Sublethal Effects of Emamectin Benzoate on Fall Armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects and Test Pesticides
2.2. Toxicity of EMB to FAW Larvae
2.3. Sublethal Effects of EMB on the FAW Parental Generation
2.4. Sublethal Effects of EMB on FAW Offspring
2.5. Statistical Analysis
3. Results
3.1. Lethal Effects of EMB on FAW Larvae
3.2. Sublethal Effects of EMB on FAW Parental Generation
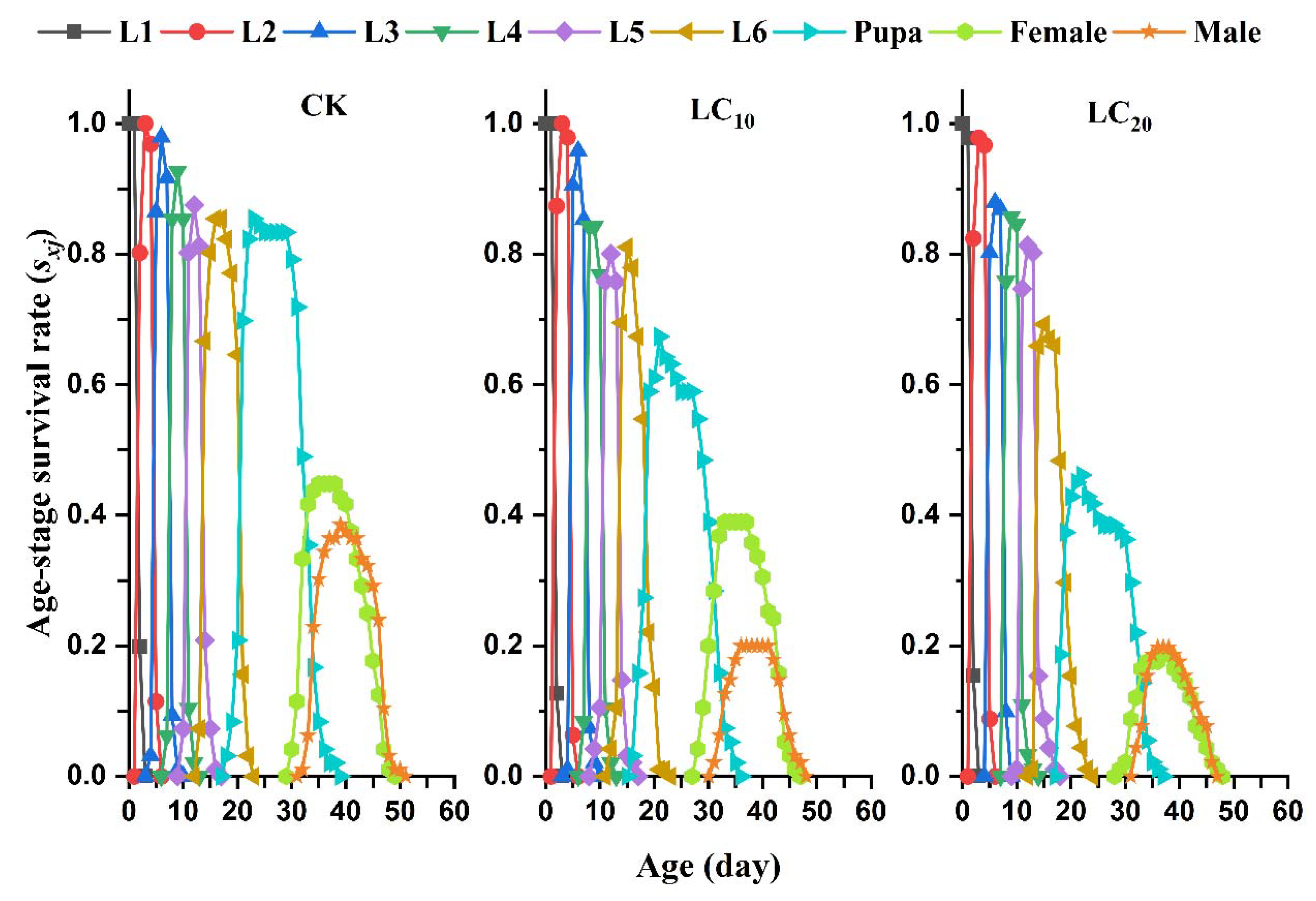
3.3. Sublethal Effects of EMB on the Mortality and Development Rate of the FAW Offspring Generation
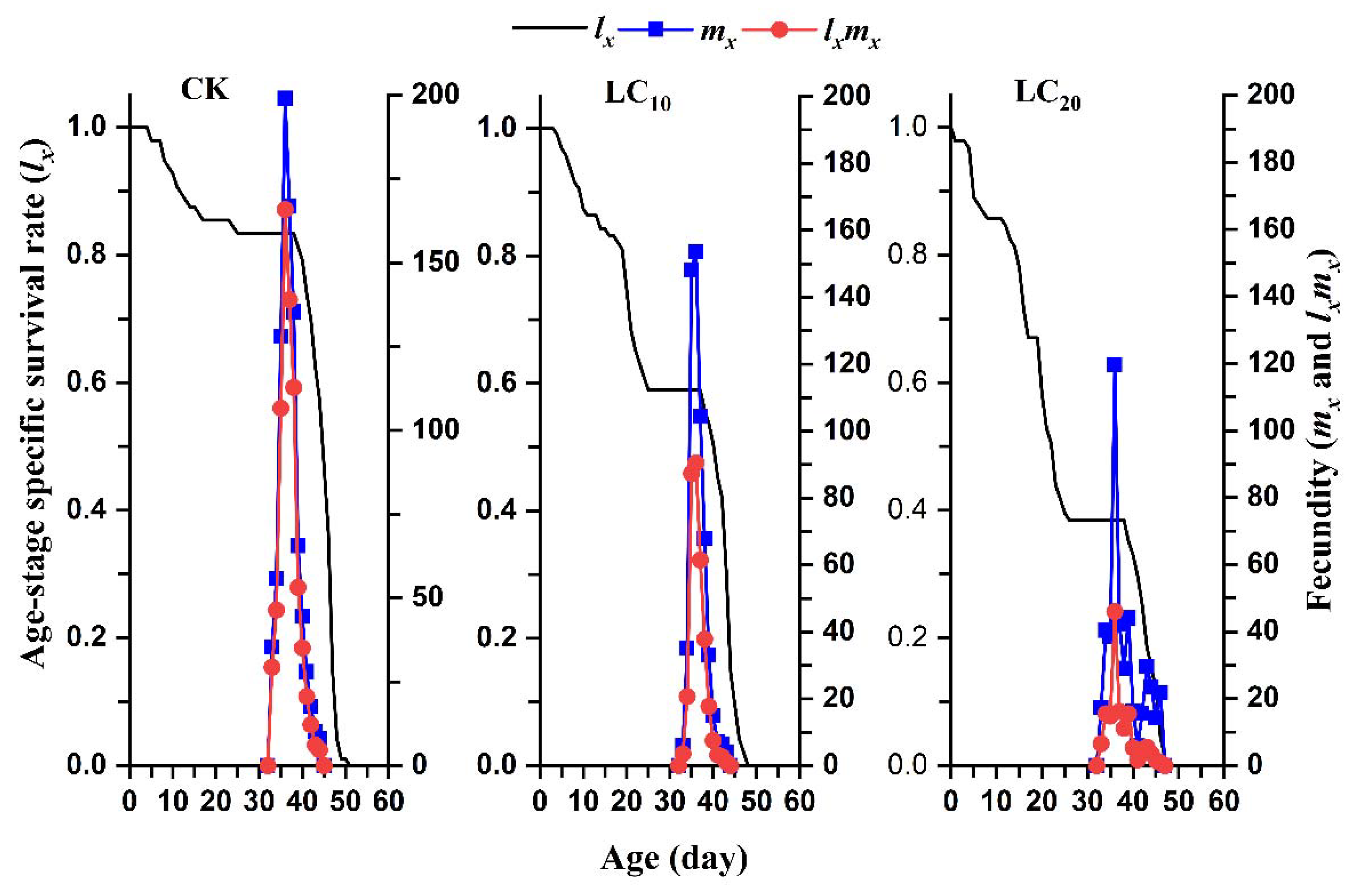
3.4. Sublethal Effects of EMB on the Reproduction Parameters of the FAW Offspring Generation
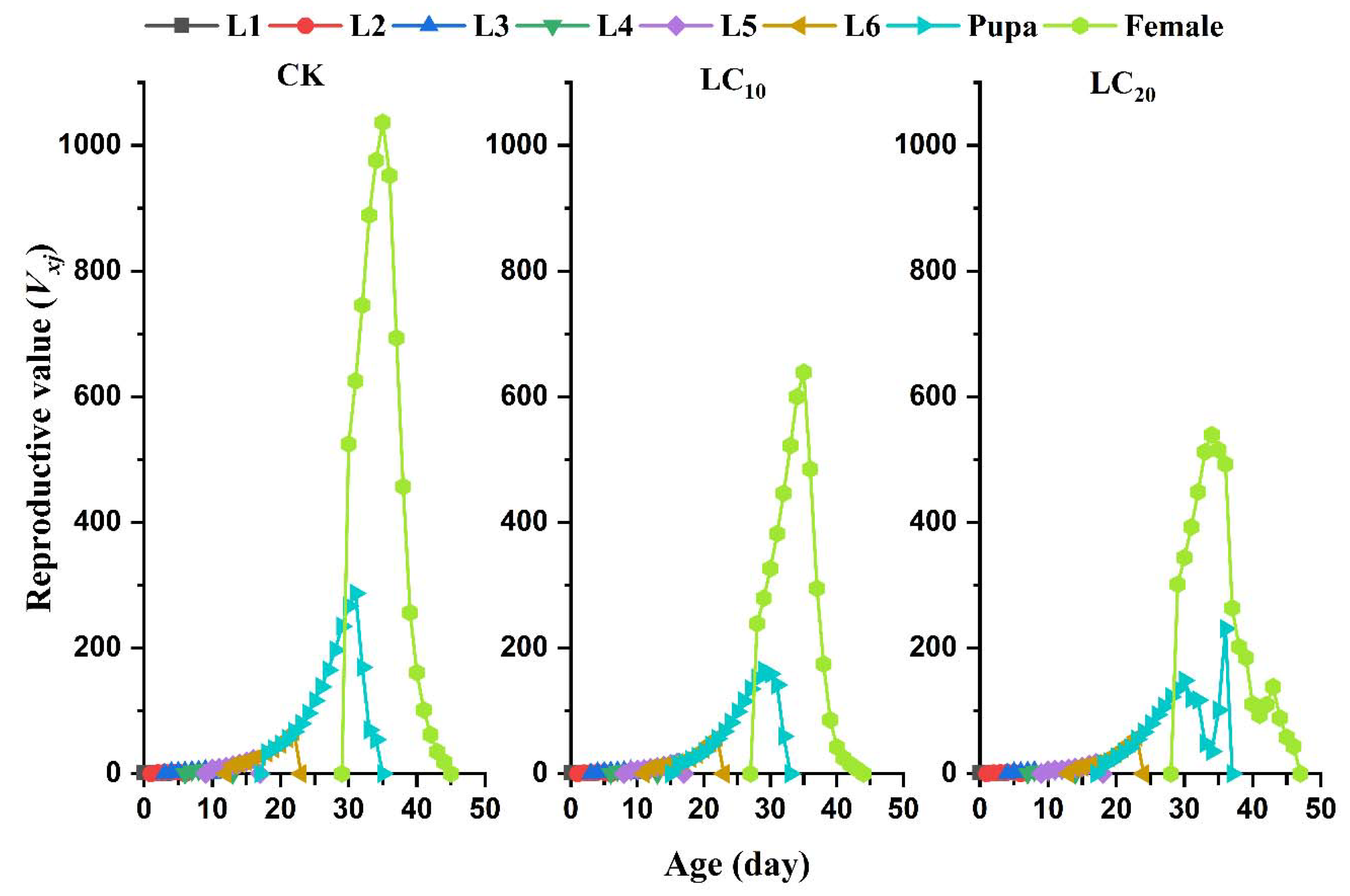
3.5. Sublethal Effects of EMB on the Population Parameters of the FAW Offspring Generation
4. Discussion
4.1. Effect of EMB on the Performance of FAW Parental Generation
4.2. Sublethal Effects of EMB on the Performance of the FAW Offspring Generation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wan, J.; Huang, C.; Li, C.; Zhou, H.; Ren, Y.; Li, Z.; Xing, L.; Zhang, B.; Qiao, X.; Liu, B.; et al. Biology, invasion and management of the agricultural invader: Fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae). J. Integr. Agric. 2021, 20, 646–663. [Google Scholar] [CrossRef]
- Sun, X.; Hu, C.; Jia, H.; Wu, Q.; Shen, X.; Zhao, S.; Jiang, Y.; Wu, K. Case study on the first immigration of fall armyworm Spodoptera frugiperda invading into China. J. Integr. Agric. 2021, 20, 664–672. [Google Scholar] [CrossRef]
- Guimapi, R.A.; Niassy, S.; Mudereri, B.T.; Abdel-Rahman, E.M.; Tepa-Yotto, G.T.; Subramanian, S.; Mohamed, S.A.; Thunes, K.H.; Kimathi, E.; Agboka, K.M.; et al. Harnessing data science to improve integrated management of invasive pest species across Africa: An application to Fall armyworm (Spodoptera frugiperda) (J.E. Smith) (Lepidoptera: Noctuidae). Glob. Ecol. Conserv. 2022, 35, e02056. [Google Scholar] [CrossRef]
- De Groote, H.; Kimenju, S.C.; Munyua, B.; Palmas, S.; Kassie, M.; Bruce, A. Spread and impact of fall armyworm (Spodoptera frugiperda J.E. Smith) in maize production areas of Kenya. Agric. Ecosyst. Environ. 2020, 292, 106804. [Google Scholar] [CrossRef] [PubMed]
- Paredes-Sánchez, F.A.; Rivera, G.; Bocanegra-García, V.; Martínez-Padrón, H.Y.; Berrones-Morales, M.; Niño-García, N.; Herrera-Mayorga, V. Advances in control strategies against Spodoptera frugiperda. A review. Molecules 2021, 26, 5587. [Google Scholar] [CrossRef]
- Li, Q.; Men, X.; Jing, C.; Yu, Y.; Zhou, X.; Dai, X.; Lu, S.; Li, L. Research progress in emergency prevention and control of Spodoptera frugiperda in China. Plant Prot. 2021, 47, 21–27. [Google Scholar]
- Zhang, L.; Liu, B.; Zheng, W.; Liu, C.; Zhang, D.; Zhao, S.; Li, Z.; Xu, P.; Wilson, K.; Withers, A.; et al. Genetic structure and insecticide resistance characteristics of fall armyworm populations invading China. Mol. Ecol. Resour. 2020, 20, 1682–1696. [Google Scholar] [CrossRef] [PubMed]
- Stavrakaki, M.; Ilias, A.; Ioannidis, P.; Vontas, J.; Roditakis, E. Investigating mechanisms associated with emamectin benzoate resistance in the tomato borer Tuta Absol. J. Pest Sci. 2022, 95, 1163–1177. [Google Scholar] [CrossRef]
- Jansson, R.K.; Brown, R.; Cartwright, B.; Cox, D.; Dunbar, D.M.; Dybas, R.A. Emamectin benzoate: A novel avermectin derivative for control of lepidopterous pests. In Proceedings of the 3rd International Workshop on Management of Diamondback Moth and Other Crucifer Pests, Kuala Lumpur, Malaysia, 29 October–1 November 1996; Vegetable Pest Management. Malaysian Agricultural Research and Development Institute: Selangor, Malaysia, 1997; pp. 1–7. [Google Scholar]
- Raja, R.A.; Patil, P.K.; Avunje, S.; Kumaran, M.; Solanki, H.G.; Jithendran, K.P.; Alavandi, S.V.; Vijayan, K.K. Efficacy of emamectin benzoate in controlling natural infestations of ectoparasites in economically important fish species of India. Aquaculture 2022, 551, 737940. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, L.; Yang, C.; Zong, M.; Huang, Q.; Tao, L. Detection on emamectin benzoate-induced apoptosis and DNA damage in Spodoptera frugiperda Sf-9 cell line. Pestic. Biochem. Physiol. 2016, 126, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Jansson, R.K.; Dybas, R.A. Avermectins: Biochemical mode of action, biological activity and agricultural importance. In Insecticides with Novel Modes of Action; Springer: Berlin/Heidelberg, Germany, 1998; pp. 152–170. [Google Scholar]
- Lu, F.; Guo, K.; Chen, A.; Chen, S.; Lin, H.; Zhou, X. Transcriptomic profiling of effects of emamectin benzoate on the pine wood nematode Bursaphelenchus xylophilus. Pest Manag. Sci. 2020, 76, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Thumar, R.K.; Zala, M.B.; Varma, H.S.; Dhobi, C.B.; Patel, B.N.; Patel, M.B.; Borad, P. Evaluation of insecticides against fall armyworm, Spodoptera frugiperda (J.E. Smith) infesting. Int. J. Chem. Stud. 2020, SP-8, 100–104. [Google Scholar]
- Sangle, S.V.; Jayewar, N.E.; Kadam, D.R. Efficacy of insecticides on larval population of fall armyworm, Spodoptera frugiperda on maize. J. Entomol. Zool. Stud. 2020, 8, 1831–1834. [Google Scholar]
- Khan, M.M.; Nawaz, M.; Hua, H.; Cai, W.; Zhao, J. Lethal and sublethal effects of emamectin benzoate on the rove beetle, Paederus fuscipes, a non-target predator of rice brown planthopper, Nilaparvata lugens. Ecotoxicol. Environ. Saf. 2018, 165, 19–24. [Google Scholar] [CrossRef]
- Shan, Y.; Zhu, Y.; Li, J.; Wang, N.; Yu, Q.; Xue, C. Acute lethal and sublethal effects of four insecticides on the lacewing (Chrysoperla sinica Tjeder ). Chemosphere 2020, 250, 126321. [Google Scholar] [CrossRef] [PubMed]
- Desneux, N.; Decourtye, A.; Delpuech, J.-M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef] [PubMed]
- de França, S.M.; Breda, M.O.; Barbosa, D.R.S.; Araujo, A.M.N.; Guedes, C.A. The sublethal effects of insecticides in insects. In Biological Control of Pest and Vector Insects; Shields, V.D.C., Ed.; BoD–Books on Demand: London, UK, 2017; pp. 23–39. [Google Scholar]
- Liu, X.; Fu, Z.; Zhu, Y.; Gao, X.; Liu, T.X.; Liang, P. Sublethal and transgenerational effects of afidopyropen on biological traits of the green peach aphid Myzus persicae (Sluzer). Pestic. Biochem. Physiol. 2022, 180, 104981. [Google Scholar] [CrossRef]
- Wang, H.; Xin, T.; Wang, J.; Zou, Z.; Zhong, L.; Xia, B. Sublethal effects of bifenazate on biological traits and enzymatic properties in the Panonychus citri (Acari: Tetranychidae). Sci. Rep. 2021, 11, 20934. [Google Scholar] [CrossRef] [PubMed]
- Mokbel, E.; Huesien, A. Sublethal effects of emamectin benzoate on life table parameters of the cotton leafworm, Spodoptera littoralis (Boisd). Bull. Natl. Res. Cent. 2020, 44, 155. [Google Scholar] [CrossRef]
- Ziaee, M.; Sohrabi, F. Lethal and sublethal effects of emamectin benzoate on the tomato leafminer Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). J. Appl. Res. Plant Prot. 2022, 10, 91–97. [Google Scholar]
- Xu, Z.; Bai, J.; Li, L.; Liang, L.; Ma, X.; Ma, L. Sublethal concentration of emamectin benzoate inhibits the growth of gypsy moth by inducing digestive dysfunction and nutrient metabolism disorder. Pest Manag. Sci. 2021, 77, 4073–4083. [Google Scholar] [CrossRef] [PubMed]
- Kandil, M.A.; Fouad, E.A.; El Hefny, D.E.; Abdel-Mobdy, Y.E. Toxicity of fipronil and emamectin benzoate and their mixtures against cotton leafworm, Spodoptera littoralis (Lepidoptera: Noctuidae) with relation to GABA content. J. Econ. Entomol. 2020, 113, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Chen, D.-F.; Yang, M.-F.; Liu, J.-F. The effect of temperatures and hosts on the life cycle of Spodoptera frugiperda (Lepidoptera: Noctuidae). Insects 2022, 13, 211. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-Y.; Su, L.; Li, S.; Li, Y.-P.; Xu, X.-L.; Cheng, W.-N.; Wang, Y.; Wu, J.-X. Insecticide resistance of the field populations of oriental armyworm, Mythimna separata (Walker) in Shaanxi and Shanxi provinces of China. J. Integr. Agric. 2018, 17, 1556–1562. [Google Scholar] [CrossRef]
- Dong, Q.; Zhou, J.; Zhu, K.; Zhang, Z.; Dong, H. A simple method for identifying sexuality of Spodoptera frugiperda (J.E. Smith) pupae and adults. Plant Prot. 2019, 45, 96–98. [Google Scholar]
- Chi, H. Life-table analysis incorporating both sexes and variable development rates among individuals. Environ. Entomol. 1988, 17, 26–34. [Google Scholar] [CrossRef]
- Chi, H.; Liu, H. Two new methods for the study of insect population ecology. Bull. Inst. Zool. Acad. Sin 1985, 24, 225–240. [Google Scholar]
- Chi, H. TWOSEX-MSChart: A Computer Program for the Age-Stage, Two-Sex Life Table Analysis; National Chung Hsing University: Taichung, Taiwan; Available online: http://140.120.197.173/Ecology/prod02.htm (accessed on 9 April 2021).
- Kumar, A.; Kanwar, R.; Mehta, S.K. Eucalyptus oil-based nanoemulsion: A potent green nanowagon for controlled delivery of emamectin benzoate. ACS Agric. Sci. Technol. 2021, 1, 76–88. [Google Scholar] [CrossRef]
- Cvetovich, R.J.; Kelly, D.H.; DiMichele, L.M.; Shuman, R.F.; Grabowski, E.J.J. Syntheses of 4’’-epi-Amino-4’’-deoxyavermectins B1. J. Org. Chem. 1994, 59, 7704–7708. [Google Scholar] [CrossRef]
- Van Le, T.; Nguyen, H.T. Dissipation dynamics and half-lives of cypermethrin, emamectin benzoate, and indoxacarb insecticides in different parts of amaranth (Amaranthus tricolor L.) and mustard greens (Brassica juncea). Trop. Agric. 2021, 98, 57–68. [Google Scholar]
- Khan, M.M.; Ali, M.W.; Hafeez, M.; Fan, Z.Y.; Ali, S.; Qiu, B.L. Lethal and sublethal effects of emamectin benzoate on life-table and physiological parameters of citrus red mite, Panonychus citri. Exp. Appl. Acarol. 2021, 85, 173–190. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Chen, Y.; He, K.; Guo, J.; Wang, Z. Sublethal effects of the microbial-derived insecticide spinetoram on the growth and fecundity of the fall armyworm ( Lepidoptera: Noctuidae). J. Econ. Entomol. 2021, 114, 1582–1587. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Rui, C.; Ren, L.; Tan, X. Effect of sublethal dose of emamectin benzoate on growth and development of Helicoverpa armigera (Hübner). Acta Phytophylacica Sin. 2011, 38, 539–544. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, C.; Jin, D.C.; Gong, M.F.; Wang, Z.; Long, G. Sublethal effects of abamectin on the development, fecundity, and wing morphs of the brown planthopper Nilaparvata lugens. J. Asia. Pac. Entomol. 2019, 22, 1180–1186. [Google Scholar] [CrossRef]
- Hamedi, N.; Fathipour, Y.; Saber, M. Sublethal effects of abamectin on the biological performance of the predatory mite, Phytoseius plumifer (Acari: Phytoseiidae). Exp. Appl. Acarol. 2011, 53, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Pakyari, H.; Enkegaard, A. Sublethal and transgenerational effects of abamectin on the biological performance of the predatory thrips Scolothrips longicornis (Thysanoptera: Thripidae). J. Econ. Entomol. 2015, 108, 559–565. [Google Scholar] [CrossRef]
- Zibaee, I.; Esmaeily, M. Effect of sublethal doses of abamectin on demographic traits of tomato leafminer Tuta absoluta (Meyrick, 1917) (Lepidoptera: Gelechiidae). J. Plant Prot. Res. 2017, 57, 256–267. [Google Scholar] [CrossRef][Green Version]
- Hedayati, M.; Sadeghi, A.; Maroufpoor, M.; Ghobari, H.; Güncan, A. Transgenerational sublethal effects of abamectin and pyridaben on demographic traits of Phytonemus pallidus (Banks) (Acari: Tarsonemidae). Ecotoxicology 2019, 28, 467–477. [Google Scholar] [CrossRef]
- Parsaeyan, E.; Saber, M.; Bagheri, M. Toxicity of emamectin benzoate and cypermethrin on biological parameters of cotton bollworm, Helicoverpa armigera (Hübner) in laboratory conditions. J. Crop Prot. 2013, 2, 477–485. [Google Scholar]
- Khani, A.; Ahmadi, F.; Ghadamyari, M. Side effects of imidacloprid and abamectin on the mealybug destroyer, Cryptolaemus montrouzieri. Trakia J. Sci. 2012, 10, 30–35. [Google Scholar]
- Yao, Q.; Xu, S.; Dong, Y.; Que, Y.; Quan, L.; Chen, B. Characterization of vitellogenin and vitellogenin receptor of conopomorpha sinensisbradley and their responses to sublethal concentrations of insecticide. Front. Physiol. 2018, 9, 1250. [Google Scholar] [CrossRef] [PubMed]
- Wise, L.D.; Allen, H.L.; Hoe, C.L.; Verbeke, D.R.; Gerson, R.J. Developmental neurotoxicity evaluation of the avermectin pesticide, emamectin benzoate, in sprague–dawley rats. Neurotoxicol. Teratol. 1997, 19, 315–326. [Google Scholar] [CrossRef]
- Lu, J.; Wang, W.; Xu, W.; Zhang, C.; Zhang, C.; Tao, L.; Li, Z.; Zhang, Y. Induction of developmental toxicity and cardiotoxicity in zebra fish embryos by emamectin benzoate through oxidative stress. Sci. Total Environ. 2022, 825, 154040. [Google Scholar] [CrossRef]
- Lin, R.; He, D.; Men, X.; Zheng, L.; Cheng, S.; Tao, L.; Yu, C. Sublethal and transgenerational effects of acetamiprid and imidacloprid on the predatory bug Orius sauteri (Poppius) (Hemiptera: Anthocoridae). Chemosphere 2020, 255, 126778. [Google Scholar] [CrossRef] [PubMed]
- Saber, M.; Ahmadi, Z.; Mahdavinia, G. Sublethal effects of spirodiclofen, abamectin and pyridaben on life-history traits and life-table parameters of two-spotted spider mite, Tetranychus urticae (Acari: Tetranychidae). Exp. Appl. Acarol. 2018, 75, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Taleh, M.; Dastjerdi, H.R.; Naseri, B.; Ebadollahi, A.; Garjan, A.S.; Jahromi, K.T. Toxicity and biochemical effects of emamectin benzoate against Tuta absoluta (Meyrick) alone and in combination with some conventional insecticides. Physiol. Entomol. 2021, 46, 210–217. [Google Scholar] [CrossRef]
- Abo-El-Mahasen, M.M. Histological effects of emamectin benzoate on larvae of the cotton leaf worm, Spodoptera littoralis (Boisd.) (Lepidoptera: Noctuidae). Egypt. J. Biol. Pest Control 2016, 26, 147–152. [Google Scholar]

| Concentration | Number of Test Insects | (%) Mortality ± SE | Corrected Mortality (%) |
|---|---|---|---|
| 0.5000 0.2500 0.1250 0.0625 0.0313 0.0156 0.0070 Control | 72 72 72 72 72 72 72 72 | 100 ± 0 a 93.06 ± 1.39 b 59.72 ± 3.68 c 37.5 ± 2.41 d 18.05 ± 2.78 e 15.28 ± 1.39 e 11.11 ± 1.39 e 8.33 ± 2.40 e | 91.74 ± 2.38 a 84.46 ± 3.91 b 51.43 ± 5.55 c 29.19 ± 4.81 d 9.73 ± 3.67 e 6.95 ± 2.78 e 2.78 ± 3.68 e / |
| Model | LC10 (mg/L) (95% CL) | LC20 (mg/L) (95% CL) | LC50 (mg/L) (95% CL) | r | χ2 | p |
|---|---|---|---|---|---|---|
| Y = 0.1266 + 1.8963x | 0.0127 (−0.0061–0.0276) | 0.0589 (0.0455–0.0714) | 0.1062 (0.0932–0.1214) | 0.9129 | 2.1852 | 0.995 |
| Parameters | Control | LC10 | LC20 |
|---|---|---|---|
| Pupation rate (%) | 74.67 ± 3.89 a | 77.33 ± 3.40 a | 64.00 ± 4.52 a |
| Pupal period (female) | 12.81 ± 0.13 a | 12.54 ± 0.19 a | 12.76 ± 0.16 a |
| Pupal period (male) | 13.75 ± 0.29 b | 14.37 ± 0.19 ab | 14.77 ± 0.19 a |
| Pupal weight (mg, female) | 2015.70 ± 52.10 a | 2003.47 ± 59.83 a | 2043.03 ± 50.48 a |
| Pupal weight (mg, male) | 2017.13 ± 71.83 b | 2169.11 ± 53.92 a | 2210.57 ± 40.49 a |
| Eclosion rate (%) | 78.67 ± 3.89 a | 69.33 ± 3.40 ab | 62.67 ± 3.40 b |
| Pre-oviposition period (day) | 3.45 ± 0.27 a | 4.00 ± 0.28 a | 3.60 ± 0.20 a |
| Oviposition period (day) | 5.55 ± 0.37 b | 6.00 ± 0.55 b | 8.50 ± 0.47 a |
| Fecundity (day) | 1161.90 ± 41.11 a | 1049.35 ± 71.26 a | 1079.70 ± 60.42 a |
| Longevity (d, female) | 9.90 ± 0.34 b | 10.00 ± 0.62 b | 13.00 ± 0.58 a |
| Longevity (d, male) | 8.75 ± 0.26 b | 9.50 ± 0.57 b | 11.55 ± 0.49 a |
| Hatching rate (%) | 95.70 ± 0.98 a | 82.00 ± 1.45 b | 79.60 ± 2.44 b |
| Immature Stages | Control | LC10 | LC20 |
|---|---|---|---|
| L1 (%) | 0 a | 0 a | 2.20 ± 1.53 a |
| L2 (%) | 2.08 ± 1.45 b | 4.21 ± 2.06 ab | 9.89 ± 3.10 a |
| L3 (%) | 4.17 ± 2.04 a | 5.26 ± 2.29 a | 2.20 ± 1.53 a |
| L4 (%) | 4.17 ± 2.04 a | 4.21 ± 2.06 a | 0 b |
| L5 (%) | 2.08 ± 1.46 a | 2.11 ± 1.48 a | 4.40 ± 2.16 a |
| L6 (%) | 2.08 ± 1.46 b | 2.11 ± 1.48 b | 15.38 ± 3.79 a |
| Pupa (%) | 2.08 ±1.46 b | 23.16 ± 4.31 a | 27.47 ± 4.67 a |
| Pre-adult (%) | 16.67 ± 3.80 c | 41.05 ± 5.03 b | 61.54 ± 5.08 a |
| Control | n | LC10 | n | LC20 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Female | Male | n | Female | Male | n | Female | n | Male | |||
| L1 (day) | 43 | 2.12 ± 0.05 a A | 37 | 2.14 ± 0.06 a A | 37 | 2.05 ± 0.04 a A | 19 | 2.05 ± 0.05 a A | 17 | 2.06 ± 0.06 a A | 18 | 2.11 ± 0.08 a A |
| L2 (day) | 43 | 2.86 ± 0.05 b A | 37 | 2.97 ± 0.03 a A | 37 | 2.95 ± 0.04 ab A | 19 | 2.95 ± 0.05 a A | 17 | 3.00 ± 0.00 a A | 18 | 3.00 ± 0.00 a A |
| L3 (day) | 43 | 2.95 ± 0.03 ab A | 37 | 2.97 ± 0.03 a A | 37 | 2.86 ± 0.06 b B | 19 | 3.05 ± 0.05 a A | 17 | 3.12 ± 0.08 a A | 18 | 3.00 ± 0.00 a A |
| L4 (day) | 43 | 2.95 ± 0.03 a B | 37 | 3.14 ± 0.06 a A | 37 | 2.92 ± 0.05 a A | 19 | 3.05 ± 0.05 ab A | 17 | 3.06 ± 0.06 a A | 18 | 3.00 ± 0.00 b A |
| L5 (day) | 43 | 3.14 ± 0.05 a A | 37 | 3.22 ± 0.07 a A | 37 | 3.00 ± 0.00 a B | 19 | 3.21 ± 0.10 a A | 17 | 3.24 ± 0.14 a A | 18 | 3.17 ± 0.12 a A |
| L6 (day) | 43 | 6.47 ± 0.10 a B | 37 | 6.78 ± 0.07 a A | 37 | 4.54 ± 0.16 c B | 19 | 5.11 ± 0.19 b A | 17 | 5.41 ± 0.24 b A | 18 | 4.89 ± 0.21 b A |
| Larva (day) | 43 | 20.49 ± 0.16 a B | 37 | 21.22 ± 0.13 a A | 37 | 18.32 ± 0.21 b B | 19 | 19.42 ± 0.28 b A | 17 | 19.88 ± 0.42 a A | 18 | 19.17 ± 0.35 b A |
| Pupa (day) | 43 | 11.51 ± 0.11 b B | 37 | 13.43 ± 0.17 c A | 37 | 12.11 ± 0.13 a B | 19 | 13.89 ± 0.15 b A | 17 | 12.12 ± 0.24 a B | 18 | 14.50 ± 0.22 a A |
| Pre-adult (day) | 43 | 32.00 ± 0.16 a B | 37 | 34.65 ± 0.25 a A | 37 | 30.43 ± 0.23 b B | 19 | 33.32 ± 0.35 b A | 17 | 32.00 ± 0.44 a B | 18 | 33.67 ± 0.28 b A |
| Adult longevity (day) | 43 | 12.47 ± 0.40 a A | 37 | 11.70 ± 0.32 a A | 37 | 12.05 ± 0.40 a A | 19 | 11.37 ± 0.31 ab A | 17 | 11.29 ± 0.55 a A | 18 | 10.11 ± 0.57 b A |
| n | Control | n | LC10 | n | LC20 | |
|---|---|---|---|---|---|---|
| Total pre-oviposition (TPOP)/d | 35 | 35.37 ± 0.22 a | 27 | 35.19 ± 0.22 a | 8 | 35.75 ± 0.98 a |
| Pre-oviposition (APOP)/d | 35 | 3.63 ± 0.16 b | 27 | 4.67 ± 0.24 a | 8 | 3.75 ± 0.37 b |
| Oviposition period/d | 35 | 6.14 ± 0.24 a | 27 | 3.96 ± 0.25 c | 8 | 5.25 ± 0.37 b |
| Fecundity (egg/female) | 35 | 2008.74 ± 78.77 a | 27 | 1174.26 ± 75.11 b | 8 | 1689.25 ± 157.74 a |
| rm (day−1) | 35 | 0.1758 ± 0.0040 a | 27 | 0.1563 ± 0.0049 a | 8 | 0.1328 ± 0.0106 a |
| λ (day−1) | 35 | 1.1923 ± 0.0048 a | 27 | 1.1692 ± 0.0058 a | 8 | 1.1420 ± 0.0121 a |
| R0 (Offspring/ individual) | 35 | 732.35 ± 102.70 a | 27 | 333.74 ± 58.28 a | 8 | 148.51 ± 51.57 a |
| T (day) | 35 | 37.51 ± 0.22 a | 27 | 37.17 ± 0.20 a | 8 | 37.66 ± 0.75 a |
| GRR (Offspring) | 35 | 893.72 ± 118.89 a | 27 | 580.16 ± 89.06 b | 8 | 457.15 ± 154.51 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.-K.; Li, X.-L.; Tan, X.-F.; Yang, M.-F.; Idrees, A.; Liu, J.-F.; Song, S.-J.; Shen, J. Sublethal Effects of Emamectin Benzoate on Fall Armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae). Agriculture 2022, 12, 959. https://doi.org/10.3390/agriculture12070959
Liu Z-K, Li X-L, Tan X-F, Yang M-F, Idrees A, Liu J-F, Song S-J, Shen J. Sublethal Effects of Emamectin Benzoate on Fall Armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae). Agriculture. 2022; 12(7):959. https://doi.org/10.3390/agriculture12070959
Chicago/Turabian StyleLiu, Zhuo-Kun, Xue-Lin Li, Xiao-Feng Tan, Mao-Fa Yang, Atif Idrees, Jian-Feng Liu, Sai-Jie Song, and Jian Shen. 2022. "Sublethal Effects of Emamectin Benzoate on Fall Armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae)" Agriculture 12, no. 7: 959. https://doi.org/10.3390/agriculture12070959
APA StyleLiu, Z.-K., Li, X.-L., Tan, X.-F., Yang, M.-F., Idrees, A., Liu, J.-F., Song, S.-J., & Shen, J. (2022). Sublethal Effects of Emamectin Benzoate on Fall Armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae). Agriculture, 12(7), 959. https://doi.org/10.3390/agriculture12070959








