Bumblebee Pollination Enhances Yield and Flavor of Tomato in Gobi Desert Greenhouses
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site, Plants and Bees
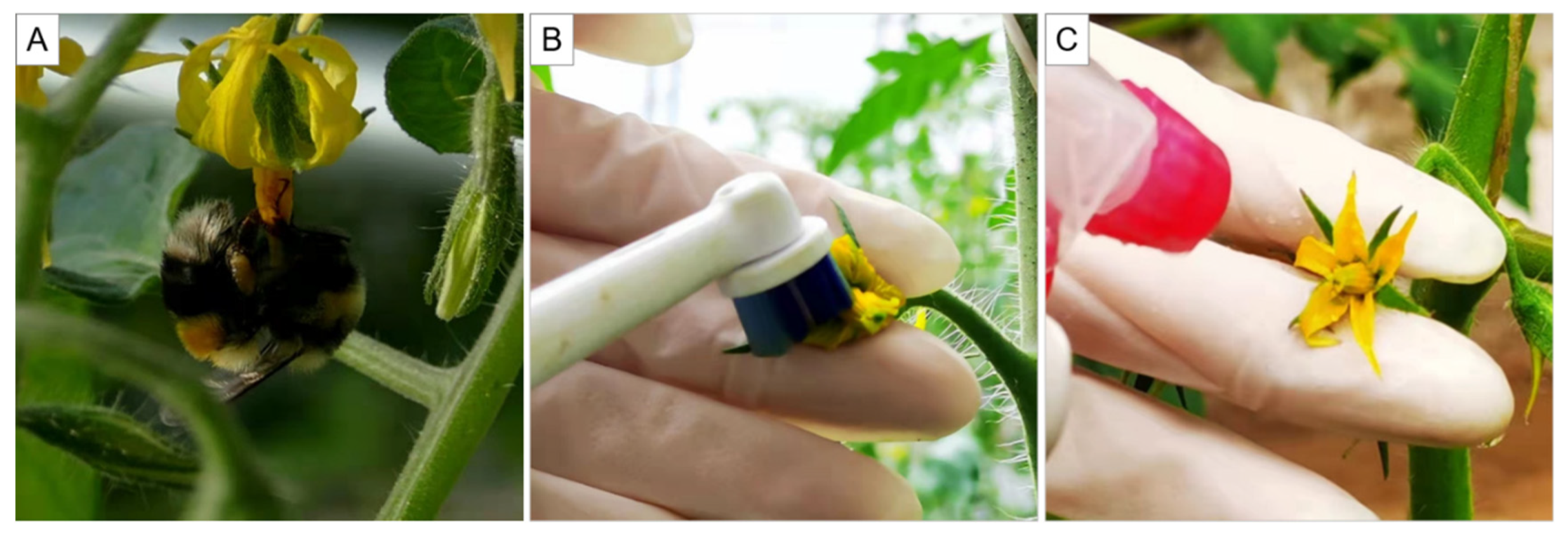
2.2. Pollination Treatment
2.3. Measure of Yield
2.4. Sample Preparation for Flavor Analysis
2.4.1. Sample Preparation for Sugar Analysis
2.4.2. Sample Preparation for Organic Acid Analysis
2.4.3. Sample Preparation for VOC Analysis
2.5. Instrument Method for Flavor Analysis
2.5.1. UHPLC-QqQ-MS/MS for Sugar Analysis
2.5.2. UHPLC-QqQ-MS/MS Condition for Organic Acid Detection
2.5.3. HS-SPME-GC-QTOF-MS Condition for VOC Detection
2.6. Statistical Analysis
3. Results
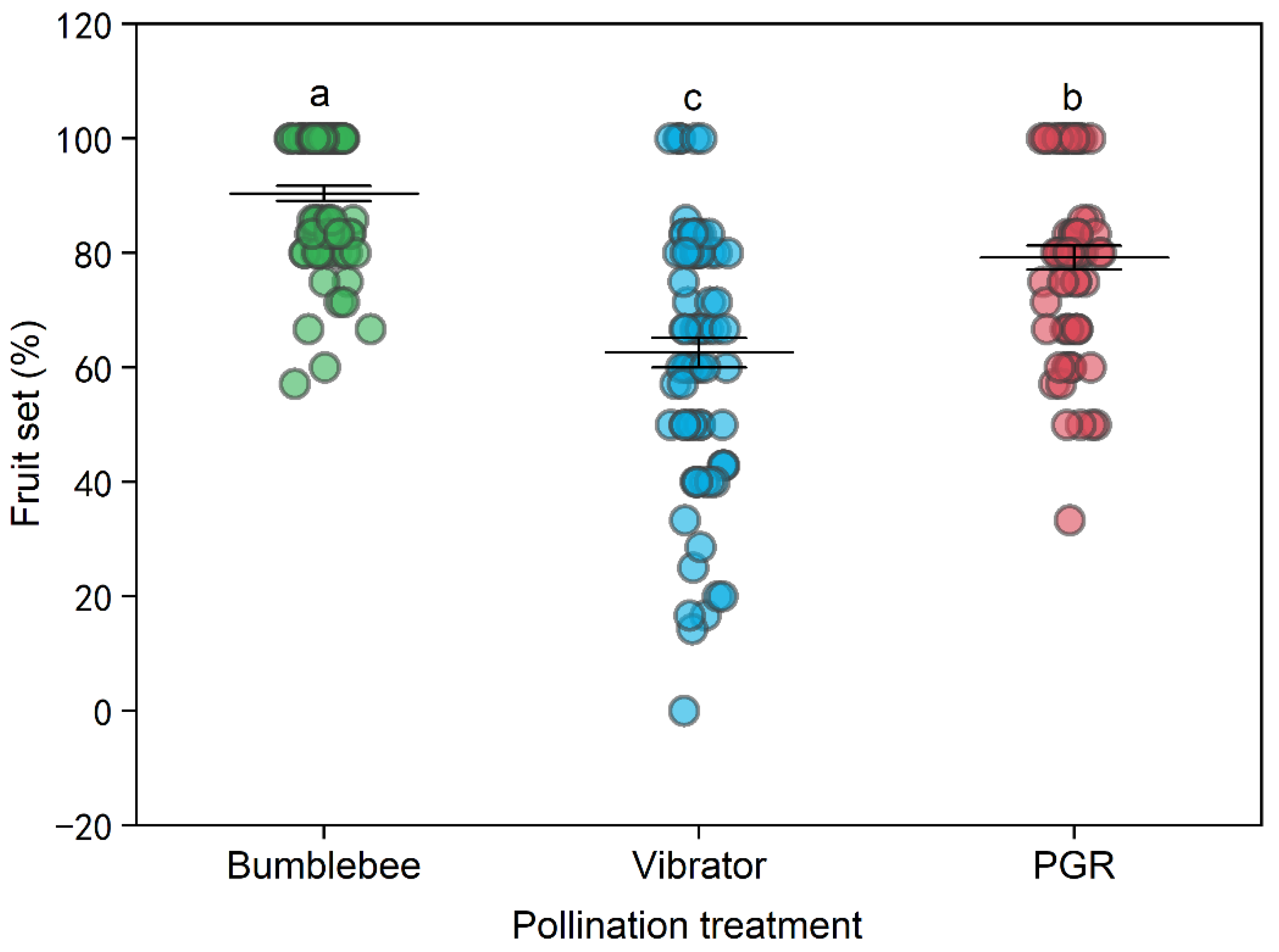
3.1. Tomato Yield
3.2. Sugars and Organic Acids in Tomato
3.3. VOCs in Tomato
4. Discussion
4.1. Bumblebee Pollination Enhanced Tomato Yield
4.2. Bumblebee Pollination Improved Tomato Flavor
4.3. Implications for Growers and Food Systems
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Food and Agricultural Organization of the United Nations. Online Statistical Database. FAOSTAT. 2021. Available online: http://faostat.fao.org/default.aspx (accessed on 1 December 2021).
- Ministry of Agriculture and Rural Affairs of the People’s Repulic of China. Summary of China National Agricultural Statistics. 2015 Statistical Division. Available online: http://www.moa.gov.cn/ (accessed on 10 May 2020).
- Klein, A.M.; Vaissiere, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of pollinators in changing landscapes for world crops. Proc. Biol. Sci. 2007, 274, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Free, J. Insect Pollination of Crops, 2nd ed.; Academic Press: London, UK, 1993. [Google Scholar]
- Bowers, K.A.W. The pollination ecology of Solanum rostratum (Solanaceae). Am. J. Bot. 1975, 62, 633–638. [Google Scholar] [CrossRef]
- Li, B.; Zhu, G. Effects of dipping flower with growth regulators and artificial vibration pollination on fruit development and occurrence of grey mould in tomato. Acta Hortic. Sin. 1999, 26, 337–338. [Google Scholar]
- Kaur, P.; Mal, D.; Sheokand, A.; Weta, S.; Singh, L.; Datta, S. Role of plant growth regulators in vegetable production: A Review. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 2177–2183. [Google Scholar] [CrossRef]
- Garibaldi, L.A.; Aizen, M.A.; Klein, A.M.; Cunningham, S.A.; Harder, L.D. Global growth and stability of agricultural yield decrease with pollinator dependence. Proc. Natl. Acad. Sci. USA 2011, 108, 5909–5914. [Google Scholar] [CrossRef]
- Fijen, T.P.M.; Scheper, J.A.; Boom, T.M.; Janssen, N.; Raemakers, I.; Kleijn, D. Insect pollination is at least as important for marketable crop yield as plant quality in a seed crop. Ecol. Lett. 2018, 21, 1704–1713. [Google Scholar] [CrossRef]
- Rollin, O.; Garibaldi, L.A. Impacts of honeybee density on crop yield: A meta-analysis. J. Appl. Ecol. 2019, 56, 1152–1163. [Google Scholar] [CrossRef]
- Reilly, J.R.; Artz, D.R.; Biddinger, D.; Bobiwash, K.; Boyle, N.K.; Brittain, C.; Brokaw, J.; Campbell, J.W.; Daniels, J.; Elle, E.; et al. Crop production in the USA is frequently limited by a lack of pollinators. Proc. R. Soc. B Biol. Sci. 2020, 287, 20200922. [Google Scholar] [CrossRef]
- Gallai, N.; Salles, J.; Settele, J.; Vaissi, E.; Re, B.E. Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecol. Econ. 2009, 68, 810–821. [Google Scholar] [CrossRef]
- Winfree, R.; Gross, B.J.; Kremen, C. Valuing pollination services to agriculture. Ecol. Econ. 2011, 71, 80–88. [Google Scholar] [CrossRef]
- Pérez Méndez, N.; Andersson, G.K.S.; Requier, F.; Hipólito, J.; Aizen, M.A.; Morales, C.L.; García, N.; Gennari, G.P.; Garibaldi, L.A. The economic cost of losing native pollinator species for orchard production. J. Appl. Ecol. 2020, 57, 599–608. [Google Scholar] [CrossRef]
- Jordan, A.; Patch, H.M.; Grozinger, C.M.; Khanna, V. Economic dependence and vulnerability of United States agricultural sector on insect-mediated pollination service. Environ. Sci. Technol. 2021, 55, 2243–2253. [Google Scholar] [CrossRef] [PubMed]
- Cribb, D.; Hand, D.; Edmondson, R. A comparative study of the effects of using the honeybee as a pollinating agent of glasshouse tomato. J. Hortic. Sci. 1993, 68, 79–88. [Google Scholar] [CrossRef]
- Sabara, H.A.; Winston, M.L. Managing honey bees (Hymenoptera: Apidae) for greenhouse tomato pollination. J. Econ. Entomol. 2003, 96, 547–754. [Google Scholar] [CrossRef]
- Higo, H.A.; Rice, N.D.; Winston, M.L.; Lewis, B. Honey bee (Hymenoptera: Apidae) distribution and potential for supplementary pollination in commercial tomato greenhouses during winter. J. Econ. Entomol. 2004, 97, 163–170. [Google Scholar] [CrossRef]
- Neiswander, R.B. Honeybees as pollinators of greenhouse tomatoes. Gleanings 1954, 82, 10–13. [Google Scholar]
- Banda, H.; Paxton, R. Pollination of greenhouse tomatoes by bees. Acta Hortic. 1991, 288, 194–198. [Google Scholar] [CrossRef]
- Bispo Dos Santos, S.; Roselino, A.; Hrncir, M.; Bego, L. Pollination of tomatoes by the stingless bee Melipona quadrifasciata and the honey bee Apis mellifera (Hymenoptera, Apidae). Genet. Mol. Res. 2009, 8, 751–757. [Google Scholar] [CrossRef]
- Zhang, H.; Shan, S.; Gu, S.; Huang, X.; Li, Z.; Khashaveh, A.; Zhang, Y. Prior experience with food reward influences the behavioral responses of the honeybee Apis mellifera and the bumblebee Bombus lantschouensis to tomato floral scent. Insects 2020, 11, 884. [Google Scholar] [CrossRef]
- Kevan, P.; Imperatriz-Fonseca, V.L.; Shipp, L.; Hrncir, M.; Nunes-Silva, P. The behaviour of Bombus impatiens (Apidae, Bombini) on tomato (Lycopersicon esculentum Mill., Solanaceae) flowers: Pollination and reward perception. J. Pollinat. Ecol. 2013, 11, 33–40. [Google Scholar] [CrossRef]
- Velthuis, H.H.; van Doorn, A. A century of advances in bumblebee domestication and the economic and environmental aspects of its commercialization for pollination. Apidologie 2006, 37, 421–451. [Google Scholar] [CrossRef]
- Vergara, C.H.; Fonseca-Buendía, P. Pollination of greenhouse tomatoes by the Mexican bumblebee Bombus ephippiatus (Hymenoptera: Apidae). J. Pollinat. Ecol. 2012, 7, 27–30. [Google Scholar] [CrossRef]
- Bashir, M.A.; Alvi, A.M.; Khan, K.A.; Rehmani, M.I.A.; Ansari, M.J.; Atta, S.; Ghramh, H.A.; Batool, T.; Tariq, M. Role of pollination in yield and physicochemical properties of tomatoes (Lycopersicon esculentum). Saudi J. Biol. Sci. 2018, 25, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Toni, H.C.; Djossa, B.A.; Ayenan, M.A.T.; Teka, O. Tomato (Solanum lycopersicum) pollinators and their effect on fruit set and quality. J. Hortic. Sci. Biotechnol. 2021, 96, 1–13. [Google Scholar] [CrossRef]
- Klee, H.J.; Tieman, D.M. The genetics of fruit flavour preferences. Nat. Rev. Genet. 2018, 19, 347–356. [Google Scholar] [CrossRef]
- Wang, D.; Seymour, G.B. Tomato flavor: Lost and found? Mol. Plant 2017, 10, 782–784. [Google Scholar] [CrossRef]
- Baldwin, E.A.; Scott, J.W.; Shewmaker, C.K.; Schuch, W. Flavor trivia and tomato aroma: Biochemistry and possible mechanisms for control of important aroma components. HortScience 2000, 35, 1013–1022. [Google Scholar] [CrossRef]
- Anthon, G.E.; LeStrange, M.; Barrett, D.M. Changes in pH, acids, sugars and other quality parameters during extended vine holding of ripe processing tomatoes. J. Sci. Food Agric. 2011, 91, 1175–1181. [Google Scholar] [CrossRef]
- Tieman, D.; Bliss, P.; McIntyre, L.M.; Blandon-Ubeda, A.; Bies, D.; Odabasi, A.Z.; Rodríguez, G.R.; van der Knaap, E.; Taylor, M.G.; Goulet, C.; et al. The chemical interactions underlying tomato flavor preferences. Curr. Biol. 2012, 22, 1035–1039. [Google Scholar] [CrossRef]
- Du, X.; Song, M.; Baldwin, E.; Rouseff, R. Identification of sulphur volatiles and GC-olfactometry aroma profiling in two fresh tomato cultivars. Food Chem. 2015, 171, 306–314. [Google Scholar] [CrossRef]
- Zou, J.; Chen, J.; Tang, N.; Gao, Y.; Hong, M.; Wei, W.; Cao, H.; Jian, W.; Li, N.; Deng, W.; et al. Transcriptome analysis of aroma volatile metabolism change in tomato (Solanum lycopersicum) fruit under different storage temperatures and 1-MCP treatment. Postharvest Biol. Technol. 2018, 135, 57–67. [Google Scholar] [CrossRef]
- Hogendoorn, K.; Bartholomaeus, F.; Keller, M.A. Chemical and sensory comparison of tomatoes pollinated by bees and by a pollination wand. J. Econ. Entomol. 2010, 103, 1286–1292. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, T. The effect of greenhouse pollination methods on consumers’ willingness to pay for tomatoes in Japan. J. Agric. Appl. Econ. 2021, 53, 186–208. [Google Scholar] [CrossRef]
- Cooley, H.; Vallejo-Marín, M. Buzz-pollinated crops: A global review and meta-analysis of the effects of supplemental bee pollination in tomato. J. Econ. Entomol. 2021, 114, 505–519. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Yu, J.; Chen, B.; Feng, Z.; Lyu, J.; Hu, L.; Gan, Y.; Siddique, K.H.M. Gobi agriculture: An innovative farming system that increases energy and water use efficiencies. A review. Agron. Sustain. Dev. 2018, 38, 62. [Google Scholar] [CrossRef]
- Zhou, Z. Measurement of Flavor Compounds and Identification of Associated Loci Associated with Tomato Fruit Flavor. Master’s Thesis, Chinese Academy of Agricultural Sciences, Beijing, China, 5 June 2020. [Google Scholar]
- Rambla, J.L.; Alfaro, C.; Medina, A.; Zarzo, M.; Primo, J.; Granell, A. Tomato fruit volatile profiles are highly dependent on sample processing and capturing methods. Metabolomics 2015, 11, 1708–1720. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, S.; Huang, Z.; Zhang, S.; Liao, Q.; Zhang, C.; Lin, T.; Qin, M.; Peng, M.; Yang, C.; et al. Rewiring of the Fruit Metabolome in Tomato Breeding. Cell 2018, 172, 249–261. [Google Scholar] [CrossRef]
- Tieman, D.; Zhu, G.; Resende, J.M.F.R.; Lin, T.; Nguyen, C.; Bies, D.; Rambla, J.L.; Beltran, K.S.O.; Taylor, M.; Zhang, B.; et al. A chemical genetic roadmap to improved tomato flavor. Science 2017, 355, 391–394. [Google Scholar] [CrossRef]
- Buttery, R.G.; Teranishi, R.; Ling, L.C.; Flath, R.A.; Stern, D.J. Quantitative studies on origins of fresh tomato aroma volatiles. J. Agric. Food Chem. 1988, 36, 1247–1250. [Google Scholar] [CrossRef]
- Nazer, I.K.; Kasrawi, M.A.; Al-Attal, Y.Z. Influence of pollination technique on greenhouse tomato production. J. Agric. Mar. Sci. 2003, 8, 21–26. [Google Scholar] [CrossRef]
- Olimpieri, I.; Siligato, F.; Caccia, R.; Soressi, G.P.; Mazzucato, A.; Mariotti, L.; Ceccarelli, N. Tomato fruit set driven by pollination or by the parthenocarpic fruit allele are mediated by transcriptionally regulated gibberellin biosynthesis. Planta 2007, 226, 877–888. [Google Scholar] [CrossRef] [PubMed]
- Serrani, J.C.; Ruiz-Rivero, O.; Fos, M.; García-Martínez, J.L. Auxin-induced fruit-set in tomato is mediated in part by gibberellins. Plant J. 2008, 56, 922–934. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.; Patrick, J.W.; Bouzayen, M.; Osorio, S.; Fernie, A.R. Molecular regulation of seed and fruit set. Trends Plant Sci. 2012, 17, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.S.; Park, S.D.; Kim, J.H. Influence of pollination methods on fruit development and sugar contents of oriental melon (Cucumis melo L. cv. Sagyejeol-Ggul). Sci. Hortic. 2007, 112, 388–392. [Google Scholar] [CrossRef]
- Mahmood, S.; Hasan, M.N.; Ali, S.M.Y.; Ripa, R.A.; Hossain, M.G. Effect of plant growth regulators on fruit-set and quality of guava. Turk. J. Agric. Food Sci. Technol. 2016, 4, 1088–1091. [Google Scholar] [CrossRef][Green Version]
- Kumari, S.; Bakshi, P.; Sharma, A.; Wali, V.K.; Jasrotia, A.; Kour, S. Use of plant growth regulators for improving fruit production in sub tropical crops. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 659–668. [Google Scholar] [CrossRef]
- Malundo, T.M.M.; Shewfelt, R.L.; Scott, J.W. Flavor quality of fresh tomato (Lycopersicon esculentum Mill.) as affected by sugar and acid levels. Postharvest Biol. Technol. 1995, 6, 103–110. [Google Scholar] [CrossRef]
- Agius, C.; von Tucher, S.; Poppenberger, B.; Rozhon, W. Quantification of sugars and organic acids in tomato fruits. MethodsX 2018, 5, 537–550. [Google Scholar] [CrossRef]
- Wang, T.; Ye, H.; Zheng, J.; Li, M. Research progress of main flavor compounds in tomato fruits. Acta Agric. Zhejiangensis 2020, 32, 1513–1522. (In Chinese) [Google Scholar]
- Zhang, H.; Huang, J.; Williams, P.H.; Vaissière, B.E.; Zhou, Z.; Gai, Q.; Dong, J.; An, J. Managed bumblebees outperform honeybees in increasing peach fruit set in China: Different limiting processes with different pollinators. PLoS ONE 2015, 10, e121143. [Google Scholar] [CrossRef]
- Li, J.; Di, T.; Bai, J. Distribution of volatile compounds in different fruit structures in four tomato cultivars. Molecules 2019, 24, 2594. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Li, Y.; Sun, D.; Chen, Q.; Fu, M.; Zhao, H.; Chen, X.; Huang, Y.; Xu, H. Odor, tastes, nutritional compounds and antioxidant activity of fresh-eating walnut during ripening. Sci. Hortic. 2022, 293, 110744. [Google Scholar] [CrossRef]
- Naeem, M.; Yuan, X.; Huang, J.; An, J. Habitat suitability for the invasion of Bombus terrestris in East Asian countries: A case study of spatial overlap with local Chinese bumblebees. Sci. Rep. 2018, 8, 11035. [Google Scholar] [CrossRef] [PubMed]
- Aizen, M.A.; Arbetman, M.P.; Chacoff, N.P.; Chalcoff, V.R.; Feinsinger, P.; Garibaldi, L.A.; Harder, L.D.; Morales, C.L.; Saez, A.; Vanbergen, A.J. Invasive bees and their impact on agriculture. Adv. Ecol. Res. 2020, 63, 49–92. [Google Scholar] [CrossRef]




| Compounds Names | Formula | CAS | Concentration (ng g−1) | Consumer Liking Effect | ||
|---|---|---|---|---|---|---|
| Bumblebee | Vibrator | PGR | ||||
| Hexanal * | C6H12O | 66-25-1 | 484.19 ± 22.33a | 296.66 ± 17.13b | 251.94 ± 10.11b | Positive |
| Methyl salicylate * | C8H8O3 | 119-36-8 | 80.77 ± 4.24a | 39.69 ± 1.83b | 57.02 ± 3.29ab | Positive |
| 6-Methyl-5-hepten-2-one * | C8H14O | 110-93-0 | 80.95 ± 2.62a | 38.01 ± 2.68b | 20.75 ± 0.89b | Positive |
| Phenylethyl alcohol * | C8H10O | 60-12-8 | 12.62 ± 0.63a | 5.68 ± 0.38b | 3.78 ± 0.38b | Positive |
| 1-Penten-3-one * | C5H8O | 1629-58-9 | 10.85 ± 0.34a | 5.30 ± 0.35c | 7.86 ± 0.23b | Positive |
| 2-Isobutylthiazole * | C7H11NS | 18640-74-9 | 10.02 ± 0.38a | 1.18 ± 0.24b | 1.25 ± 0.17b | Positive |
| (E)-2-Octenal * | C8H14O | 2548-87-0 | 6.10 ± 0.18a | 3.54 ± 0.12b | 2.71 ± 0.12b | Positive |
| 1-Penten-3-ol * | C5H10O | 616-25-1 | 4.47 ± 0.17a | 1.92 ± 0.14b | 1.54 ± 0.11b | Positive |
| Heptanal * | C7H14O | 111-71-7 | 2.21 ± 0.23a | 1.46 ± 0.13ab | 0.97 ± 0.05b | Positive |
| Citral * | C10H16O | 5392-40-5 | 2.01 ± 0.08a | 1.01 ± 0.05b | 0.86 ± 0.05b | Positive |
| β-Ionone * | C13H20O | 14901-07-6 | 0.70 ± 0.02a | 0.30 ± 0.04b | 0.49 ± 0.03ab | Positive |
| Acetone * | C3H6O | 67-64-1 | 0.58 ± 0.04a | 0.27 ± 0.02b | 0.25 ± 0.01b | Positive |
| (Z)-3-Hexen-1-ol | C6H12O | 33467-74-2 | 1.86 | n.d. | 0.77 | Positive |
| 3-Pentanone | C5H10O | 96-22-0 | 1.14 ± 0.14 | 0.31 | 0.80 ± 0.12 | Positive |
| Butanoic acid, 3-methyl- | C5H10O2 | 503-74-2 | 0.86 ± 0.05 | 0.66 ± 0.12 | 0.56 ± 0.09 | Positive |
| β-Cyclocitral | C10H16O | 432-25-7 | 0.62 ± 0.03 | 0.41 ± 0.02 | 0.49 ± 0.02 | Positive |
| α-Phellandrene | C10H16 | 99-83-2 | 0.46 ± 0.03 | 0.15 ± 0.03 | 0.19 ± 0.04 | Positive |
| α-Terpineol | C10H18O | 98-55-5 | 0.19 ± 0.01 | 0.18 ± 0.02 | 0.19 ± 0.01 | Positive |
| 1-Hexanol | C6H14O | 111-27-3 | 0.17 ± 0.01 | 0.11 ± 0.01 | 0.07 ± 0.01 | Positive |
| (E)-2-Hexenal * | C6H10O | 6728-26-3 | 1432.35 ± 57.81a | 789.39 ± 48.14b | 843.77 ± 23.20b | Negative |
| 1-Butanol, 2-methyl- * | C5H12O | 137-32-6 | 7.76 ± 0.61a | 6.06 ± 0.56a | 0.76 ± 0.21b | Negative |
| Benzeneacetaldehyde * | C8H8O | 122-78-1 | 1.47 ± 0.06a | 0.62 ± 0.06b | 0.78 ± 0.03b | Negative |
| Benzaldehyde, 2-hydroxy- * | C7H6O2 | 90-02-8 | 0.31 ± 0.02a | 0.14 ± 0.01b | 0.19 ± 0.01b | Negative |
| 2,4-Decadienal, (E,E)- * | C10H16O | 25152-84-5 | 1.04 ± 0.04a | 0.42 ± 0.03b | 0.49 ± 0.03b | Negative |
| 2-Methoxy-phenol | C7H8O2 | 90-05-1 | 4.31 ± 0.19 | 2.24 ± 0.22 | 4.70 ± 0.44 | Negative |
| Eugenol | C10H12O2 | 97-53-0 | 0.68 ± 0.03 | 0.62 ± 0.05 | 0.70 ± 0.03 | Negative |
| Disulfide, dimethyl | C2H6S2 | 624-92-0 | 0.19 ± 0.03 | 0.53 ± 0.10 | 0.08 ± 0.00 | Negative |
| Furfural | C5H4O2 | 98-01-1 | 0.04 ± 0.002 | 0.03 ± 0.01 | 0.03± 0.01 | Negative |
| Ethyl acetate | C4H8O2 | 141-78-6 | n.d. | 0.08 | 0.24 | Negative |
| Dimethyl sulfide | C2H6S | 75-18-3 | n.d. | 0.02 | 0.02 | Negative |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Han, C.; Breeze, T.D.; Li, M.; Mashilingi, S.K.; Hua, J.; Zhang, W.; Zhang, X.; Zhang, S.; An, J. Bumblebee Pollination Enhances Yield and Flavor of Tomato in Gobi Desert Greenhouses. Agriculture 2022, 12, 795. https://doi.org/10.3390/agriculture12060795
Zhang H, Han C, Breeze TD, Li M, Mashilingi SK, Hua J, Zhang W, Zhang X, Zhang S, An J. Bumblebee Pollination Enhances Yield and Flavor of Tomato in Gobi Desert Greenhouses. Agriculture. 2022; 12(6):795. https://doi.org/10.3390/agriculture12060795
Chicago/Turabian StyleZhang, Hong, Chao Han, Tom D. Breeze, Mengdan Li, Shibonage K. Mashilingi, Jun Hua, Wenbin Zhang, Xuebin Zhang, Shiwen Zhang, and Jiandong An. 2022. "Bumblebee Pollination Enhances Yield and Flavor of Tomato in Gobi Desert Greenhouses" Agriculture 12, no. 6: 795. https://doi.org/10.3390/agriculture12060795
APA StyleZhang, H., Han, C., Breeze, T. D., Li, M., Mashilingi, S. K., Hua, J., Zhang, W., Zhang, X., Zhang, S., & An, J. (2022). Bumblebee Pollination Enhances Yield and Flavor of Tomato in Gobi Desert Greenhouses. Agriculture, 12(6), 795. https://doi.org/10.3390/agriculture12060795






