Experimental Study on the Droplet Size and Charge-to-Mass Ratio of an Air-Assisted Electrostatic Nozzle
Abstract
:1. Introduction
2. Materials and Methods
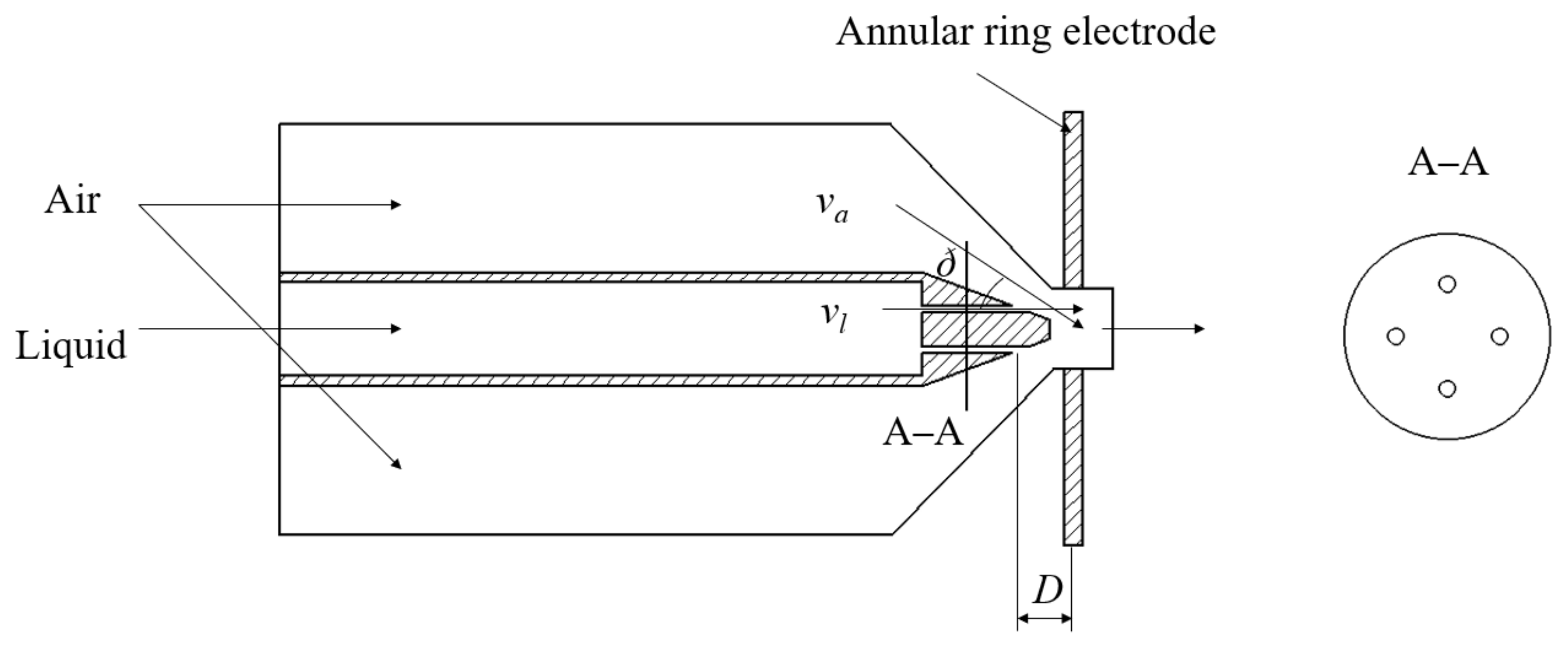
2.1. Air-Assisted Electrostatic Nozzle Design
2.2. Electrostatic Spray System Design
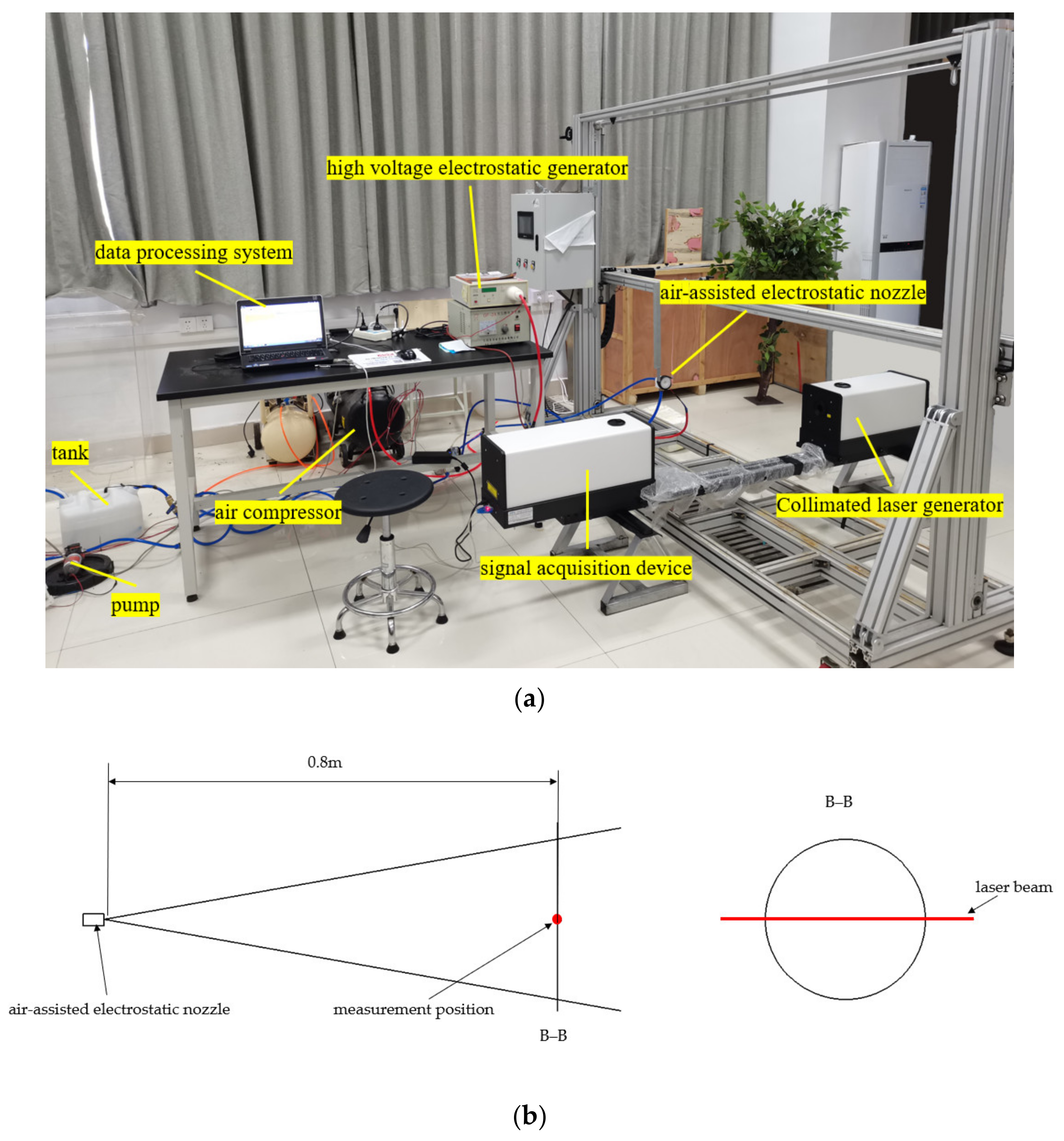
2.3. Experimental Set-Up to Measure the Droplet Size
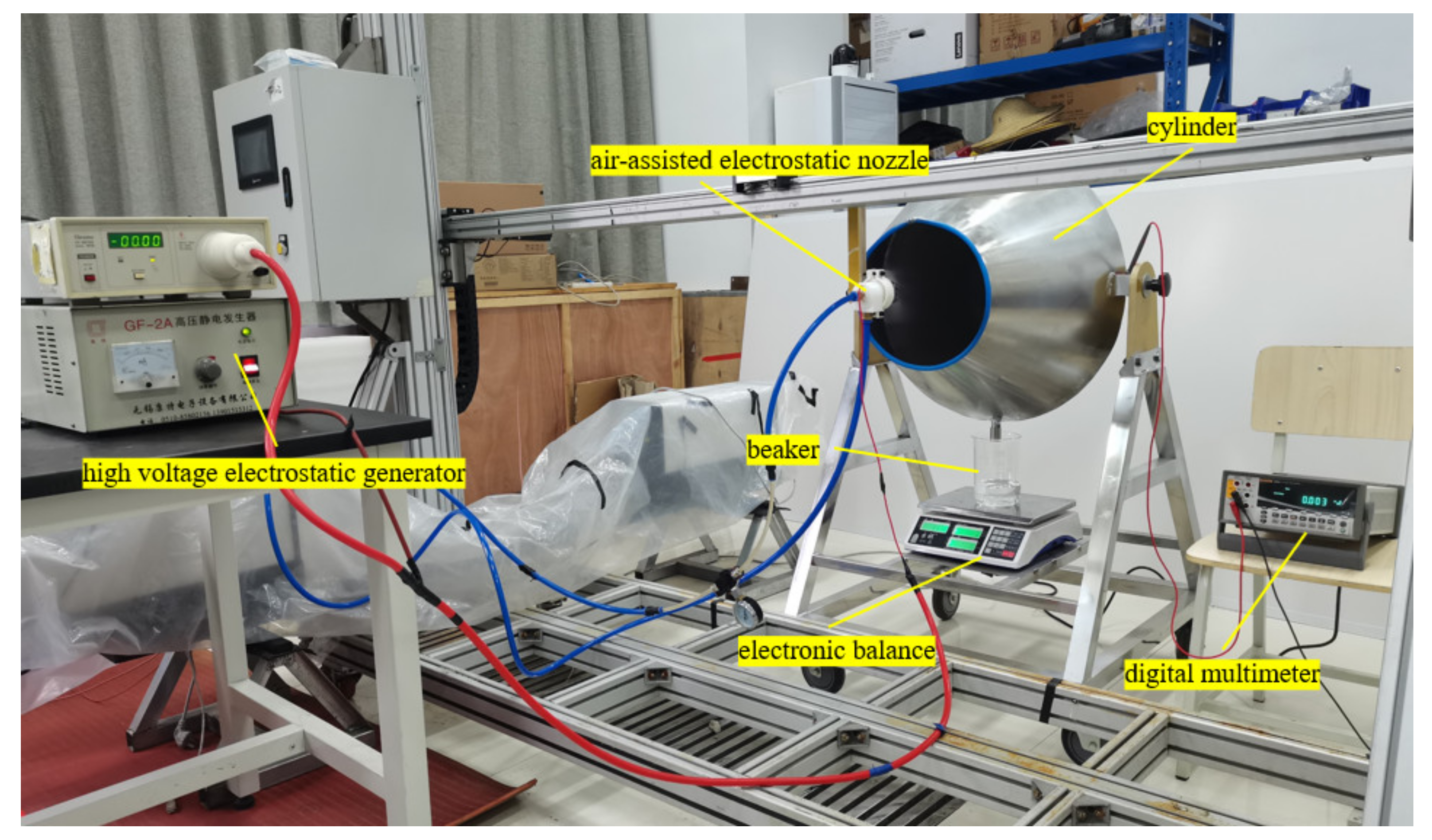
2.4. Experimental Set-Up to Measure the Charge-to-Mass Ratio
2.5. Laboratory Test
3. Results and Discussion
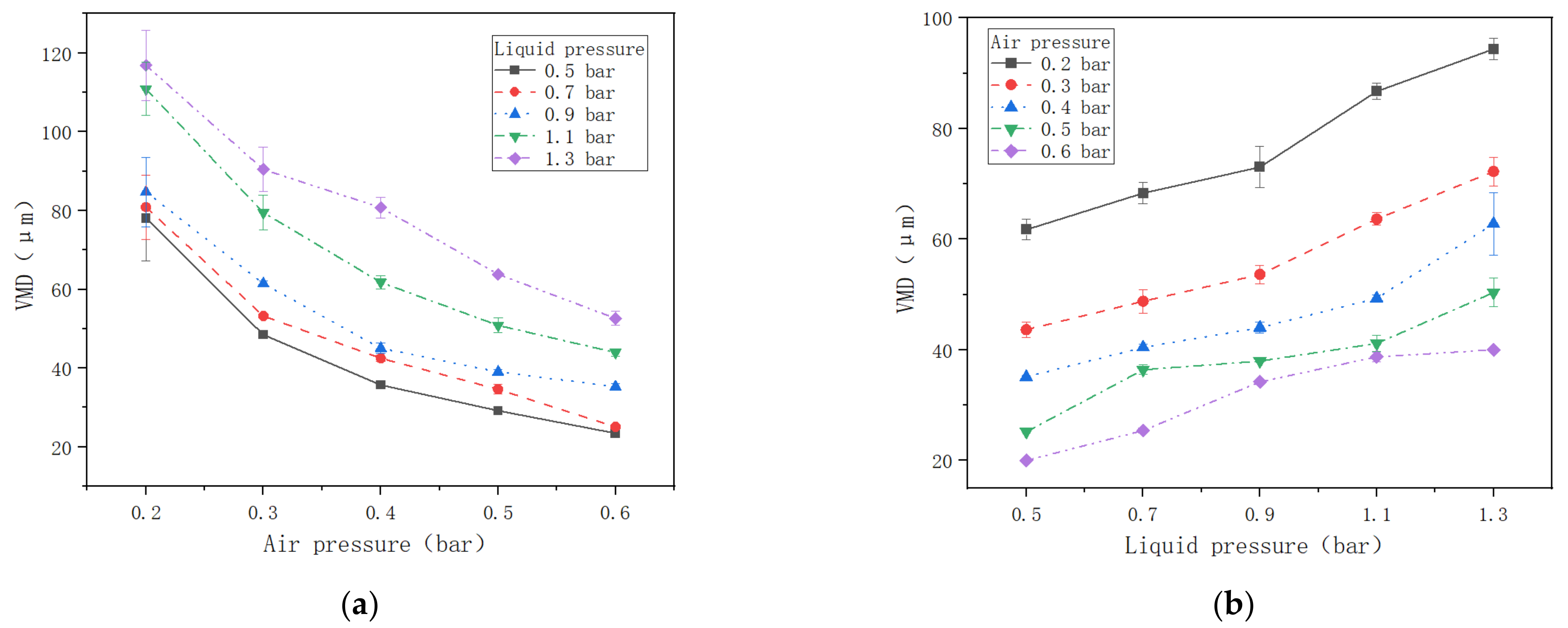
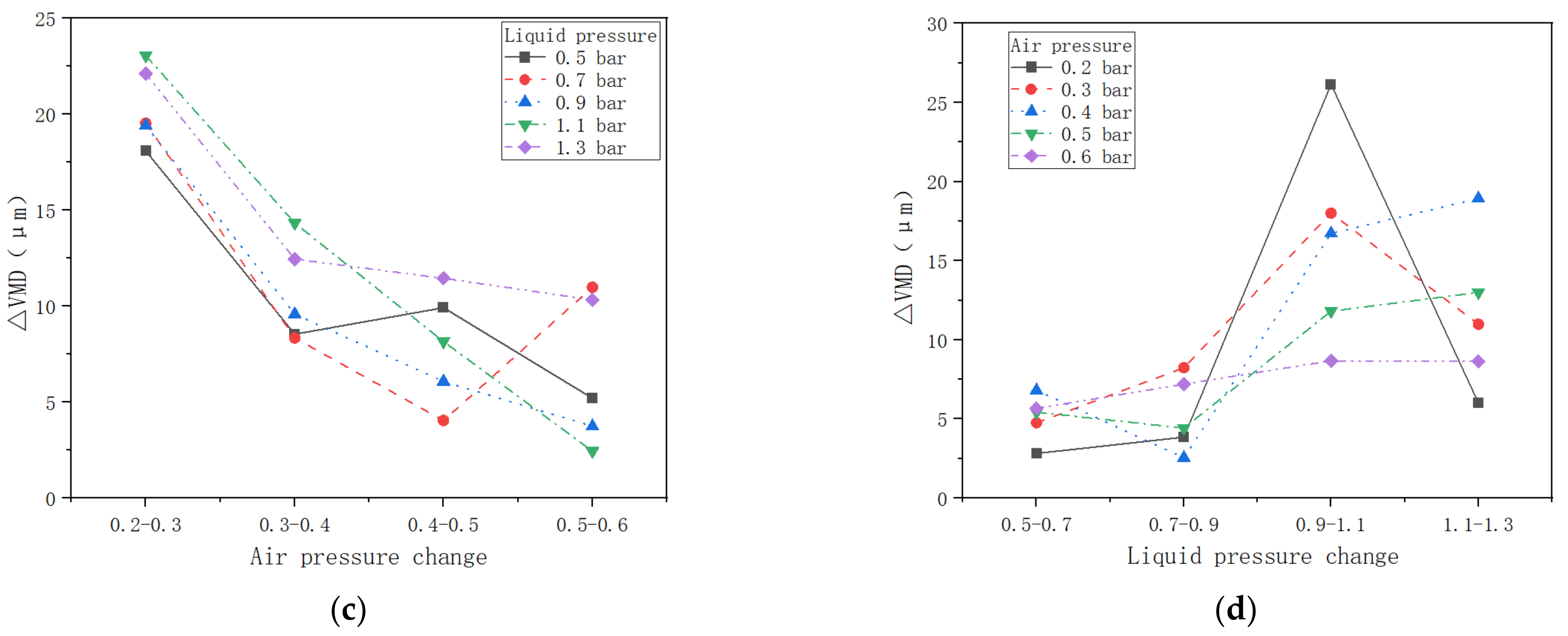
3.1. VMD Variation with Air Pressure and Liquid Pressure
3.2. VMD Variation with the Applied Voltage
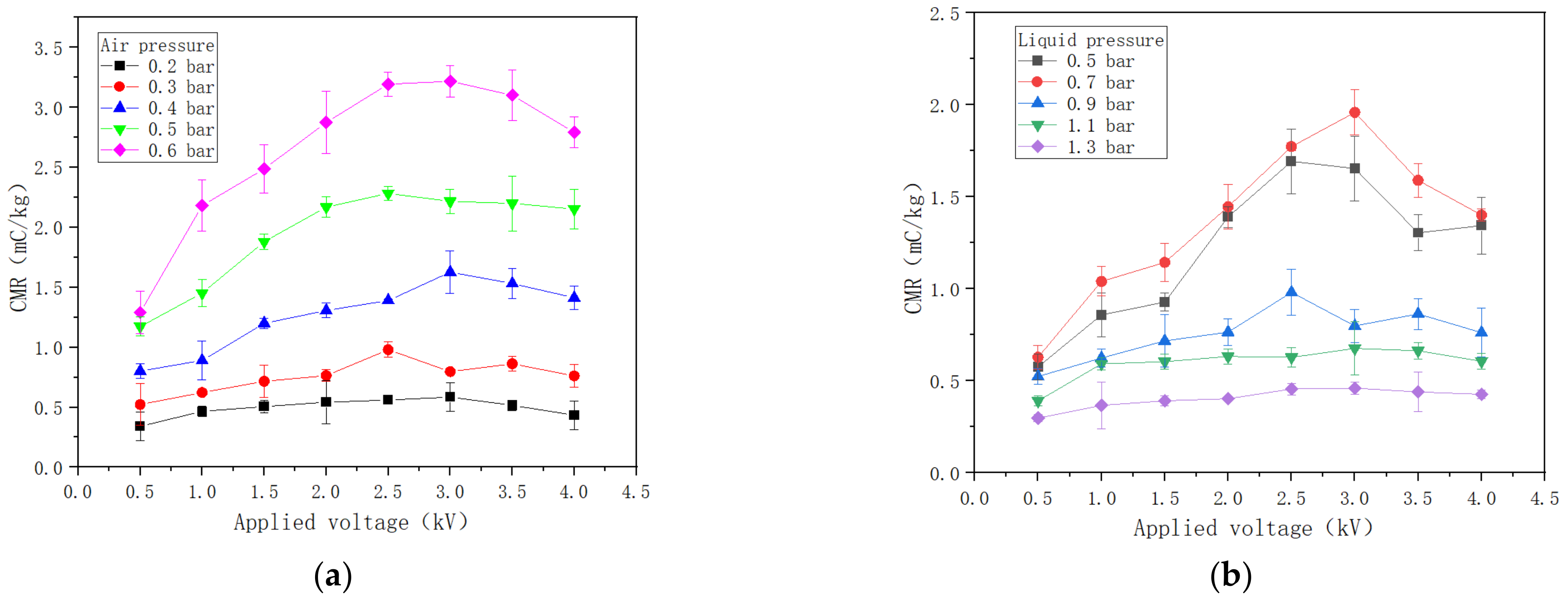
3.3. CMR Variation with the Applied Voltage
3.4. Relationship between the VMD and CMR
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chambers, J.E.; Greim, H.; Kendall, R.J.; Segner, H.; Sharpe, R.M.; Van Der Kraak, G. Human and ecological risk assessment of a crop protection chemical: A case study with the azole fungicide epoxiconazole. Crit. Rev. Toxicol. 2014, 44, 176–210. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Kang, C.; Jia, W.; Yang, W.; Wang, Y. The effect of spray structure of oil-based emulsion spray on the droplet characteristics. Biosyst. Eng. 2020, 198, 78–90. [Google Scholar] [CrossRef]
- Huang, Y. Characterization of spray deposition and drift from a low drift nozzle for aerial application at different application altitudes. Electron. Lett. 2011, 38, 967–968. [Google Scholar]
- Sinha, R.; Ranjan, R.; Khot, L.R.; Hoheisel, G.A.; Grieshop, M.J. Comparison of within canopy deposition for a solid set canopy delivery system (SSCDS) and an axial-fan airblast sprayer in a vineyard. Crop Prot. 2020, 132, 105124. [Google Scholar] [CrossRef]
- Maski, D.; Durairaj, D. Effects of charging voltage, application speed, target height, and orientation upon charged spray deposition on leaf abaxial and adaxial surfaces. Crop Prot. 2010, 29, 134–141. [Google Scholar] [CrossRef]
- Patel, M.K.; Sahoo, H.K.; Nayak, M.K.; Kumar, A.; Ghanshyam, C.; Kumar, A. Electrostatic Nozzle: New Trends in Agricultural Pesticides Spraying. In Proceedings of the National Conference on Emerging Fields in Engineering and Sciences (EFES-2015), Kobe, Japan, 18–20 November 2015. [Google Scholar]
- Patel, M.K.; Sharma, T.; Nayak, M.K.; Ghanshyam, C. Computational modeling and experimental evaluation of the effects of electrode geometry and deposition target on electrostatic spraying processes. Int. J. Comput. Appl. 2015, 124, 10e15. [Google Scholar]
- Almekinders, H.; Ozkan, H.E.; Reichard, D.L.; Carpenter, T.G.; Brazee, R.D. Spray Deposit Patterns of an Electrostatic Atomizer. Trans. Asae 1992, 35, 1361–1367. [Google Scholar] [CrossRef]
- Palumbo, J.C.; Coates, W.E. Air-assisted electrostatic application of pyrethroid and endosulfan mixtures for sweetpotato whitefly (Homoptera: Aleyrodidae) control and spray deposition in cauliflower. J. Econ. Entomol. 1996, 89, 970–980. [Google Scholar] [CrossRef]
- Patel, M.K.; Ghanshyam, C.; Kapur, P. Characterization of electrode material for electrostatic spray charging: Theoretical and engineering practices. J. Electrost. 2013, 71, 55–60. [Google Scholar] [CrossRef]
- Patel, M.K.; Kundu, M.; Sahoo, H.K.; Nayak, M.K. Enhanced performance of an air-assisted electrostatic nozzle: Role of electrode material and its dimensional considerations in spray charging. Eng. Agric. Environ. Food 2016, 9, 332–338. [Google Scholar] [CrossRef]
- Appah, S.; Zhou, H.; Wang, P.; Ou, M.; Jia, W. Charged monosized droplet behaviour and wetting ability on hydrophobic leaf surfaces depending on surfactant-pesticide concentrate formulation. J. Electrost. 2019, 100, 103356. [Google Scholar] [CrossRef]
- Laryea, G.N.; No, S.Y. Development of electrostatic pressure-swirl nozzle for agricultural applications. J. Electrost. 2003, 57, 129–142. [Google Scholar] [CrossRef]
- Gan-Mor, S.; Ronen, B.; Ohaliav, K. The effect of air velocity and proximity on the charging of sprays from conventional hydraulic nozzles. Biosyst. Eng. 2014, 121, 200–208. [Google Scholar] [CrossRef]
- Zhao, S.; Castle, G.; Adamiak, K. Factors affecting deposition in electrostatic pesticide spraying. J. Electrost. 2008, 66, 594–601. [Google Scholar] [CrossRef]
- Krupa, A.; Jaworek, A.; Sobczyk, A.T.; Marchewicz, A.; Szudyga, M.; Antes, T. Charged spray generation for gas cleaning applications. J. Electrost. 2013, 71, 260–264. [Google Scholar] [CrossRef]
- Rayleigh, L. On the Equilibrium of Liquid Conducting Masses Charged with Electricity. Phil. Mag. J. Sci. 1882, 14, 87. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Wang, J.; Wang, Z.; Yu, K.; Xu, H.; Wang, D.; Zhang, W. Impact dynamics of a charged droplet onto different substrates. Phys. Fluids 2021, 33, 102111. [Google Scholar] [CrossRef]
- Liu, L.; Fu, Q.; Yang, L. Theoretical Atomization Model of Liquid Sheet Generated by Coaxial Swirl Injectors. Int. J. Multiph. Flow 2021, 142, 103725. [Google Scholar] [CrossRef]
- Patel, M.K.; Praveen, B.; Sahoo, H.K.; Patel, B.; Kumar, A.; Singh, M.; Nayak, M.K.; Rajan, P. An advance air-induced air-assisted electrostatic nozzle with enhanced performance. Comput. Electron. Agric. 2017, 127, 280–288. [Google Scholar] [CrossRef]
- Kacprzyk, R.; Lewandowski, M. Post-dispersion electrification of droplets. J. Electrost. 2016, 79, 33–37. [Google Scholar] [CrossRef]
- Biris, A.S.; Saini, D.; Srirama, P.K.; Mazumder, M.K.; Sims, R.A.; Calle, C.I.; Buhler, C.R. Electrodynamic removal of contaminant particles and its applications. In Proceedings of the Industry Applications Conference, Seattle, WA, USA, 3–7 October 2004. [Google Scholar]
- Chen, H.; Zhao, Y. Electrostatic Induction Field and Charging Property of Droplet in Electrostatic Spraying Process. High Volt. Eng. 2010, 36, 2519–2524. [Google Scholar]
- Lan, Y.B.; Zhang, H.Y.; Wen, S.; Li, S.H. Analysis and Experiment on Atomization Characteristics and Spray Deposition of Electrostatic Nozzle. Trans. Chin. Soc. Agric. Mach. 2018, 49, 130–139. [Google Scholar]
- Beck-Broichsitter, M. Polymer-coated aperture plates for tailored atomization processes. Mater. Sci. Eng. C 2020, 110, 110666. [Google Scholar] [CrossRef] [PubMed]
- Pontinen, P.J.; Airaksinen, O. Comparative effects of exposure to different light sources (He-Ne laser, InGaAl diode laser, a specific type of noncoherent LED) on skin blood flow for the head. Acupunct. Electro-Ther. Res. 1996, 21, 105–118. [Google Scholar]
- Kumar, S.S.; He, Z.; Hogan, C.J.; Fredericks, S.A.; Hong, J. Evaluation of laser diffraction-based particle size measurements using digital inline holography. Meas. Sci. Technol. 2020, 31, 125201. [Google Scholar] [CrossRef]
- Patel, M.K.; Khanchi, A.; Chauhan, A.; Kumar, A.; Akkireddi, S.R.; Jangra, A.; Kanawade, R.; Manivannan, N.; Mitchell, G.R. Real-time measurement of droplet size and its distribution of an air-induced air-assisted electrostatic nozzle. J. Electrost. 2022, 115, 103665. [Google Scholar] [CrossRef]
- Law, S.E.; Bailey, A.G. Perturbations of Charged-Droplet Trajectories Caused by Induced Target Corona: LDA Analysis. Industry Applications. IEEE Trans. Ind. Appl. 1984, IA-20, 1613–1622. [Google Scholar] [CrossRef]
- Wang, S.; He, X.; Song, J.; Zhong, C.; Wang, Z.; Qi, P.; Ling, Y. Charging and spraying performance test of bipolar contact electrostatic spraying system for unmanned aerial vehicle. Trans. Chin. Soc. Agric. Eng. 2018, 34, 82–89. [Google Scholar]
- Appah, S.; Wang, P.; Ou, M.; Gong, C.; Jia, W. Review of electrostatic system parameters, charged droplets characteristics and substrate impact behavior from pesticides spraying. Int. J. Agric. Biol. Eng. 2019, 12, 9. [Google Scholar] [CrossRef]
- Appah, S.; Jia, W.; Ou, M.; Wang, P.; Gong, C. Investigation of Optimum Applied Voltage, Liquid Flow Pressure, and Spraying Height for Pesticide Application by Induction Charging. Appl. Eng. Agric. 2019, 35, 795–804. [Google Scholar] [CrossRef]
- Nukiyama, S.; Tanasawa, Y. Experiments on the Atomization of Liquids in an Air Stream; Report 3: On the Droplet-Size Distribution in An Atomized Jet. 1950. Available online: https://www.researchgate.net/publication/284091443_Experiments_on_the_atomization_of_liquids_in_an_airstream (accessed on 6 May 2022).
- Cao, J. Liquid Sprays; China Machine Press: Beijing, China, 2005. [Google Scholar]
- Ma, X.; Guo, L.; Wen, Z. Atomization characteristics of multi-nozzle electrostatic spray and field experiment. Trans. Chin. Soc. Agric. Eng. 2020, 36, 73–82. [Google Scholar]
- Jia, W.; Zhu, H. Dynamics of water droplet impact and spread on soybean leaves. Trans. Asabe 2015, 58, 1009–1016. [Google Scholar]
- Grimm, R.L.; Beauchamp, J.L. Evaporation and Discharge Dynamics of Highly Charged Multicomponent Droplets Generated by Electrospray Ionization. J. Phys. Chem. A 2009, 114, 1411–1419. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.F.; Huang, J.W.; Wang, Z.T.; Wang, X.Y.; Hang, Q.M. Analysis of charged state of droplet during evaporation process. High Volt. Eng. 2010, 36, 2531–2536. [Google Scholar]
- Pascuzzi, S.; Cerruto, E. Spray deposition in “tendone” vineyards when using a pneumatic electrostatic sprayer. Crop Prot. 2015, 68, 1–11. [Google Scholar] [CrossRef]





| No. | Air Pressure (bar) | Liquid Pressure (bar) | Applied Voltage 1 1 (kV) | Applied Voltage 2 2 (kV) |
|---|---|---|---|---|
| 1 | 0.2 | 0.5 | 0 | 0.5 |
| 2 | 0.3 | 0.7 | 1.0 | 1.0 |
| 3 | 0.4 | 0.9 | 2.0 | 1.5 |
| 4 | 0.5 | 1.1 | 3.0 | 2.0 |
| 5 | 0.6 | 1.3 | 4.0 | 2.5 |
| 6 | 3.0 | |||
| 7 | 3.5 | |||
| 8 | 4.0 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, S.; Zhang, J.; Jia, W.; Ou, M.; Zhou, H.; Dong, X.; Chen, H.; Wang, M.; Chen, Y.; Yang, S. Experimental Study on the Droplet Size and Charge-to-Mass Ratio of an Air-Assisted Electrostatic Nozzle. Agriculture 2022, 12, 889. https://doi.org/10.3390/agriculture12060889
Dai S, Zhang J, Jia W, Ou M, Zhou H, Dong X, Chen H, Wang M, Chen Y, Yang S. Experimental Study on the Droplet Size and Charge-to-Mass Ratio of an Air-Assisted Electrostatic Nozzle. Agriculture. 2022; 12(6):889. https://doi.org/10.3390/agriculture12060889
Chicago/Turabian StyleDai, Shiqun, Jiayao Zhang, Weidong Jia, Mingxiong Ou, Huitao Zhou, Xiang Dong, Hong Chen, Ming Wang, Yu Chen, and Shuai Yang. 2022. "Experimental Study on the Droplet Size and Charge-to-Mass Ratio of an Air-Assisted Electrostatic Nozzle" Agriculture 12, no. 6: 889. https://doi.org/10.3390/agriculture12060889
APA StyleDai, S., Zhang, J., Jia, W., Ou, M., Zhou, H., Dong, X., Chen, H., Wang, M., Chen, Y., & Yang, S. (2022). Experimental Study on the Droplet Size and Charge-to-Mass Ratio of an Air-Assisted Electrostatic Nozzle. Agriculture, 12(6), 889. https://doi.org/10.3390/agriculture12060889





