Assessing the Effect of Physicochemical Properties of Saline and Sodic Soil on Soil Microbial Communities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling Sites and Collection
2.2. Analysis of Soil Physicochemical Properties
2.3. DNA Extraction
2.4. PCR Amplification
2.5. Sequencing Analysis
2.6. Statistical Analyses
3. Results
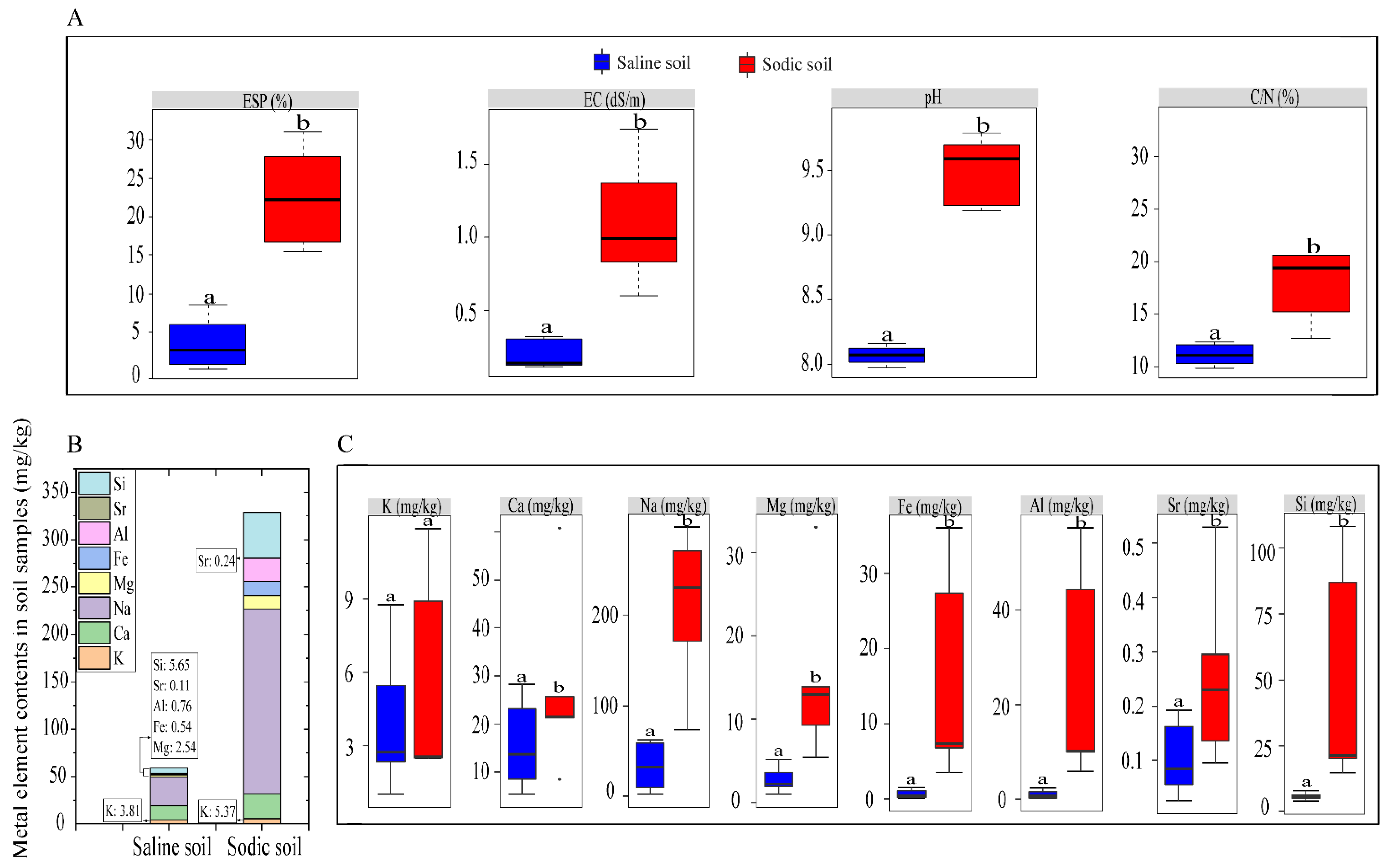
3.1. Differences in Soil Physicochemical Properties
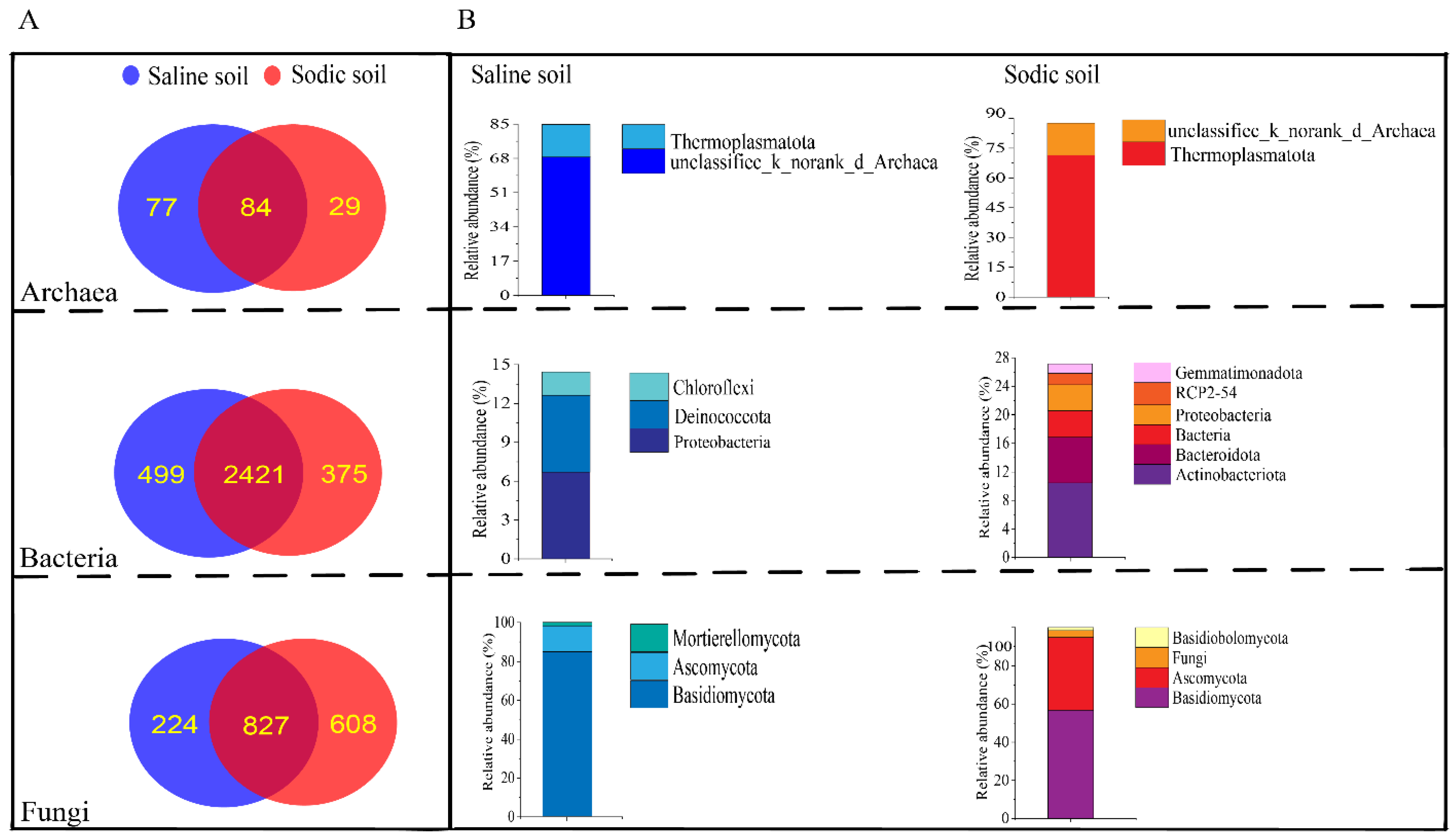
3.2. Sequencing Data and Alpha Diversity of Saline and Sodic Soil
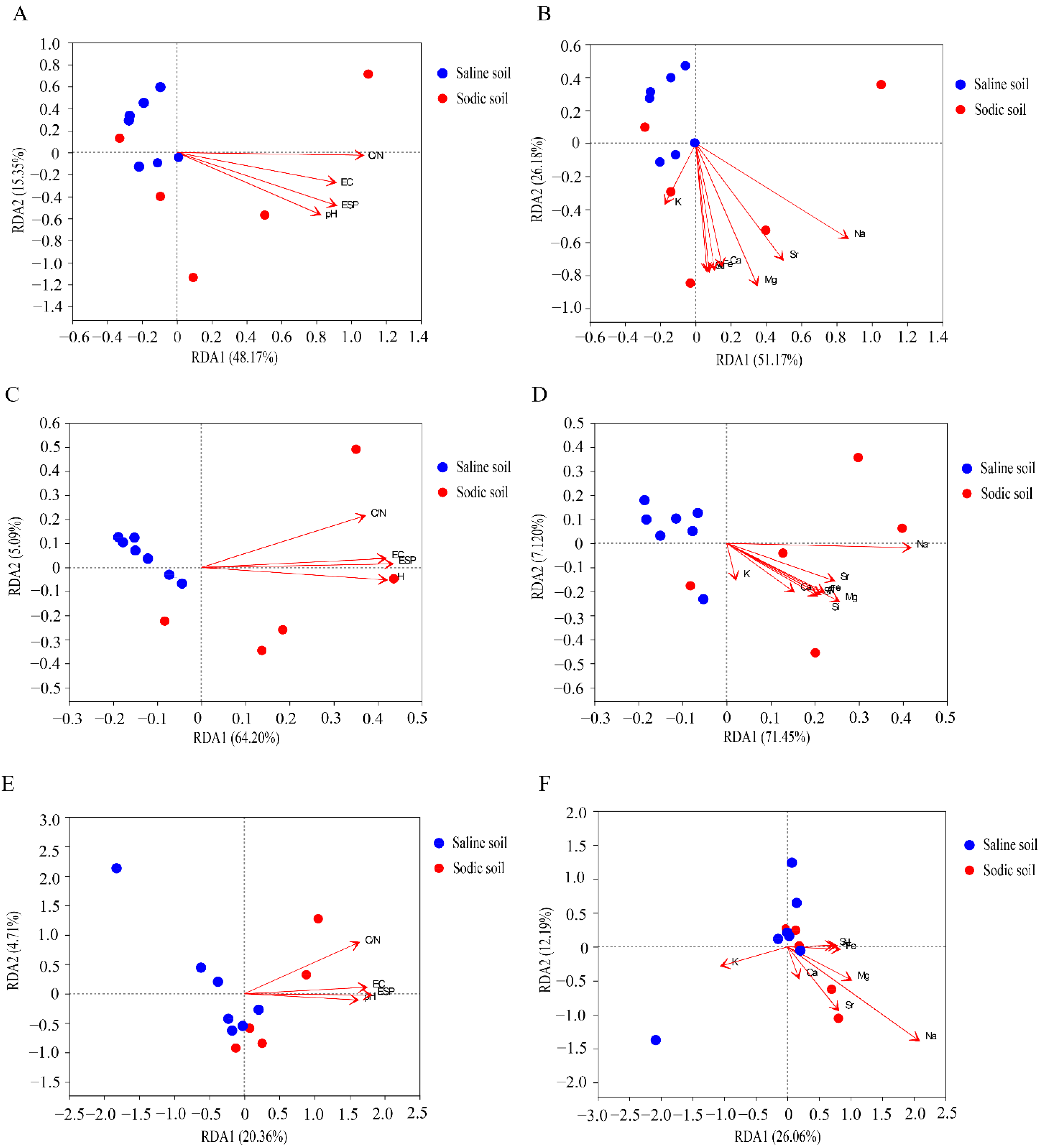
3.3. Beta Diversity of Saline and Sodic Soil
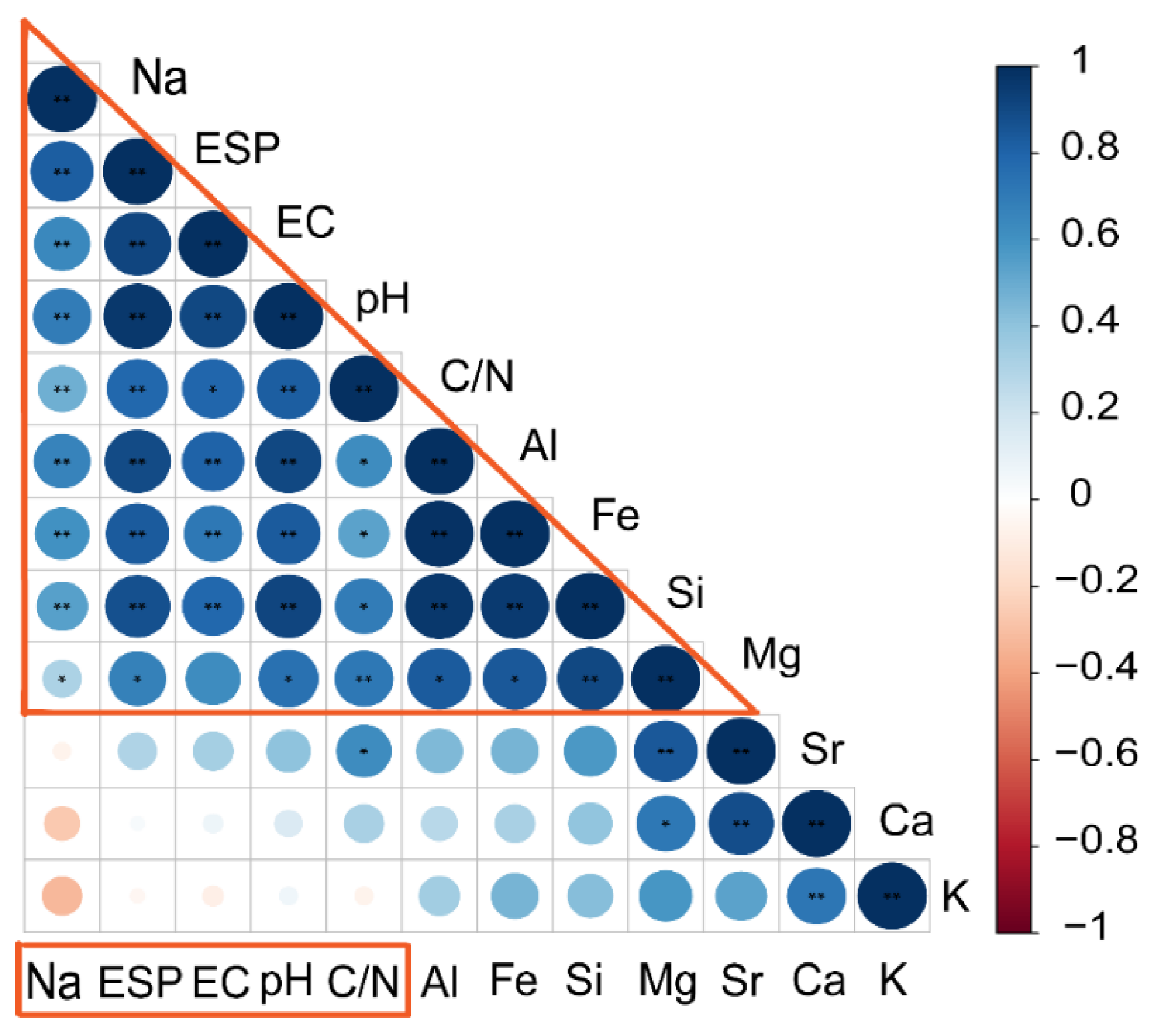
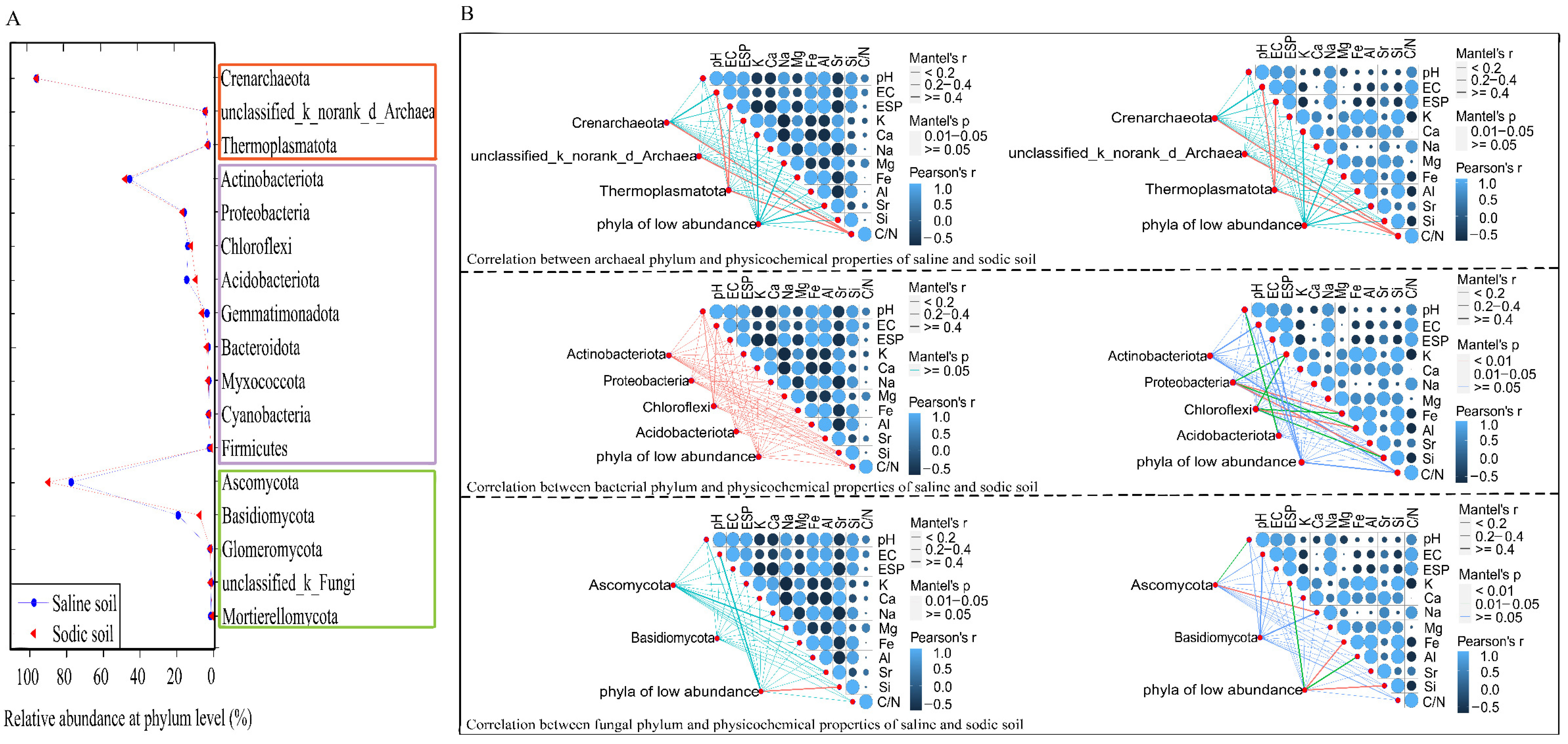
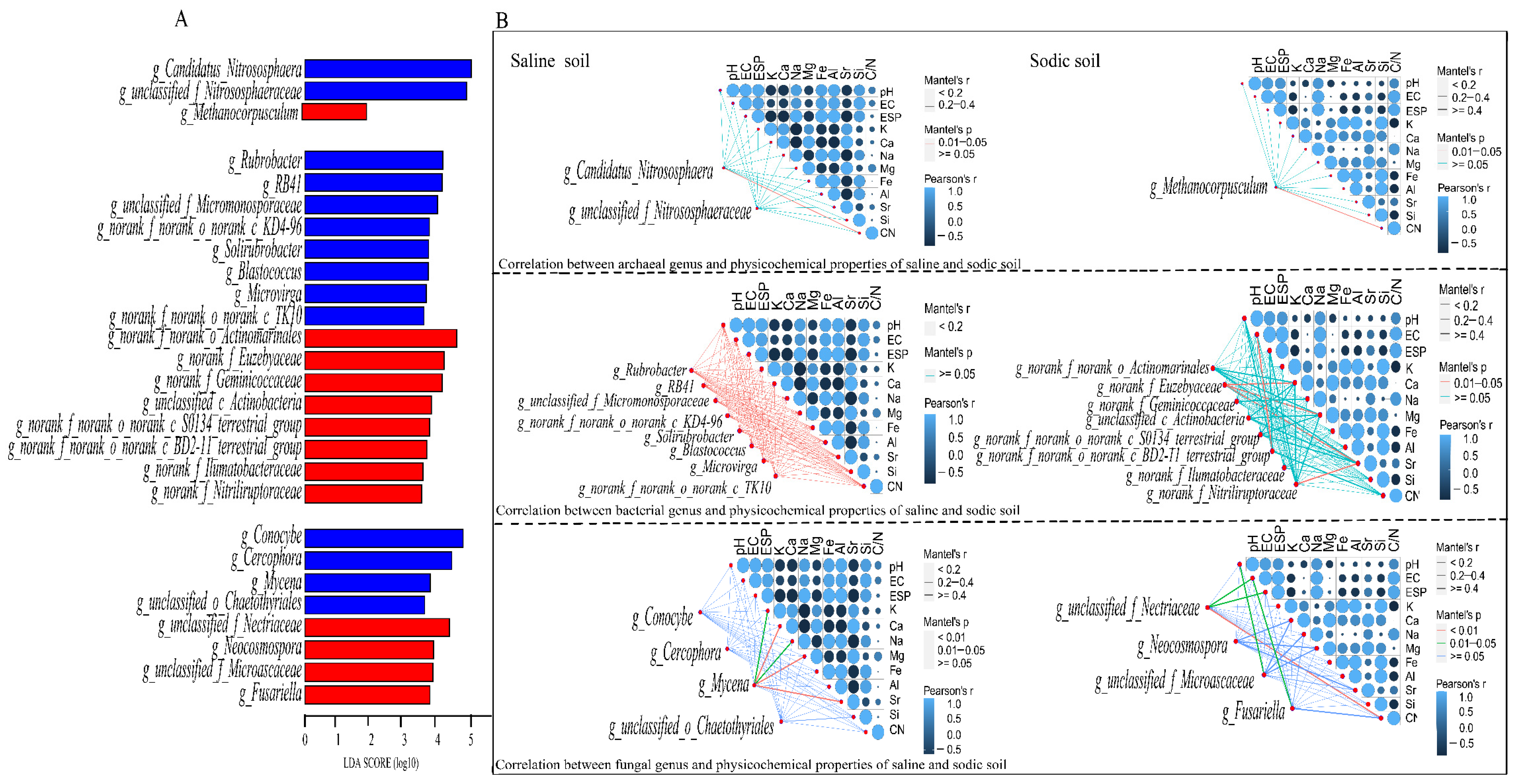
3.4. Microbial Composition of Soil and Driving Factors in Soil Physicochemical Properties
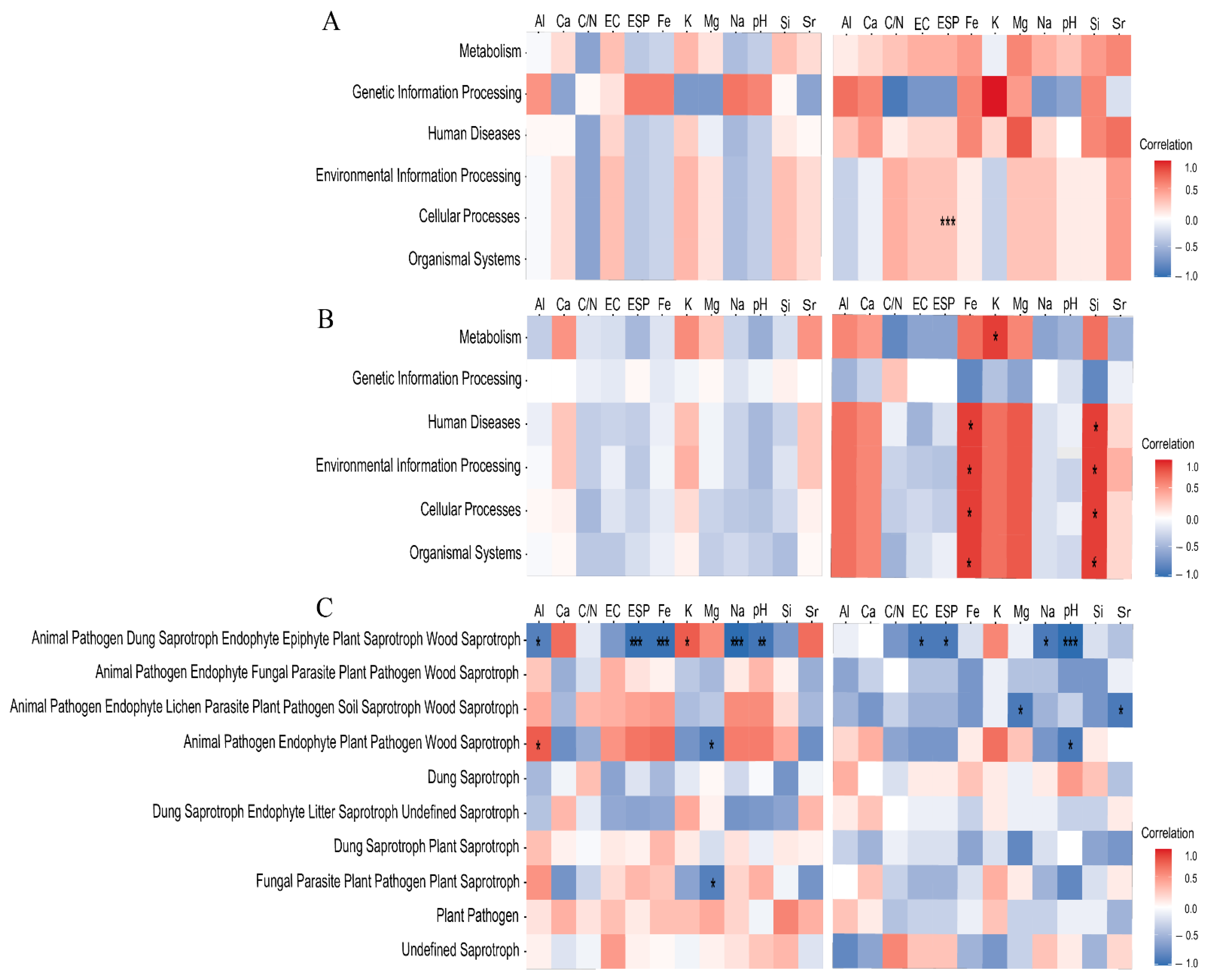
3.5. The Effect of Soil Physicochemical Properties on Functional Prediction of Microbiota
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Z.C.; Feng, S.C.; Luo, J.Q.; Hao, B.H.; Diao, F.W.; Li, X.; Jia, B.B.; Wang, L.X.; Bao, Z.H.; Guo, W. Evaluation of microbial assemblages in various saline-alkaline soils driven by soluble salt ion components. J. Agric. Food Chem. 2021, 69, 3390–3400. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.; Kumari, S.; Chakraborty, A.; Gupta, A.; Chakrabarti, K.; Bandyapadhyay, B.K. Microbial biomass and its activities in salt-affected coastal soils. Biol. Fertil. Soils 2005, 42, 273–277. [Google Scholar] [CrossRef]
- Shahid, S.A.; Zaman, M.; Heng, L. Soil salinity: Historical perspectives and a world overview of the problem. In Guideline for Salinity Assessment, Mitigation and Adaptation Using Nuclear and Related Techniques; Springer: New York, NY, USA, 2018; pp. 43–53. [Google Scholar]
- Wicke, B.; Smeets, E.; Dornburg, V.; Vashev, B.; Gaiser, T.; Turkenburg, W.; Faaij, A. The global technical and economic potential of bioenergy from salt-affected soils. Energy Environ. Sci. 2011, 4, 2669–2681. [Google Scholar] [CrossRef] [Green Version]
- Bai, W. Sustainable Development of Animal Husbandry in Inner Mongolia. Master’s Thesis, Minzu University of China, Beijing, China, 2007. [Google Scholar]
- Egamberdieva, D.; Kamilova, F.; Validov, S.; Gafurova, L.; Kucharova, Z.; Lugtenberg, B. High incidence of plant growth-stimulating bacteria associated with the rhizosphere of wheat grown on salinated soil in Uzbekistan. Environ. Microbiol. 2008, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; Abdullah, I.; Xu, T.T.; Zhu, W.Y.; Gao, Y.; Wang, L. Effects of mowing disturbance and competition on spatial expansion of the clonal plant Leymus chinensis into saline-alkali soil patches. Environ. Exp. Bot. 2019, 168, 103890. [Google Scholar] [CrossRef]
- Cheng, B.; Zhang, H.H. Information extraction of saline-alkali soil in the west of jilin province based on fully polarimetric sar-a case study in qian an county. In Proceedings of the 2nd International Conference on Computer Science and Application Engineering of the Conference, Hohhot, China, 22–24 October 2018. [Google Scholar]
- Allison, L.; Richards, L.A. Diagnosis and Improvement of Saline and Alkali Soils; Soil and Water Conservative Research Branch, Agricultural Research Service: Washington, DC, USA, 1954. [Google Scholar]
- Chandra, N.; Kumar, S. Antibiotics producing soil microorganisms. In Antibiotics and Antibiotics Resistance Genes in Soils; Springer: New York, NY, USA, 2017; pp. 1–18. [Google Scholar]
- Mataix-Solera, J.; Guerrero, C.; García-Orenes, F.; Bárcenas, G.M.; Torres, M.P. Forest fire effects on soil microbiology. In Fire Effects on Soils and Restoration Strategies; CRC Press: Boca Raton, FL, USA, 2009; pp. 149–192. [Google Scholar]
- Rashid, M.I.; Mujawar, L.H.; Shahzad, T.; Almeelbi, T.; Ismail, I.M.I.; Oves, M. Bacteria and fungi can contribute to nutrients bioavailability and aggregate formation in degraded soils. Microbiol. Res. 2016, 183, 26–41. [Google Scholar] [CrossRef]
- Muñoz-Rojas, M.; Erickson, T.E.; Dixon, K.W.; Merritt, D.J. Soil quality indicators to assess functionality of restored soils in degraded semiarid ecosystems. Restor. Ecol. 2016, 24, S43–S52. [Google Scholar] [CrossRef]
- Mendes, L.W.; Tsai, S.M.; Navarrete, A.A.; De Hollander, M.; Van Veen, J.A.; Kuramae, E.E. Soil-Borne Microbiome: Linking Diversity to Function. Microb. Ecol. 2015, 70, 255–265. [Google Scholar] [CrossRef]
- Wagg, C.; Bender, S.F.; Widmer, F.; van der Heijden, M.G.A. Soil biodiversity and soil community composition determine ecosystem multifunctionality. Proc. Natl. Acad. Sci. USA 2014, 111, 5266–5270. [Google Scholar] [CrossRef] [Green Version]
- Caton, T.M.; Witte, L.R.; Ngyuen, H.D.; Buchheim, J.A.; Schneegurt, M.A. Halotolerant Aerobic Heterotrophic Bacteria from the Great Salt Plains of Oklahoma. Microb. Ecol. 2004, 48, 449–462. [Google Scholar] [CrossRef]
- Hollister, E.B.; Engledow, A.S.; Hammett, A.J.M.; Provin, T.L.; Wilkinson, H.H.; Gentry, T.J. Shifts in microbial community structure along an ecological gradient of hypersaline soils and sediments. ISME J. 2010, 4, 829–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lozupone, C.A.; Knight, R. Global patterns in bacterial diversity. Proc. Natl. Acad. Sci. USA 2007, 104, 11436–11440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, K.P.; Shi, Y.; Cui, X.Q.; Yue, P.; Li, K.H.; Liu, X.J.; Tripathi, B.M.; Chu, H.Y. Salinity Is a Key Determinant for Soil Microbial Communities in a Desert Ecosystem. mSystems 2019, 4, e00225-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banda, J.F.; Zhang, Q.; Ma, L.Q.; Pei, L.X.; Du, Z.R.; Hao, C.B.; Dong, H.L. Both pH and salinity shape the microbial communities of the lakes in Badain Jaran Desert, NW China. Sci. Total Environ. 2021, 791, 148108. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Sun, J. Soil Salinity Drives the Distribution Patterns and Ecological Functions of Fungi in Saline-Alkali Land in the Yellow River Delta, China. Front. Microbiol. 2020, 11, 594284. [Google Scholar] [CrossRef] [PubMed]
- Andronov, E.E.; Petrova, S.N.; Pinaev, A.G.; Pershina, E.V.; Rakhimgaliyeva, S.Z.; Akhmedenov, K.M.; Gorobets, A.V.; Sergaliev, N.K. Analysis of the structure of microbial community in soils with different degrees of salinization using T-RFLP and real-time PCR techniques. Eurasian Soil Sci. 2012, 45, 147–156. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, J.J.; Banerjee, S.; Zhou, N.; Zhao, Z.Y.; Zhang, K.; Tian, C.Y. Soil pH is equally important as salinity in shaping bacterial communities in saline soils under halophytic vegetation. Sci. Rep. 2018, 8, 4550. [Google Scholar] [CrossRef]
- Dakouré, M.Y.S.; Mermoud, A.; Yacouba, H.; Boivin, P. Impacts of irrigation with industrial treated wastewater on soil properties. Geoderma 2013, 200–201, 31–39. [Google Scholar] [CrossRef]
- Feng, H.J.; Wang, S.Y.; Gao, Z.D.; Wang, Z.K.; Ren, X.Q.; Hu, S.W.; Pan, H. Effect of land use on the composition of bacterial and fungal communities in saline-sodic soils. Land Degrad Dev. 2019, 30, 1851–1860. [Google Scholar] [CrossRef]
- Rietz, D.N.; Haynes, R.J. Effects of irrigation-induced salinity and sodicity on soil microbial activity. Soil Biol. Biochem. 2003, 35, 845–854. [Google Scholar] [CrossRef]
- Yang, X.; Shen, Y.; Badgery, W.B.; Guo, Y.P.; Zhang, Y.J. Arbuscular mycorrhizal fungi alter plant community composition along a grazing gradient in Inner Mongolia Steppe. Basic Appl. Ecol. 2018, 32, 53–65. [Google Scholar] [CrossRef]
- Yin, Y.; Liu, H.Y.; He, S.Y.; Zhao, F.J.; Zhu, J.L.; Wang, H.Y.; Liu, G.; Wu, X.C. Patterns of local and regional grain size distribution and their application to Holocene climate reconstruction in semi-arid Inner Mongolia, China. Palaeogeogr. Palaeoclim. Palaeoecol. 2011, 307, 168–176. [Google Scholar] [CrossRef]
- Miao, L.J.; Jiang, C.; Xue, B.L.; Liu, Q.; He, B.; Nath, R.; Cui, X.F. Vegetation dynamics and factor analysis in arid and semi-arid Inner Mongolia. Environ. Earth Sci. 2014, 73, 2343–2352. [Google Scholar] [CrossRef]
- Sun, G.N.; Wang, M.H. Study on relation and distribution between vegetative coverage and land degradation in Inner Mongolia. Arid Land Resour. Environ. 2008, 22, 140–144. [Google Scholar]
- Cao, Y.P.; Pang, Y.J.; Pang, X.J. The characteristics of climate change in Xilingol league from 1956 to 2017. Agric. Res. Arid. Areas 2019, 37, 284–290. [Google Scholar]
- Luo, X.X.; Liu, G.C.; Xia, Y.; Chen, L.; Jiang, Z.X.; Zheng, H.; Wang, Z.Y. Use of biochar-compost to improve properties and productivity of the degraded coastal soil in the Yellow River Delta, China. J. Soils Sediments 2016, 17, 780–789. [Google Scholar] [CrossRef]
- Irvine, S.A.; Reid, D.J. Field prediction of sodicity in dryland agriculture in Central Queensland, Australia. Soil Res. 2001, 39, 1349–1357. [Google Scholar] [CrossRef]
- Liu, C.; Li, H.; Zhang, Y.Y.; Si, D.D.; Chen, Q.W. Evolution of microbial community along with increasing solid concentration during high-solids anaerobic digestion of sewage sludge. Bioresour. Technol. 2016, 216, 87–94. [Google Scholar] [CrossRef] [Green Version]
- Xu, N.; Tan, G.C.; Wang, H.Y.; Gai, X.P. Effect of biochar additions to soil on nitrogen leaching, microbial biomass and bacterial community structure. Eur. J. Soil Biol. 2016, 74, 1–8. [Google Scholar] [CrossRef]
- Adams, R.I.; Miletto, M.; Taylor, J.W.; Bruns, T.D. Dispersal in microbes: Fungi in indoor air are dominated by outdoor air and show dispersal limitation at short distances. ISME J. 2013, 7, 1262–1273. [Google Scholar] [CrossRef] [Green Version]
- Eren, A.M.; Sogin, M.L.; Morrison, H.G.; Vineis, J.H.; Fisher, J.C.; Newton, R.J.; McLellan, S.L. A single genus in the gut microbiome reflects host preference and specificity. ISME J. 2015, 9, 90–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [Green Version]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [Green Version]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [Green Version]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [Green Version]
- Langille, M.G.I.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Thurber, R.L.V.; Knight, R.; et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Song, Z.W.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- Lombard, N.; Prestat, E.; Van Elsas, J.D.; Simonet, P. Soil-specific limitations for access and analysis of soil microbial communities by metagenomics. FEMS Microbiol. Ecol. 2011, 78, 31–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chowdhury, N.; Marschner, P.; Burns, R.G. Soil microbial activity and community composition: Impact of changes in matric and osmotic potential. Soil Biol. Biochem. 2011, 43, 1229–1236. [Google Scholar] [CrossRef]
- Wang, J.J.; Yang, D.M.; Zhang, Y.; Shen, J.; van der Gast, C.; Hahn, M.W.; Wu, Q.L. Do Patterns of Bacterial Diversity along Salinity Gradients Differ from Those Observed for Macroorganisms? PLoS ONE 2011, 6, e27597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bissett, A.; Richardson, A.E.; Baker, G.; Thrall, P.H. Long-term land use effects on soil microbial community structure and function. Appl. Soil Ecol. 2011, 51, 66–78. [Google Scholar] [CrossRef]
- Ushio, M.; Kitayama, K.; Balser, T.C. Tree species-mediated spatial patchiness of the composition of microbial community and physicochemical properties in the topsoils of a tropical montane forest. Soil Biol. Biochem. 2010, 42, 1588–1595. [Google Scholar] [CrossRef]
- Lin, W.; Wang, Y.Z.; Gorby, Y.; Nealson, K.; Pan, Y.X. Integrating niche-based process and spatial process in biogeography of magnetotactic bacteria. Sci. Rep. 2013, 3, 1643. [Google Scholar] [CrossRef] [Green Version]
- Logares, R.; Bråte, J.; Bertilsson, S.; Clasen, J.L.; Shalchian-Tabrizi, K.; Rengefors, K. Infrequent marine–freshwater transitions in the microbial world. Trends Microbiol. 2009, 17, 414–422. [Google Scholar] [CrossRef]
- de Vries, F.T.; Liiri, M.E.; Bjørnlund, L.; Bowker, M.A.; Christensen, S.; Setälä, H.M.; Bardgett, R.D. Land use alters the resistance and resilience of soil food webs to drought. Nat. Clim. Change 2012, 2, 276–280. [Google Scholar] [CrossRef]
- Ayton, J.; Aislabie, J.; Barker, G.M.; Saul, D.; Turner, S. Crenarchaeota affiliated with group 1.1 b are prevalent in coastal mineral soils of the Ross Sea region of Antarctica. Environ. Microbiol. 2010, 12, 689–703. [Google Scholar] [CrossRef]
- Canfora, L.; Bacci, G.; Pinzari, F.; Lo Papa, G.; Dazzi, C.; Benedetti, A. Salinity and Bacterial Diversity: To What Extent Does the Concentration of Salt Affect the Bacterial Community in a Saline Soil? PLoS ONE 2014, 9, e106662. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, V.; Verma, M.K.; Gupta, S.; Mandhan, V.; Chauhan, N.S. Metagenomic Profiling of Soil Microbes to Mine Salt Stress Tolerance Genes. Front. Microbiol. 2018, 9, 159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rousk, J.; Bååth, E.; Brookes, P.C.; Lauber, C.L.; Lozupone, C.; Caporaso, J.G.; Knight, R.; Fierer, N. Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J. 2010, 4, 1340–1351. [Google Scholar] [CrossRef] [PubMed]
- Osono, T.; Takeda, H. Fungal decomposition of Abiesneedle and Betulaleaf litter. Mycologia 2006, 98, 172–179. [Google Scholar] [CrossRef]
- Drenovsky, R.E.; Steenwerth, K.L.; Jackson, L.E.; Scow, K.M. Land use and climatic factors structure regional patterns in soil microbial communities. Glob. Ecol. Biogeogr. 2009, 19, 27–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oren, A. Thermodynamic limits to microbial life at high salt concentrations. Environ. Microbiol. 2010, 13, 1908–1923. [Google Scholar] [CrossRef]
- Coban, O.; De Deyn, G.B.; van der Ploeg, M. Soil microbiota as game-changers in restoration of degraded lands. Science 2022, 375, abe0725. [Google Scholar] [CrossRef]
- Wang, M.; Chen, S.B.; Chen, L.; Wang, D. Responses of soil microbial communities and their network interactions to saline-alkaline stress in Cd-contaminated soils. Environ. Pollut. 2019, 252, 1609–1621. [Google Scholar] [CrossRef]
| Sample Classifications | Observed OTUs | Chao 1 Estimator | Shannon Index |
|---|---|---|---|
| Archaea | |||
| Saline soil | 52 ± 14 a | 64 ± 24 a | 2.16 ± 0.14 a |
| Sodic soil | 74 ± 11 b | 90 ± 20 b | 2.04 ± 0.12 a |
| Bacteria | |||
| Saline soil | 1780 ± 104 a | 2227 ± 142 a | 6.18 ± 0.09 a |
| Sodic soil | 1634 ± 204 a | 2073 ± 252 a | 5.91 ± 0.29 b |
| Fungi | |||
| Saline soil | 585 ± 76 a | 118 ± 48 a | 3.74 ± 0.83 a |
| Sodic soil | 444 ± 16 a | 193 ± 30 b | 3.82 ± 0.57 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, J.; Zhao, Q.; Chang, D.; Ndayisenga, F.; Yu, Z. Assessing the Effect of Physicochemical Properties of Saline and Sodic Soil on Soil Microbial Communities. Agriculture 2022, 12, 782. https://doi.org/10.3390/agriculture12060782
Gao J, Zhao Q, Chang D, Ndayisenga F, Yu Z. Assessing the Effect of Physicochemical Properties of Saline and Sodic Soil on Soil Microbial Communities. Agriculture. 2022; 12(6):782. https://doi.org/10.3390/agriculture12060782
Chicago/Turabian StyleGao, Junzhi, Qingzhou Zhao, Dongdong Chang, Fabrice Ndayisenga, and Zhisheng Yu. 2022. "Assessing the Effect of Physicochemical Properties of Saline and Sodic Soil on Soil Microbial Communities" Agriculture 12, no. 6: 782. https://doi.org/10.3390/agriculture12060782
APA StyleGao, J., Zhao, Q., Chang, D., Ndayisenga, F., & Yu, Z. (2022). Assessing the Effect of Physicochemical Properties of Saline and Sodic Soil on Soil Microbial Communities. Agriculture, 12(6), 782. https://doi.org/10.3390/agriculture12060782







