Abstract
The aim of this study was to examine variations in cow milk composition as a function of breeding system and seasonality. This study was carried out in 16 dairy farms located in the Abruzzo region (Central Italy), equally distributed between farms that adopt grazing in the spring and summer months, and farms where the intensive system is exploited. Milk was sampled in all seasons in each of the farms involved and was analyzed with particular attention given to the quality of the lipid and protein fractions. A lower concentration of saturated fatty acids and an increase in rumenic, vaccenic and oleic acids were registered for milk samples coming from outdoor grazing, in which was also observed the greatest presence of α and β caseins. The opposite result was instead observed for κ casein, which showed the highest values from intensive farming. Evaluations also focused on retinol, which significantly increased in concentration during summer in both breeding systems. The present results suggest positive insights into the role of the outdoor breeding system in improving the main qualitative trait of bovine milk in warm seasons.
1. Introduction
Milk and dairy products are an excellent source of different components of most diets. Milk composition can be affected, directly or indirectly, by several aspects. Some of these factors include animal health, farm management, variations in feeding systems and the impact of seasonal changes and environmental conditions [1,2].
An important aspect related to milk quality concerns the fatty acids composition; milk fat is one of the most elaborate components, being made up of 150 different fatty acids, it has a proportion equal to about two-thirds of saturated fatty acids and one-third of unsaturated fatty acids and a specific high proportion of low molecular weight volatile fatty acids, which can be influenced by nutrition [3].
Other aspects, such as somatic cell count, nutrition stage and genetic variations in caseins can cause changes in the milk protein fraction, which can significantly change the properties of milk during technological processes [4]. Milk is characterized by two categories of proteins: 80% represented by the casein fraction and 20% by whey proteins. Caseins are found in the form of spherical particles known as casein micelles with a diameter ranging from 30 to 300 nm. The other fraction is represented by whey protein, which includes about 17% of the nitrogenous substances in cow’s milk and are also called soluble proteins [5]. In particular, α-lactalbumin (α-La) and β-lactoglobulin (β-Lg) have a balanced nutritional profile, as they are made up of a high quantity of sulfur amino acids (methionine, cystine and cysteine) and have technological properties, which include the ability to form and stabilize gels, emulsions and foams [6]. In the last decades, several studies focused their attention on the role of diet in influencing the above-mentioned parameters.
Outdoor grazing is a common feeding method for dairy cows, usually supplemented with a low volume of concentrate offered only at the extremes of the pasture-growing season [7]. The composition of the forage essences of each pasture depends on the pedo-climatic conditions and human interventions, such as sowing, crop rotations, grazing intensity and the administration of fertilizers. Pastures, in general, are considered important both from a naturalistic point of view, given the great variety of essences they contain, and from a zootechnical point of view for feeding the animals. Nowadays, the most widespread farming system is the intensive one. In this type of farming, grazing is not provided, cows are stabulated in boxes throughout the production cycle.
Furthermore, climatic variations greatly influence dairy cows, particularly their welfare and their capacity to produce milk [8]. It has been shown [9] that the season of the year has a substantial impact on the components and properties of milk. Bourauoi et al. [10] observed a significant decrease in the lipidic and proteic fractions and a significant increase in the somatic cell count (SCC) in the lactation of Holstein cows during the summer compared to spring. Larsen et al. [11] studied the influence of climatic conditions and the season on the composition of milk from 20 farms located in central and southern Sweden. These authors found less milk fat in summer than in winter and these dissimilarities were attributed to climatic differences. Given the increasing attention that consumers are placing on the qualitative characteristics of milk, the main objective of this work is to analyze the effect of the season and the differences between outdoor grazing (ODG) and intensive feeding (IFG) on the quality of bovine milk, observing the variation in chemical composition, fatty acids, proteins and retinol.
2. Materials and Methods
2.1. Experimental Design, Feeding Strategies and Sampling
The trial followed the guidelines reported in the Directive 2010/63/EU of the European Parliament (European Union, 2010, Brussels, Belgium) and Directive 86/609/EEC (European Economic Community, 1986, Brussels, Belgium), in which is guaranteed animal protection used for scientific purposes.
The investigation was carried out on bulk milk of 16 Friesian dairy farms located in the Abruzzo region (Central Italy), each of which is characterized by a herd ranging from 30 to 50 animals. Eight of these farms kept cows on pasture (i.e., an open-air area with a meadow that allows for grazing), in spring and summer, while the remaining half, exploited the intensive system. All information about animal feeding was collected through questionnaires that were filled out by the farmers; in the modules regarding the feeding of the animals, this included the composition of pasture and the type of feeding in the barn. During the entire experimental period: dairy farms that exploited outdoor grazing, mainly used legumes, in particular, alfalfa (Medicago sativa L.) and clover (Trifolium L.); for the time that they were in the stables, they were fed with hay and concentrates such as: common wheat (Triticum aestivum L.), barley (Hordeum vulgare L.), maize (Zea mays L.) and ryegrass (Lolium perenne L.); meanwhile, intensive animal farms received food in the form of “unifeed”.
During each season, bulk milk samples were collected for each farm following the milking in the morning and part of this milk was immediately analyzed for chemical composition (fat, protein, casein and lactose) and somatic cell counts (SCC). The remaining milk was properly aliquoted and stored at −20 °C until analysis. These samples were specifically used for the characterization of the fatty acids, proteins profiles and retinol dosage. For the fatty acids and protein analyses, all four seasons throughout the year were taken into consideration; meanwhile, for milk composition and retinol concentration, only two seasons were considered, winter and summer, leaving out the transitional seasons.
2.2. Fatty Acids Analysis in Milk
The milk lipid fraction was extracted according to the AOAC official method [12]. For the fatty acid characterization in milk, 50 mg of extracted lipids were weighed and reconstituted with 1 mL of hexane and methylated with 500 μL of 2 N sodium methoxide in methanol. The detection of the fatty acid methyl esters (FAME) was performed by a gas chromatograph (GC) coupled with a flame ionization detector (FID) equipped with a capillary column (Restek rt-2560 Column, fused silica 100 m × 0.25 mm highly polar phase; Restek Corporation, Bellefonte, PA, USA). A carrier gas, hydrogen, was used as the mobile phase at a flow rate of 1 mL/min. The thermal program was characterized by an initial phase at 80 °C for 10 min, thereafter a temperature of 172 °C at 4 °C/min for 30 min, finally increased to 190 °C at 4 °C/min for 10 min. The FAMEs were identified by using Chrome Card software and even by comparison with the registered retention times with FAME analytical standards (FIM-FAME-7-Mix; Matreya LLC, State College, PA, USA). The individual fatty acids were expressed as mean relative percentages of the total FAME identified. Desaturation indices (DI) for C14, C16, C18 and rumenic acid were calculated using the formula suggested by Brogna et al. [13].
2.3. Protein Extraction and Separation by Sodium Dodecyl Sulfate Polyacrilamide Gel Electrophoresis (SDS-PAGE)
Each milk sample (15 mL) was centrifugated at 4 °C for 15 min at 10,000× g in order to remove fat and other insoluble solids, and Whatman filter paper was used to recover and filter the supernatant.
The extracted proteins were quantified using the Bradford method [14] with bovine serum albumin (BSA; Sigma Aldrich, Milan, Italy) as the standard. Volumes of each sample, containing 20 μg of total extracted proteins, were diluted in a 2 X sample buffer (62.5 mM Tris-HCl pH 6.8, 2% SDS, 10% glycerol, 5% β-mercaptoethanol and bromophenol blue. Subsequently, samples were heated for 5 min to promote the protein denaturation and loaded onto a 12% polyacrylamide gel. The electrophoresis was performed in a mini-protean III dual slab cell (Bio-Rad Laboratories, Wartford, UK) at a constant voltage of 120 V. At the end of the run, gels were placed for 30 min in a staining solution containing 40% methanol, 10% acetic acid and 0.1% Comassie Brillant Blue G-250 (Bio-Rad Laboratories, Wartford, UK). The densitometric analysis of displayed bands was performed by using the ImageJ software [15].
2.4. Protein Identification by Western Blotting (WB)
For the Western blotting, protein samples (20 μg) were resolved by a 12% SDS-PAGE as previously described. Subsequently, proteins were conveyed onto a polyvinylidene difluoride (PVDF) membrane to allow the immune-recognition. In order to avoid non-specific signals, membranes were blocked overnight at 4°C, using TBS-T (10 mM Tris-HCl pH 7.4, 150 mM NaCl, 0.05% Tween 20), and then the incubation with primary antibodies for the identification of κ-casein (CSN3 antibody; Biorbyt Ltd., Cambridge, UK) and β-casein (CSN2 antibody; Biorbyt Ltd., Cambridge, UK) was performed. After washing with TBS-T, the membranes were incubated in TBS-T containing the secondary antibody IgG (1:5000) (donkey anti-rabbit antibody HRP; Biorbyt Ltd., Cambridge, UK) for 1 h at room temperature. After washing in TBS-T, membranes were incubated with enhanced chemiluminescent (ECL) system (WESTAR ηC Ultra 2.0; Bologna, Italy) and proteins were visualized using Azure c400 (Azure Biosystems, Dublin, CA, USA). The densitometric values of the immunoreactive bands were analyzed by the ImageJ software [15].
2.5. High-Performance Liquid Chromatography (HPLC) Analysis of Retinol in Milk
Briefly, 1 mL of milk was moved into a 15 mL tube in which there was the addition of 1 mL potassium hydroxide (KOH) solution (50% w/v), and 2 mL of ethanol (containing ascorbic acid 0.1% as internal standard). Tubes were then capped and placed in a thermostated bath at 80 °C. After 2 h, 10 mL of an ethyl ether and petroleum ether solution (50:50) was added, and the mixture was then centrifuged at 10,000× g for 10 min at 4 °C. The upper layers were collected with a glass Pasteur pipette and the extraction process was repeated three times. During the last wash, the solutions were transferred into a 50 mL round-bottomed flask and dried with a Strike-Rotating Evaporator (Steroglass s.r.l., Perugia, Italy). The residue was then recuperated with 1 mL of methanol and a 20 μL aliquot was inserted into the chromatographic system.
Retinol concentration in milk samples was analyzed using an HPLC chromatographic system (Varian, Harbor City, CA, USA) equipped with a Supelcosil LC-18 HPLC column (25 cm × 4.6 mm, 5 µm; Sigma-Aldrich, Milan, Italy). Isocratic conditions with a mobile phase containing 95% methanol and 5% water were used. The flow rate of the mobile phase was 0.7 mL/min, and the column temperature was set at 40 ± 0.1°C. The peaks were detected at 325 nm. Retinol (Sigma-Aldrich, Switzerland) was used as the standard to obtain a calibration curve that was linear in the range of concentration from 0.5 to 500 μg/mL (R2 = 0.9967).
2.6. Statistical Analysis
The statistical analysis was performed using the software SAS software of the JMP 14 program (SAS Institute, Cary, NC, USA). All data have been processed with ANOVA (Analysis of Variance) to analyze the impact of seasons and different kinds of breading on the fatty acid profile, protein content, chemical composition and retinol. Sample means were assessed by HSD Tukey’s test and differences were declared significant for p-values lower than 0.05 and 0.01. All data were tested by the Shapiro–Wilk test. Pearson correlation was applied to all major protein components.
3. Results
3.1. Chemical Composition of Milk
In Table 1 is reported the chemical composition of cow milk referring to ODG and IFG. Considering seasonality and the feeding system there are significant changes only in the ODG farms between the two seasons, with a higher level of lactose (p < 0.01) and lipids (p < 0.01). No variations were detected with respect to total caseins, total protein and somatic cells.

Table 1.
Chemical composition in bovine milk samples collected in two seasons (summer and winter) from two different feeding systems.
3.2. Milk Fatty Acid Profile
The characterization of the fatty acids profile evidenced several significant differences among the analyzed samples (Table 2). Palmitic acid (C16:0) showed higher in IFG compared to ODG in particular in summer (p < 0.01); palmitoleic acid (C16:1) resulted higher in IFG compared to ODG (p < 0.01) except for ODG in winter; stearic (C18:0) and vaccenic (C18:1 trans 11) were significantly higher value in ODG compared to IFG (p < 0.01) in particular in spring and summer; α-linolenic (C18:3) and rumenic acid (CLA) acids, in addition to the sum of monounsaturated fatty acids (MUFA) and the sum of polyunsaturated fatty acids (PUFA) showed higher values in ODG, specifically in summer (p < 0.01); the sum of saturated fatty acids (SFA) showed instead to be lower in the ODG samples (p < 0.01).

Table 2.
Fatty acid profile in bovine milk samples collected in four seasons from two different farming systems.
3.3. Caseins and Whey Protein Separation by Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE)
As shown in Figure 1, the SDS-PAGE was useful for the identification of caseins α, β and κ and two whey proteins (β-Lg and α-La). Diversity is found in all proteins: α-casein, β-casein and β-Lg with the highest values in ODG compared to IFG, in particular, in spring and summer (p < 0.01). Opposite results were shown for κ-casein and α-La with higher concentrations in IFG compared to ODG (p < 0.01) (Table 3).
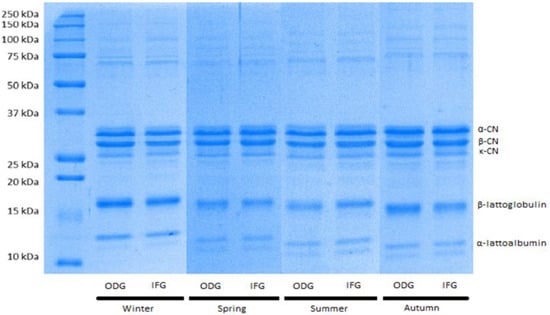
Figure 1.
A representative Sodium Dodecyl Sulphate Polyacrylamide Gel Electrophoresis (SDS-PAGE) of caseins and whey proteins resulting from bovine milk samples obtained in four different seasons from two different farming systems.

Table 3.
Quantification of protein bands in milk samples in four seasons between two different farming systems.
The trend of major protein fractions was evaluated and confirmed by Pearson correlation (Table 4).

Table 4.
Pearson correlation coefficient between caseins fraction and whey proteins.
3.4. Caseins Identification by Immunoblotting
Specific antibodies were used to verify the presence of β-casein and κ-casein in milk samples (Figure 2). As represented in Figure 3, a significant increase in β and κ-casein was observed in ODG in summer. On the contrary, in IFG samples, higher levels of β-casein were observed in winter, while for κ-casein, the same trend was observed as evidenced by the ODG system. All variations showed the same significance (p < 0.01).

Figure 2.
A representative Western Blotting of caseins β and κ resulting from milk samples obtained in four different seasons between two different farming systems. ODG: Outdoor grazing, IFG: intensive feeding.
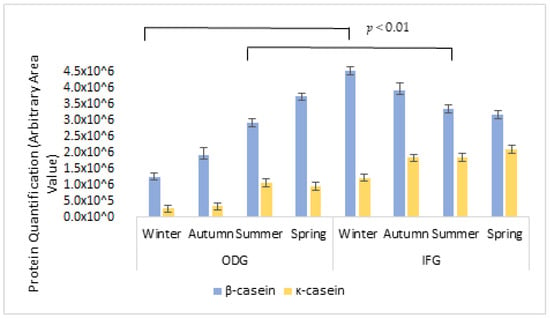
Figure 3.
Immunoblotting of cow milk caseins during all seasons between ODG and IFG farming systems. Data are reported as area percentage ± S.D. of the total retinol concentration in each sample. p < 0.01. ODG: Outdoor grazing, IFG: intensive feeding.
3.5. Retinol Analysis
The retinol (Vitamin A) concentrations are shown in Figure 4. Statistical significance was found between the ODG system and the IFG system (p-value < 0.01). Concerning the ODG samples, the higher values were recorded during summer (23.88 ± 3.9 µg/mL) and the lower in winter (20.33 ± 4.69 µg/mL; p < 0.01). Regarding the IFG samples, the higher concentrations were instead reported during summer (11.2 ± 0.82 µg/mL) and the lower in winter (8.23 ± 2.69 µg/mL; p < 0.01).
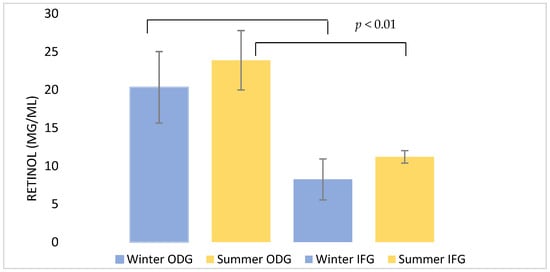
Figure 4.
Retinol concentrations in milk during the winter and summer seasons between the two different farming systems. Data are reported as mean (µg/mL) ± S.D. of the total retinol concentration in each sample. p < 0.01. ODG: Outdoor grazing, IFG: intensive feeding.
4. Discussion
The aim of this study was to examine changes in milk composition as a function of feeding practice and seasonality. Particular attention was paid to chemical composition, fatty acid composition, protein profile and retinol concentration.
The concentrations of fat, protein and lactose were affected by the feeding system and the interaction with seasonal variability. In the IFG system, the chemical composition is similar among all seasons. On the contrary, in ODG, significant variations in chemical composition related to the seasons were detected between summer and winter. Higher fat and lactose percentages were shown in winter compared to the summer; however, no significant changes in protein percentages were detected among the seasons. The lowest level of lipids was observed in ODG summer milk could be due to the intake of unsaturated fatty acids that are typically found in forage. Some studies reported that high concentration of these fatty acids in the rumen can inhibit some microbial species in the rumen, with the production of CLA isomers produced in the rumen inhibiting fatty acid synthesis, inducing a low concentration of fat in milk [16,17]. During the summer season in ODG, a decrease was shown in the lactose content, compared to the winter season, while in IFG, the percentages were similar between the two seasons. Lactose is correlated to α-La, a coenzyme required for its production. This whey protein has specific production in the mammary gland. Inside the Golgi apparatus of the mammary gland’s epithelial cells, α-La interacts with β-1,4-galactosyltransferase, to allow lactose-synthase enzyme formation. α-La modifies the substrate specificity of β-1,4-galactosyltransferase, allowing the formation of lactose from glucose and UDP-galactose [18]. Our α-La results seem to confirm this trend. In fact, in IFG, there is no significance between winter and summer, while in ODG, there is a significance with a decrease in α-La in summer compared to the winter season.
Seasonality and feeding strategies have a decisive influence on the fat, which can influence milk fatty acid profiles as a result of the balance between body fat mobilization and de novo synthesis in the mammary glands. In the present research, in pasture-fed cows compared to IFG, it was possible to highlight a significant increase in the concentration of conjugates of linoleic acid (CLA), whose production is correlated to the activity of stearoyl CoA desaturase (SCD), which converts vaccenic acid (C18:1 trans-11) into rumenic acid (C18:2 cis-9, trans 11) [19]. A higher desaturase index in ODG milk compared to IFG milk suggests a possible increase in SCD activity as reported by Lock [20] in cows fed with fresh grass. The production of vaccenic acid and stearic acid is correlated to α-linoleic (ALA) taken from the diet, according to Leiber et al. [21], which reported an increase in α-linoleic acid in the milk of cows with pasture feeding. In fact, this also happened in our study, where there was a higher concentration of ALA in ODG, with a possible increase in the synthesis of vaccenic and stearic acid. These data just discussed are of considerable interest if we consider the high health value associated with these fatty acids. High intake of ALA, rumenic and vaccenic acids is usually associated with an improvement in the consumer’s health. CLA has been shown to exert potent physiological functions such as antihypertensive, antidiabetic and anti-carcinogenic properties [22]. In order to support our thesis, differences were also highlighted by the concentration of palmitic acid (C16:0), higher in IFG than ODG during summer. This type of fatty acid is generally influenced by the animal’s feeding. Since there is a higher presence of C16:0 and, hypothetically, higher enzymatic activity of SCD, there is a greater conversation from C16:0 to C16:1. These differences in palmitic acid could be due to the enzymatic activity of fatty acid synthetase (FAS), which is fundamental in the process for de novo production of palmitate [23].
The trend of casein and whey protein during the seasons in ODG and IFG was monitored through the use of SDS-PAGE. Milk proteins were separated into three caseins (α, β and κ) and two whey proteins (α-La and β-Lg) from all analyzed samples. Under the experimental condition, the protein profile showed a major concentration of α and β caseins in ODG compared to the IFG, with the highest value in summer with a trend to decrease in winter. Meanwhile, κ casein showed a major concentration in IFG compared to ODG, with the same trend. This finding has been previously demonstrated to be strongly related to an increase in the dairy yield [24], an aspect of great interest for our study.
With specific regard to whey proteins, our hypothesis is that the observed variations can be attributed to the proteolytic activity associated with the lactation stage of animals. Many farmers make the final stage of lactation and dry period coincide with the winter season, lowering the protein content in milk, while they prefer to have animals grazing during the maximum lactation peak. Proteinase activity increases with advancing lactation; this enzyme promotes more plasmin and plasminogen to enter the milk. This is in agreement with Gina et al. [25], who claim that this increase in the protein fraction should not solely be because of increased plasminogen activation, but also be dependent on the lactation period of animals. This, associated with a higher intake and a predominantly protein pasture, leads to the cows taking in more energy. Consequently, the milk will have a higher casein content. With the reduction in the grazing period and the feeding of animals with silage or hay, the availability of essential amino acids (EAAs) useful for the synthesis of casein is reduced [26]. This reduction in the quality of animal feed would tend to increase even the synthesis and presence of whey proteins in milk [27]. The availability of EAAs is a key factor regulating protein synthesis in milk [28,29]. An increase in EAAs would favor the expression of some signaling pathways such as JAK2/STAT5 and mTOR [30], which would stimulate cow mammary epithelial cells. This increased ingestion of EAAs is attributable to pasture, mainly composed of alfalfa (Medicago sativa L.), a legume rich in these EAAs. In fact, our ODG pastures are particularly rich in this plant variety. All these results are in accordance with this study. The presence of β-casein and κ-caseins was confirmed with immunoblotting (WB).
The fat-soluble vitamin A is the main vitamin in milk, which is incorporated in the form of retinol and carotenoids. In forage essences, we find carotenoids; once ingested they are transformed into retinol, which is found only in animal tissues. Vitamin A has a key role in the positive functionality of the immune system and is also essential in vision, cell differentiation, reproduction and growth. On retinol analysis, the highest levels are normally found during summer, presumably because fresh pasture is rich in vitamins, which is ingested by cows [31]. In agreement with Ellis et al. [32], in our study, the highest concentration of retinol was found during the summer season, while the lower concentration was found in winter in ODG. Moreover, retinol concentration in IFG is lower compared to the ODG seasons. Presumably, this happens because milk vitamin content is reduced in silage, which is predominantly used in IFG, as previously described by Hulshof et al. [33]. An analogous seasonal effect on milk retinol concentration was reported by Revilla et al. [34]; they reported a lower retinol concentration in the winter period and a higher retinol concentration in summer, and claimed that the green fodder in the grazing period increased the retinol content. Another hypothesis is that a small percentage of retinol and carotenoids is associated with whey proteins and/or their concentration in the milk fat globule membrane [35].
5. Conclusions
The first finding coming from our survey is related to the fact that in the extensive farming system there were significant differences between summer and winter as opposed to intensive farming. Therefore, nutrition represents the most influential factor. However, we did find a few differences within the intensive system, thus confirming a role also for seasonality.
The obtained results specifically suggested the positive role of an outdoor grazing system on the milk composition compared to an indoor feeding system. In particular, it was possible to observe an increase in bioactive fatty acids, such as rumenic and oleic acids and the concentration of vitamin A, in pasture-fed cows. Moreover, caseins showed a major concentration in summer and a decrease in winter; these caseins could favor an increase in cheese-making yields. The nature of the forage consumed by dairy cows may have a large effect on both nutritional and sensorial characteristics of milk and dairy products. Further analysis should be performed in order to improve knowledge of the chemical mechanism at the source of these findings.
Author Contributions
Conceptualization, G.M.; methodology, M.F., C.G., F.B. and L.G.; investigation, M.F. and C.G.; resources, G.M.; data curation, F.B. and A.I.; writing—original draft preparation, M.F.; writing—review and editing, C.G.; supervision, G.M. and A.I.; project administration, G.M.; funding acquisition, G.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research is part a project funded by the Italian Ministry of University and Research (MIUR), entitled “Studio di processi per la produzione di nuovi prodotti idonei a migliorare la qualità e la sicurezza degli alimenti nel settore lattiero caseario”; CIPE-MIUR DM61317 (Scientific coordinator: Giuseppe Martino).
Institutional Review Board Statement
Ethical review and approval were not necessary for this study, because no breeding practices other than those normally adopted by the farm were introduced.
Data Availability Statement
The data presented in this study are available on reasonable request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- O’Callaghan, T.F.; Mannion, D.T.; Hennessy, D.; McAulie, S.; O’Sullivan, M.G.; Leeuwendaal, N.; Ross, R.P. Effect of pasture versus indoor feeding systems on quality characteristics, nutritional composition, and sensory and volatile properties of full-fat Cheddar cheese. J. Dairy Sci. 2017, 100, 6053–6073. [Google Scholar] [CrossRef] [PubMed]
- Kljajevic, N.V.; Tomasevic, I.B.; Miloradovic, Z.N.; Nedeljkovic, A.; Miocinovic, J.B.; Jovanovic, S.T. Seasonal variations of Saanen goat milk composition and the impact of climatic conditions. J. Food Sci. Technol. 2018, 55, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Parodi, P.W. Milk fat in human nutrition. Aust. J. Dairy Technol. 2004, 59, 3–59. [Google Scholar]
- Bernabucci, U.; Lacetera, N.; Ronchi, B.; Nardone, A. Effects of the hot season on milk protein fractions in Holstein cows. Anim. Res. 2002, 51, 25–33. [Google Scholar] [CrossRef]
- Del Prato, O.S. Tecnologie Del Latte; Edagricole: Bologna, Italy, 2005. [Google Scholar]
- Davis, J.P.; Foegeding, E.A. Foaming and Interfacial Properties of Polymerized Whey Protein Isolate. Food Chem. Toxicol. 2004, 69, C404–C410. [Google Scholar] [CrossRef]
- Gulati, A.; Galvin, N.; Lewis, E.; Hennessy, D.; O’Donovan, M.; McManus, J.J.; Fenelon, M.A.; Guinee, T.P. Outdoor grazing of dairy cows on pasture versus indoor feeding on total mixed ration: Effects on gross composition and mineral content milk during lactation. J. Dairy Sci. 2017, 101, 2710–2723. [Google Scholar] [CrossRef]
- Renna, M.; Lussiana, C.; Malfatto, V.; Mimosi, A.; Battaglini, L.M. Effect of exposure to heat stress conditions on milk yield and quality of dairy cows grazing on Alpine pasture. In Proceedings of the 9th European IFSA Symposium, Vienna, Austria, 4–8 July 2010; pp. 1338–1348. [Google Scholar]
- Cziszter, L.T.; Acatnincăi, S.; Neciu, F.C.; Neamt, R.; Ilie, D.E.; Costin, L.; Tripon, I. The influence of season on the cow milk quantity, quality and hygiene. Anim. Sci. Biotechnol. 2012, 45, 305–312. [Google Scholar]
- Bourauoi, R.; Lahmar, M.; Majdoub, A.; Djemali, M.; Belyea, R. The relantionship of temperature-humidity index with milk production of dairy cows in a Mediterranean climate. Anim. Res. 2002, 51, 479–491. [Google Scholar] [CrossRef]
- Larsen, M.K.; Nielsen, J.H.; Butler, G.; Leifert, C.; Slots, T.; Kristiansen, G.H.; Gustafsson, A.H. Milk quality as affected by feeding regimens in a country with climatic variation. J. Dairy Sci. 2010, 93, 2863–2873. [Google Scholar] [CrossRef]
- AOAC International. Official Methods of Analysis, 17th ed.; AOAC International: Washington, DC, USA, 2000. [Google Scholar]
- Brogna, D.; Nasri, S.; Salem, H.; Mele, M.; Serra, A.; Bella, M.; Priolo, A.; Makkar, H.P.S.; Vasta, V. Effect of dietary saponins from Quillaja saponaria L. on fatty acid composition and cholesterol content in muscle longissimus dorsi of lambs. Animal 2011, 5, 1124–1130. [Google Scholar] [CrossRef][Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of 377 protein dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Rasband, W.S. ImageJ Software; National Institutes of Health: Bethesda, MD, USA, 1997. [Google Scholar]
- Jenkins, T.C.; Harvatine, K.J. Lipid Feeding and Milk Fat Depression. Vet. Clin. N. Am.-Food Anim. Pract. 2014, 30, 623–642. [Google Scholar] [CrossRef] [PubMed]
- Erickson, P.S.; Kalscheurb, K.F. Nutrition and feeding of dairy cattle. In Animal Agriculture; Academic Press: Cambridge, MA, USA, 2020; pp. 157–180. [Google Scholar] [CrossRef]
- Farrell, H.M., Jr.; Jimenez-Flores, R.; Bleck, G.T.; Brown, E.M.; Butler, J.E.; Creamer, L.K.; Hicks, C.L.; Hollar, C.M.; Ng-Kwai-Hang, K.F.; Swaisgood, H.E. Nomenclature of the Proteins of Cows’ Milk—Sixth Revision. J. Dairy Sci. 2004, 87, 1641–1674. [Google Scholar] [CrossRef]
- Destaillats, F.; Trottier, J.P.; Galvez, J.M.G.; Angers, P. Analysis of α-Linolenic Acid Biohydrogenation Intermediates in Milk Fat with Emphasis on Conjugated Linolenic Acids. J. Dairy Sci. 2005, 88, 3231–3239. [Google Scholar] [CrossRef]
- Lock, A.L.; Garnsworthy, P.C. Seasonal variation in milk conjugated linoleic acid and Δ9-desaturase activity in dairy cows. Livest. Prod. Sci. 2003, 79, 47–59. [Google Scholar] [CrossRef]
- Leiber, F.; Kreuzer, M.; Nigg, D.; Wettstein, H.-R.; Scheeder, M.R.L. A study on the causes for the elevated n−3 fatty acids in cows’ milk of alpine origin. Lipids 2005, 40, 191–202. [Google Scholar] [CrossRef]
- Kazunori, K.; Teruyoshi, Y. Health benefits of conjugated linoleic acid (CLA). Obes. Res. Clin. 2014, 8, e525–e532. [Google Scholar] [CrossRef]
- Wakil, S.J. Fatty acid synthase, a proficient multifunctional enzyme. Biochemistry 1989, 28, 4523–4530. [Google Scholar] [CrossRef]
- Cipolat-Gotet, C.; Cecchinato, A.; Malacarne, M.; Bittante, G.; Summer, A. Variations in milk protein fractions affect the efficiency of the cheese-making process. J. Dairy Sci. 2018, 101, 8788–8804. [Google Scholar] [CrossRef]
- Gina, D.N.; Auldist, M.J.; Molan, P.C.; Stelwagen, K.; Prosser, C.G. Effects of stage of lactation and time of year on plasmin-derived proteolytic activity in bovine milk in New Zealand. J. Dairy Res. 2002, 69, 533–540. [Google Scholar] [CrossRef]
- Auldist, M.J.; Walsh, B.J.; Thomson, N.A. Seasonal and lactational influences on bovine milk composition in New Zealand. J. Dairy Res. 1998, 65, 401–411. [Google Scholar] [CrossRef]
- Gray, R.M.; MacKenzie, D.D.S. Effect of plane of nutrition on the concentration and yield of whey proteins in bovine milk. N. Z. J. Dairy Sci. 1987, 22, 157–165. [Google Scholar]
- Backwell, F.R.; Bequette, B.J.; Wilson, D.; Metcalf, J.A.; Franklin, M.F.; Beever, D.E.; Lobley, G.E.; MacRae, J.C. Evidence for the utilization of peptides for milk protein synthesis in the lactating dairy goat in vivo. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1996, 271, R955–R960. [Google Scholar] [CrossRef] [PubMed]
- Bobe, G.; Beitz, D.C.; Freeman, A.E.; Lindberg, G.L. Effect of Milk Protein Genotypes on Milk Protein Composition and Its Genetic Parameter Estimates. J. Dairy Sci. 1999, 82, 2797–2804. [Google Scholar] [CrossRef]
- Zhang, M.C.; Zhao, S.G.; Wang, S.S.; Luo, C.C.; Gao, H.N.; Zheng, N.; Wang, J.Q. d-Glucose and amino acid deficiency inhibits casein synthesis through JAK2/STAT5 and AMPK/mTOR signaling pathways in mammary epithelial cells of dairy cows. J. Dairy Sci. 2018, 101, 1737–1746. [Google Scholar] [CrossRef]
- Sauvant, P.; Grolier, P.; Azais-Braesco, V. Vitamin A, Nutritional significance. In Encyclopedia of Dairy Sciences; Academic Press: Cambridge, MA, USA, 2002. [Google Scholar]
- Ellis, K.A.; Monteiro, A.; Innocent, G.T.; Grove-White, D.; Cripps, P.; Graham McLean, W.; Howard, C.V.; Mihm, M. Investigation of the vitamins A and E and β-carotene content in milk from UK organic and conventional dairy farms. J. Dairy Res. 2007, 74, 484–491. [Google Scholar] [CrossRef]
- Nozière, P.; Graulet, B.; Lucas, A.; Martin, B.; Grolier, P.; Doreau, M. Carotenoids for ruminants: From forages to dairy products. Anim. Feed Sci. Technol. 2006, 131, 418–450. [Google Scholar] [CrossRef]
- Hulshof, P.J.M.; van Roekel-Jansen, T.; van de Bovenkamp, P.; West, C.E. Variation in retinol and carotenoid content of milk and milk products in The Netherlands. J. Food Compos. Anal. 2006, 19, 67–75. [Google Scholar] [CrossRef]
- Revilla, I.; Lobos-Ortega, I.; Vivar-Quintana, A.; González-Martín, M.I.; Hernández-Hierro, J.M.; González-Pérez, C. Variations in the contents of vitamins A and E during the ripening of cheeses with different compositions. Czech J. Food Sci. 2014, 32, 342–347. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).