Friability of Maize Shoot (Zea mays L.) in Relation to Cell Wall Composition and Physical Properties
Abstract
1. Introduction
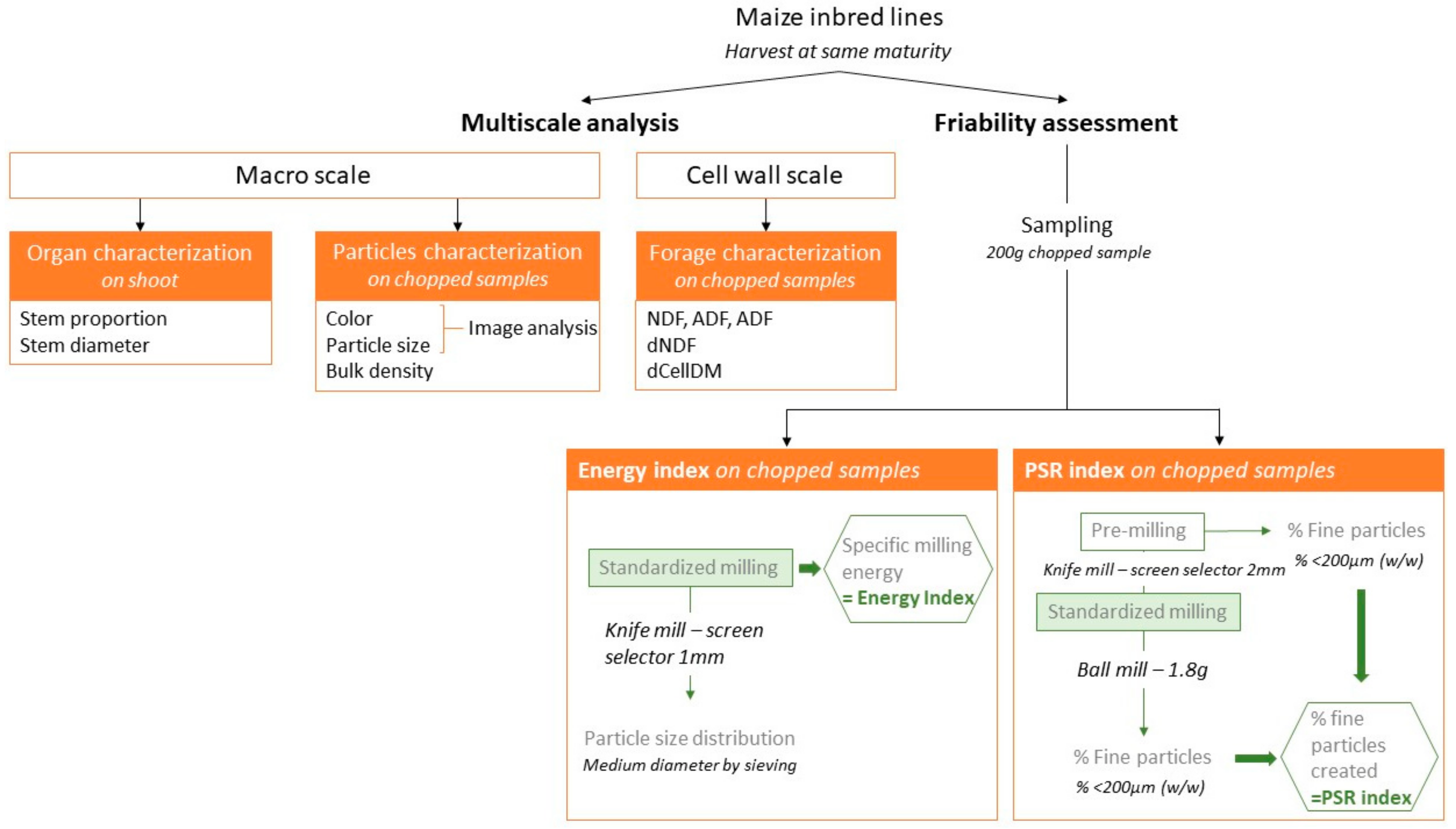
2. Materials and Methods
2.1. Plant Material
2.2. Chemical Analysis and Digestibility Estimates
2.3. Bulk Density
2.4. Color and Particle Size Analysis of Chopped Maize at Macroscopic Scale
2.4.1. Sampling and Image Acquisition
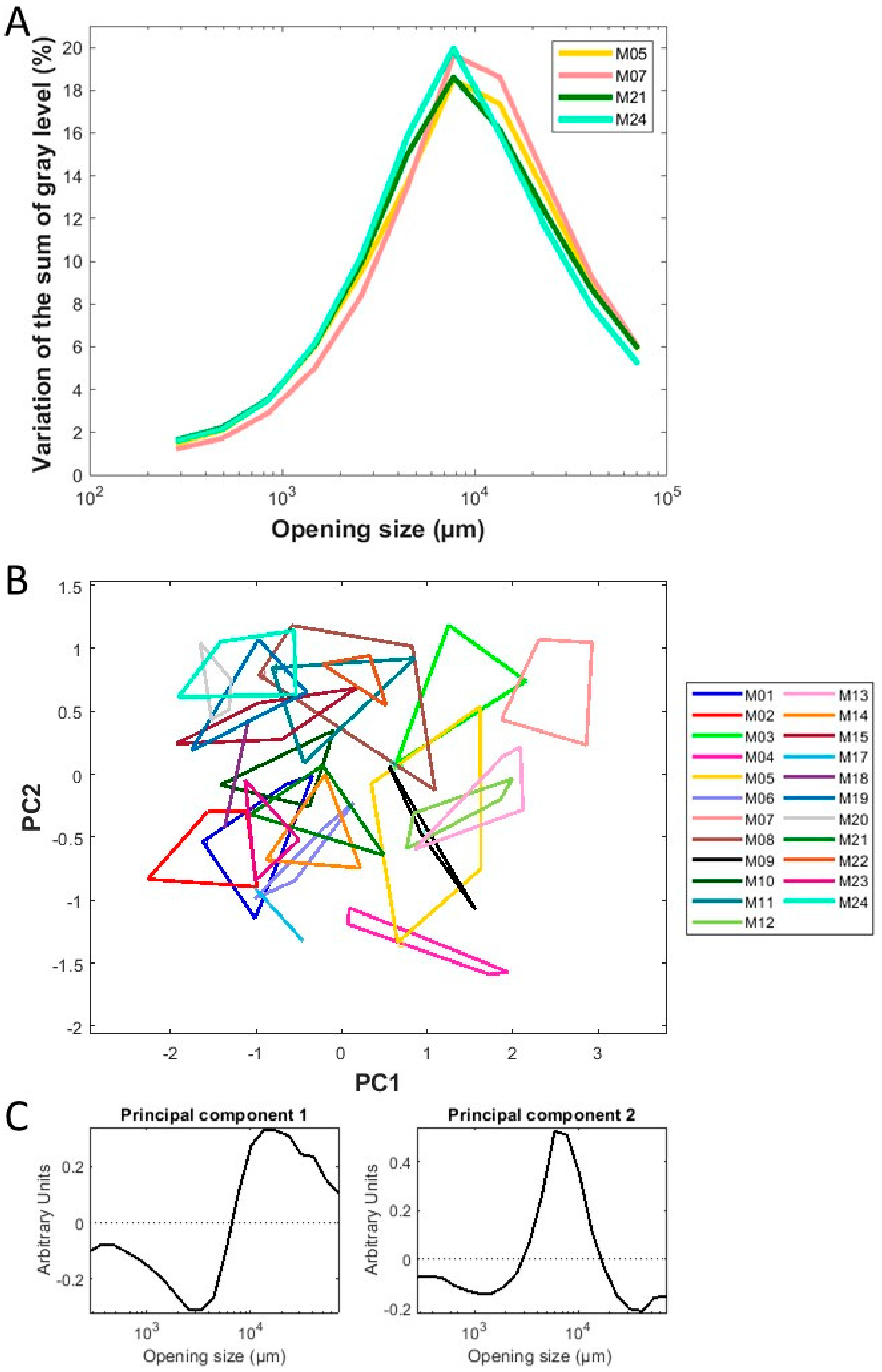
2.4.2. Particle Size Analysis by Gray Level Mathematical Morphology
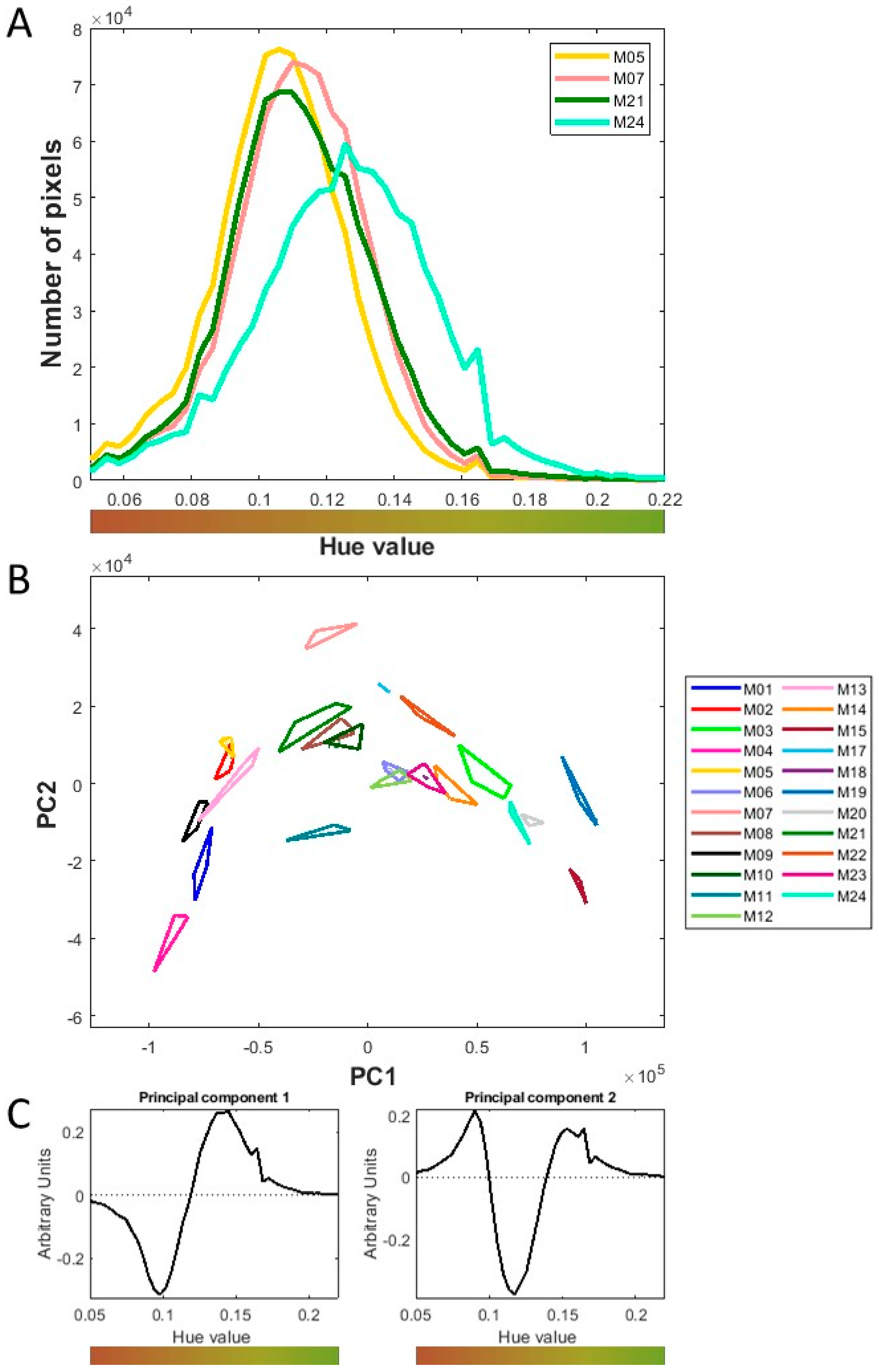
2.4.3. Color Analysis
2.5. Energy Index
2.6. Particle Size Reduction Index
2.7. Data Analysis
3. Results
3.1. Multi-Scale Characterization of the Inbred Maize Lines
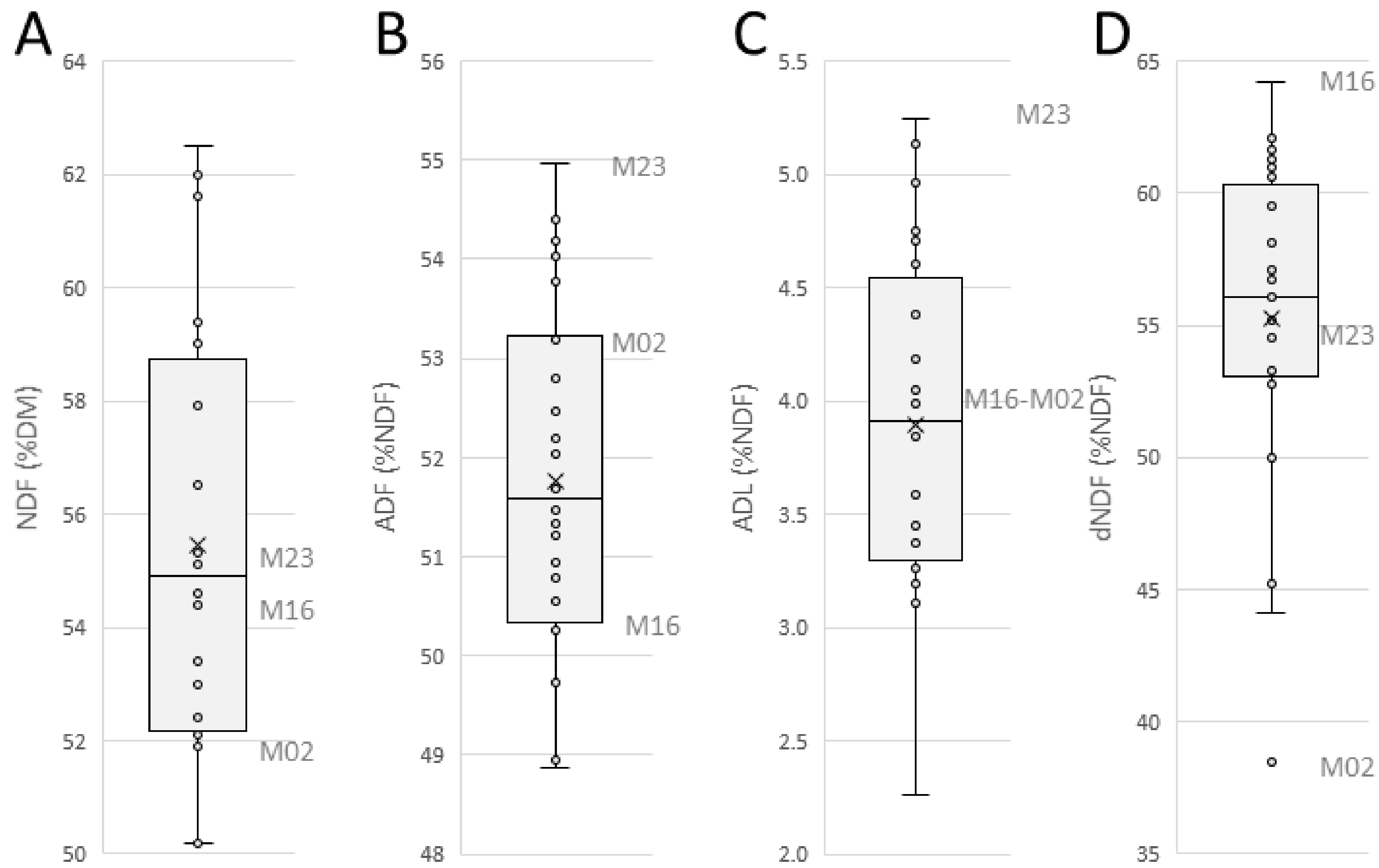
3.1.1. Characterization of Chopped Maize at Cell-Wall Scale
3.1.2. Physical Properties of Chopped Maize at Macroscopic Scale
3.2. Definition of Two Friability Indices
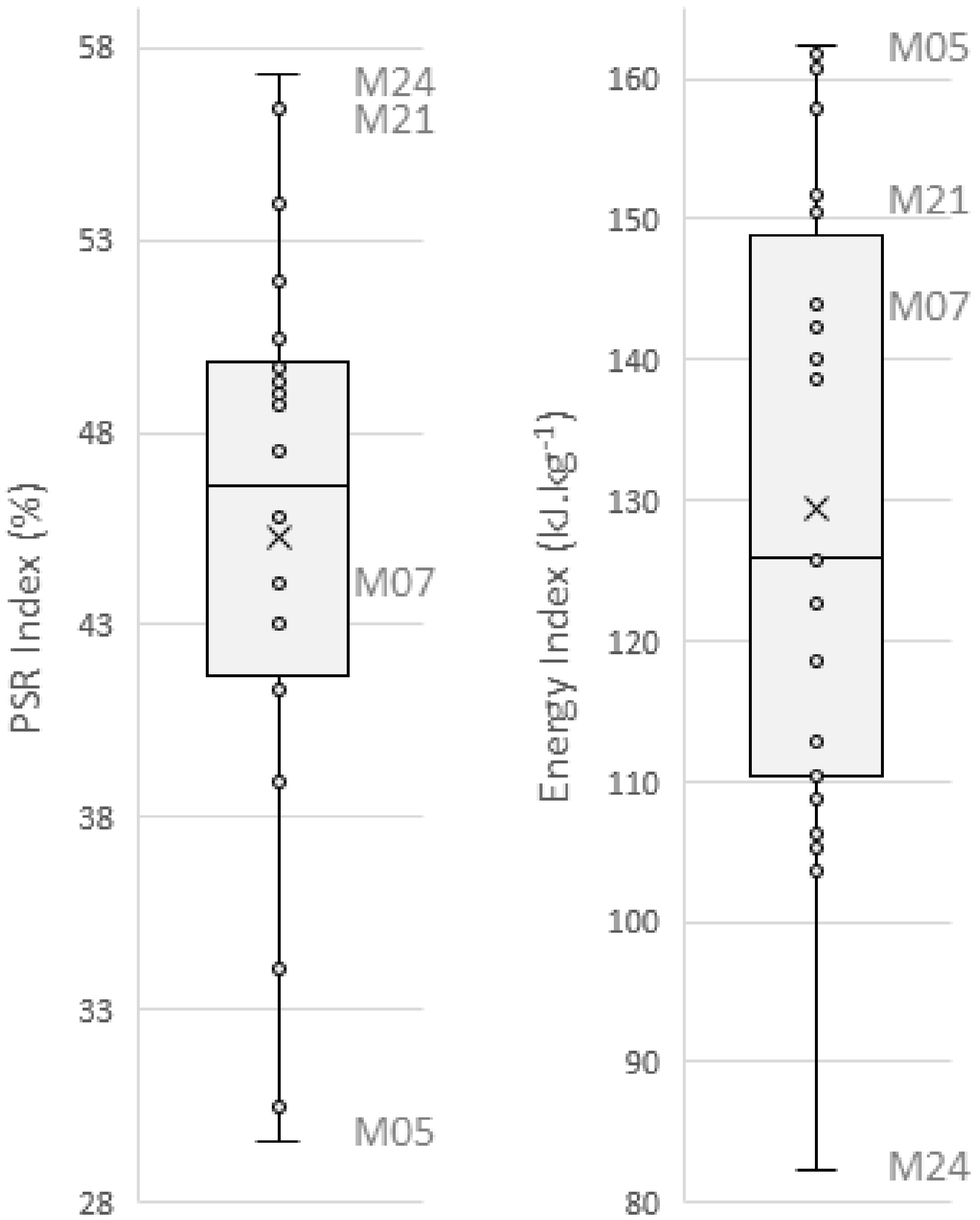
3.2.1. PSR Index Measurement
3.2.2. Energy Index Measurement
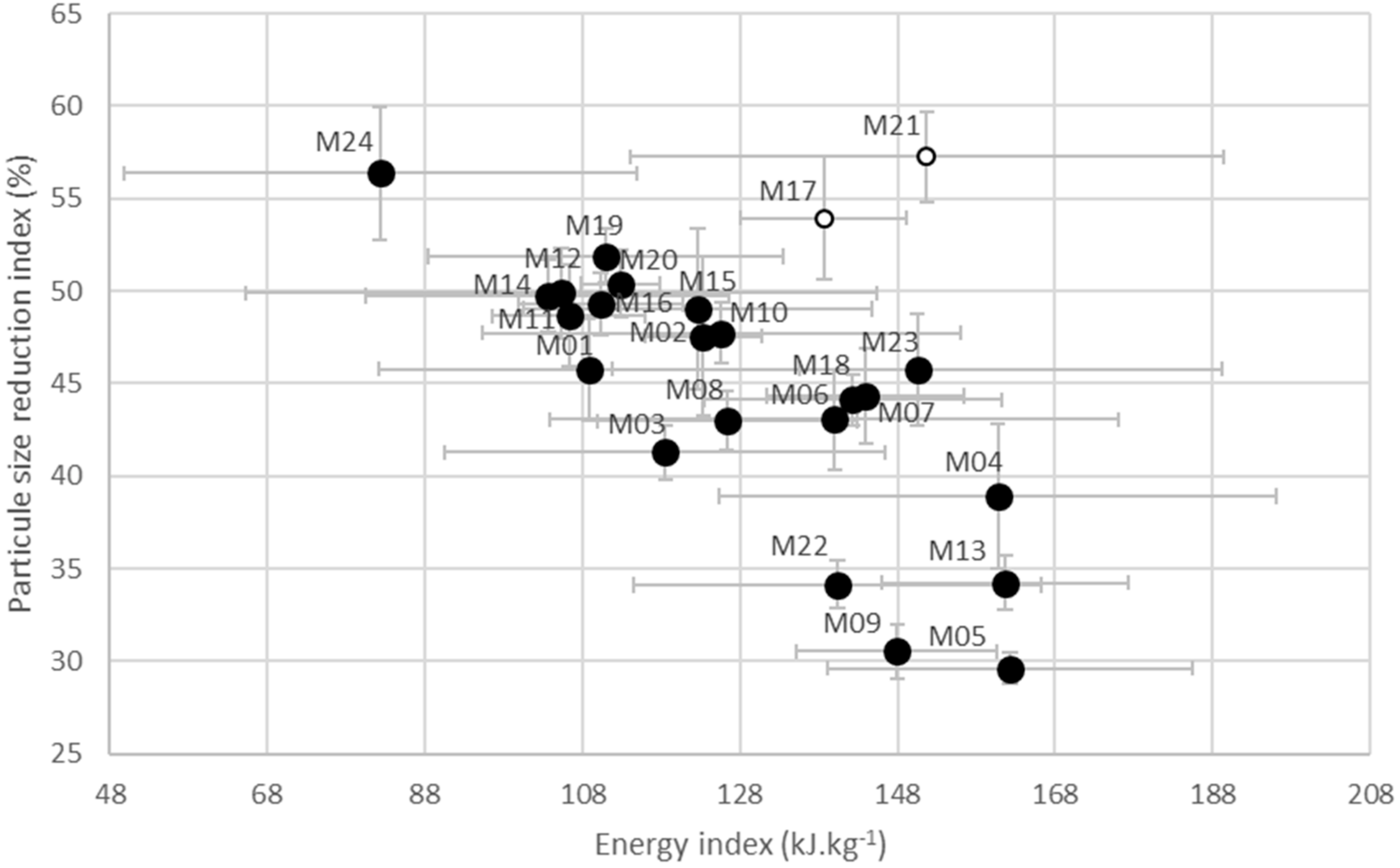
3.3. Maize Forage Friability
3.3.1. PSR Index
3.3.2. Energy Index
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amon, T.; Amon, B.; Kryvoruchko, V.; Zollitsch, W.; Mayer, K.; Gruber, L. Biogas production from maize and dairy cattle manure—Influence of biomass composition on the methane yield. Agric. Ecosyst. Environ. 2007, 118, 173–182. [Google Scholar] [CrossRef]
- Barrière, Y.; Mechin, V.; Lafarguette, F.; Manicacci, D.; Guillon, F.; Wang, H.; Lauressergues, D.; Pichon, M.; Bosio, M.; Tatout, C. Toward the discovery of maize cell wall genes involved in silage quality and capacity to biofuel production. Maydica 2009, 54, 161–198. [Google Scholar]
- Barros-Rios, J.; Santiago, R.; Malvar, R.A.; Jung, H.-J.G. Chemical composition and cell wall polysaccharide degradability of pith and rind tissues from mature maize internodes. Anim. Feed Sci. Technol. 2012, 172, 226–236. [Google Scholar] [CrossRef]
- Barriere, Y. Brown-midrib genes in maize and their efficiency in dairy cow feeding. Perspectives for breeding improved silage maize targeting gene modifications in the monolignol and p-hydroxycinnamate pathways. Maydica 2017, 62, 1–19. [Google Scholar]
- Casler, M.D.; Vogel, K.P. Accomplishments and impact from breeding for increased forage nutritional value. Crop Sci. 1999, 39, 12–20. [Google Scholar] [CrossRef]
- Barrière, Y.; Guillet, C.; Goffner, D.; Pichon, M. Genetic variation and breeding strategies for improved cell wall digestibility in annual forage crops. A review. Anim. Res. 2003, 52, 193–228. [Google Scholar] [CrossRef]
- Barriere, Y.; Alber, D.; Dolstra, O.; Lapierre, C.; Motto, M.; Ordas Perez, A.; Van Waes, J.; Vlasminkel, L.; Welcker, C.; Monod, J.P. Past and prospects of forage maize breeding in Europe. I. The grass cell walls as a basis of genetic variation and future improvements in feeding value. Maydica 2005, 50, 259–274. [Google Scholar]
- Ertiro, B.T.; Twumasi-Afriyie, S.; Blümmel, M.; Friesen, D.; Negera, D.; Worku, M.; Abakemal, D.; Kitenge, K. Genetic variability of maize stover quality and the potential for genetic improvement of fodder value. Field Crops Res. 2013, 153, 79–85. [Google Scholar] [CrossRef]
- Pino, F.; Mitchell, L.K.; Jones, C.M.; Heinrichs, A.J. Comparison of diet digestibility, rumen fermentation, rumen rate of passage, and feed efficiency in dairy heifers fed ad-libitum versus precision diets with low and high quality forages. J. Appl. Anim. Res. 2018, 46, 1296–1306. [Google Scholar] [CrossRef]
- Barrière, Y.; Emile, J.-C.; Surault, F. Genetic variation of maize silage ingestibility in dairy cattle. Anim. Res. 2003, 52, 489–500. [Google Scholar] [CrossRef][Green Version]
- Fahey, G., Jr.; Hussein, H. Forty years of forage quality research: Accomplishments and impact from an animal nutrition perspective. Crop Sci. 1999, 39, 4–12. [Google Scholar] [CrossRef]
- Crampton, E.W.; Donefer, E.; Lloyd, L.E. A nutritive value index for forages. J. Anim. Sci. 1960, 19, 538–544. [Google Scholar] [CrossRef]
- Waghorn, G.C. Changes in rumen digesta of cows during a restricted feeding period when offered fresh red clover, lucerne, or lucerne hay. N. Z. J. Agric. Res. 1986, 29, 233–241. [Google Scholar] [CrossRef]
- Waghorn, G.C.; Reid, C.S.W.; Ulyatt, M.J.; John, A. Feed comminution, particle composition and distribution between the four compartments of the stomach in sheep fed chaffed lucerne hay at two feeding frequencies and intake levels. J. Agric. Sci. 1986, 106, 287–296. [Google Scholar] [CrossRef]
- Bauchart, D.; Legay-Carmier, F.; Doreau, M. Distribution des bactéries adhérentes en fonction de la taille des particules dans les contenus du rumen et du feuillet chez la vache laitière. Reprod. Nutr. Dev. 1987, 27, 341–342. [Google Scholar] [CrossRef][Green Version]
- Weston, R. Some aspects of constraint to forage consumption by ruminants. Aust. J. Agric. Res. 1996, 47, 175–197. [Google Scholar] [CrossRef]
- Wilson, J.; Kennedy, P.M. Plant and animal constraints to voluntary feed intake associated with fibre characteristics and particle breakdown and passage in ruminants. Aust. J. Agric. Res. 1996, 47, 199–225. [Google Scholar] [CrossRef]
- Casler, M.D. Agricultural fitness of smooth bromegrass populations selected for divergent particle-size reduction index. Crop Sci. 2008, 48, 1793–1798. [Google Scholar] [CrossRef]
- Mertens, D.R. Regulation of Forage Intake; Fahey, G.C., Jr., Ed.; Wiley Online Library: Hoboken, NJ, USA, 1994; pp. 450–493. [Google Scholar] [CrossRef]
- Surprenant, J.; Barnes, D.K.; Busch, R.H.; Marten, G.C. Bidirectional selection for neutral detergent fiber and yield in reed canarygrass. Can. J. Plant Sci. 1988, 68, 705–712. [Google Scholar] [CrossRef]
- Han, L.; Casler, M.; Grau, C. Responses to divergent selection for fiber concentration at two disease potentials in smooth bromegrass. Crop Sci. 2001, 41, 30–39. [Google Scholar] [CrossRef][Green Version]
- Troelsen, J.E.; Bigsby, F.W. Artificial mastication—A new approach for predicting voluntary forage consumption by ruminants. J. Anim. Sci. 1964, 23, 1139–1142. [Google Scholar] [CrossRef]
- Chenost, M. L’indice de fibrosité des foins: Mesure et relations avec la valeur alimentaire. Anim. Res. 1966, 15, 253–257. [Google Scholar] [CrossRef]
- Laredo, M.; Minson, D. The voluntary intake, digestibility, and retention time by sheep of leaf and stem fractions of five grasses. Aust. J. Agric. Res. 1973, 24, 875–888. [Google Scholar] [CrossRef]
- Prasad, J.; Gupta, C. Mechanical properties of maize stalk as related to harvesting. J. Agric. Eng. Res. 1975, 20, 79–87. [Google Scholar] [CrossRef]
- Al-Zube, L.; Sun, W.; Robertson, D.; Cook, D. The elastic modulus for maize stems. Plant Methods 2018, 14, 11. [Google Scholar] [CrossRef]
- Casler, M.; Schneider, D.; Combs, D. Development and application of a selection criterion for particle size breakdown of smooth bromegrass leaves. Anim. Feed Sci. Technol. 1996, 61, 57–71. [Google Scholar] [CrossRef]
- Chenost, M.; Grenet, E. Fibre Index of Forages: Its Significance and Use for Predicting Feeding Value of Forages; Annales de Zootechnie: Paris, France, 1971. [Google Scholar]
- Mani, S.; Tabil, L.G.; Sokhansanj, S. Grinding performance and physical properties of wheat and barley straws, corn stover and switchgrass. Biomass Bioenergy 2004, 27, 339–352. [Google Scholar] [CrossRef]
- Cheeke, P.R. Natural Toxicants in Feeds, Forages, and Poisonous Plants; Interstate Publishers, Inc.: Danville, IL, USA, 1998. [Google Scholar]
- McDonald, P. Animal Nutrition; Pearson Education India: Bengaluru, India, 2002. [Google Scholar]
- Chundawat, S.P.; Venkatesh, B.; Dale, B.E. Effect of particle size based separation of milled corn stover on AFEX pretreatment and enzymatic digestibility. Biotechnol. Bioeng. 2006, 96, 219–231. [Google Scholar] [CrossRef]
- Casler, M.; Culvenor, R.; Combs, D. Divergent selection for two laboratory predictors of voluntary intake: Relationships among the predictors and leaf morphology variables. Anim. Feed Sci. Technol. 2000, 84, 107–119. [Google Scholar] [CrossRef]
- Miao, Z.; Grift, T.; Hansen, A.; Ting, K. Energy requirement for comminution of biomass in relation to particle physical properties. Ind. Crops Prod. 2011, 33, 504–513. [Google Scholar] [CrossRef]
- Mayer-Laigle, C.; Blanc, N.; Rajaonarivony, R.K.; Rouau, X. Comminution of dry lignocellulosic biomass, a review: Part I. from fundamental mechanisms to milling behaviour. Bioengineering 2018, 5, 41. [Google Scholar] [CrossRef] [PubMed]
- Méchin, V.; Argillier, O.; Menanteau, V.; Barrière, Y.; Mila, I.; Pollet, B.; Lapierre, C. Relationship of cell wall composition to in vitro cell wall digestibility of maize inbred line stems. J. Sci. Food Agric. 2000, 80, 574–580. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Aufrère, J.; Michalet-Doreau, B. In vivo digestibility and prediction of digestibility of some by-products. In Proceedings of the EEC Seminar, Melle-Gontrode, Belgium, 26–29 September 1983. [Google Scholar]
- Devaux, M.-F.; Bouchet, B.; Legland, D.; Guillon, F.; Lahaye, M. Macro-vision and grey level granulometry for quantification of tomato pericarp structure. Postharvest Biol. Technol. 2008, 47, 199–209. [Google Scholar] [CrossRef]
- Devaux, M.F.; Legland, D. Grey level granulometry for histological image analysis of plant tissues. In Microscopy; 2014; p. 624. Available online: https://hal.inrae.fr/hal-01195487 (accessed on 2 June 2022).
- Dinca, M.; Moiceanu, G.; Paraschiv, G.; Voicu, G.; Negoita, O.; Chitoiu, M.; Tudor, P. Energy consumption at size reduction of lignocellulose biomass for bioenergy. Sustainability 2019, 11, 2477. [Google Scholar] [CrossRef]
- Aufrere, J.; Michalet-Doreau, B. Comparison of methods for predicting digestibility of feeds. Anim. Feed Sci. Technol. 1988, 20, 203–218. [Google Scholar] [CrossRef]
- Aufrere, J.; Baumont, R.; Delaby, L.; Peccatte, J.R.; Andrieu, J.; Andrieu, J.P.; Dulphy, J.P. Prévision de la digestibilité des fourrages par la méthode pepsine-cellulase. Le point sur les équations proposées. INRAE Prod. Anim. 2007, 20, 129–136. [Google Scholar] [CrossRef]
- Baumont, R.; Aufrere, J.; Niderkorn, V.; Andueza, D.; Surault, F.; Peccatte, J.R.; Delaby, L.; Pelletier, P. Specific diversity in forages: Its consequences on the feeding value. Fourrages 2008, 194, 189–206. [Google Scholar]
- Zhang, Y.; Legland, D.; Hage, F.E.; Devaux, M.-F.; Guillon, F.; Reymond, M.; Méchin, V. Changes in cell walls lignification, feruloylation and p-coumaroylation throughout maize internode development. PLoS ONE 2019, 14, e0219923. [Google Scholar] [CrossRef]
- Wolf, D.P.; Coors, J.G.; Albrecht, K.A.; Undersander, D.J.; Carter, P.R. Forage quality of maize genotypes selected for extreme fiber concentrations. Crop Sci. 1993, 33, 1353–1359. [Google Scholar] [CrossRef]
- Khan, N.A.; Yu, P.; Ali, M.; Cone, J.W.; Hendriks, W.H. Nutritive value of maize silage in relation to dairy cow performance and milk quality. J. Sci. Food Agric. 2014, 95, 238–252. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Cone, J.W.; Hendriks, W.H.; Dijkstra, J. Relationships between chemical composition and in vitro gas production parameters of maize leaves and stems. J. Anim. Physiol. Anim. Nutr. 2019, 104, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Van Soest, P.J.; Robertson, J.B.; Hall, M.B.; Barry, M.C. Klason lignin is a nutritionally heterogeneous fraction unsuitable for the prediction of forage neutral-detergent fibre digestibility in ruminants. Br. J. Nutr. 2020, 124, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Sokhansanj, S.; Turhollow, A.; Cushman, J.; Cundiff, J. Engineering aspects of collecting corn stover for bioenergy. Biomass Bioenergy 2002, 23, 347–355. [Google Scholar] [CrossRef]
- Ghorbani, Z.; Masoumi, A.; Hemmat, A. Specific energy consumption for reducing the size of alfalfa chops using a hammer mill. Biosyst. Eng. 2010, 105, 34–40. [Google Scholar] [CrossRef]
- Rajaonarivony, K.; Rouau, X.; Lampoh, K.; Delenne, J.-Y.; Mayer-Laigle, C. Fine comminution of pine bark: How does mechanical loading influence particles properties and milling efficiency? Bioengineering 2019, 6, 102. [Google Scholar] [CrossRef]
- Bitra, V.S.; Womac, A.R.; Igathinathane, C.; Miu, P.I.; Yang, Y.T.; Smith, D.R.; Chevanan, N.; Sokhansanj, S. Direct measures of mechanical energy for knife mill size reduction of switchgrass, wheat straw, and corn stover. Bioresour. Technol. 2009, 100, 6578–6585. [Google Scholar] [CrossRef]
- Yu, M.; Womac, A.R.; Pordesimo, L.O. Review of biomass size reduction technology. In 2003 ASAE Annual Meeting; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2003. [Google Scholar]
- Temmerman, M.; Jensen, P.D.; Von Hébert, J. Rittinger theory adapted to wood chip and pellet milling, in a laboratory scale hammermill. Biomass Bioenergy 2013, 56, 70–81. [Google Scholar] [CrossRef]
- von Rittinger, P.R. Taschenbuch der Aufbereitungskunde; Ernst & Korn: Berlin, Germany, 1867. [Google Scholar]
- Himmel, M.; Tucker, M.; Baker, J.; Rivard, C.; Oh, K.; Grohmann, K. Comminution of biomass: Hammer and knife mills. In Proceedings of the 7th Symposium on Biotechnology for Fuels and Chemicals, Gatlinburg, Tennessee, 14–17 May 1985. [Google Scholar]
- Cadoche, L.; López, G.D. Assessment of size reduction as a preliminary step in the production of ethanol from lignocellulosic wastes. Biol. Wastes 1989, 30, 153–157. [Google Scholar] [CrossRef]
- Mayer-Laigle, C.; Rajaonarivony, R.K.; Blanc, N.; Rouau, X. Comminution of dry lignocellulosic biomass: Part II. Technologies, improvement of milling performances, and security issues. Bioengineering 2018, 5, 50. [Google Scholar] [CrossRef] [PubMed]
- Kratky, L.; Jirout, T. Biomass size reduction machines for enhancing biogas production. Chem. Eng. Technol. 2011, 34, 391–399. [Google Scholar] [CrossRef]
- Oyedeji, O.; Gitman, P.; Qu, J.; Webb, E. Understanding the impact of lignocellulosic biomass variability on the size reduction process: A review. ACS Sustain. Chem. Eng. 2020, 8, 2327–2343. [Google Scholar] [CrossRef]
- Pickering, K.L.; Aruan Efendy, M.G.; Le, T.M. A review of recent developments in natural fibre composites and their mechanical performance. Compos. Part A Appl. Sci. Manuf. 2016, 83, 98–112. [Google Scholar] [CrossRef]
- Berger, M.; Devaux, M.-F.; Legland, D.; Barron, C.; Delord, B.; Guillon, F. Darkfield and fluorescence macrovision of a series of large images to assess anatomical and chemical tissue variability in whole cross-sections of maize stems. Front. Plant Sci. 2021, 12, 792981. [Google Scholar] [CrossRef]
- Legland, D.; El-Hage, F.; Méchin, V.; Reymond, M. Histological quantification of maize stem sections from FASGA-stained images. Plant Methods 2017, 13, 84. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Du, J.; Zhao, Y.; Lu, X.; Wen, W.; Gu, S.; Fan, J.; Wang, C.; Wu, S.; et al. Dissecting the phenotypic components and genetic architecture of maize stem vascular bundles using high-throughput phenotypic analysis. Plant Biotechnol. J. 2020, 19, 35–50. [Google Scholar] [CrossRef]

| Chopped | With Pre-Milling | |||||
|---|---|---|---|---|---|---|
| Milling Energy | Median Diameter | Milling Energy | Median Diameter | |||
| kJ.kg−1 | RSD (%) | RSD (%) | kJ.kg−1 | RSD (%) | RSD (%) | |
| M01 | 109 | 25 | 3.1 | 60.9 | 4.3 | 0.3 |
| M04 | 161 | 25 | 2.8 | 77.4 | 23.9 | 3.8 |
| M10 | 126 | 24 | 3.1 | 46.1 | 5.5 | 0.5 |
| M12 | 105 | 38 | 1.1 | 54 | 8.0 | 2.2 |
| Variables | PSR Index | Energy Index | |
|---|---|---|---|
| Physical properties | Bulk density (kg.m−3) | 0.291 | −0.447 * |
| Stem proportion (% fresh matter) | −0.314 | 0.326 | |
| Stem diameter (mm) | −0.134 | −0.153 | |
| Particle size (granulometry PC1 values) | −0.559 ** | 0.428 | |
| Particle size heterogeneity (granulometry PC2 values) | 0.110 | −0.456 * | |
| Particle color (hue PC1 values) | 0.508 * | −0.539 ** | |
| Particle color heterogeneity (hue PC2 values) | 0.012 | 0.224 | |
| Composition | NDF (%dm) | −0.586 ** | 0.585 ** |
| Hemicellulose (% NDF) | 0.629 ** | −0.471 * | |
| Cellulose (% NDF) | −0.702 ** | 0.491 * | |
| ADL (% NDF) | 0.150 | −0.030 | |
| dNDF (%NDF) | −0.049 | 0.222 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berger, M.; Devaux, M.-F.; Mayer-Laigle, C.; Réau, A.; Delord, B.; Guillon, F.; Barron, C. Friability of Maize Shoot (Zea mays L.) in Relation to Cell Wall Composition and Physical Properties. Agriculture 2022, 12, 951. https://doi.org/10.3390/agriculture12070951
Berger M, Devaux M-F, Mayer-Laigle C, Réau A, Delord B, Guillon F, Barron C. Friability of Maize Shoot (Zea mays L.) in Relation to Cell Wall Composition and Physical Properties. Agriculture. 2022; 12(7):951. https://doi.org/10.3390/agriculture12070951
Chicago/Turabian StyleBerger, Marie, Marie-Françoise Devaux, Claire Mayer-Laigle, Adrien Réau, Benoit Delord, Fabienne Guillon, and Cécile Barron. 2022. "Friability of Maize Shoot (Zea mays L.) in Relation to Cell Wall Composition and Physical Properties" Agriculture 12, no. 7: 951. https://doi.org/10.3390/agriculture12070951
APA StyleBerger, M., Devaux, M.-F., Mayer-Laigle, C., Réau, A., Delord, B., Guillon, F., & Barron, C. (2022). Friability of Maize Shoot (Zea mays L.) in Relation to Cell Wall Composition and Physical Properties. Agriculture, 12(7), 951. https://doi.org/10.3390/agriculture12070951







