Rapeseed Meal and Its Application in Pig Diet: A Review
Abstract
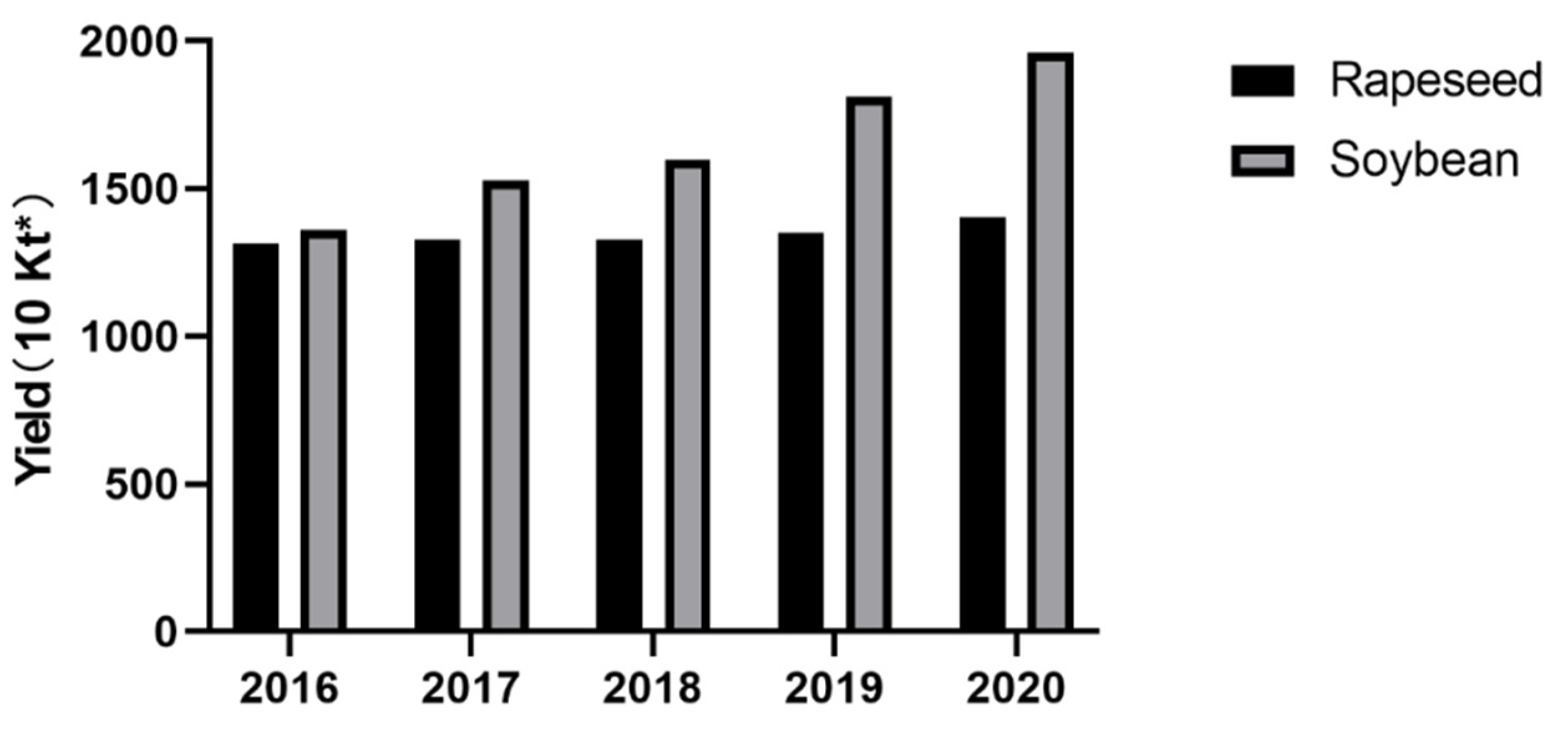
1. Introduction
2. Composition and Characteristics of Rapeseed Meal
2.1. Crude Protein and Amino Acids
2.2. Crude Fat
2.3. Carbohydrate
2.4. Minerals and Vitamins
2.5. Anti-Nutritional Factors
2.5.1. Glucosinolates
2.5.2. Phytic Acid
2.5.3. Tannin
3. Improvements to the Nutritive Values of Rapeseed Meal
3.1. Optimize Processing Conditions
3.2. Reduce Fiber Content
3.3. Biological Method to Improve Utilization of Rapeseed Meals
3.3.1. Enzymic Method
3.3.2. Microbiological Fermentation
4. Use of Dietary Rapeseed Meal in Pig Nutrition
4.1. Growth Performance and Meat Quality
4.2. Reproduction of Sows
5. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- United States Department of Agriculture. Oilseeds: World Markets and Trade; United States Department of Agriculture: Washington, DC, USA, 2021; pp. 1–38.
- Wickramasuriya, S.S.; Yi, Y.J.; Yoo, J.; Kang, N.K.; Heo, J.M. A review of canola meal as an alternative feed ingredient for ducks. J. Anim. Sci. Technol. 2015, 57, 29. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.L.; Pan, Y.Y.; Wang, C.; Li, X.C.; Lu, Y.Q.; Tian, Z.; Kuang, L.Q.; Wang, X.F.; Dun, X.L.; Wang, H.Z. Multi-functional development and utilization of rapeseed: Comprehensive analysis of the nutritional value of rapeseed sprouts. Food 2022, 11, 778. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. Nutrient Requirements of Swine; National Research Council: Washington, DC, USA, 2012. [Google Scholar]
- Wang, L.; Hu, Q.; Li, P.; Lai, C.; Li, D.; Zang, J.; Ni, S. Development and validation of equations for predicting the metabolizable energy value of double-low rapeseed cake for growing pigs. Animals 2021, 11, 1168. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, H.; Han, B.; Li, H.Y.; Liu, X.L. Improvement of nutritional value, molecular weight patterns (soluble peptides), free amino acid patterns, total phenolics and antioxidant activity of fermented extrusion pretreatment rapeseed meal with Bacillus subtilis YY-1 and Saccharomyces cerevisiae YY-2. LWT 2022, 160, 113280. [Google Scholar]
- Shi, C.; He, J.; Wang, J.; Yu, J.; Yu, B.; Mao, X.; Zheng, P.; Huang, Z.; Chen, D. Effects of Aspergillus niger fermented rapeseed meal on nutrient digestibility, growth performance and serum parameters in growing pigs. Anim. Sci. J. 2016, 87, 557–563. [Google Scholar] [CrossRef]
- Oliveira, A.M.; Yu, P. Research progress and future study on physicochemical, nutritional, and structural characteristics of canola and rapeseed feedstocks and co-products from bio-oil processing and nutrient modeling evaluation methods. Crit. Rev. Food Sci. Nutri. 2022, 1–7. [Google Scholar] [CrossRef]
- Radfar, M.; Rogiewicz, A.; Slominski, B.A. Chemical composition and nutritive value of canola-quality Brassica juncea meal for poultry and the effect of enzyme supplementation. Anim. Feed Sci. Tech. 2017, 225, 97–108. [Google Scholar] [CrossRef]
- Slominski, B.A.; Jia, W.; Rogiewicz, A.; Nyachoti, C.M.; Hickling, D. Low-fiber canola. Part 1. Chemical and nutritive composition of the meal. J. Agric. Food Chem. 2012, 60, 12225–12230. [Google Scholar]
- Mejicanos, G.A. Tail End Dehulling of Canola Meal Chemical Composition and Nutritive Value of Dehulled Meal for Brolier Chicken and Weaned Pigs; University of Manitoba: Winnipeg, MB, Canada, 2015. [Google Scholar]
- Mosenthin, R.; Messerschmidt, U.; Sauer, N.; Carre, P.; Quinsac, A.; Schone, F. Effect of the desolventizing/toasting process on chemical composition and protein quality of rapeseed meal. J. Anim. Sci. Biotechnol. 2016, 7, 205–216. [Google Scholar]
- Li, P.; Wang, F.; Wu, F.; Wang, J.; Liu, L.; Lai, C. Chemical composition, energy and amino acid digestibility in double-low rapeseed meal fed to growing pigs. J. Anim. Sci. Biotechnol. 2015, 6, 1–10. [Google Scholar]
- Banaszkiexicz, T.; Nutritional Value of Soybean Meal. Soybean and Nutrition. 2011, pp. 1–20. Available online: www.intechopen.com/books/nutritional-value-of-soybean-meal (accessed on 20 April 2022).
- Nega, T. Review on nutritional limitations and opportunities of using rapeseed meal and other rape seed byproducts in animal feeding. J. Nutr. Health Food Engl. 2018, 8, 43–48. [Google Scholar]
- Khajali, F.; Slominski, B.A. Factors that affect the nutritive value of canola meal for poultry. Poult. Sci. 2012, 91, 2564–2575. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Villanea, S.; Bruininx, E.M.; Gruppen, H.; Hendriks, W.H.; Carre, P.; Quinsac, A.; van der Poel, A.F. Physical and chemical changes of rapeseed meal proteins during toasting and their effects on in vitro digestibility. J. Anim. Sci. Biotechnol. 2016, 7, 62. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Y.; Li, P.L.; Liu, L.; Zhang, S.; Li, J.T.; Zhang, L.X.; Li, D.F. Ether extract and acid detergent fibre but not glucosinolates are determinants of the digestible and metabolizable energy of rapeseed meal in growing pigs. J. Appl. Anim. Res. 2020, 48, 384–389. [Google Scholar] [CrossRef]
- Adewole, D.I.; Rogiewicz, A.; Dyck, B.; Slominski, B.A. Chemical and nutritive characteristics of canola meal from Canadian processing facilities. Anim. Feed Sci. Technol. 2016, 222, 17–30. [Google Scholar] [CrossRef]
- Theodoridou, K.; Yu, P. Effect of processing conditions on the nutritive value of canola meal and presscake. Comparison of the yellow and brown-seeded canola meal with the brown-seeded canola presscake. J. Sci. Food Agric. 2013, 93, 1986–1995. [Google Scholar] [CrossRef]
- Bojanowska, M. Changes in chemical composition of rapeseed meal during storage, influencing nutritional value of its protein and lipid fractions. J. Anim. Feed Sci. 2017, 26, 157–164. [Google Scholar] [CrossRef]
- Slominski, B.A.; Campbell, L.D. Non-starch Polysaccharides of Canola Meal: Qulification, Digestibility in Poutry and Potential Benefit of Dietary Enzyme Supplementation. J. Sci. Food Agric. 1990, 53, 175–184. [Google Scholar] [CrossRef]
- Pustjens, A.M.; Vries, S.D.; Schols, H.A.; Gruppen, H.; Gerrits, W.J.; Kabel, M.A. Understanding carbohydrate structures fermented or resistant to fermentation in broilers fed rapeseed (Brassica napus) meal to evaluate the effect of acid treatment and enzyme addition. Poult. Sci. 2014, 93, 926–934. [Google Scholar] [CrossRef]
- Chmielewska, A.; Kozlowska, M.; Rachwal, D.; Wnukowski, P.; Amarowicz, R.; Nebesny, E.; Rosicka-Kaczmarek, J. Canola/rapeseed protein—nutritional value, functionality and food application: A review. Crit. Rev. Food Sci. Nutr. 2020, 61, 3836–3856. [Google Scholar] [CrossRef]
- Carre, P.; Citeau, M.; Robin, G.; Estorges, M. Hull content and chemical composition of whole seeds, hulls and germs in cultivars of rapeseed (Brassica napus). OCL 2016, 23, A302. [Google Scholar] [CrossRef]
- Beyzi, E.; Gunes, A.; Beyzi, S.B.; Konca, Y. Changes in fatty acid and mineral composition of rapeseed (Brassica napus ssp. oleifera L.) oil with seed sizes. Ind. Crops Prod. 2019, 129, 10–14. [Google Scholar] [CrossRef]
- Summer, J.D.; Bedford, M.; Spratt, D. Amino acid supplementation of canola meal. Can. J. Anim. Sci. 1989, 69, 469–475. [Google Scholar] [CrossRef]
- Szydlowska-Czerniak, A. Rapeseed and its products--sources of bioactive compounds: A review of their characteristics and analysis. Crit. Rev. Food Sci. Nutr. 2013, 53, 307–330. [Google Scholar] [CrossRef]
- Vuorela, S.; Meyer, A.S.; Heinonen, M. Impact of isolation method on the antioxidant activity of rapeseed meal phenolics. J. Agric. Food Chem. 2004, 52, 8202–8207. [Google Scholar] [CrossRef]
- Chen, S.; Andreasson, E. Update on glucosinolate metabolism and transport. Plant Physiol. Biochem. 2001, 39, 743–758. [Google Scholar] [CrossRef]
- Konkol, D.; Szmigiel, I.; Domzal-Kedzia, M.; Kulazynski, M.; Krasowska, A.; Opalinski, S.; Korczynski, M.; Lukaszewicz, M. Biotransformation of rapeseed meal leading to production of polymers, biosurfactants, and fodder. Bioorganic Chem. 2019, 93, 102865. [Google Scholar] [CrossRef]
- Lee, J.W.; Woyengo, T.A. Growth performance, organ weights, and blood parameters of nursery pigs fed diets containing increasing levels of cold-pressed canola cake. J. Anim. Sci. 2018, 96, 4704–4712. [Google Scholar] [CrossRef]
- Goyal, A.; Tanwar, B.; Sihag, M.K.; Kumar, V.; Sharma, V.; Soni, S. Rapeseed Canola (Brassica napus) Seed. In Oilseeds: Health Attributes and Food Applications; Springer: Singapore, 2021; pp. 56–57. [Google Scholar]
- Landero, J.L.; Beltranena, E.; Zijlstra, R.T. Diet nutrient digestibility and growth performance of weaned pigs fed solvent-extracted Brassica juncea canola meal. Anim. Feed Sci. Technol. 2013, 180, 64–72. [Google Scholar] [CrossRef]
- Zhou, Q.; Tang, H.; Jia, X.; Zheng, C.; Huang, F.H.; Zhang, M. Distribution of glucosinolate and pungent odors in rapeseed oils from raw and microwaved seeds. Int. J. Food Prop. 2018, 21, 2296–2308. [Google Scholar] [CrossRef]
- Lee, J.W.; Wang, S.; Huang, Y.; Seefeldt, T.; Donkor, A.; Logue, B.A.; Woyengo, T.A. Toxicity of canola-derived glucosinolates in pigs fed resistant starch-based diets. J. Anim. Sci. 2020, 98, 1–10. [Google Scholar] [CrossRef]
- Tripathi, M.K.; Mishra, A.S. Glucosinolates in animal nutrition A review. Anim. Feed Sci. Technol. 2007, 132, skaa111. [Google Scholar] [CrossRef]
- Velayudhan, D.E.; Schuh, K.; Woyengo, T.A.; Sands, J.S.; Nyachoti, C.M. Effect of expeller extracted canola meal on growth performance, organ weights, and blood parameters of growing pigs. J. Anim. Sci. 2017, 95, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Seneviratne, R.W.; Young, M.G.; Beltranena, E.; Goonewardene, L.A.; Newkirk, R.W.; Zijlstra, R.T. The nutritional value of expeller-pressed canola meal for grower-finisher pigs. J. Anim. Sci. 2010, 88, 2073–2083. [Google Scholar] [CrossRef]
- Gresse, R.; Chaucheyras-Durand, F.; Fleury, M.A.; Van de Wiele, T.; Forano, E.; Blanquet-Diot, S. Gut Microbiota dysbiosis in postweaning piglets: Understanding the keys to health. Trends Microbiol. 2017, 25, 851–873. [Google Scholar] [CrossRef]
- Moeser, A.J.; Pohl, C.S.; Rajput, M. Weaning stress and gastrointestinal barrier development: Implications for lifelong gut health in pigs. Anim. Nutr. 2017, 3, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Pluske, J.R.; Turpin, D.L.; Kim, J.C. Gastrointestinal tract (gut) health in the young pig. Anim. Nutr. 2018, 4, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.J.; Wang, Y.P.; Liu, S.Y.; Huang, J.J.; Zhai, Z.X.; He, C.; Ding, J.M.; Wang, J.; Wang, H.J.; Fan, W.B.; et al. The dynamic distribution of porcine microbiota across different ageds and gastrointestinal tract segments. PLoS ONE 2015, 10, e0117441. [Google Scholar]
- Jacela, J.Y.; Derouchey, J.M.; Tokach, M.D.; Goodband, R.D.; Nelssen, J.L. Feed additives for swine: Fact sheets—flavors and mold inhibitors, mycotoxin binders, and antioxidants. J. Swine Health Prod. 2010, 18, 27–32. [Google Scholar] [CrossRef]
- Nissar, J.; Ahad, T.; Naik, H.R.; Hussain, S.Z. A review phytic acid as antinutrient or nutraceutical. J. Pharmacogn. Phytochem. 2017, 6, 1554–1560. [Google Scholar]
- Hashmi, S.I.; Satwadhar, P.N.; Khotpal, R.R.; Deshpande, H.W.; Syed, K.A.; Vibhute, B.P. Rapeseed meal nutraceuticals. J. Oilseed Brassica 2010, 1, 43–54. [Google Scholar]
- Tanwar, B.; Modgil, R.; Goyal, A. Antinutritional factors and hypocholesterolemic effect of wild apricot kernel (Prunus armeniaca L.) as affected by detoxification. Food Funct. 2018, 9, 2121–2135. [Google Scholar] [CrossRef] [PubMed]
- Woyengo, T.A.; Kiarie, E.; Nyachoti, C.M. Energy and amino acid utilization in expeller-extracted canola meal fed to growing pigs. J. Anim. Sci. 2010, 88, 1433–1441. [Google Scholar] [CrossRef] [PubMed]
- Seneviratne, R.W.; Beltranena, E.; Newkirk, R.W.; Goonewardene, L.A.; Zijlstra, R.T. Processing conditions affect nutrient digestibility of cold-pressed canola cake for grower pigs. J. Anim. Sci. 2011, 89, 2452–2461. [Google Scholar] [CrossRef]
- Toghyani, M.; Rodgers, N.; Barekatain, M.R.; Iji, P.A.; Swick, R.A. Apparent metabolizable energy value of expeller-extracted canola meal subjected to different processing conditions for growing broiler chickens. Poult. Sci. 2014, 93, 2227–2236. [Google Scholar] [CrossRef]
- Ton, L.B.; Neik, T.X..; Batley, J. The Use of Genetic and Gene Technologies in Shaping Modern Rapeseed Cultivars (Brassica napus L.). Genes 2020, 11, 1116. [Google Scholar] [CrossRef]
- Slominski, B.A.; Simbaya, J.; Campbell, L.D.; Rakow, G.; Guenter, W. Nutritive value for broilers of meals derived from newly. Anim. Feed Sci. Technol. 1999, 79, 249–262. [Google Scholar] [CrossRef]
- Kracht, W.; Danicke, S.; Kluge, H.; Keller, K.; Matzke, W.; Hennig, U.; Schumann, W. Effect of dehulling of rapeseed on feed value and nutrient digestibility of rape products in pigs. Arch. Anim. Nutr. 2004, 58, 389–404. [Google Scholar] [CrossRef]
- Zhou, X.; Zijlstra, R.T.; Beltranena, E. Nutrient digestibility of solvent-extracted Brassica napus and Brassica juncea. J. Anim. Sci. 2015, 93, 217–228. [Google Scholar] [CrossRef][Green Version]
- Hansen, J.O.; Skrede, A.; Mydland, L.T.; Overland, M. Fractionation of rapeseed meal by milling, sieving and air classification-effect on crude protein, amino acids and fiber content and digestibility. Ani. Feed Sci. Technol. 2017, 230, 143–153. [Google Scholar] [CrossRef]
- Zhang, Z.B.; Kornegay, E.T.; Radcliffe, J.S.; Wilson, J.H.; Veit, H.P. Comparison of phytase from genetically engineered Aspergillus and canola in weanling pig diets. J. Anim. Sci. 2000, 78, 2868–2878. [Google Scholar] [CrossRef] [PubMed]
- Kasprowicz-Potocka, M.; Zaworska-Zakrzewska, A.; Rutkowski, A. Effect of Phytase on Digestibility and Performance of Growing and Finishing Pigs Fed Diets with Lupins and Rapeseed Meal. J. Agric. Sci. Technol. A 2020, 10, 216–227. [Google Scholar] [CrossRef]
- Maison, T.; Liu, Y.; Stein, H.H. Apparent and standardized total tract digestibility by growing pigs of phosphorus in canola meal from North America and 00-rapeseed meal and 00-rapeseed expellers from Europe without and with microbial phytase. J. Anim. Sci. 2015, 93, 3494–3502. [Google Scholar] [CrossRef]
- Velayudhan, D.E.; Hossain, M.M.; Regassa, A.; Nyachoti, C.M. Effect of canola meal inclusion as a major protein source in gestation and lactation sow diets with or without enzymes on reproductive performance, milk composition, fecal bacterial profile and nutrient digestibility. Anim. Feed Sci. Technol. 2018, 241, 141–150. [Google Scholar] [CrossRef]
- Li, P.; Lyu, Z.Q.; Wang, L.; Huang, B.B.; Lai, C.H.; Miglior, F. Nutritive values of double-low rapeseed expellers and rapeseed meal with or without supplementation of multi-enzyme in pigs. Can. J. Anim. Sci. 2020, 100, 729–738. [Google Scholar] [CrossRef]
- Torres-Pitarch, A.; McCormack, U.M.; Beattie, V.E.; Magowan, E.; Gardiner, G.E.; Pérez-Vendrell, A.M.; Torrallardona, D.; O’Doherty, J.V.; Lawlor, P.G. Effect of phytase, carbohydrase, and protease addition to a wheat distillers dried grains with solubles and rapeseed based diet on in vitro ileal digestibility, growth, and bone mineral density of grower-finisher pigs. Livest. Sci. 2018, 216, 94–99. [Google Scholar] [CrossRef]
- Long, C.; Venema, K. Pretreatment of Rapeseed Meal Increases Its Recalcitrant Fiber Fermentation and Alters the Microbial Community in an in vitro Model of Swine Large Intestine. Front. Microbiol. 2020, 11, 58826. [Google Scholar] [CrossRef]
- Velayudhan, D.E.; Hossain, M.M.; Stein, H.H.; Nyachoti, C.M. Standardized ileal digestibility of amino acids in canola meal fed to gestating and lactating sows1. J. Anim. Sci. 2019, 97, 4219–4226. [Google Scholar] [CrossRef]
- Czech, A.; Grela, E.R.; Kiesz, M. Dietary fermented rapeseed or/and soybean meal additives on performance and intestinal health of piglets. Sci. Rep. 2021, 11, 16952. [Google Scholar] [CrossRef]
- Shi, C.; He, J.; Yu, J.; Yu, B.; Mao, X.; Zheng, P.; Huang, Z.; Chen, D. Amino acid, phosphorus, and energy digestibility of Aspergillus niger fermented rapeseed meal fed to growing pigs. J. Anim. Sci. 2015, 93, 2916–2925. [Google Scholar] [CrossRef]
- Tomaszewska, E.; Muszyński, S.; Dobrowolski, P.; Kamiński, D.; Czech, A.; Grela, E.R.; Wiącek, D.; Tomczyk-Warunek, A. Dried fermented post-extraction rapeseed meal given to sows as an alternative protein source for soybean meal during pregnancy improves bone development of their offspring. Livest. Sci. 2019, 224, 60–68. [Google Scholar] [CrossRef]
- Zhu, X.F.; Wang, L.Y.; Zhang, Z.; Ding, L.R.; Hang, S.Q. Combination of fiber-degrading enzymatic hydrolysis and lactobacilli fermentation enhances utilization of fiber and protein in rapeseed meal as revealed in simulated pig digestion and fermentation in vitro. Anim. Feed Sci. Technol. 2021, 278, 115001. [Google Scholar] [CrossRef]
- Rao, D.E.; Rao, K.V.; Reddy, T.P.; Reddy, V.D. Molecular characterization, physicochemical properties, known and potential applications of phytases: An overview. Crit. Rev. Biotechnol. 2009, 29, 182–198. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, I.M.; Carvalho, M.G.V.; Rocha, J.M. Increase of protein extraction yield from rapeseed meal through a pretreatment with phytase. J. Sci. Food Agric. 2017, 97, 2641–2646. [Google Scholar] [CrossRef]
- Cheng, L.; Rosch, C.; Vries, S.D.; Schols, H.; Venema, K. Cellulase and alkaline treatment improve intestinal microbial degradation of recalcitrant fibers of rapeseed meal in pigs. J. Agric. Food Chem. 2020, 68, 11011–11025. [Google Scholar]
- Sethy, K.; Mishra, S.K.; Mohanty, P.P.; Agarawal, J.; Meher, P.; Satapathy, D.; Satapathy, D.; Sahoo, J.K.; Panda, S.; Nayak, S.M. An Overview of Non-Starch Polysaccharide. J. Anim. Nutr. Physiol. 2015, 1, 17–22. [Google Scholar]
- Zhang, Z.Y.; Wen, M.; Chang, Y.Q. Degradation of glucosinolates in rapeseed meal by Lactobacillus delbrueckii and Bacillus subtilis. Grain Oil Sci. Technol. 2020, 3, 70–76. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Wei, F.H.; Liu, X.L.; Yi, C.X.; Zhang, Y.G. Improvement of the nutritional value, sensory properties and bioavailability of rapeseed meal fermented with mixed microorganisms. LWT 2019, 112, 1082338. [Google Scholar] [CrossRef]
- Vig, A.P.; Walia, A. Beneficial effects of Rhizopus oligosporus fermentation on reduction of glucosinolates, fibre and phytic acid in rapeseed (Brassica napus) meal. Bioresour. Technol. 2001, 78, 309–312. [Google Scholar]
- Olukomaiya, O.O.; Fernando, W.C.; Mereddy, R.; Li, X.H.; Sultanbawa, Y. Solid-state fermentation of canola meal with Aspergillus sojae, Aspergillus ficuum and their co-cultures: Effects on physicochemical, microbiological and functional properties. LWT 2020, 127, 109362. [Google Scholar] [CrossRef]
- Tie, Y.; Li, L.; Liu, J.; Liu, C.; Fu, J.; Xiao, X.; Wang, G.; Wang, J. Two-step biological approach for treatment of rapeseed meal. J. Food Sci. 2020, 85, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Lucke, F.K.; Fritz, V.; Tannhauser, K.; Arya, A. Controlled fermentation of rapeseed presscake by Rhizopus, and its effect on some components with relevance to human nutrition. Food Res. Int. 2019, 120, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Wang, Z.; Zou, Y.; He, R.; Ju, X.; Yuan, J. Effect of static-state fermentation on volatile composition in rapeseed meal. J. Sci. Food Agric. 2020, 100, 2145–2152. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, H.A.; Piao, M.; Ma, T.; Huo, R.Y.; Tu, Y. Enhancing the Quality of Total Mixed Ration Containing Cottonseed or Rapeseed Meal by Optimization of Fermentation Conditions. Fermentation 2021, 7, 234. [Google Scholar] [CrossRef]
- Do, S.H.; Kim, B.O.; Fang, L.H.; You, D.H.; Hong, J.S.; Kim, Y.Y. Various levels of rapeseed meal in weaning pig diets from weaning to finishing periods. Asian-Australas J. Anim. Sci. 2017, 30, 1292–1302. [Google Scholar] [CrossRef]
- Choi, H.B.; Jeong, J.H.; Kim, D.H.; Lee, Y.; Kwon, H.; Kim, Y.Y. Influence of rapeseed meal on growth performance, blood profiles, nutrient digestibility and economic benefit of growing-finishing pigs. Asian-Australas J. Anim. Sci. 2015, 28, 1345–1353. [Google Scholar] [CrossRef]
- Zmudzinska, A.; Bigorowski, B.; Banaszak, M.; Roslewska, A.; Adamski, M.; Hejdysz, M. The effect of diet based on legume seeds and rapeseed meal on pig performance and meat quality. Animals 2020, 10, 1084. [Google Scholar] [CrossRef]
- Grabez, V.; Egelandsdal, B.; Kjos, N.P.; Hakenasen, I.M.; Mydland, L.T.; Vik, J.O.; Hallenstvedt, E.; Devle, H.; Overland, M. Replacing soybean meal with rapeseed meal and faba beans in a growing-finishing pig diet: Effect on growth performance, meat quality and metabolite changes. Meat Sci. 2020, 166, 108134. [Google Scholar] [CrossRef]
- Landero, J.L.; Wang, L.F.; Beltranena, E.; Bench, C.J.; Zijlstra, R.T. Feed preference of weaned pigs fed diets containing soybean meal, Brassica napus canola meal, or Brassica juncea canola meal. J. Anim. Sci. 2018, 96, 600–611. [Google Scholar] [CrossRef]
- Hansen, J.; Øverland, M.; Skrede, A.; Anderson, D.M.; Collins, S.A. A meta-analysis of the effects of dietary canola/double low rapeseed meal on growth performance of weanling and growing-finishing pigs. Anim. Feed Sci. Technol. 2020, 259, 114302. [Google Scholar] [CrossRef]
- Skugor, A.; Kjos, N.P.; Sundaram, A.Y.M.; Mydland, L.T.; Anestad, R.; Tauson, A.H.; Overland, M. Effects of long-term feeding of rapeseed meal on skeletal muscle transcriptome, production efficiency and meat quality traits in Norwegian Landrace growing-finishing pigs. PLoS ONE 2019, 14, e0220441. [Google Scholar] [CrossRef] [PubMed]
- Skoufos, I.; Tzora, A.; Giannenas, I.; Bonos, E.; Papagiannis, N.; Tsinas, A.; Christaki, E.; Florou-Paneri, P. Dieraty inclusion of rapeseed meal as soybean meal subtitute on growth performance, gut microbiota, oxidative stability and fatty acid profile in growing-fattening pigs. Asian J. Anim. Vet. Adv. 2016, 11, 89–97. [Google Scholar] [CrossRef][Green Version]
- Tan, C.Q.; Wei, H.K.; Sun, H.Q.; Long, G.; Ao, J.T.; Jiang, S.W.; Peng, J. Effects of supplementing sow diets during two gestations with konjac flour and Saccharomyces boulardii on constipation in peripartal period, lactation feed intake and piglet performance. Anim. Feed Sci. Technol. 2015, 210, 254–262. [Google Scholar] [CrossRef]
- Grela, E.R.; Czech, A.; Kiesz, M.; Wlazlo, L.; Nowakowicz-Debek, B. A fermented rapeseed meal additive: Effects on production performance, nutrient digestibility, colostrum immunoglobulin content and microbial flora in sows. Anim. Nutr. 2019, 5, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Quiniou, N.; Quinsac, A.; Crepon, K.; Evrard, J.; Peyronnet, C.; Bourdillon, A.; Royer, E.; Etienne, M. Effects of feeding 10% rapeseed meal (Brassica napus) during gestation and lactation over three reproductive cycles on the performance of hyperprolific sows and their litters. Can. J. Ani. Sci. 2012, 92, 513–524. [Google Scholar] [CrossRef]
- Czech, A.; Grela, E.R.; Kiesz, M.; Kłys, S. Biochemical andhaematological blood parameters of sows and piglets fed a diet with a dried fermented rapeseed meal. Ann. Anim. Sci. 2020, 20, 535–550. [Google Scholar] [CrossRef]
- Czech, A.; Stepniowska, A.; Kiesz, M. Effect of fermented rapeseed meal as a feed component on the redox and immune system of pregnant sows and their offspring. Ann. Anim. Sci. 2022, 1, 201–219. [Google Scholar] [CrossRef]
- Park, C.S.; Helmbrecht, A.; Htoo, J.K.; Adeola, O. Comparison of digestibility of amino acids in full-fat soybean, soybean meal, and peanut flour between broiler chickens and pigs. J. Ani. Sci. 2017, 95, 3110–3111. [Google Scholar] [CrossRef]
- Bowland, J.P.; Hardin, R.T. Rapeseed meal as apartial replacement for soybean meal in the diets of growing gilts and of sows for up to three reproduction cucles. Can. J. Anim. Sci. 1973, 53, 355–363. [Google Scholar] [CrossRef]
- Hanczakowska, E.; Weglarzy, K.; Bereza, M. Effectiveness of Rapeseed Press Cake (RPC) in Sow Feeding in Two Reproduction Cycles. Ann. Anim. Sci. 2012, 12, 95–104. [Google Scholar] [CrossRef][Green Version]
| Rapeseed Meal | Double-Low Rapeseed Meal | Soybean Meal | |
|---|---|---|---|
| Crude protein (%) | 37.6~38.2 | 39.4~43.6 | 43.8~49.9 |
| Arginine (g/kg) | 20.6~22.1 | 20.8~24.1 | 34.9~37.8 |
| Histidine (g/kg) | 10.0~10.1 | 10.4~12.0 | 12.1~13.2 |
| Isoleucine (g/kg) | 14.6~15.3 | 13.8~15.6 | 21.5~27.8 |
| Leucine (g/kg) | 26.6~27.0 | 25.4~27.6 | 36.6~39.2 |
| Lysine (g/kg) | 17.2~19.5 | 19.4~24.1 | 29.9~32.2 |
| Methionine (g/kg) | 7.4~7.6 | 7.6~9.7 | 6.0~6.9 |
| Phenylalanine (g/kg) | 15.1~15.3 | 12.2~13.6 | 23.5~30.0 |
| Threonine (g/kg) | 17.5~17.6 | 17.6~19.1 | 18.9~20.3 |
| Tryptophan (g/kg) | 5.0~5.1 | 4.6~5.4 | 6.6~7.5 |
| Valine (g/kg) | 18.6~19.7 | 21.5~23.8 | 22.4~26.7 |
| Mosenthin et al. [12] | Li et al. [13] | Banaszkiexicz [14] |
| Method | Treatment | Animal | Main Results | Reference |
|---|---|---|---|---|
| Enzymic method | Aspergillus ficuum phytase | Weanling pig | Increase digestibilities of P and Ca and improve bone structure | Zhang et al. [56] |
| Quantum Blue phytase | Growing and finishing pig | Improve P and Ca digestibility, and reduce P excretion | Małgorzata et al. [57] Maison et al. [58] | |
| Carbohydrases and phytase | Sow | Reduce the body weight loss and improve P digestibility post-farrowing | Velayudhan et al. [59] | |
| Cellulose, xylanase, glucanase and protease | Growing and finishing pig | Increased the standardized ileal digestibility of crude protein and all amino acids and enhanced fiber degradation | Li et al. [60] Torres et al. [61] | |
| Cellulose and pectinase | Growing pig | Change microbial community and increase the abundance of microbial fibre-degrading enzymes and pathways | Long and Venema [62] | |
| Cellulase, pectinase, amylase, protease and phytase | Gestating and lactating sow | Improve the standard ileal digestibility of amino acid | Velayudhan et al. [63] | |
| Microbiological fermentation | Bacillus subtilis and Lactobacillus fermentum | Weaned piglet | Reduce the incidence of diarrhea and improve the gut microbiota | Czech et al. [64] |
| Aspergillus niger 41258 | Growing barrow | Increase P digestibility and digestible amino acid content and decrease P excretion | Shi et al. [65] | |
| Lactobacillus | Pregnant sow | Improve the structure and mechanical properties of compact bone in offspring | Tomaszewska et al. [66] | |
| Lactobacillus, cellulose and pectinase | Growing pig | Increase reducing sugars and reduce the glucosinolate, total short-chain fatty acid and acetic acid content | Zhu et al. [67] |
| Source | Glucosinolates Content (μmol/g) | Treatment | Degradation Ratio | Reference |
|---|---|---|---|---|
| Canola meal | 9.31 | Aspergillus sojae and Aspergillus icuum | 30% | Olukomaiya et al. [75] |
| Rapeseed meal | 16.45 | Aspergillus niger | 43.07% | Shi et al. [7] |
| Rapeseed meal | 23.79 | Aspergillus niger | 30.6% | Tie et al. [76] |
| Rapeseed press cake | 32.1 | Rhizupus | 15.9% | Lucke et al. [77] |
| Rapeseed meal | 64.6 | Lactobacillus delbrueckii and Bacillus subtilis | 94.62% | Zhang et al. [72] |
| Rapeseed meal | 203.7 | Bacillus subtilis and Actinomucor elegans | 45.26% | Hao et al. [78] |
| Animal | Source | Results | References |
|---|---|---|---|
| Weanling pig | Rapeseed meal | No adverse effects on the growth performance with up to 8% rapeseed meal | Do et al. [80] |
| Weaned pig | Brassica napus and Brassica juncea canola meal | No difference in feed intake, BWG and FCR | Landero et al. [84] |
| Growing pig | Rapeseed meal fermented by Aspergillus niger | No adverse effects on performance, when replaced with rapeseed meal up to 10% | Shi et al. [7] |
| Growing pig | Canola/double low rapeseed meal/expeller | No difference in growth performance with rapeseed meal up to 5% | Hansen et al. [85] |
| Growing-finishing pig | Rapeseed meal | No adverse effects on performance, when rapeseed meal was provided up to 9% | Choi et al. [81] |
| Finishing pig | Extracted rapeseed meal and legume plant | No adverse effects on pork quality; Reduce fatness; daily BWG↓ | Zmudzinska et al. [82] |
| Growing-finishing pig | Comercial expeller pressed rapeseed | ADG↓; generally no difference in meat quality with rapeseed meal up to 20% | Skugor et al. [86] |
| Growing-finishing pig | Rapeseed meal | FCR↑; glucose level, lightness and yellowness of meat↓; oxidative stress↓; free amino acids, sweet tasting metabolites and flavor attributes↑ | Grabez et al. [83] |
| Growing-finishing pig | Rapeseed meal | FCR↑; total MUFA↑, SFA and PUFA↓ in the steak cuts↓; modified the microbial balance in the digestive tract | Skoufos et al. [87] |
| Sources | Appending Proportion (%) | Main Results | References |
|---|---|---|---|
| Rapeseed meal | 10% | Not affect piglet weight at birth or weaning, survival and litter weight gain | Quiniou et al. [90] |
| Rapeseed meal | 12% | No detrimental effects on reproductive performance and growth their progeny | Park et al. [93] |
| Rapeseed meal | 6% | No detrimental effects on growth and production | Bowland and Hardin [94] |
| Canola meal | 30% | Gut lactic acid bacteria↑; sow body weight and plasma urea nitrogen↓; No adverse effects on milk composition and nutrient digestibility | Velayudhan et al. [59] |
| Rapeseed press cake | 8~14% | Body weight of piglet↑; piglet growth rate↑; | Hanczakowska et al. [95] |
| Fermented rapeseed meal | 4~9% | Stimulate immune and antioxidant system; | Czech et al. [92] |
| Fermented rapeseed meal | 4~9% | Litter size and litter weight↑; nutrient digestibility↑; maleficent bacteria↓ | Grela et al. [89] |
| Fermented rapeseed meal | 4~9% | Plasma content of Ht, Hb, RBC and mineral↑; plasma content of total cholesterol and triacylglycerols↓; liver enzyme activity↓ | Czech et al. [91] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, H.; Liu, X.; Xiao, Q.; Zhang, F.; Liu, N.; Tang, L.; Wang, J.; Ma, X.; Tan, B.; Chen, J.; et al. Rapeseed Meal and Its Application in Pig Diet: A Review. Agriculture 2022, 12, 849. https://doi.org/10.3390/agriculture12060849
Cheng H, Liu X, Xiao Q, Zhang F, Liu N, Tang L, Wang J, Ma X, Tan B, Chen J, et al. Rapeseed Meal and Its Application in Pig Diet: A Review. Agriculture. 2022; 12(6):849. https://doi.org/10.3390/agriculture12060849
Chicago/Turabian StyleCheng, Hao, Xiang Liu, Qingrui Xiao, Fan Zhang, Nian Liu, Lizi Tang, Jing Wang, Xiaokang Ma, Bie Tan, Jiashun Chen, and et al. 2022. "Rapeseed Meal and Its Application in Pig Diet: A Review" Agriculture 12, no. 6: 849. https://doi.org/10.3390/agriculture12060849
APA StyleCheng, H., Liu, X., Xiao, Q., Zhang, F., Liu, N., Tang, L., Wang, J., Ma, X., Tan, B., Chen, J., & Jiang, X. (2022). Rapeseed Meal and Its Application in Pig Diet: A Review. Agriculture, 12(6), 849. https://doi.org/10.3390/agriculture12060849








