca2+ Treatments on the Activity of Defensive Enzymes in Maize Seeds
Plants secrete a large amount of chitinase in response to pathogen infection and synthesize a large amount of SOD, POD, and CAT to cope with the oxidative stress caused by pathogen infection. The activities of these four enzymes in the seeds inoculated with F. graminearum and treated with Ca2+ channel inhibitors/chelators were determined. Ca2+ channel inhibitor/chelator treatment significantly reduced the activity of chitinase at 24 h after inoculation, the activity of SOD at 24 and 48 h after inoculation, and the activities of POD and CAT at all three time points after inoculation in both high- and low-vigor seeds (Figure 11 and Figure 12). RR had the highest inhibitory effect on the activity of these enzymes, which was consistent with the previously mentioned germination data (Figure 5 and Figure 6). Exogenous Ca2+ treatments increased the activity of the four enzymes mentioned above in both high- and low-vigor seeds, and the differences were significant for most of the treatments (Figure 13 and Figure 14).
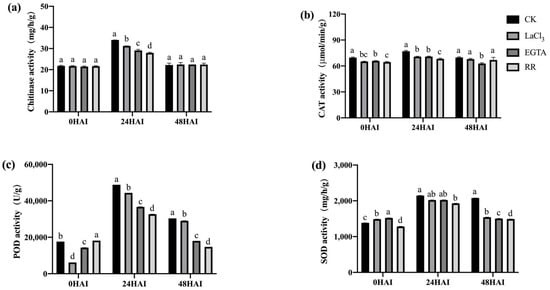
Figure 11.
Ca2+ chelating agent/channel inhibitor treatments inhibited the activities of chitinase (a), CAT (b), POD (c), and SOD (d) in high-vigor maize seeds under F. graminearum infection. EGTA, ethylene glycol-bis(beta-aminoethyl ether)-N-tetraacetic acid; RR, Ruthenium Red; CK, control; HAI, hours after inoculation. Different letters indicate significant differences among different lines (Tukey’s test).
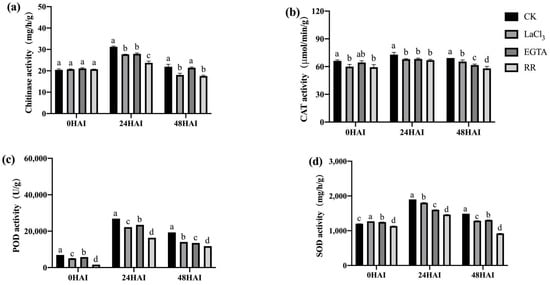
Figure 12.
Ca2+ chelating agent/channel inhibitor treatments inhibited the activities of chitinase (a), CAT (b), POD (c), and SOD (d) in low-vigor maize seeds under F. graminearum infection. EGTA, ethylene glycol-bis(beta-aminoethyl ether)-N-tetraacetic acid; RR, Ruthenium Red; CK, control; HAI, hours after inoculation. Different letters indicate significant differences among different lines (Tukey’s test).
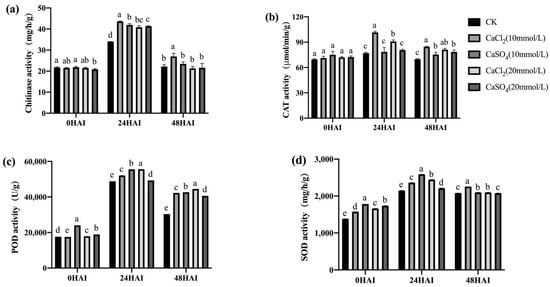
Figure 13.
Exogenous Ca2+ enhanced the activities of chitinase (a), CAT (b), POD (c), and SOD (d) in high-vigor maize seeds under F. graminearum infection. HAI, hours after inoculation. Different letters indicate significant differences among different lines (Tukey’s test).
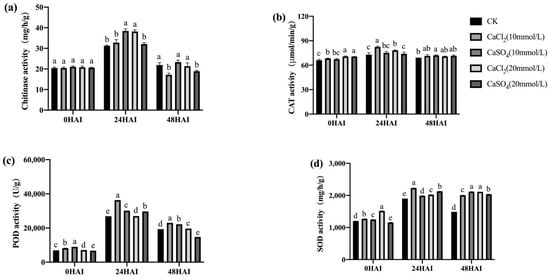
Figure 14.
Exogenous Ca2+ enhanced the activities of chitinase (a), CAT (b), POD (c), and SOD (d) in low-vigor maize seeds under F. graminearum infection. HAI, hours after inoculation. Different letters indicate significant differences among different lines (Tukey’s test).
These results indicate that the inhibition of Ca2+ entry into the cytoplasm and the exogenous application of Ca2+ significantly affected the activities of chitinase, SOD, POD, and CAT in maize seeds, which then affected the resistance of the seeds to F. graminearum infection.
4. Discussion
Seed vigor is an important indicator of seed quality. High-vigor seeds have a high level of resistance to various adverse environmental conditions. In this study, the newly harvested high-vigor seeds and seeds with low vigor caused by an artificially accelerated aging treatment were placed in a F. graminearum suspension to imbibe it for 24 h and then subjected to a germination test. F. graminearum infection did not cause distinct damage to high-vigor seeds, while F. graminearum infection significantly inhibited low-vigor seed-related germination and seedling growth (Figure 1). Why are high-vigor seeds more resistant to F. graminearum infection?
Ca2+ plays a key role in promoting plant development and resistance to biotic and abiotic stress. Previous studies showed that Ca2+ is involved in the GA-induced synthesis of α-amylase in rice seeds [] and that Ca2+ activates protease in germinated mung bean seeds []. Ca2+ is involved in regulating early axis growth during the germination of mung bean seeds []. Exogenous application of CaCl2 reduced the effect of salt stress on Phragmites karka and Festuca ovina [,]. However, as far as we know, there is no report on the effect of Ca2+ on seed germination under pathogen infection. In this study, transgenic maize seeds expressing the aequorin gene were used to examine the concentration of free Ca2+ in the cytoplasm and nuclei. The results showed that the concentration of free Ca2+ in the embryo cells of high-vigor seeds was significantly higher than that of low-vigor seeds at different time points of imbibition (Figure 4a). After the infection of embryos with F. graminearum, the free Ca2+ concentration in the embryo cells of both the high- and low-vigor seeds increased rapidly and significantly, and the free Ca2+ concentration in the seed embryo cells of the high-vigor seeds was higher than that of the low-vigor seeds (Figure 4b).
To clarify the effect of Ca2+ on seed germination under F. graminearum infection, we used three Ca2+ antagonists (LaCl3, EGTA, and RR) and two exogenous calcium solutions (CaCl2 and CaSO4) to treat maize seeds with high and low vigor, respectively. Inhibiting the entry of extracellular or vacuolar Ca2+ into the cytoplasm significantly inhibited seed germination and seedling growth (Figure 5 and Figure 6) and significantly reduced the activity of chitinase, CAT, POD, and SOD (Figure 11 and Figure 12), while exogenous Ca2+ treatment promoted seed germination and seedling growth and significantly increased the activities of the four enzymes (Figure 9, Figure 10, Figure 13 and Figure 14). The results indicated that Ca2+ played an important role in the resistance of maize seeds to F. graminearum infection.
Singh et al. [] and Verma et al. [] demonstrated that the Ca2+ required for the early germination of mung bean (Vigna radiata) seeds came from the apoplastic calcium store. Our previous study showed that during the imbibition of maize seeds, Ca2+ in embryo cells flowed from the extracellular calcium pool into cells, and the influx rate of Ca2+ in the embryos of high-vigor seeds was higher than that of low-vigor seeds []. In the present study, although the treatments with all the three inhibitors significantly inhibited seed germination and seedling growth, in the presence of F. graminearum infection, the vacuolar Ca2+ channel inhibitor RR showed the highest inhibitory effect (Figure 5 and Figure 6), while in the absence of F. graminearum infection, the inhibitor LaCl3 showed the highest inhibitory effect (Figure 7). The results indicated that in the case of F. graminearum infection, the required Ca2+ mainly came from the vacuolar calcium pool, and in the absence of F. graminearum infection, the required Ca2+ mainly came from the apoplastic calcium store. The vacuole and apoplast are the major calcium pools in plants. The concentrations of free Ca2+ were 0.2–0.5 mM, 0.33–1 mM, and 50–100 nM in the vacuole, apoplast, and cytoplasm, respectively []. The concentration of free Ca2+ in vacuoles and apoplasts is much higher than that in the cytoplasm. Therefore, when F. graminearum infection occurs, both the vacuolar and apoplast calcium pools can theoretically provide sufficient Ca2+ to form a Ca2+ signal to initiate the expression of downstream disease resistance genes. Then, why did Ca2+ mainly come from the vacuolar calcium pool during fungal infection? The answer might be that Ca2+ release from vacuolar is faster than Ca2+ influx from the apoplast upon pathogen infection. The Ca2+ signal responds quickly to pathogen infection. About 30 s to 2 min after the plant perceived MAMPs/DAMPs, Ca2+ began to flow into the cytoplasm, and in about 4 to 6 min, the Ca2+ content in the cytoplasm reached a peak []. Therefore, calcium pools need to respond quickly to pathogen infection. The hypothesis needs to be verified in further studies.
Diseases are highly harmful to agricultural production. Toxic chemicals are usually used to control diseases. In this study, we found that soaking seeds in 10 and 20 mmol/L CaCl2 or 10 and 20 mmol/L CaSO4 solution significantly promoted seed germination and seedling growth (Figure 9 and Figure 10) and improved the resistance to F. graminearum infection. In addition, applying exogenous CaCl2 to plants can alleviate the damage to plants caused by stress due to abiotic factors, such as high temperature, drought, freezing, salt, and heavy metals [,,,,]. Furthermore, CaCl2 is a safe, environmentally friendly, and inexpensive chemical. It is expected that CaCl2, as a seed treatment agent, may play a more important role in sustainable agricultural production in the future; of course, field trials are needed to confirm its practical value in agriculture.
5. Conclusions
Low-vigor seed-related germination and seedling growth were significantly inhibited by F. graminearum infection, while those related to high-vigor seed were not. The concentration of free Ca2+ in the cytoplasm and nuclei of the embryo cells of the high-vigor seeds was significantly higher than that of the low-vigor seeds prior to and after F. graminearum infection. Inhibiting extracellular or vacuolar Ca2+ into the cytoplasm significantly inhibited seed germination and seedling growth and significantly reduced the activity of chitinase, CAT, POD, and SOD. Treatments with exogenous Ca2+ promoted seed germination and seedling growth and significantly increased the activities of chitinase, CAT, POD, and SOD. In the presence of F. graminearum infection, the Ca2+ required for seed germination and seedling growth mainly came from the vacuolar calcium pool, while in the absence of F. graminearum infection, the required Ca2+ mainly came from the apoplastic calcium store.
Author Contributions
Conceptualization, Y.L. and C.Z.; methodology, Y.L.; formal analysis, B.X., X.L. and X.S.; investigation, B.X., X.L., Q.G. and Y.Y.; writing—original draft preparation, B.X. and Y.L.; writing—review and editing, Y.L.; funding acquisition, Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number 31971997, and the Modern Agricultural Industry Technology System Innovation Team of Shandong Province (SDAIT-02-02).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in this article.
Acknowledgments
We thank LetPub (Shanghai, China) for its linguistic assistance during the preparation of this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tian, W.; Wang, C.; Gao, Q.; Li, L.; Luan, S. Calcium spikes, waves and oscillations in plant development and biotic interactions. Nat. Plants 2020, 6, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Wallrad, L.; Almutairi, B.O.; Kudla, J. Ca2+ signaling in plant responses to abiotic stresses. J. Integr. Plant Biol. 2022, 64, 287–300. [Google Scholar]
- Kudla, J.; Becker, D.; Grill, E.; Hedrich, R.; Hippler, M.; Kummer, U.; Parniske, M.; Romeis, T.; Schumacher, K. Advances and current challenges in calcium signaling. New Phytol. 2018, 218, 414–431. [Google Scholar] [CrossRef]
- Kong, D.; Ju, C.; Parihar, A.; Kim, S.; Cho, D.; Kwak, J.M. Arabidopsis glutamate receptor homolog3.5 modulates cytosolic Ca2+ level to counteract effect of abscisic acid in seed germination. Plant Physiol. 2015, 167, 1630–1642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fallah, S.; Malekzadeh, S.; Pessarakli, M. Seed priming improves seedling emergence and reduces oxidative stress in Nigella sativa under soil moisture stress. J. Plant Nutr. 2018, 41, 29–40. [Google Scholar] [CrossRef]
- Singh, K.L.; Mukherjee, A.; Kar, R.K. Early axis growth during seed germination is gravitropic and mediated by ROS and calcium. J. Plant Physiol. 2017, 216, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Basra, S.; Hafeez, K. Seed invigoration by osmohardening in coarse and fine rice. Seed Sci. Technol. 2006, 34, 181–187. [Google Scholar] [CrossRef]
- Guan, Y.; Li, Z.; He, F.; Huang, Y.; Song, W.; Hu, J. “On-Off” thermoresponsive coating agent containing salicylic acid applied to maize seeds for chilling tolerance. PLoS ONE 2015, 10, e0120695. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Pan, B.; Wang, Y.; Xu, W.; Zhang, S. Exogenous calcium improved resistance to Botryosphaeria dothidea by increasing autophagy activity and salicylic acid level in pear. Mol. Plant Microbe Interact. 2020, 33, 1150–1160. [Google Scholar] [CrossRef]
- Tan, W.; Meng, Q.W.; Brestic, M.; Olsovska, K.; Yang, X. Photosynthesis is improved by exogenous calcium in heat-stressed tobacco plants. J. Plant Physiol. 2011, 168, 2063–2071. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Ye, T.; Zhong, B.; Liu, X.; Chan, Z. Comparative proteomic and metabolomic analyses reveal mechanisms of improved cold stress tolerance in bermudagrass (Cynodon dactylon (L.) Pers.) by exogenous calcium. J. Integr. Plant Biol. 2014, 56, 1064–1079. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Yin, H.; Peng, S.; Khan, F.A.; Khan, F.; Sameeullah, M.; Hussain, H.A.; Huang, J.; Cui, K.; Nie, L. Comparative transcriptional profiling of primed and non-primed rice seedlings under submergence stress. Front. Plant Sci. 2016, 7, 1125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valivand, M.; Amooaghaie, R.; Ahadi, A. Seed priming with H2S and Ca2+ trigger signal memory that induces cross-adaptation against nickel stress in zucchini seedlings. Plant Physiol. Biochem. 2019, 143, 286–298. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xi, M.; Li, Y.; Cheng, Z.; Wang, S.; Kong, F. Improvement in salt tolerance of Iris pseudacorus L. in constructed wetland by exogenous application of salicylic acid and calcium chloride. J. Environ. Manag. 2021, 300, 113703. [Google Scholar] [CrossRef] [PubMed]
- Finch-Savage, W.E.; Bassel, G.W. Seed vigour and crop establishment: Extending performance beyond adaptation. J. Exp. Bot. 2016, 67, 567–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, K.; Zhang, Y.; Sun, J.; Meng, J.; Tao, J. Deterioration of orthodox seeds during ageing: Influencing factors, physiological alterations and the role of reactive oxygen species. Plant Physiol. Biochem. 2021, 158, 475–485. [Google Scholar] [CrossRef]
- Han, C.; Zhen, S.; Zhu, G.; Bian, Y.; Yan, Y. Comparative metabolome analysis of wheat embryo and endosperm reveals the dynamic changes of metabolites during seed germination. Plant Physiol. Biochem. 2017, 115, 320–327. [Google Scholar] [CrossRef]
- Sych, T.; Mély, Y.; Römer, W. Lipid self-assembly and lectin-induced reorganization of the plasma membrane. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018, 373, 20170117. [Google Scholar] [CrossRef]
- Tilden, R.L.; West, S.H. Reversal of the effects of aging in soybean seeds. Plant Physiol. 1985, 77, 584–586. [Google Scholar] [CrossRef]
- Jones, J.D.G.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [Green Version]
- Kadota, Y.; Shirasu, K.; Zipfel, C. Regulation of the NADPH oxidase RBOHD during plant immunity. Plant Cell Physiol. 2015, 56, 1472–1480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Guo, Y.; Ma, C.; Zhang, D.; Wang, C.; Yang, Q. Transcriptome analysis of maize resistance to Fusarium graminearum. BMC Genom. 2016, 17, 477. [Google Scholar]
- Nomura, H.; Komori, T.; Uemura, S.; Kanda, Y.; Shimotani, K.; Nakai, K.; Furuichi, T.; Takebayashi, K.; Sugimoto, T.; Sano, S.; et al. Chloroplast-mediated activation of plant immune signalling in Arabidopsis. Nat. Commun. 2012, 3, 926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Figueroa, M.; Hammond-Kosack, K.E.; Solomon, P.S. A review of wheat diseases-a field perspective. Mol. Plant Pathol. 2018, 19, 1523–1536. [Google Scholar] [CrossRef]
- Mehlmer, N.; Parvin, N.; Hurst, C.H.; Knight, M.R.; Teige, M.; Vothknecht, U.C. A toolset of aequorin expression vectors for in planta studies of subcellular calcium concentrations in Arabidopsis thaliana. J. Exp. Bot. 2012, 63, 1751–1761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tu, X.; Mejía-Guerra, M.K.; Valdes Franco, J.A.; Tzeng, D.; Chu, P.Y.; Shen, W.; Wei, Y.; Dai, X.; Li, P.; Buckler, E.S.; et al. Reconstructing the maize leaf regulatory network using ChIP-seq data of 104 transcription factors. Nat. Commun. 2020, 11, 5089. [Google Scholar] [CrossRef] [PubMed]
- Mittal, D.; Mishra, S.; Prajapati, R.; Vadassery, J. Forward genetic screen using transgenic calcium reporter aequorin to identify novel targets in calcium signaling. J. Vis. Exp. 2020, 162, e61259. [Google Scholar] [CrossRef]
- Wang, M.; Qu, H.; Zhang, H.; Liu, S.; Li, Y.; Zhang, C. Hormone and RNA-seq analyses reveal the mechanisms underlying differences in seed vigour at different maize ear positions. Plant Mol. Biol. 2019, 99, 461–476. [Google Scholar] [CrossRef]
- Zhu, P.; Song, X.; Mao, Y.; Li, Y.; Zhang, C. The flux rate of Ca2+ into embryo can be used to evaluate the vigour level of maize seeds. Qual. Assur. Saf. Crop. Foods 2020, 12, 81–88. [Google Scholar] [CrossRef]
- Kaneko, M.; Itoh, H.; Ueguchi-Tanaka, M.; Ashikari, M.; Matsuoka, M. The α-amylase induction in endosperm during rice seed germination is caused by gibberellin synthesized in epithelium. Plant Physiol. 2002, 128, 1264–1270. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.; Verma, G.; Sharma, S. A novel Ca2+-activated protease from germinating Vigna radiata seeds and its role in storage protein mobilization. J. Plant Physiol. 2010, 167, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Zehra, A.; Gul, B.; Ansari, R.; Khan, M.A. Role of calcium in alleviating effect of salinity on germination of Phragmites karka seeds. S. Afr. J. Bot. 2012, 78, 122–128. [Google Scholar] [CrossRef] [Green Version]
- Salahshoor, F.; Kazemi, F. Effect of calcium on reducing salt stress in seed germination and early growth stage of Festuca ovina L. Plant Soil Environ. 2016, 62, 460–466. [Google Scholar] [CrossRef] [Green Version]
- Verma, G.; Khan, S.; Agarwal, S.K.; Sharma, S. Role of apoplastic calcium during germination and initial stages of seedling establishment in Vigna radiata seeds. J. Plant Physiol. 2019, 236, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Pirayesh, N.; Giridhar, M.; Khedher, A.B.; Vothknecht, U.C.; Chigri, F. Organellar calcium signaling in plants: An update. BBA-Mol. Cell Res. 2021, 1868, 118948. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).