Seasonality of Carabid Beetles on an Organic Agricultural Field and Its Effect on Foraging Use
Abstract
:1. Introduction
2. Methods and Sites
3. Results
3.1. Seasonal Occurrence and Migration Speed
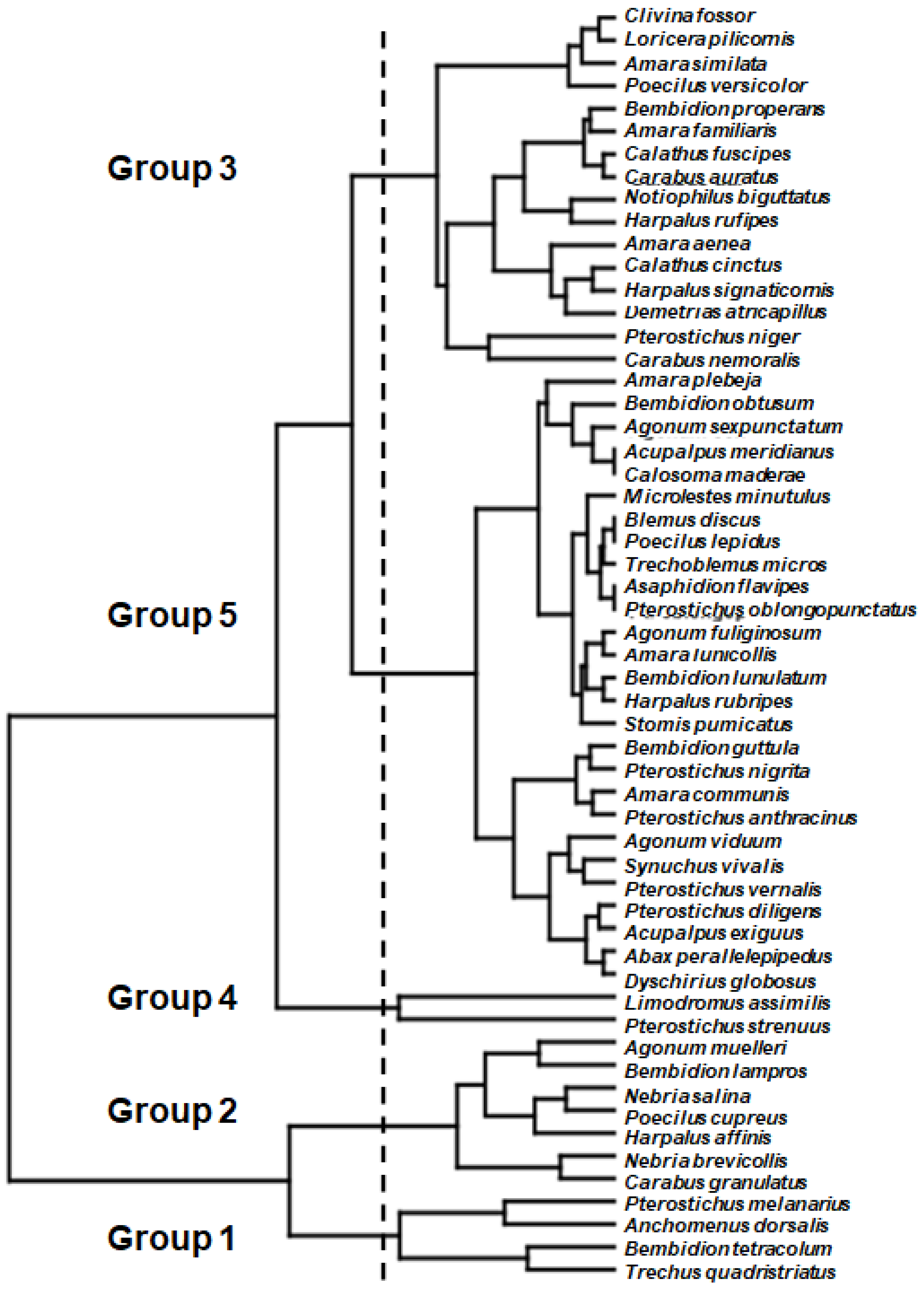
3.2. Time and Space Related Foraging Potentials
4. Discussion
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilson, E.O. Biodiversity; National Academic Press: Washington, DC, USA, 1988. [Google Scholar]
- Niemelä, J.; Kotze, J.; Ashworth, A.; Brandmayr, P.; Desender, K.; New, T.; Penev, L.; Samways, M.J.; Spence, J. The search for common anthropogenic impacts on biodiversity: A global network. J. Insect Conserv. 2000, 4, 3–9. [Google Scholar] [CrossRef]
- Siebenhüner, B. Administrator of global biodiversity: The secretariat of the convention on biological diversity. Biodivers. Conserv. 2006, 16, 259–274. [Google Scholar] [CrossRef]
- Tscharntke, T.; Klein, A.M.; Kruess, A.; Steffan-Dewenter, I.; Thies, C. Landscape perspectives on agricultural intensification. Ecol. Lett. 2005, 8, 857–874. [Google Scholar] [CrossRef]
- Poschlod, P.; Bakker, J.; Kahmen, S. Changing land use and its impact on biodiversity. Basic Appl. Ecol. 2005, 6, 93–98. [Google Scholar] [CrossRef]
- Bengtsson, J.; Ahnström, J.; Weibull, A.-C. The effects of organic agriculture on biodiversity and abundance: A meta-analysis. J. Appl. Ecol. 2005, 42, 261–269. [Google Scholar] [CrossRef]
- Irmler, U. Which carabid species (Coleoptera: Carabidae) profit from organic farming after a succession of 15 years? Agric. Ecosyst. Environ. 2018, 263, 1–6. [Google Scholar] [CrossRef]
- Irmler, U. The succession of Staphylinidae (Coleoptera) after 15 years of conversion from conventional to organic farming. Biodivers. Conserv. 2018, 27, 3233–3246. [Google Scholar] [CrossRef]
- Schröter, L.; Irmler, U. Organic cultivation reduces barrier effect of arable fields on species diversity. Agric. Ecosyst. Environ. 2013, 164, 176–180. [Google Scholar] [CrossRef]
- Irmler, U. Changes in earthworm populations during conversion from conventional to organic farming. Agric. Ecosyst. Environ. 2010, 135, 194–198. [Google Scholar] [CrossRef]
- Irmler, U.; Koop, B.; Schrautzer, A. Entwicklung der Lebensgemeinschaften nach der Konversion vom konventionellen zum ökologischen Landbau. Nat. Landsch. 2020, 95, 253–262. [Google Scholar]
- Irmler, U. Veränderung der Laufkäfergemeinschaften (Carabidae) in 15 Jahren Sukzession Nach der Umstellung vom Konventionellen auf Ökologischen Landbau auf Hof Ritzerau; Faunistisch-Ökologische Mitteilungen Supplement; Kiel University Publishing: Kiel, Germany, 2020; Volume 41, pp. 1–97. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software. Palaeontol. Electron. 2012, 4, 1–300. [Google Scholar]
- Lukasiewicz, J. Predation by the beetle Carabus granulatus L. (Coleoptera, Carabidae) on soil macrofauna in grassland on drained peats. Pedobiologia 1996, 40, 364–376. [Google Scholar]
- Cornell, H.V.; Lawton, J.H. Species interactions, local and regional processes, and limits to the richness of ecological communities: A theoretical persepective. J. Anim. Ecol. 1992, 61, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Duelli, P.; Obrist, K. Regional biodiversity in an agricultural landscape: The contribution of seminatural habitat islands. Basic Appl. Ecol. 2003, 4, 129–138. [Google Scholar] [CrossRef]
- Denys, C.; Tscharnke, T. Plant-insect communities and predator-prey ratios in field margin strips, adjacent crop field, and fallows. Oecologia 2002, 130, 315–324. [Google Scholar] [CrossRef]
- Levin, S. Dispersion and Population Interactions. Am. Nat. 1974, 108, 207–228. [Google Scholar] [CrossRef]
- Desender, K. Wing polymorphism and reproductive biology in the halobiont carabid beetle Pogonus chalceus (Marsham) (Coleoptera, Carabidae). Biol. Jaarb. Dodonaea 1985, 53, 89–100. [Google Scholar]
- De Vries, H.H.; Boer, P.J.D.; Van Dijk, T.S. Ground beetle species in heathland fragments in relation to survival, dispersal, and habitat preference. Oecologia 1996, 107, 332–342. [Google Scholar] [CrossRef]
- Baars, M.A. Patterns of movement of radioactive carabid beetles. Oecologia 1979, 44, 125–140. [Google Scholar] [CrossRef]
- Griffiths, E.; Wratten, S.; Vickerman, G.P. Foraging by the carabid Agonum dorsale in the field. Ecol. Èntomol. 1985, 10, 181–189. [Google Scholar] [CrossRef]
- Hůrka, K.; Jarošík, V. Larval omnivory in Amara aenea (Coleoptera: Carabidae). Eur. J. Èntomol. 2003, 100, 329–335. [Google Scholar] [CrossRef] [Green Version]
- Saska, P.; Jarošík, V. Laboratory study of larval food requirements in nine species of Amara (Coleoptera: Carabidae). Plant Prot. Sci. 2001, 37, 103–110. [Google Scholar] [CrossRef] [Green Version]
- Fawki, S.; Toft, S. Food preferences and the value of animal food for the carabid beetle Amara similata (Gyll.) (Col., Carabidae). J. Appl. Èntomol. 2005, 129, 551–556. [Google Scholar] [CrossRef]
- Hengeveld, R. Quantitative and quantitative species of food of ground beetles (Coleoptera, Carabidae): A review. Neth. J. Zool. 1980, 30, 555–563. [Google Scholar] [CrossRef]
- Oberholzer, F.; Frank, T. Predation by the carabid beetles Pterostichus melanarius and Poecilus cupreus on slugs and slug eggs. Biocontrol Sci. Technol. 2003, 13, 99–110. [Google Scholar] [CrossRef]
- Charrier, S.; Petit, S.; Burel, F. Movements of Abax parallelepipedus (Coleoptera, Carabidae) in woody habitats of hedgerow netwotk landscape: A radio-tracing study. Agric. Ecosyst. Environ. 1997, 61, 133–144. [Google Scholar] [CrossRef]
- Tischendorf, L.; Irmler, U.; Hingst, R. A simulation experiment on the potential of hedgerows as movement corridors for forest carabids. Ecol. Model. 1998, 106, 107–118. [Google Scholar] [CrossRef]
- Chaabane, K.; Josens, H.; Loreau, M. Quantitative assessment of consumption by Abax ater Villiers (Coleoptera, Carabidae). Pedobiologia 1993, 37, 291–302. [Google Scholar]

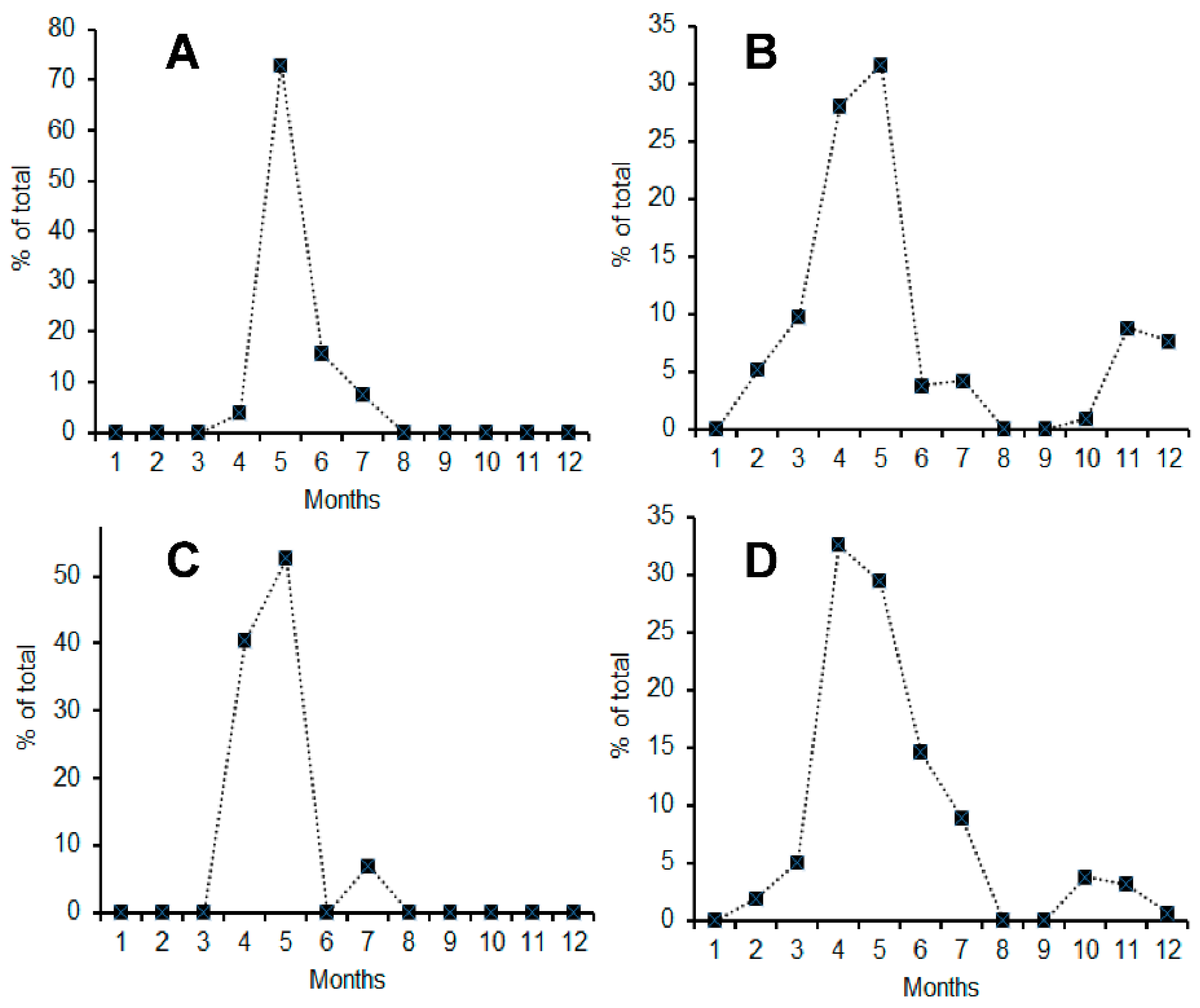
| Species | W. Mean | Slope | 100 m | p | Beginning at | Ending at | ||
|---|---|---|---|---|---|---|---|---|
| (Months *) | S.d. | (d) | m | d Group | m | d Group | ||
| no seasonal difference among distances | ||||||||
| Bembidion obtusum | 2.8/11.6 | 0.18 | ||||||
| Poecilus versicolor | 5.4 | 0.05 | ||||||
| Poecilus cupreus | 5.5 | 0.03 | ||||||
| Carabus auratus | 5.5 | 0.07 | ||||||
| Amara aenea | 5.8 | 0.06 | ||||||
| Loricera pilicornis | 6.0 | 0.07 | ||||||
| Bembidion lampros | 6.1 | 0.15 | ||||||
| Harpalus affinis | 6.1 | 0.07 | ||||||
| Agonum mülleri | 6.1 | 0.09 | ||||||
| Harpalus rubripes | 6.2 | 0.39 | ||||||
| Poecilus lepidus | 6.3 | 0.22 | ||||||
| Calosoma maderae | 6.3 | 0.27 | ||||||
| Amara lunicollis | 6.3 | 0.19 | ||||||
| Pterostichus melanarius | 6.8 | 0.09 | ||||||
| Pterostichus niger | 7.3 | 0.06 | ||||||
| Calathus cinctus | 3.6/10.5 | 0.11 | ||||||
| Nebria salina | 4.9/10.7 | 0.16 | ||||||
| Trechus quadristriatus | 3.4/10.8 | 0.19 | ||||||
| seasonal occurrence increasing from center to margin | ||||||||
| Acupalpus meridianus | 5.0/11.6 | −0.012 | 35.4 | 0.037 | 5.2 | 240 | 8.0 | 0 |
| Clivina fossor | 5.9 | −0.002 | 5.1 | 0.001 | 5.6 | 240 | 6.0 | 0 |
| Harpalus rufipes | 6.7 | −0.002 | 6.3 | 0.001 | 6.3 | 240 | 6.8 | 0 |
| Harpalus signaticornis | 6.7 | −0.003 | 9.9 | 0.050 | 6.0 | 240 | 6.8 | 0 |
| Synuchus vivalis | 7.1 | −0.004 | 11.7 | 0.001 | 6.5 | 240 | 7.5 | 0 |
| Blemus discus | 7.5 | −0.003 | 9.1 | 0.001 | 7.2 | 240 | 7.9 | 0 |
| Calathus fuscipes | 7.6 | −0.002 | 5.1 | 0.001 | 7.4 | 240 | 7.8 | 0 |
| seasonal occurrence increasing from margin to center | ||||||||
| Asaphidion flavipes | 4.4 | 0.0082 | 24.6 | 0.030 | 4.1 | 0 | 6.0 | 240 |
| Nebria brevicollis | 4.9/10.0 | 0.0051 | 15.3 | 0.001 | 4.2 | 0 | 5.5 | 240 |
| Bembidion properans | 5.4/10.7 | 0.0017 | 5.4 | 0.003 | 5.1 | 0 | 5.5 | 240 |
| Carabus nemoralis | 5.4 | 0.0017 | 5.4 | 0.007 | 5.4 | 0 | 5.8 | 240 |
| Agonum sexpunctatum | 5.5 | 0.0029 | 8.7 | 0.048 | 5.2 | 0 | 5.9 | 240 |
| Pterostichus anthracinus | 5.6 | 0.0044 | 13.2 | 0.007 | 5.4 | 0 | 6.4 | 240 |
| Carabus granulatus | 5.7 | 0.0016 | 4.7 | 0.050 | 5.6 | 0 | 6.0 | 240 |
| Limodromus assimilis | 5.7 | 0.0012 | 3.5 | 0.050 | 5.6 | 0 | 5.8 | 240 |
| Anchomenus dorsalis | 5.9 | 0.002 | 2.0 | 0.006 | 5.8 | 0 | 6.0 | 240 |
| Stomis pumicatus | 6.0 | 0.0019 | 5.7 | 0.040 | 5.7 | 0 | 6.1 | 240 |
| Abax parallelpipedus | 6.0 | 0.0073 | 21.9 | 0.001 | 5.8 | 0 | 7.6 | 240 |
| Amara similata | 6.4 | 0.0002 | 2.0 | 0.001 | 6.3 | 0 | 6.4 | 240 |
| Agonum viduum | 6.4 | 0.0133 | 39.9 | 0.001 | 5.6 | 0 | 8.7 | 240 |
| Amara plepebja | 7.3 | 0.002 | 4.8 | n.s. | 7.1 | 0 | 7.5 | 240 |
| logarithmic (ln) increase from margin to center | ||||||||
| Bembidion tetracolum | 4.4/11.0 | 0.0986 | 13.6 | 0.011 | 4.0 | 0 | 4.6 | 240 |
| Demetrias atricapillus | 5.2 | 0.1896 | 6.3 | 0.001 | 4.6 | 0 | 5.6 | 240 |
| Amara familiaris | 5.8 | 0.1457 | 20.1 | 0.001 | 5.2 | 0 | 5.9 | 240 |
| Trechoblemus micros | 6.3 | 0.5331 | 73.6 | 0.009 | 4.4 | 0 | 7.3 | 240 |
| Microlestes minutulus | 6.4 | 0.1299 | 17.9 | 0.020 | 5.9 | 0 | 6.3 | 240 |
| seasonal occurrence increasing from margin but not reaching center | ||||||||
| Pterostichus strenuus | 4.7/10.8 | 0.0071 | 21.3 | 0.001 | 4.6 | 0 | 5.5 | 120 |
| Bembidion lunulatum | 4.7 | 0.0032 | 9.6 | 0.004 | 4.6 | 0 | 5.3 | 120 |
| Acupalpus exiguus | 5.3/11.6 | 0.0081 | 29.2 | 0.044 | 5.2 | 0 | 6.1 | 120 |
| Pterostichus oblongipunctatus | 5.4 | 0.066 | 16.5 | 0.050 | 5.1 | 0 | 5.7 | 120 |
| Pterostichus diligens | 5.4 | 0.0073 | 21.9 | 0.025 | 5.0 | 0 | 5.8 | 120 |
| Bembidion guttula | 5.6 | 0.0129 | 38.7 | 0.001 | 5.2 | 0 | 6.2 | 120 |
| Notiophilus biguttatus | 6.1 | 0.0028 | 8.4 | 0.001 | 6.0 | 0 | 6.7 | 120 |
| Amara communis | 6.6 | 0.012 | 37.5 | 0.003 | 5.8 | 0 | 7.3 | 120 |
| Dyschirius globosus | 6.6 | 0.0051 | 15.3 | 0.001 | 6.1 | 0 | 6.7 | 120 |
| Pterostichus nigrita | 6.8 | 0.0067 | 45.9 | 0.011 | 6.0 | 0 | 7.8 | 120 |
| Pterostichus vernalis | 7.3 | 0.0068 | 20.4 | 0.050 | 7.2 | 0 | 7.9 | 120 |
| Agonum fuliginosum | 5.3/10.7 | 0.0177 | 53.1 | 0.047 | 5.1 | 0 | 6.2 | 60 |
| Margin | Field | Potential Use | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Species | Points (%) | Space (ha) | AM | ST | Points (%) | Space (ha) | AM | ST | Margin (%) | Field (%) |
| Group 1 with equal use of margin and field | ||||||||||
| Trechus quadristriatus | 100 | 18 | 9.4 | 14.1 | 100 | 180 | 10.1 | 151.1 | 52 | 84 |
| Bembidion tetracolum | 100 | 18 | 10.0 | 15.1 | 100 | 180 | 9.3 | 139.1 | 56 | 77 |
| Pterostichus melanarius | 100 | 18 | 7.8 | 11.7 | 100 | 180 | 8.3 | 124.7 | 43 | 69 |
| Anchomenus dorsalis | 100 | 18 | 8.1 | 12.2 | 100 | 180 | 7.0 | 105.3 | 45 | 59 |
| Group 2 with equal use of margin and field and lower foraging effect | ||||||||||
| Agonum mülleri | 100 | 18 | 5.4 | 8.2 | 100 | 180 | 7.4 | 111.7 | 30 | 62 |
| Bembidion lampros | 96 | 17 | 5.5 | 7.8 | 100 | 180 | 6.6 | 99.7 | 29 | 55 |
| Nebria brevicollis | 100 | 18 | 6.6 | 9.9 | 100 | 180 | 6.0 | 89.5 | 37 | 50 |
| Carabus granulatus | 100 | 18 | 6.1 | 9.1 | 100 | 180 | 5.5 | 82.5 | 34 | 46 |
| Nebria salina | 93 | 17 | 3.8 | 5.4 | 100 | 180 | 6.5 | 98.1 | 20 | 55 |
| Poecilus cupreus | 100 | 18 | 4.4 | 6.6 | 100 | 180 | 6.4 | 96.1 | 24 | 53 |
| Harpalus affinis | 100 | 18 | 3.1 | 4.7 | 100 | 180 | 5.9 | 87.8 | 17 | 49 |
| Group 3 with small distribution and small foraging effect | ||||||||||
| Clivina fossor | 93 | 17 | 3.5 | 5.0 | 99 | 178 | 4.2 | 62.6 | 18 | 35 |
| Poecilus versicolor | 96 | 17 | 4.0 | 5.7 | 100 | 180 | 4.1 | 61.7 | 21 | 34 |
| Loricera pilicornis | 100 | 18 | 3.0 | 4.6 | 100 | 180 | 4.1 | 60.9 | 17 | 34 |
| Amara similata | 100 | 18 | 3.4 | 5.2 | 100 | 180 | 3.8 | 57.7 | 19 | 32 |
| Bembidion properans | 59 | 11 | 2.0 | 1.8 | 95 | 171 | 3.5 | 49.3 | 7 | 27 |
| Amara familiaris | 85 | 15 | 2.0 | 2.5 | 100 | 180 | 3.1 | 47.2 | 9 | 26 |
| Notiophilus biguttatus | 78 | 14 | 4.1 | 4.8 | 95 | 171 | 3.3 | 47.1 | 18 | 26 |
| Harpalus rufipes | 89 | 16 | 2.7 | 3.7 | 99 | 178 | 3.1 | 46.1 | 14 | 26 |
| Calathus fuscipes | 74 | 13 | 1.9 | 2.1 | 92 | 165 | 3.1 | 43.3 | 8 | 24 |
| Carabus auratus | 81 | 15 | 1.6 | 1.9 | 95 | 171 | 3.0 | 43.1 | 7 | 24 |
| Amara aenea | 52 | 9 | 0.9 | 0.7 | 93 | 167 | 2.5 | 35.3 | 3 | 20 |
| Calathus cinctus | 63 | 11 | 1.2 | 1.1 | 80 | 144 | 2.4 | 29.1 | 4 | 16 |
| Harpalus signaticornis | 67 | 12 | 1.7 | 1.7 | 91 | 163 | 2.1 | 28.6 | 6 | 16 |
| Demetrias atricapillus | 78 | 14 | 2.1 | 2.5 | 91 | 163 | 1.8 | 24.5 | 9 | 14 |
| Pterostichus niger | 100 | 18 | 4.3 | 6.5 | 100 | 180 | 2.6 | 38.8 | 24 | 22 |
| Limodromus assimilis | 85 | 15 | 3.9 | 5.0 | 78 | 141 | 1.8 | 21.1 | 18 | 12 |
| Group 4 with high foraging effects in the margin | ||||||||||
| Pterostichus strenuous | 100 | 18 | 6.6 | 9.9 | 54 | 98 | 1.0 | 8.5 | 37 | 5 |
| Carabus nemoralis | 96 | 17 | 6.7 | 9.5 | 95 | 171 | 3.1 | 44.4 | 35 | 25 |
| Group 5 with small foraging effects margin and field | ||||||||||
| Amara plepebja | 81 | 15 | 1.7 | 2.1 | 74 | 133 | 1.4 | 15.5 | 8 | 9 |
| Bembidion obtusum | 59 | 11 | 1.3 | 1.1 | 65 | 116 | 1.7 | 16.5 | 4 | 9 |
| Agonum sexpunctatum | 56 | 10 | 1.1 | 0.9 | 68 | 122 | 1.1 | 11.1 | 3 | 6 |
| Acupalpus meridianus | 33 | 6 | 0.5 | 0.2 | 58 | 105 | 1.3 | 11.0 | 1 | 6 |
| Calosoma maderae | 33 | 6 | 0.4 | 0.2 | 61 | 111 | 1.2 | 10.7 | 1 | 6 |
| Microlestes minutulus | 26 | 5 | 0.4 | 0.1 | 47 | 84 | 0.7 | 4.8 | 1 | 3 |
| Blemus discus | 19 | 3 | 0.2 | 0.1 | 32 | 58 | 0.5 | 2.6 | 0 | 1 |
| Poecilus lepidus | 4 | 1 | 0.0 | 0.0 | 29 | 53 | 0.5 | 2.1 | 0 | 1 |
| Bembidion guttula | 93 | 17 | 4.4 | 6.2 | 45 | 81 | 0.6 | 4.3 | 23 | 2 |
| Pterostichus nigrita | 89 | 16 | 4.3 | 5.7 | 44 | 79 | 0.8 | 5.3 | 21 | 3 |
| Amara communis | 85 | 15 | 1.7 | 2.2 | 15 | 65.6 | 0.5 | 2.6 | 8 | 1 |
| Pterostichus anthracinus | 78 | 14 | 4.1 | 4.8 | 30 | 54 | 0.6 | 2.7 | 18 | 1 |
| Agonum viduum | 100 | 18 | 2.6 | 3.9 | 22 | 39 | 0.3 | 0.9 | 15 | 0 |
| Synuchus vivalis | 56 | 10 | 0.9 | 0.7 | 10 | 96 | 0.8 | 6.2 | 3 | 3 |
| Pterostichus vernalis | 89 | 16 | 2.7 | 3.6 | 60 | 109 | 1.0 | 9.4 | 13 | 5 |
| Pterosticus diligens | 81 | 15 | 2.3 | 2.9 | 9 | 17 | 0.1 | 0.1 | 11 | 0 |
| Acupalpus exiguus | 78 | 14 | 2.3 | 2.7 | 29 | 53 | 0.4 | 2.0 | 10 | 1 |
| Abax parallelpipedus | 67 | 12 | 2.2 | 2.2 | 29 | 53 | 0.4 | 1.5 | 8 | 1 |
| Dyschirius globosus | 70 | 13 | 2.1 | 2.2 | 11 | 21 | 0.1 | 0.2 | 8 | 0 |
| Agonum fuliginosum | 63 | 11 | 1.6 | 1.5 | 5 | 9 | 0.1 | 0.1 | 6 | 0 |
| Amara lunicollis | 67 | 12 | 1.4 | 1.4 | 21 | 38 | 0.3 | 0.8 | 5 | 0 |
| Stomis pumicatus | 63 | 11 | 1.1 | 1.0 | 46 | 83 | 0.7 | 4.9 | 4 | 3 |
| Bembidion lunulatum | 41 | 7 | 1.2 | 0.7 | 15 | 26 | 0.4 | 0.8 | 3 | 0 |
| Harpalus rubripes | 56 | 10 | 0.9 | 0.7 | 34 | 62 | 0.4 | 2.2 | 3 | 1 |
| Asaphidion flavipes | 37 | 7 | 0.6 | 0.3 | 15 | 26 | 0.1 | 0.3 | 1 | 0 |
| Pt. oblongopunctatus | 37 | 7 | 0.5 | 0.3 | 7 | 13 | 0.1 | 0.1 | 1 | 0 |
| Trechoblemus micros | 37 | 7 | 0.4 | 0.2 | 32 | 58 | 0.5 | 2.3 | 1 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Irmler, U. Seasonality of Carabid Beetles on an Organic Agricultural Field and Its Effect on Foraging Use. Agriculture 2022, 12, 596. https://doi.org/10.3390/agriculture12050596
Irmler U. Seasonality of Carabid Beetles on an Organic Agricultural Field and Its Effect on Foraging Use. Agriculture. 2022; 12(5):596. https://doi.org/10.3390/agriculture12050596
Chicago/Turabian StyleIrmler, Ulrich. 2022. "Seasonality of Carabid Beetles on an Organic Agricultural Field and Its Effect on Foraging Use" Agriculture 12, no. 5: 596. https://doi.org/10.3390/agriculture12050596
APA StyleIrmler, U. (2022). Seasonality of Carabid Beetles on an Organic Agricultural Field and Its Effect on Foraging Use. Agriculture, 12(5), 596. https://doi.org/10.3390/agriculture12050596






