Antioxidant and Anti-Inflammatory Effects of Ethanol Extract from Whole Onion (Allium cepa L.) with Leaves
Abstract
1. Introduction
2. Materials and Methods
2.1. Antioxidant Effects of WOEE
2.1.1. Plant Material and Sample Preparation for Functional Analysis
2.1.2. Total Polyphenol Content
2.1.3. DPPH Radical-Scavenging Activity
2.1.4. ABTS Radical-Scavenging Activity
2.1.5. Measurement of Superoxide Dismutase Activity
2.1.6. Measurement of Catalase Activity
2.2. Anti-Inflammatory Effects of WOEE
2.2.1. Cell Viability Assay
2.2.2. Nitric Oxide Assay
2.2.3. Analysis of Cytokine Concentration in the Supernatant of the Cells Cultured with WOEE
2.2.4. Measurement of Cytokine Gene Expression Using a Real-Time PCR
2.3. Statistical Analysis
3. Results and Discussion
3.1. Antioxidant Activity of WOEE
3.1.1. Total Polyphenol Concentration
3.1.2. DPPH and ABTS Radical Scavenging Activity
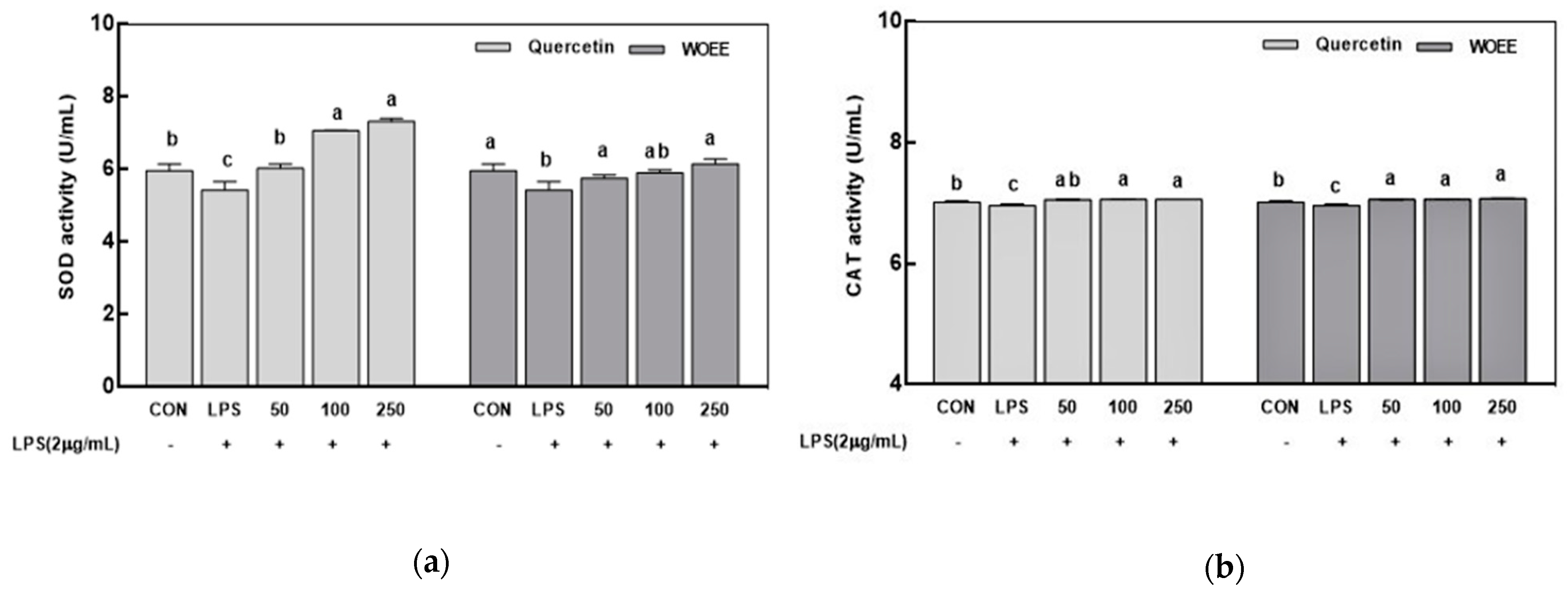
3.1.3. Superoxide Dismutase and Catalase Activities
3.2. Anti-Inflammatory Effects of WOEE
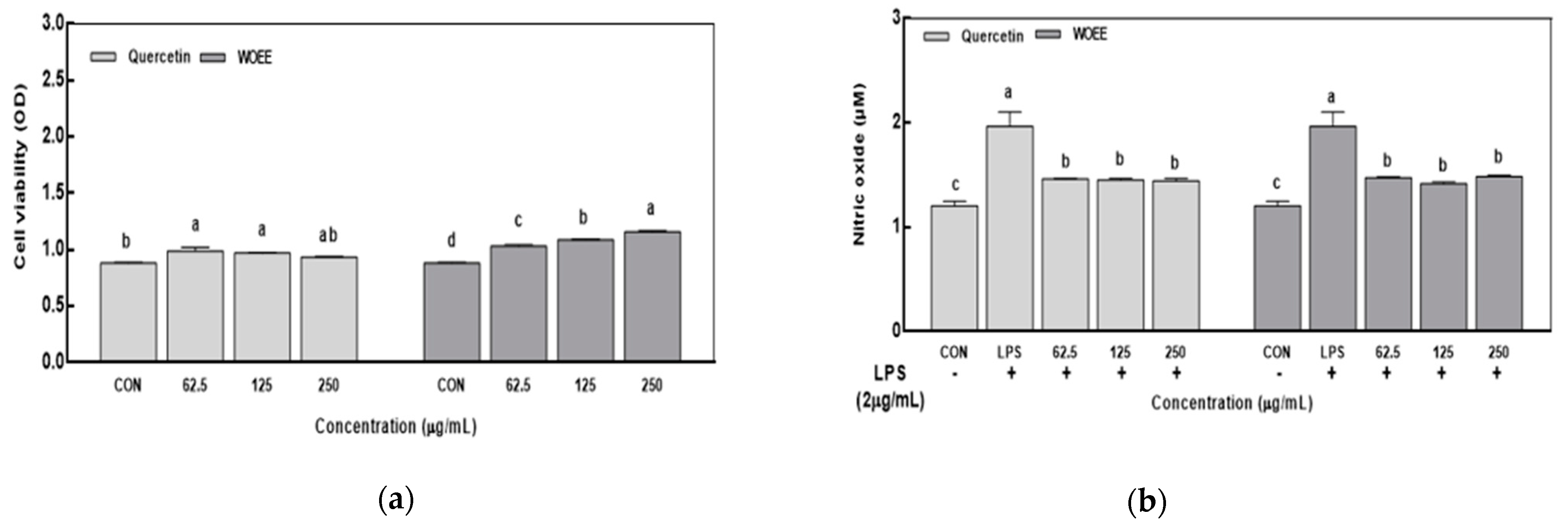
3.2.1. Cell Viability
3.2.2. Nitric Oxide Production
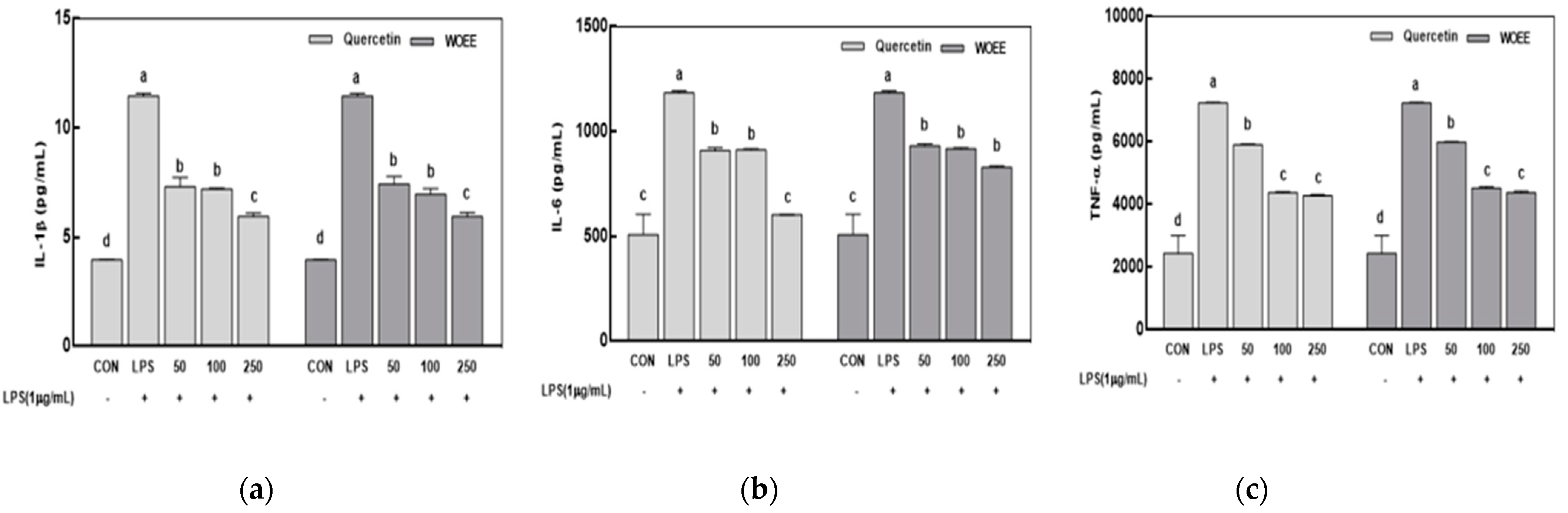
3.2.3. Inflammatory Cytokines Levels in the Supernatant of the RAW 264.7 Cells Cultured with WOEE
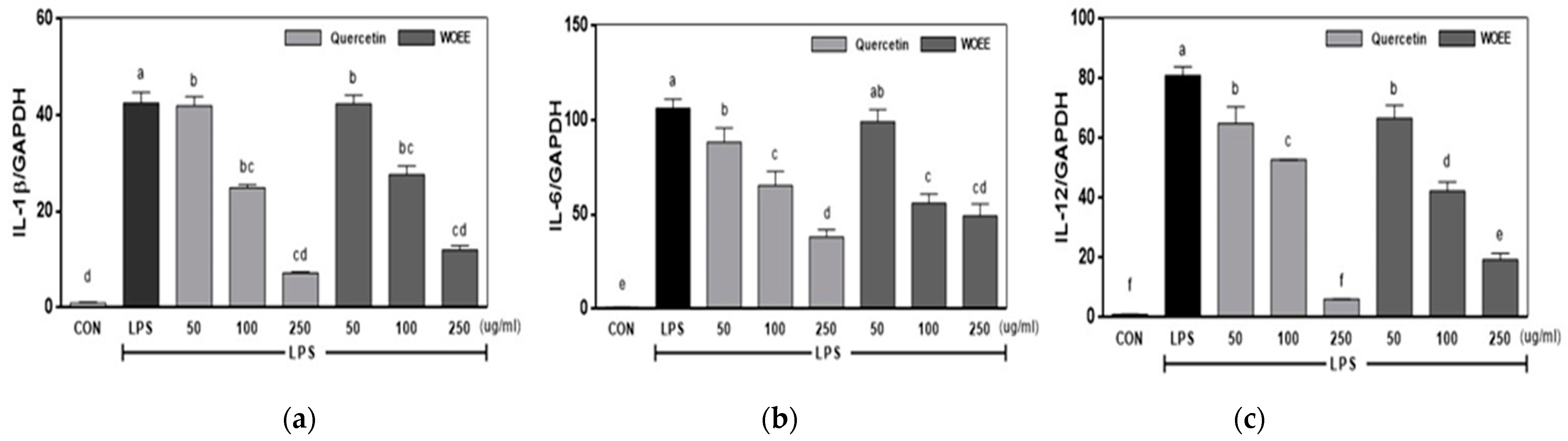
3.2.4. IL-1β, IL-6, and IL-12 mRNA Expression in the Cells Cultured with WOEE
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cho, H.Y.; Noh, K.H.; Cho, M.K.; Jang, J.H.; Lee, M.O.; Kim, S.H.; Song, Y.S. Anti-oxidative and anti-inflammatory effects of genistein in BALB/c mice injected with LPS. J. Korean. Soc. Food Sci. Nutr. 2008, 37, 1126–1135. [Google Scholar] [CrossRef][Green Version]
- Kim, J.H.; Yu, Y.B.; Choi, J.H. Toxic effects on bioaccumulation, hematological parameters, oxidative stress, immune responses and neurotoxicity in fish exposed to microplastics: A review. J. Hazard. Mater. 2021, 413, 125423. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Lee, E.B.; Jang, H.H.; Cha, Y.S.; Park, Y.S.; Lee, S.H. Allium hookeri extracts improve scopolamine-induced cognitive impairment via activation of the cholinergic system and anti-neuroinflammation in mice. Nutrients 2021, 13, 2890. [Google Scholar] [CrossRef] [PubMed]
- Ferrero-Miliani, L.; Nielsen, O.H.; Andersen, P.S.; Girardin, S. Chronic inflammation: Importance of NOD2 and NALP3 in interleukin-1β generation. Clin. Exp. Immunol. 2007, 147, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.B.; Choi, J.H.; Kim, S.H.; Jang, H.H.; Lee, S.H. Effects of Black rice (Oryza sativa L.) aleurone layer on cyclophosphamide-induced immunosuppression in mice. Korean J. Pharmacogn. 2021, 52, 170–176. [Google Scholar]
- Yang, J.; Liu, L.; Li, M.; Huang, X.; Yang, H.; Li, K. Naringenin inhibits pro-inflammatory cytokine production in macrophages through inducing MT1G to suppress the activation of NF-κB. Mol. Immunol. 2021, 137, 155–162. [Google Scholar] [CrossRef]
- Ko, Y.J.; Song, S.M.; Hyun, W.C.; Yang, S.K.; Song, C.K.; Lee, D.S.; Yoon, W.J. Anti-inflammatory effect of Castanopsis cuspidata extracts in murine macrophage RAW 264.7 cells. Korean J. Plant Res. 2014, 27, 439–446. [Google Scholar] [CrossRef]
- Jeon, S.Y.; Baek, J.H.; Jeong, E.J.; Cha, Y.J. Potential of onion peel extract as a functional ingredient for functional foods. J. Life Sci. 2012, 22, 1207–1213. [Google Scholar] [CrossRef][Green Version]
- Pérez-Gregorio, M.R.; Regueiro, J.; González-Barreiro, C.; Rial-Otero, R.; Simal-Gándara, J. Changes in antioxidant flavonoids during freeze-drying of red onions and subsequent storage. Food Control 2011, 22, 1108–1113. [Google Scholar] [CrossRef]
- Arshad, M.S.; Sohaib, M.; Nadeem, M.; Saeed, F.; Imran, A.; Javed, A.; Batool, S.M. Status and trends of nutraceuticals from onion and onion by-products: A critical review. Cogent Food Agric. 2017, 3, 1280254. [Google Scholar] [CrossRef]
- Novilla, A.; Djamhuri, D.S.; Nurhayati, B.; Rihibiha, D.D.; Afifah, E.; Widowati, W. Anti-inflammatory properties of oolong tea (Camellia sinensis) ethanol extract and epigallocatechin gallate in LPS-induced RAW 264.7 cells. Asian Pac. J. Trop. Biomed. 2017, 7, 1005–1009. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Ramachandraiah, K.; Wu, Z.; Li, S.; Eun, J.B. Impact of ball-milling time on the physical properties, bioactive compounds, and structural characteristics of onion peel powder. Food Biosci. 2020, 36, 100630. [Google Scholar] [CrossRef]
- Pannu, A.; Jalwal, P.; Singh, B. Extraction of phenolic compounds and evaluation of the antioxidant and phenolic compounds of onion (Allium cepa L.). Int. Food Res. J. 2019, 8, 4853–4859. [Google Scholar]
- Griffiths, K.; Aggarwal, B.B.; Singh, R.B.; Buttar, H.S.; Wilson, D.; De Meester, F. Food Antioxidants and Their Anti-inflammatory properties: A potential role in cardiovascular diseases and cancer prevention. Diseases 2016, 4, 28. [Google Scholar] [CrossRef]
- Folin, O.; Denis, W. On phosphotungastic-phosphomolybetic compounds as color reagents. J. Biol. Chem. 1912, 12, 239–249. [Google Scholar] [CrossRef]
- Brand, W.W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT- Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Bystrická, J.; Kavalcová, P.; Musilová, J.; Tomáš, J.; Tóth, T.; Orsák, M. Selenium and its influence on the content of polyphenol compounds in onion (Allium cepa L.). J. Microbiol. Biotechnol. Food Sci. 2015, 4, 23–26. [Google Scholar] [CrossRef]
- Lu, X.; Wang, J.; Al-Qadiri, H.M.; Ross, C.F.; Powers, J.R.; Tang, J.; Rasco, B.A. Determination of total phenolic content and antioxidant capacity of onion (Allium cepa) and shallot (Allium oschaninii) using infrared spectroscopy. Food Chem. 2011, 129, 637–644. [Google Scholar] [CrossRef]
- Prakash, D.; Singh, B.N.; Upadhyay, G. Antioxidant and free radical scavenging activities of phenols from onion (Allium cepa). Food Chem. 2007, 102, 1389–1393. [Google Scholar] [CrossRef]
- Floegel, A.; Kim, D.O.; Chung, S.J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compost. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Sarvinehbaghi, M.B.; Ahmadi, M.; Shiran, M.; Azizkhani, M. Antioxidant and antimicrobial activity of red onion (Allium cepa L.) extract nanoencapsulated in native seed gums coating and its effect on shelf-life extension of beef fillet. J. Food Meas. Charact. 2021, 15, 4771–4780. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexandria J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Bulkley, G.B. The role of oxygen free radicals in human disease processes. Surgery 1983, 94, 407–411. [Google Scholar]
- Yasui, K.; Baba, A. Therapeutic potential of superoxide dismutase (SOD) for resolution of inflammation. Inflamm. Res. 2006, 55, 359–363. [Google Scholar] [CrossRef]
- Atli, G.; Alptekin, Ö.; Tükel, S.; Canli, M. Response of catalase activity to Ag+, Cd2+, Cr6+, Cu2+ and Zn2+ in five tissues of freshwater fish Oreochromis niloticus. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2006, 143, 218–224. [Google Scholar] [CrossRef]
- Lee, H.A.; Han, J.S. Anti-inflammatory effect of Perilla frutescens (L.) Britton var. frutescens extract in LPS-stimulated RAW 264.7 macrophages. Prev. Nutr. Food Sci. 2012, 17, 109. [Google Scholar] [CrossRef]
- Pak, W.M.; Kim, B.R.; Park, J.H.; Bae, N.Y.; Ahn, D.H. Anti-inflammatory Effect of onion (Allium cepa) peel hot water extract in vitro and in vivo. KSBB J. 2015, 30, 148–154. [Google Scholar]
- Yamasaki, M.; Yamasaki, Y.; Furusho, R.; Kimura, H.; Kamei, I.; Sonoda, H.; Nishiyama, K. Onion (Allium cepa L.)-derived nanoparticles inhibited LPS-induced nitrate production, however, their intracellular incorporation by endocytosis was not involved in this effect on RAW264 cells. Molecules 2021, 26, 2763. [Google Scholar] [CrossRef]
- Ippoushi, K.; Itou, H.; Azuma, K.; Higashio, H. Effect of naturally occurring organosulfur compounds on nitric oxide production in lipopolysaccharide-activated macrophages. Life Sci. 2002, 71, 411–419. [Google Scholar] [CrossRef]
- Jakaria, M.; Azam, S.; Cho, D.Y.; Haque, M.; Kim, I.S.; Choi, D.K. The methanol extract of Allium cepa L. protects inflammatory markers in LPS-induced BV-2 microglial cells and upregulates the antiapoptotic gene and antioxidant enzymes in N27-A cells. Antioxidants 2019, 8, 348. [Google Scholar] [CrossRef] [PubMed]
- Sami, R.; Elhakem, A.; Alharbi, M.; Almatrafi, M.; Benajiba, N.; Ahmed Mohamed, T.; Helal, M. In-Vitro Evaluation of the antioxidant and anti-inflammatory activity of volatile compounds and minerals in five different onion varieties. Separations 2021, 8, 57. [Google Scholar] [CrossRef]
- Block, M.L.; Zecca, L.; Hong, J.S. Microglia-mediated neurotoxicity: Uncovering the molecular mechanisms. Nat. Rev. Neurol. 2007, 8, 57–69. [Google Scholar] [CrossRef]
- Ren, K.; Torres, R. Role of interleukin-1β during pain and inflammation. Brain Res. 2009, 60, 57–64. [Google Scholar] [CrossRef]
- Zhou, Y.Q.; Liu, Z.; Liu, Z.H.; Chen, S.P.; Li, M.; Shahveranov, A.; Tian, Y.K. Interleukin-6: An emerging regulator of pathological pain. J. Neuroinflamm. 2016, 13, 141. [Google Scholar] [CrossRef]
- Zhou, X.L.; Wang, Y.; Zhang, C.J.; Yu, L.N.; Cao, J.L.; Yan, M. COX-2 is required for the modulation of spinal nociceptive information related to ephrinB/EphB signalling. Eur. J. Pain 2015, 19, 1277–1287. [Google Scholar] [CrossRef]
- Endale, M.; Park, S.C.; Kim, S.; Kim, S.H.; Yang, Y.; Cho, J.Y.; Rhee, M.H. Quercetin disrupts tyrosine-phosphorylated phosphatidylinositol 3-kinase and myeloid differentiation factor-88 association, and inhibits MAPK/AP-1 and IKK/NF-κB-induced inflammatory mediators production in RAW 264.7 cells. Immunobiology 2013, 218, 1452–1467. [Google Scholar] [CrossRef]
- Lee, S.; Han, H.; Yoo, J.; Nam, M.S.; Kim, K. Anti-oxidant and anti-inflammatory effect of Allium Hookeri water extracts in RAW 264.7 cells. Korea J. Herbol. 2020, 35, 37–43. [Google Scholar]
- Gregor, M.F.; Hotamisligil, G.S. Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 2011, 29, 415–445. [Google Scholar] [CrossRef]
- Seo, M.Y.; Kim, K.R.; Lee, J.J.; Ryu, G.; Lee, S.H.; Hong, S.D.; Kim, H.Y. Therapeutic effect of topical administration of red onion extract in a murine model of allergic rhinitis. Sci. Rep. 2019, 9, 2883. [Google Scholar] [CrossRef] [PubMed]
- Popa, C.; Netea, M.G.; Van Riel, P.L.; Van Der Meer, J.W.; Stalenhoef, A.F. The role of TNF-α in chronic inflammatory conditions, intermediary metabolism, and cardiovascular risk. J. Lipid Res. 2007, 48, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Gabler, N.K.; Ostrowska, E.; Sterling, S.J.; Tatham, B.G.; Jones, R.B.; Eagling, D.R.; Jois, M.; Dunshea, F.R. Consumption of two raw brown onion varieties reduce the risk factors of cardiovascular disease. J. Sci. Food Agric. 2005, 85, 154–160. [Google Scholar] [CrossRef]
- Ostrowska, E.; Gabler, N.K.; Sterling, S.J.; Tatham, B.G.; Jones, R.B.; Eagling, D.R.; Jois, M.; Dunshea, F.R. Consumption of onions influences lipid blood chemistry of healthy pigs with responses varying with dose and onion type. Br. J. Nutr. 2004, 91, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Gabler, N.K.; Ostrowska, E.; Imsic, M.; Eagling, D.R.; Jois, M.; Tatham, B.G.; Dunshea, F.R. Dietary onion intake as part of a typical high fat diet improves indices of cardiovascular health using the mixed sex pig model. Plant Foods Hum. Nutr. 2006, 61, 179–185. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-H.; Kim, J.-S.; Kim, S.-H.; Jeong, S.-H.; Jeong, U.-Y.; Jung, J.-E.; Lee, S.-K.; Lee, S.-H. Antioxidant and Anti-Inflammatory Effects of Ethanol Extract from Whole Onion (Allium cepa L.) with Leaves. Agriculture 2022, 12, 963. https://doi.org/10.3390/agriculture12070963
Kim J-H, Kim J-S, Kim S-H, Jeong S-H, Jeong U-Y, Jung J-E, Lee S-K, Lee S-H. Antioxidant and Anti-Inflammatory Effects of Ethanol Extract from Whole Onion (Allium cepa L.) with Leaves. Agriculture. 2022; 12(7):963. https://doi.org/10.3390/agriculture12070963
Chicago/Turabian StyleKim, Ju-Hui, Ji-Su Kim, Si-Hyun Kim, Su-Hyeon Jeong, Un-Yul Jeong, Ji-Eun Jung, Sun-Kyung Lee, and Sung-Hyen Lee. 2022. "Antioxidant and Anti-Inflammatory Effects of Ethanol Extract from Whole Onion (Allium cepa L.) with Leaves" Agriculture 12, no. 7: 963. https://doi.org/10.3390/agriculture12070963
APA StyleKim, J.-H., Kim, J.-S., Kim, S.-H., Jeong, S.-H., Jeong, U.-Y., Jung, J.-E., Lee, S.-K., & Lee, S.-H. (2022). Antioxidant and Anti-Inflammatory Effects of Ethanol Extract from Whole Onion (Allium cepa L.) with Leaves. Agriculture, 12(7), 963. https://doi.org/10.3390/agriculture12070963





