Salinity in Jatropha curcas: A Review of Physiological, Biochemical, and Molecular Factors Involved
Abstract
1. Introduction
2. Physiological, Biochemical, and Molecular Responses of Jatropha curcas L. to Abiotic Stress
3. How Does Salinity Modulate Physiological, Biochemical and Molecular Responses in J. curcas?
3.1. Advantages and Disadvantages of Wastewater in Agriculture
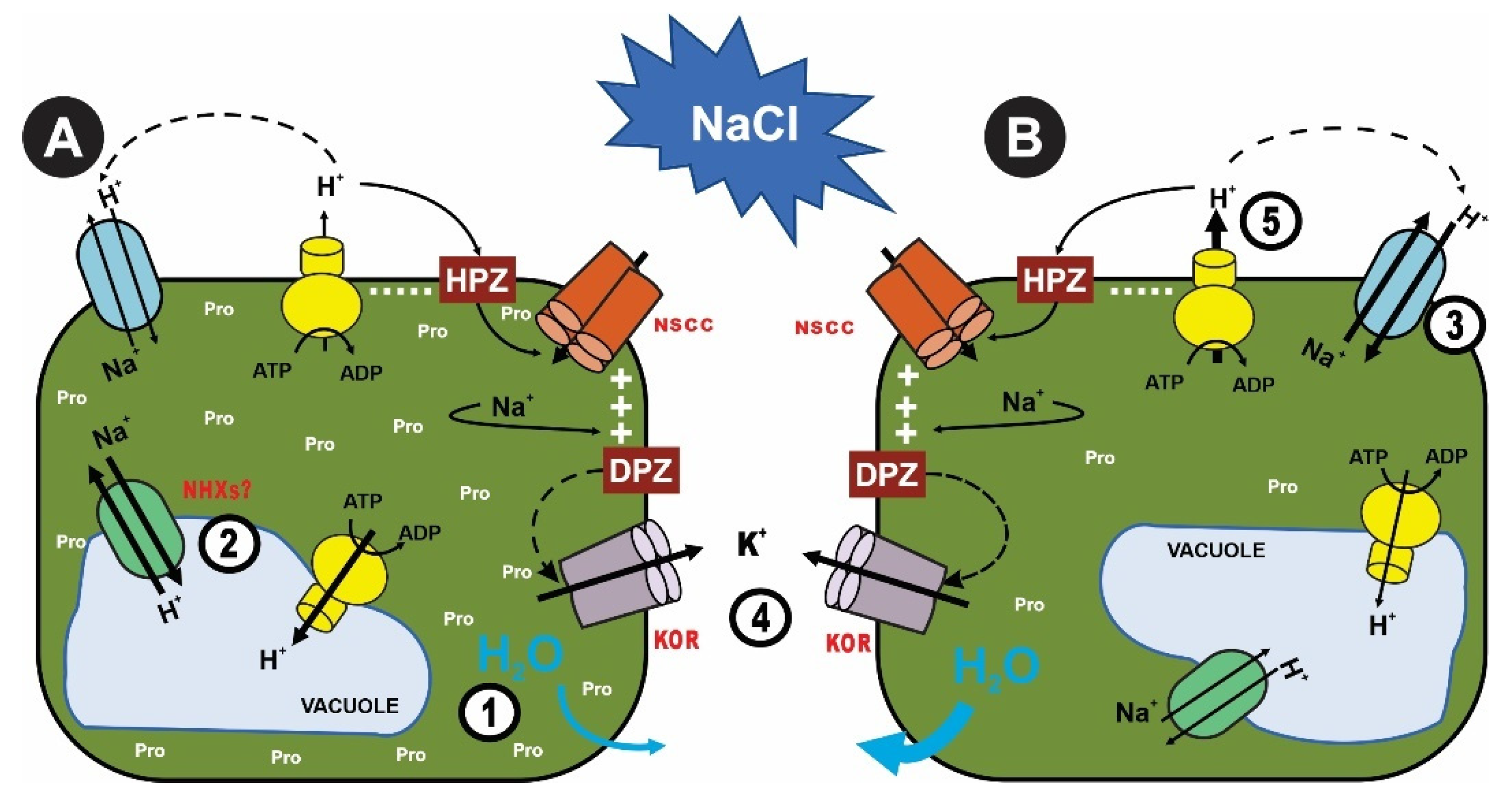
3.2. Sodium (Na+) and Chloride (Cl−) Compartmentalization Related to Salt Stress Tolerance
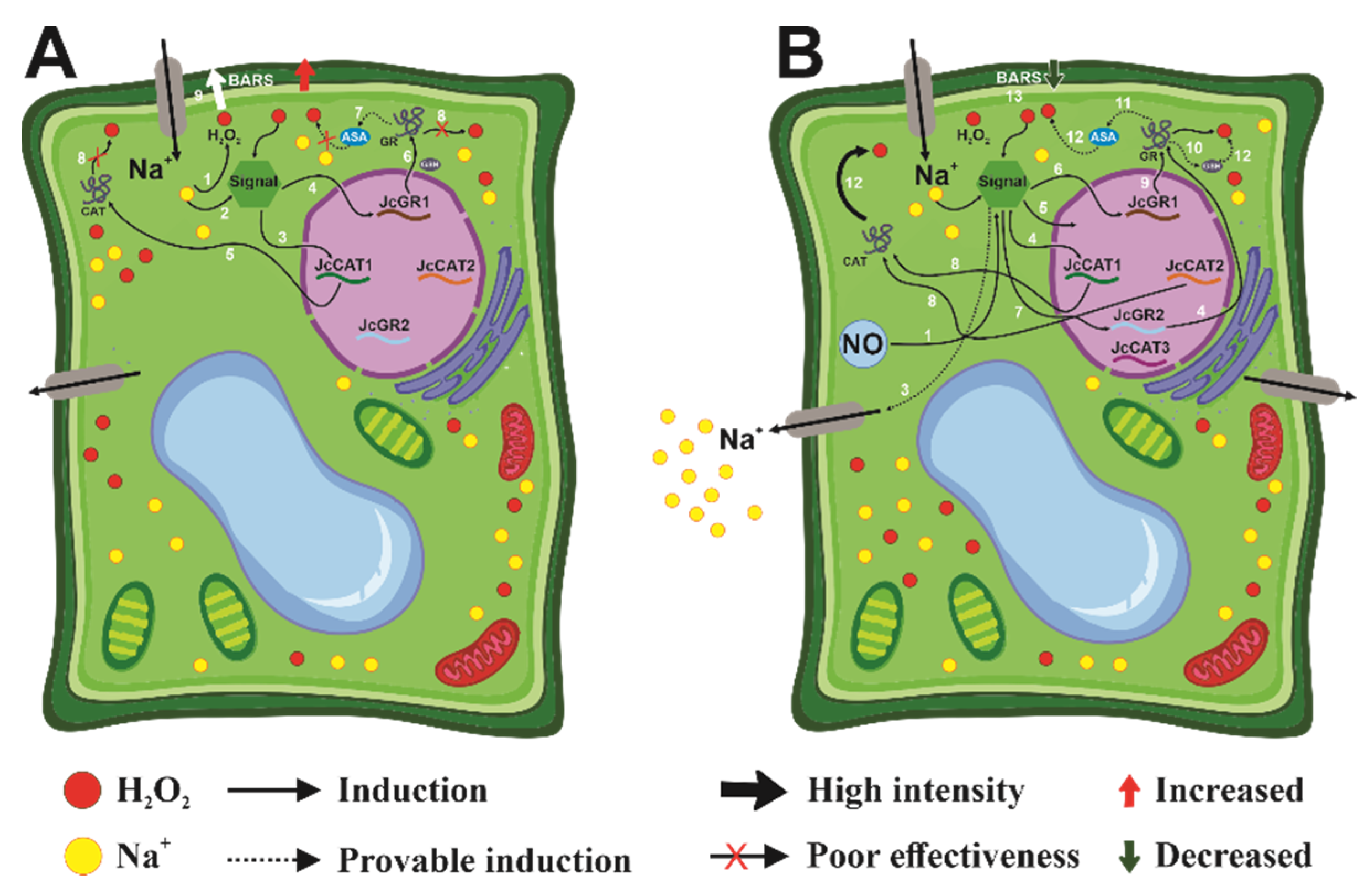
3.3. Effect of Salt on Cellular Metabolism and Defense
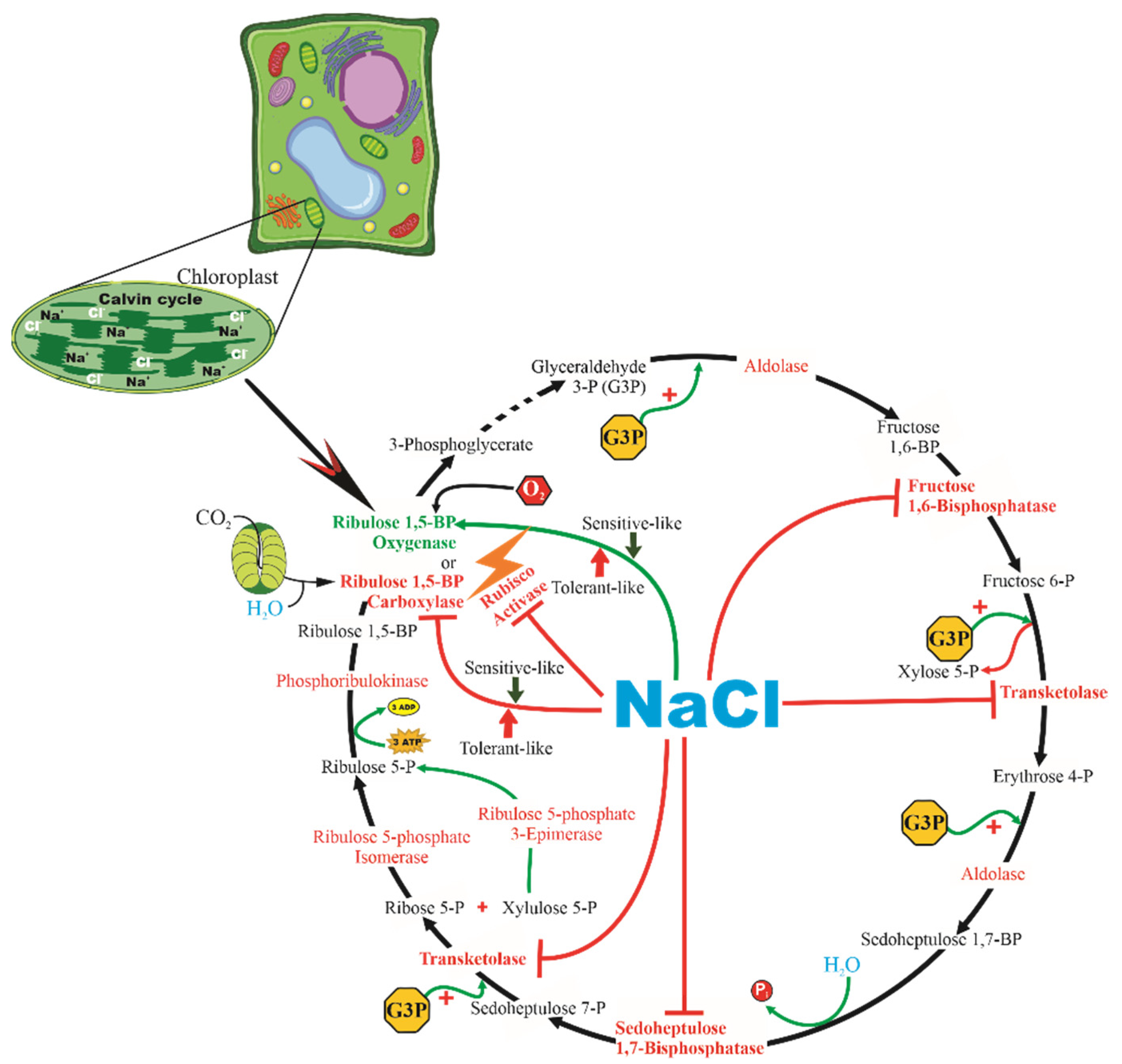
3.4. Metabolite Profile of Salt-Stressed J. curcas Plants
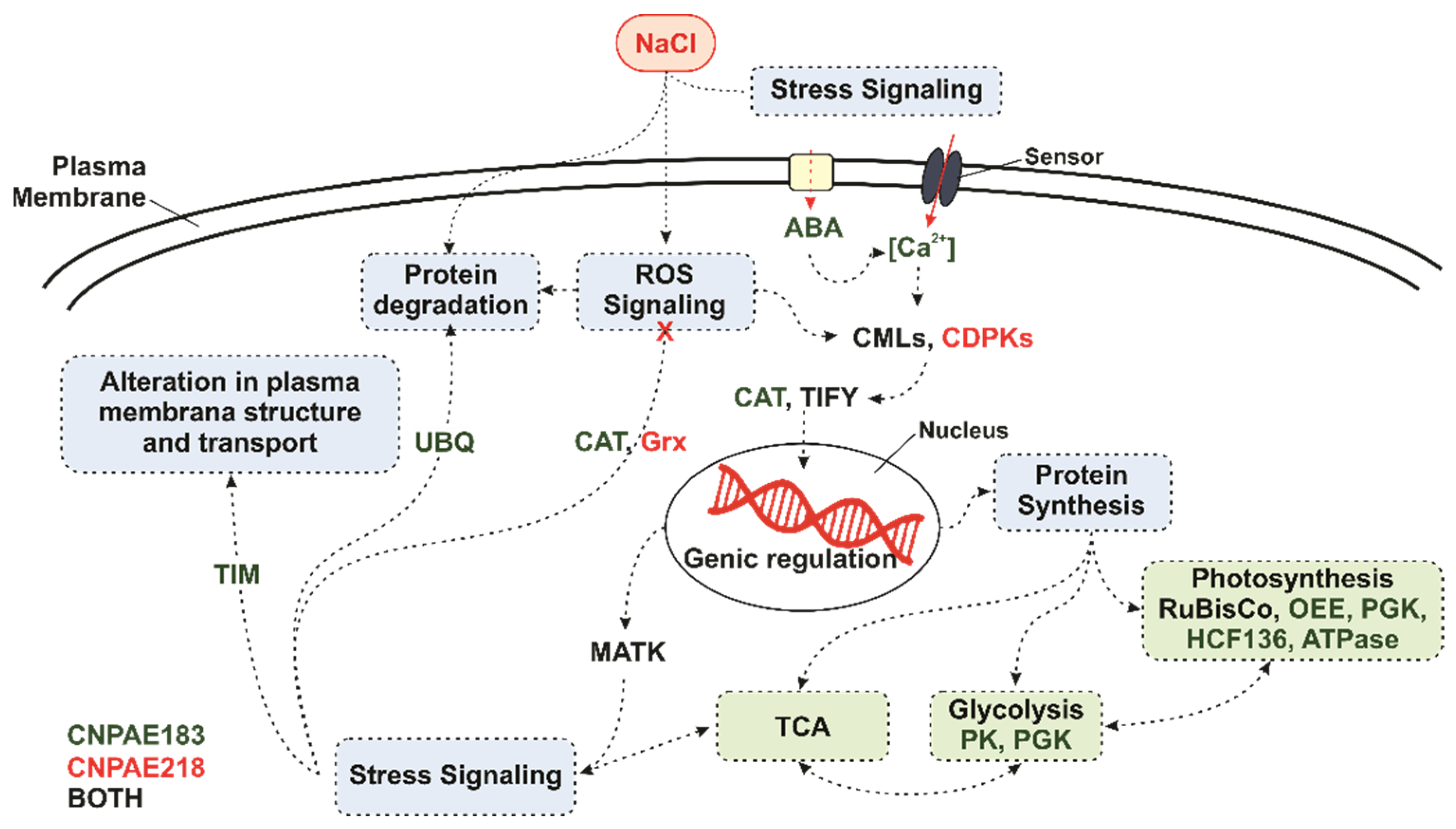
3.5. Transcriptome Analysis of Water and Salt-Stressed J. curcas Plants
3.6. Transgenic J. curcas Plants for Cultivation on Saline Conditions
4. Toxicity and Medical Use of Jatropha curcas Seeds
5. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mitra, S.; Ghose, A.; Gujre, N.; Senthilkumar, S.; Borah, P.; Paul, A.; Rangan, L. A review on environmental and socioeconomic perspectives of three promising biofuel plants Jatropha curcas, Pongamia pinnata and Mesua ferrea. Biomass Bioenergy 2021, 151, 106173. [Google Scholar] [CrossRef]
- Satterthwaite, D. Cities’ contribution to global warming: Notes on the allocation of greenhouse gas emissions. Environ. Urban 2008, 20, 539–549. [Google Scholar] [CrossRef]
- IPCC. Deforestation Accounts for about 20% of CO2 Emissions Globally; Climate Central: Princeton, NJ, USA, 2021; Available online: https://www.climatecentral.org/what-we-do#wwd (accessed on 23 September 2021).
- Rodrigues, A.S.; Rocha, A.C.P.; Da Costa, A.S.V.; Lopes, I.A.P. Prospects of raw materials for the production of biodiesel in Brazil. Int. J. Res.-Granthaalayah 2020, 8, 133–143. [Google Scholar] [CrossRef]
- Alherbawi, M.; AlNouss, A.; McKay, G.; Al-Ansari, T. Optimum sustainable utilisation of the whole fruit of Jatropha curcas: An energy, water and food nexus approach. Renew. Sustain. Energy Rev. 2021, 137, 110605. [Google Scholar] [CrossRef]
- Alherbawi, M.; McKay, G.; Mackey, H.R.; Al-Ansari, T. A novel integrated pathway for Jet Biofuel production from whole energy crops: A Jatropha curcas case study. Energy Convers. Manag. 2021, 229, 113662. [Google Scholar] [CrossRef]
- Achten, W.; Verchot, L.; Franken, Y.; Mathijs, E.; Singh, V.; Aerts, R.; Muys, B. Jatropha bio-diesel production and use. Biomass Bioenergy 2008, 32, 1063–1084. [Google Scholar] [CrossRef]
- Becker, K.; Makkar, H.P.S. Jatropha curcas: A potential source for tomorrow’s oil and biodiesel. Lipid Technol. 2008, 20, 104–107. [Google Scholar] [CrossRef]
- Klein-Marcuschamer, D.; Turner, C.; Allen, M.; Gray, P.; Dietzgen, R.G.; Gresshoff, P.M.; Hankamer, B.; Heimann, K.; Scott, P.T.; Stephens, E.; et al. Technoeconomic analysis of renewable aviation fuel from microalgae, Pongamia pinnata, and sugarcane. Biofuels Bioprod. Biorefin. 2013, 7, 416–428. [Google Scholar] [CrossRef]
- Wang, W.-C. Techno-economic analysis for evaluating the potential feedstocks for producing hydro-processed renewable jet fuel in Taiwan. Energy 2019, 179, 771–783. [Google Scholar] [CrossRef]
- Tao, L.; Milbrandt, A.; Zhang, Y.; Wang, W.-C. Techno-economic and resource analysis of hydroprocessed renewable jet fuel. Biotechnol. Biofuels 2017, 10, 261. [Google Scholar] [CrossRef]
- IATA. Jet Fuel Price Monitor; International Air Transport Association: Montreal, QC, Canada, 2021; Available online: https://www.iata.org/en/publications/economics/fuel-monitor/ (accessed on 22 September 2021).
- BRASIL. Lei No. 11.097, 13 January 2005. In Diário Oficial da União; Civil, C., Ed.; Imprensa Nacional: Rio de Janeiro, Brazil, 2005. [Google Scholar]
- Chiappini, G.; Gaudarde, G. Ministro Bento Albuquerque Garante Mistura Obrigatória de Biodiesel. Agência EPBR. 2021. Available online: https://epbr.com.br/ministro-bento-albuquerque-garante-mistura-obrigatorio-de-biodiesel/ (accessed on 3 April 2021).
- ANP. Agência Nacional de Petróleo, Anuário Estatístico 2020; ANP: Brasília, Brazil, 2020. [Google Scholar]
- Maes, W.; Trabucco, A.; Achten, W.; Muys, B. Climatic growing conditions of Jatropha curcas L. Biomass Bioenergy 2009, 33, 1481–1485. [Google Scholar] [CrossRef]
- Divakara, B.N.; Upadhyaya, H.D.; Wani, S.P.; Gowda, C.L. Biology and genetic improvement of Jatropha curcas L.: A review. Appl. Energy 2010, 87, 732–742. [Google Scholar] [CrossRef]
- Pompelli, M.F.; Barata-Luís, R.; Vitorino, H.S.; Gonçalves, E.R.; Rolim, E.V.; Santos, M.G.; Almeida-Cortez, J.S.; Ferreira, V.M.; Lemos, E.E.; Endres, L. Photosynthesis, photoprotection and antioxidant activity of purging nut under drought deficit and recovery. Biomass Bioenergy 2010, 34, 1207–1215. [Google Scholar] [CrossRef]
- Reubens, B.; Achten, W.; Maes, W.; Danjon, F.; Aerts, R.; Poesen, J.; Muys, B. More than biofuel? Jatropha curcas root system symmetry and potential for soil erosion control. J. Arid Environ. 2011, 75, 201–205. [Google Scholar] [CrossRef]
- Bekalu, Y.; Fekad, M. Review on economic importance of Jatropha apart from its use as a biofuel. Int. J. Res. Dev. 2020, 5, 24–33. [Google Scholar] [CrossRef]
- Corte-Real, N.; da Cunha de Miranda, P.V.V.; Endres, L.; de Souza, E.R.; Pompelli, M.F. Tolerance to salinity in Jatropha curcas are genotype-dependent. Braz. J. Dev. 2019, 5, 22169–22199. [Google Scholar] [CrossRef][Green Version]
- Tedla, A.; Minale, M.; Eshetu, R. Determination of the upper limit age of Jatropha curcas plantation for optimum yield production. Asian Bus. Rev. 2020, 10, 37–42. [Google Scholar] [CrossRef]
- Zavala-Hernández, J.T.; Córdova-Téllez, L.; Martínez-Herrera, J.; Molina-Moreno, J.C. Fruit and seed development of Jatropha curcas L. and physiological indicators of seed maturity. Rev. Fitotec. Mex. 2015, 38, 275–284. [Google Scholar]
- Jonas, M.; Ketlogetswe, C.; Gandure, J. Variation of Jatropha curcas seed oil content and fatty acid composition with fruit maturity stage. Heliyon 2020, 6, e03285. [Google Scholar] [CrossRef]
- Jongschaap, R.E.E.; Corré, W.J.; Bindraban, P.S.; Brandenburg, W.A. Claims and Facts on Jatropha curcas L; Wageningen UR, Plant Research International: Wageningen, The Netherlands, 2007; 66p. [Google Scholar]
- Lane, J. Jatropha Biofuel Around the World: A 13-Country Tour of Development Activity. Available online: https://www.renewableenergyworld.com/articles/2014/09/jatropha-biofuel-around-the-world-a-13-country-tour-of-development-activity.html (accessed on 29 July 2019).
- Rodríguez, O.A.V.; Vázquez, A.P.; Gamboa, C.M. Drivers and Consequences of the First Jatropha curcas Plantations in Mexico. Sustainability 2014, 6, 3732–3746. [Google Scholar] [CrossRef]
- Soto, I.; Ellison, C.; Kenis, M.; Diaz, B.; Muys, B.; Mathijs, E. Why do farmers abandon jatropha cultivation? The case of Chiapas, Mexico. Energy Sustain. Dev. 2018, 42, 77–86. [Google Scholar] [CrossRef]
- Hsie, B.S.; Mendes, K.R.; Antunes, W.C.; Endres, L.; Campos, M.L.; Souza, F.C.; Santos, N.D.; Singh, B.; Arruda, E.C.; Pompelli, M.F. Jatropha curcas L. (Euphorbiaceae) modulates stomatal traits in response to leaf-to-air vapor pressure deficit. Biomass Bioenergy 2015, 81, 273–281. [Google Scholar] [CrossRef]
- Pompelli, M.F.; Ferreira, P.P.; Chaves, A.R.; Figueiredo, R.C.; Martins, A.O.; Jarma-Orozco, A.; Bhatt, A.; Batista-Silva, W.; Endres, L.; Araújo, W.L. Physiological, metabolic, and stomatal adjustments in response to salt stress in Jatropha curcas. Plant Physiol. Biochem. 2021, 168, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Pompelli, M.F.; França, S.C.S.; Tigre, R.C.; Oliveira, M.T.; Sacilot, M.; Pereira, E.C.G. Spectrophotometric determinations of chloroplastidic pigments in acetone, ethanol and dimethylsulphoxide. Braz. J. Biosc. 2013, 11, 52–58. [Google Scholar]
- Campos, M.L.O.; Hsie, B.S.; Granja, J.A.A.; Correia, R.M.; Silva, S.R.S.; Almeida-Cortez, J.S.; Pompelli, M.F. Photosynthesis and antioxidant activity mechanisms in Jatropha curcas L. under salt stress. Braz. J. Plant Physiol. 2012, 24, 55–67. [Google Scholar] [CrossRef]
- Silva, E.N.; Ferreira-Silva, S.L.; Fontenele, A.D.V.; Ribeiro, R.V.; Viégas, R.A.; Silveira, J.A.G. Photosynthetic changes and protective mechanisms against oxidative damage subjected to isolated and combined drought and heat stresses in Jatropha curcas plants. J. Plant Physiol. 2010, 167, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.C. Turgor maintenance by osmotic adjustment: 40 years of progress. J. Exp. Bot. 2018, 69, 3223–3233. [Google Scholar] [CrossRef]
- Quintal, E.B.; Velarde-Buerdía, A.; Ku-González, A.; Carillo-Pech, M.; Ortega, D.; Echevarría-Machado, I.; Epottosin, I.; Martínez-Estévez, M. Mechanisms of salt tolerance in habanero pepper plants (Capsicum chinense Jacq.): Proline accumulation, ions dynamics and sodium root-shoot partition and compartmentation. Front. Plant Sci. 2014, 5, 605. [Google Scholar] [CrossRef]
- Yang, L.; Ma, C.; Wang, L.; Chen, S.; Li, H. Salt stress induced proteome and transcriptome changes in sugar beet monosomic addition line M14. J. Plant Physiol. 2012, 169, 839–850. [Google Scholar] [CrossRef]
- Silva, E.N.; Ferreira-Silva, S.L.; Viégas, R.A.; Silveira, J.A.G. The role of organic and inorganic solutes in the osmotic adjustment of drought-stressed Jatropha curcas plants. Environ. Exp. Bot. 2010, 69, 279–285. [Google Scholar] [CrossRef]
- Wang, H.; Ao, P.; Yang, S.; Zou, Z.; Wang, S.; Gong, M. Molecular Cloning and Expression Analysis of the Gene Encoding Proline Dehydrogenase from Jatropha curcas L. Appl. Biochem. Biotechnol. 2015, 175, 2413–2426. [Google Scholar] [CrossRef] [PubMed]
- Naliwajski, M.; Skłodowska, M. The Relationship between the Antioxidant System and Proline Metabolism in the Leaves of Cucumber Plants Acclimated to Salt Stress. Cells 2021, 10, 609. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.; Xiao, X.; Zhang, W.; Lang, D.; Li, Z.; Zhang, X. Exogenous silicon relieve drought stress and salt stress of Glycyrrhiza uralensis seedlings by regulating proline metabolism and nitrogen assimilation. J. Hortic. Sci. Biotechnol. 2021, 96, 728–737. [Google Scholar] [CrossRef]
- Masoudniaragh, A.; Oraei, M.; Gohari, G.; Akbari, A.; Faramarzi, A. Using halloysite nanotubes as carrier for proline to alleviate salt stress effects in sweet basil (Ocimum basilicum L.). Sci. Hortic. 2021, 285, 110202. [Google Scholar] [CrossRef]
- Hare, P.D.; Cress, W.A.; van Staden, J. Proline synthesis and degradation: A model system for elucidating stress-related signal transduction. J. Exp. Bot. 1999, 50, 413–434. [Google Scholar] [CrossRef]
- Drake, P.L.; Froend, R.H.; Franks, P.J. Smaller, faster stomata: Scaling of stomatal size, rate of response, and stomatal conductance. J. Exp. Bot. 2013, 64, 495–505. [Google Scholar] [CrossRef]
- Ehleringer, J.R.; Sandquist, D. Photosynthesis: Physiological and ecological considerations. In Plant Physiology, 5th ed.; Taiz, L., Zeiger, E., Eds.; Sinauer Associates Inc.: Sunderland, MA, USA, 2010; pp. 244–371. [Google Scholar]
- Buchanan, B.B.; Wolosiuk, R.A. Photosynthesis: The carbon reactions. In Plant Physiology, 5th ed.; Taiz, L., Zeiger, E., Eds.; Sinauer Associates Inc.: Sunderland, MA, USA, 2010; pp. 199–242. [Google Scholar]
- Blankenship, R.E. Photosynthesis: The light reactions. In Plant Physiology and Development, 6th ed.; Taiz, L., Zeiger, E., Møller, I.M., Murphy, A., Eds.; Sinauer Associates, Inc.: Sunderland, MA, USA, 2014; pp. 163–197. [Google Scholar]
- Mittler, R. ROS Are Good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef]
- Sivakumar, P.; Sharmila, P.; Saradhi, P.P. Proline Alleviates Salt-Stress-Induced Enhancement in Ribulose-1,5-Bisphosphate Oxygenase Activity. Biochem. Biophys. Res. Commun. 2000, 279, 512–515. [Google Scholar] [CrossRef]
- Shabala, S.; Cuin, T.A. Potassium transport and plant salt tolerance. Physiol. Plant. 2008, 133, 651–669. [Google Scholar] [CrossRef]
- Babita, M.; Maheswari, M.; Rao, L.; Shanker, A.; Rao, D.G. Osmotic adjustment, drought tolerance and yield in castor (Ricinus communis L.) hybrids. Environ. Exp. Bot. 2010, 69, 243–249. [Google Scholar] [CrossRef]
- Leandro, D.D.S.; Tessio, A.D.S.; Priscila, S.D.O.; Bruno, G.L.; Marcio, G.C.D.C.; Alan, F.D.A.A.; Fabio, P.G. Abscisic acid-mediated stomatal closure and antioxidant defenses in Jatropha curcas L. seedlings submitted to moderate water deficit. Afr. J. Agric. Res. 2016, 11, 2806–2816. [Google Scholar] [CrossRef]
- Tominaga, J.; Inafuku, S.; Coetzee, T.; Kawamitsu, Y. Diurnal regulation of photosynthesis in Jatropha curcas under drought during summer in a semi-arid region. Biomass Bioenergy 2014, 67, 279–287. [Google Scholar] [CrossRef]
- Yang, S.L.; Chen, K.; Wang, S.S.; Gong, M. Osmoregulation as a key factor in drought hardening-induced drought tolerance in Jatropha curcas. Biol. Plant. 2015, 59, 529–536. [Google Scholar] [CrossRef]
- Silva, E.N.; Ribeiro, R.V.; Ferreira-Silva, S.L.; Vieira, S.A.; Ponte, L.F.A.; Silveira, J.A.G. Coordinate changes in photosynthesis, sugar accumulationand antioxidative enzymes improve the performance of Jatropha curcas plants under drought stress. Biomass Bioenergy 2012, 45, 270–279. [Google Scholar] [CrossRef]
- Silva, E.N.; Silveira, J.A.G.; Aragão, R.M.; Vieira, C.F.; Carvalho, F. Photosynthesis impairment and oxidative stress in Jatropha curcas exposed to drought are partially dependent on decreased catalase activity. Acta Physiol. Plant. 2019, 41, 4. [Google Scholar] [CrossRef]
- Silva, E.N.; Silveira, J.A.G.; Ribeiro, R.V.; Oliveira, J.; Cardoso, R.A. Photosynthetic and Antioxidant Responses of Jatropha curcas Plants to Heat Stress: On the Relative Sensitivity of Shoots and Roots. J. Plant Growth Regul. 2018, 37, 255–265. [Google Scholar] [CrossRef]
- Imlay, J.A. Pathways of Oxidative Damage. Annu. Rev. Microbiol. 2003, 57, 395–418. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Beis, A.; Patakas, A. Relative contribution of photoprotection and anti-oxidative mechanisms to differential drought adaptation ability in grapevines. Environ. Exp. Bot. 2012, 78, 173–183. [Google Scholar] [CrossRef]
- Kramer, P.J.; Koslowski, T. Physiology of Wood Plants; Academic Press: New York, NY, USA, 1989; 811p. [Google Scholar]
- Hasegawa, P.M.; Bressan, R.A.; Zhu, J.-K.; Bohnert, H.J. Plant cellular and molecular responses to high salinity. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2000, 51, 463–499. [Google Scholar] [CrossRef] [PubMed]
- Rajaona, A.; Brueck, H.; Seckinger, C.; Asch, F. Effect of salinity on canopy water vapor conductance of young and 3-year old Jatropha curcas L. J. Arid Environ. 2012, 87, 35–41. [Google Scholar] [CrossRef]
- Pompelli, M.F. Benefits and Harms of Wastewater in Agriculture. Acta Sci. Agric. 2021, 5, 112–113. [Google Scholar] [CrossRef]
- Da Fonseca, A.F.; Melfi, A.J.; Montes, C.R. Maize Growth and Changes in Soil Fertility After Irrigation with Treated Sewage Effluent. I. Plant Dry Matter Yield and Soil Nitrogen and Phosphorus Availability. Commun. Soil Sci. Plant Anal. 2005, 36, 1965–1981. [Google Scholar] [CrossRef]
- Ribeiro, G.A.S. Acompanhamento da Safra Brasileira de Grãos; Companhia Nacional de Abastecimento: Brasília, Brazil, 2022. [Google Scholar]
- Asano, T.; Maeda, M.; Takaki, M. Wastewater reclamation and reuse in Japan: Overview and implementation examples. Water Sci. Technol. 1996, 34, 219–226. [Google Scholar] [CrossRef]
- Dorta-Santos, M.; Tejedor, M.; Jiménez, C.; Hernández-Moreno, J.M.; Palacios-Díaz, M.P.; Díaz, F.J. Recycled Urban Wastewater for Irrigation of Jatropha curcas L. in Abandoned Agricultural Arid Land. Sustainability 2014, 6, 6902–6924. [Google Scholar] [CrossRef]
- Dorta-Santos, M.; Tejedor, M.; Jiménez, C.; Hernández-Moreno, J.M.; Díaz, F.J. Using marginal quality water for an energy crop in arid regions: Effect of salinity and boron distribution patterns. Agric. Water Manag. 2016, 171, 142–152. [Google Scholar] [CrossRef]
- Valdes-Rodriguez, O.A.; Sánchez-Sánchez, O.; Pérez-Vázquez, A.; Ruiz-Bello, R. Soil texture effects on the development of Jatropha seedlings—Mexican variety ‘piñón manso’. Biomass Bioenergy 2011, 35, 3529–3536. [Google Scholar] [CrossRef]
- Ahmed, A.; Campion, B.B.; Gasparatos, A. Biofuel development in Ghana: Policies of expansion and drivers of failure in the jatropha sector. Renew. Sustain. Energy Rev. 2017, 70, 133–149. [Google Scholar] [CrossRef]
- Silva, E.N.; Silveira, J.A.; Ribeiro, R.V.; Vieira, S.A. Photoprotective function of energy dissipation by thermal processes and photorespiratory mechanisms in Jatropha curcas plants during different intensities of drought and after recovery. Environ. Exp. Bot. 2015, 110, 36–45. [Google Scholar] [CrossRef]
- Isla, F.L.; Campos, M.L.; Endres, L.; Bezerra-Neto, E.; Pompelli, M.F. Effects of seed storage time and salt stress on the germination of Jatropha curcas L. Ind. Crop. Prod. 2018, 118, 214–224. [Google Scholar] [CrossRef]
- Cabral, G.; Binneck, E.; De Souza, M.C.P.; Da Silva, M.D.; Neto, J.R.C.F.; Pompelli, M.F.; Endres, L.; Kido, É.A. First Expressed TFome of Physic Nut (Jatropha curcas L.) After Salt Stimulus. Plant Mol. Biol. Rep. 2020, 38, 189–208. [Google Scholar] [CrossRef]
- Corte-Real, N.; Oliveira, M.S.; Jarma-Orozco, A.; Fernandes, D.; dos Santos, M.A.; Endres, L.; Junior, T.C.; Pompelli, M.F. Comparative analysis of salt-induced changes in the leaves proteome of two contrasting Jatropha curcas genotypes. Braz. J. Dev. 2020, 6, 39845–39872. [Google Scholar] [CrossRef]
- Liang, J.; Zhou, M.; Zhou, X.; Jin, Y.; Xu, M.; Lin, J. JcLEA, a Novel LEA-Like Protein from Jatropha curcas, Confers a High Level of Tolerance to Dehydration and Salinity in Arabidopsis thaliana. PLoS ONE 2013, 8, e83056. [Google Scholar] [CrossRef]
- de Souza, M.C.P.; da Silva, M.D.; Binneck, E.; Cabral, G.; Iseppon, A.M.B.; Pompelli, M.F.; Endres, L.; Kido, É.A. RNA-Seq transcriptome analysis of Jatropha curcas L. accessions after salt stimulus and unigene-derived microsatellite mining. Ind. Crop. Prod. 2020, 147, 112168. [Google Scholar] [CrossRef]
- Gadelha, C.G.; Miranda, R.D.S.; Alencar, N.L.M.; Costa, J.H.; Prisco, J.T.; Gomes-Filho, E. Exogenous nitric oxide improves salt tolerance during establishment of Jatropha curcas seedlings by ameliorating oxidative damage and toxic ion accumulation. J. Plant Physiol. 2017, 212, 69–79. [Google Scholar] [CrossRef]
- Muchate, N.S.; Nikalje, G.C.; Rajurkar, N.S.; Suprasanna, P.; Nikam, T.D. Plant Salt Stress: Adaptive Responses, Tolerance Mechanism and Bioengineering for Salt Tolerance. Bot. Rev. 2016, 82, 371–406. [Google Scholar] [CrossRef]
- Díaz-López, L.; Gimeno, V.; Lidon, V.; Simón, I.; Martinez, V.; Garcia-Sanchez, F. The tolerance of Jatropha curcas seedlings to NaCl: An ecophysiological analysis. Plant Physiol. Biochem. 2012, 54, 34–42. [Google Scholar] [CrossRef]
- Pan, T.; Liu, M.; Kreslavski, V.D.; Zharmukhamedov, S.K.; Nie, C.; Yu, M.; Kuznetsov, V.V.; Allakhverdiev, S.I.; Shabala, S. Non-stomatal limitation of photosynthesis by soil salinity. Crit. Rev. Environ. Sci. Technol. 2020, 51, 791–825. [Google Scholar] [CrossRef]
- Sairam, R.K.; Rao, K.; Srivastava, G. Differential response of wheat genotypes to long term salinity stress in relation to oxidative stress, antioxidant activity and osmolyte concentration. Plant Sci. 2002, 163, 1037–1046. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Jiang, L.; Xu, Y.; Wang, Y.; Lu, D.; Chen, F. Aquaporin JcPIP2 is Involved in Drought Responses in Jatropha curcas. Acta Biochim. Biophys. Sin. 2007, 39, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, C.; Santos-Rosa, M.J.; Shirasu, K. The U-box protein family in plants. Trends Plant Sci. 2001, 6, 354–358. [Google Scholar] [CrossRef]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Ni, Z.; Chen, Q.; Qu, Y. The wheat salinity-induced R2R3-MYB transcription factor TaSIM confers salt stress tolerance in Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 2017, 491, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Jha, B.; Mishra, A.; Jha, A.; Joshi, M. Developing Transgenic Jatropha Using the SbNHX1 Gene from an Extreme Halophyte for Cultivation in Saline Wasteland. PLoS ONE 2013, 8, e71136. [Google Scholar] [CrossRef]
- Apse, M.P.; Aharon, G.S.; Snedden, W.A.; Blumwald, E. Salt Tolerance Conferred by Overexpression of a Vacuolar Na+/H+ Antiport in Arabidopsis. Science 1999, 285, 1256–1258. [Google Scholar] [CrossRef]
- Katschnig, D.; Bliek, T.; Rozema, J.; Schat, H. Constitutive high-level SOS1 expression and absence of HKT1;1 expression in the salt-accumulating halophyte Salicornia dolichostachya. Plant Sci. 2015, 234, 144–154. [Google Scholar] [CrossRef]
- Zheng, L.; Dang, Z.; Li, H.; Zhang, H.; Wu, S.; Wang, Y. Isolation and characterization of a Δ1-pyrroline-5-carboxylate synthetase (NtP5CS) from Nitraria tangutorum Bobr. and functional comparison with its Arabidopsis homologue. Mol. Biol. Rep. 2014, 41, 563–572. [Google Scholar] [CrossRef]
- Niu, L.; Liao, W. Hydrogen Peroxide Signaling in Plant Development and Abiotic Responses: Crosstalk with Nitric Oxide and Calcium. Front. Plant Sci. 2016, 7, 230. [Google Scholar] [CrossRef]
- Camejo, D.; Romero-Puertas, M.C.; Rodríguez-Serrano, M.; Sandalio, L.M.; Lázaro, J.J.; Jiménez, A.; Sevilla, F. Salinity-induced changes in S-nitrosylation of pea mitochondrial proteins. J. Proteom. 2013, 79, 87–99. [Google Scholar] [CrossRef]
- Takahashi, S.; Badger, M.R. Photoprotection in plants: A new light on photosystem II damage. Trends Plant Sci. 2011, 16, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Allakhverdiev, S.; Murata, N. Environmental stress inhibits the synthesis de novo of proteins involved in the photodamage–repair cycle of Photosystem II in Synechocystis sp. PCC 6803. Biochim. Biophys. Acta 2004, 1657, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Aragão, R.M.; Silva, E.N.; Vieira, C.F.; Silveira, J.A.G. High supply of NO3 − mitigates salinity effects through an enhancement in the efficiency of photosystem II and CO2 assimilation in Jatropha curcas plants. Acta Physiol. Plant. 2012, 34, 2135–2143. [Google Scholar] [CrossRef]
- Voss, I.; Sunil, B.; Scheibe, R.; Raghavendra, A.S. Emerging concept for the role of photorespiration as an important part of abiotic stress response. Plant Biol. 2013, 15, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Singh, S.; Parihar, P.; Mishra, R.K.; Tripathi, D.K.; Singh, V.P.; Chauhan, D.K.; Prasad, S.M. Reactive Oxygen Species (ROS): Beneficial Companions of Plants’ Developmental Processes. Front. Plant Sci. 2016, 7, 1299. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Li, J.P.; Yuan, F.; Yang, Z.; Wang, B.S.; Chen, M. Transcriptome profiling of genes involved in photosynthesis in Elaeagnus angustifolia L. under salt stress. Photosynthetica 2018, 56, 998–1009. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, C.; Wu, P.; Chen, Y.; Li, M.; Jiang, H.; Wu, G. Global Analysis of Gene Expression Profiles in Physic Nut (Jatropha curcas L.) Seedlings Exposed to Salt Stress. PLoS ONE 2014, 9, e97878. [Google Scholar] [CrossRef]
- Sapeta, H.; Lourenco, T.; Lorenz, S.; Grumaz, C.; Kirstahler, P.; Barros, P.M.; Costa, J.M.; Sohn, K.; Oliveira, M.M. Transcriptomics and physiological analyses reveal co-ordinated alteration of metabolic pathways in Jatropha curcas drought tolerance. J. Exp. Bot. 2016, 67, 845–860. [Google Scholar] [CrossRef]
- Xiong, W.; Xu, X.; Zhang, L.; Wu, P.; Chen, Y.; Li, M.; Jiang, H.; Wu, G. Genome-wide analysis of the WRKY gene family in physic nut (Jatropha curcas L.). Gene 2013, 524, 124–132. [Google Scholar] [CrossRef]
- Kaur, R.; Bhardwaj, R.; Sharma, R.; Kapoor, D.; Kohli, S.; Kumar, V.; Kaur, P. Hormonal regulation of drought stress in plants. In Water Stress and Crop Plants: A Sustainable Approach; Ahmad, P., Ed.; John Wiley & Sons, Ltd.: West Sussex, UK, 2016; Volume I, pp. 582–599. [Google Scholar]
- Wani, S.H.; Tripathi, P.; Zaid, A.; Challa, G.S.; Kumar, A.; Kumar, V.; Upadhyay, J.; Joshi, R.; Bhatt, M. Transcriptional regulation of osmotic stress tolerance in wheat (Triticum aestivum L.). Plant Mol. Biol. 2018, 97, 469–487. [Google Scholar] [CrossRef]
- Wang, L.; Wu, Y.; Tian, Y.; Dai, T.; Xie, G.; Xu, Y.; Chen, F. Overexpressing Jatropha curcas CBF2 in Nicotiana benthamiana improved plant tolerance to drought stress. Gene 2020, 742, 144588. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Xu, X.; Xiong, W.; Wu, P.; Chen, Y.; Li, M.; Wu, G.; Jiang, H. Genome-Wide Analysis of the NAC Gene Family in Physic Nut (Jatropha curcas L.). PLoS ONE 2015, 10, e0131890. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Peng, X.; Liu, H.; Wang, D.; Shen, S. Genome-wide identification of the Jatropha curcas MYB family and functional analysis of the abiotic stress responsive gene JcMYB2. BMC Genom. 2016, 17, 251. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Liu, K.; Zhang, J.; Li, X.; Xu, K.; Zhang, Y.; Qi, J.; Yu, D.; Wang, J.; Li, C. JcDREB2, a Physic Nut AP2/ERF Gene, Alters Plant Growth and Salinity Stress Responses in Transgenic Rice. Front. Plant Sci. 2017, 8, 306. [Google Scholar] [CrossRef] [PubMed]
- Reijnders, L.; Huijbregts, M.A.J. Biofuels for Road Transport: A Seed to Wheel Perspective; Springer: Dordrecht, The Netherlands, 2009. [Google Scholar]
- Moniruzzaman, M.; Yaakob, Z.; Shahinuzzaman, M.; Khatun, R.; Aminul Islam, A.K.M. Jatropha Biofuel Industry: The Challenges. In Frontiers in Bioenergy and Biofuels, 1st ed.; Jacob-Lopes, E., Queiroz, L.Q., Eds.; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Montes, J.M.; Melchinger, A.E. Domestication and Breeding of Jatropha curcas L. Trends Plant Sci. 2016, 21, 1045–1057. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Tao, Y.-B.; Xu, Z.-F. Genetic transformation and transgenics of Jatropha curcas, a biofuel plant. In Jatropha, Challenges for a New Energy Crop; Mulpuri, S., Carels, N., Bahadur, B., Eds.; Springer: Singapore, 2019; pp. 79–92. [Google Scholar]
- Liu, B.; Wang, W.; Gao, J.; Chen, F.; Wang, S.; Xu, Y.; Tang, L.; Jia, Y. Molecular cloning and characterization of a jasmonate biosynthetic pathway gene for allene oxide cyclase from Jatropha curcas. Acta Physiol. Plant. 2010, 32, 531–539. [Google Scholar] [CrossRef]
- Tsuchimoto, S.; Cartagena, J.; Khemkladngoen, N.; Singkaravanit, S.; Kohinata, T.; Wada, N.; Sakai, H.; Morishita, Y.; Suzuki, H.; Shibata, D.; et al. Development of transgenic plants in jatropha with drought tolerance. Plant Biotechnol. 2012, 29, 137–143. [Google Scholar] [CrossRef]
- Chacuttayapong, W.; Enoki, H.; Nabetani, Y.; Matsui, M.; Oguchi, T.; Motohashi, R. Transformation of Jatropha curcas L. for production of larger seeds and increased amount of biodiesel. Plant Biotechnol. 2021, 38, 247–256. [Google Scholar] [CrossRef]
- King, A.; He, W.; Cuevas, J.A.; Freudenberger, M.; Ramiaramanana, D.; Graham, I.A. Potential of Jatropha curcas as a source of renewable oil and animal feed. J. Exp. Bot. 2009, 60, 2897–2905. [Google Scholar] [CrossRef]
- Tosin, O.V.; Ojonogecha, M.S.; Oloyede, T.L.; Gabriel, S.S.; Chidiebere, A.C.; Bolong, A.-M.A. Dietary implications of toasted Jatropha curcas seed: Insight on zootechnical and hematological parameters of Clarias gariepinus. Comp. Clin. Pathol. 2022, 31, 81–90. [Google Scholar] [CrossRef]
- Devappa, R.K.; Makkar, H.P.S.; Becker, K. Jatropha Toxicity—A Review. J. Toxicol. Environ. Health Part B 2010, 13, 476–507. [Google Scholar] [CrossRef] [PubMed]
- Rakshit, K.; Darukeshwara, J.; Raj, K.R.; Narasimhamurthy, K.; Saibaba, P.; Bhagya, S. Toxicity studies of detoxified Jatropha meal (Jatropha curcas) in rats. Food Chem. Toxicol. 2008, 46, 3621–3625. [Google Scholar] [CrossRef] [PubMed]
- Francis, G.; Makkar, H.P.S.; Becker, K. Antinutritional factors present in plant-derived alternate fish feed ingredients and their effects in fish. Aquaculture 2001, 199, 197–227. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, W.; Wang, Y.; Xu, Y.; Chen, F. The effect of curcin from Jatropha curcas on apoptosis of mouse sarcoma-180 cells. Fitoterapia 2012, 83, 849–852. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, B.J.; Vanegas, Y.O.; Pompelli, M.F.; Garrido, C.G.; Bezerra Neto, E.; Orozco, A.J. Detoxification of Jatropha curcas L. seed meal as possible alternative livestock feed in the Colombian Caribbean. Rev. Actual. Divulg. Científica 2014, 17, 171–178. [Google Scholar]
- Das, M.; Uppal, H.; Singh, R.; Beri, S.; Mohan, K.; Gupta, V.C.; Adholeya, A. Co-composting of physic nut (Jatropha curcas) deoiled cake with rice straw and different animal dung. Bioresour. Technol. 2011, 102, 6541–6546. [Google Scholar] [CrossRef]
- Saha, S.; Ghosh, K. Evaluation of Nutritive Value of Raw and Fermented De-oiled Physic Nut, Jatropha curcas Seed Meal in the Formulated Diets for Rohu, Labeo rohita (Hamilton) Fingerlings. Proc. Zool. Soc. 2013, 66, 41–50. [Google Scholar] [CrossRef]
- Hassaan, M.; Goda, A.-S.; Kumar, V. Evaluation of nutritive value of fermented de-oiled physic nut, Jatropha curcas, seed meal for Nile tilapia Oreochromis niloticus fingerlings. Aquac. Nutr. 2017, 23, 571–584. [Google Scholar] [CrossRef]
- Hisano, H.; Della Flora, M.A.L.; Pilecco, J.L.; Mendonça, S. Apparent digestibility of nutrients, energy, and amino acid of nontoxic and detoxified physic nut cakes for Nile tilapia. Pesquisa Agropecuária Brasileira 2015, 50, 849–853. [Google Scholar] [CrossRef][Green Version]
- Mendonça, S.; Laviola, B.G. Uso potencial e toxidez da torta de pinhão-manso; Embrapa: Brasília, Brazil, 2009. [Google Scholar]
- Gandhi, V.; Cherian, K.; Mulky, M. Toxicological studies on ratanjyot oil. Food Chem. Toxicol. 1995, 33, 39–42. [Google Scholar] [CrossRef]
- Ratnadass, A.; Wink, M. The Phorbol Ester Fraction from Jatropha curcas Seed Oil: Potential and Limits for Crop Protection against Insect Pests. Int. J. Mol. Sci. 2012, 13, 16157–16171. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Yan, F.; Tang, L.; Chen, F. Antitumor effects of curcin from seeds of Jatropha curcas. Acta Pharmacol. Sin. 2003, 24, 241–246. [Google Scholar] [PubMed]
- Huang, M.-X.; Hou, P.; Wei, Q.; Xu, Y.; Chen, F. A ribosome-inactivating protein (curcin 2) induced from Jatropha curcas can reduce viral and fungal infection in transgenic tobacco. Plant Growth Regul. 2008, 54, 115–123. [Google Scholar] [CrossRef]
- Wu, L.; Goh, M.L.; Tian, D.; Gu, K.; Hong, Y.; Yin, Z. Isolation and characterization of curcin genes with distinct expression patterns in leaves and seeds of Jatropha curcas L. Plant Gene 2017, 9, 34–44. [Google Scholar] [CrossRef]
- Laviola, B.G.; Rodrigues, C.M.; Mendonça, S. Pinhão-Manso Pode ser usado Como Alimentação Animal; Infobibos: Campinas, Brazil, 2012; Available online: http://www.infobibos.com/ (accessed on 17 April 2022).
- Eswaran, N.; Parameswaran, S.; Anantharaman, B.; Raja Krishna Kumar, G.; Sathram, R.K.; Johnson, T.S. Generation of an expressed sequence tag (EST) library from salt stressed roots of Jatropha curcas for identification of abiotic stress-responsive genes. Plant Biol. 2012, 14, 428–437. [Google Scholar] [CrossRef]
- Liu, D.; Wang, L.; Zhai, H.; Song, X.; He, S.; Liu, Q. A novel α/β-hydrolase gene IbMas enhances salt tolerance in transgenic sweet potato. PLoS ONE 2014, 9, e115128. [Google Scholar] [CrossRef]
- Liu, D.; He, S.; Song, X.; Zhai, H.; Liu, N.; Zhang, D.; Liu, Q. IbSIMT1, a novel salt induced methyltransferase gene from Ipomoea batatas, is involved in salt tolerance. Plant Cell Tissue Organ. Cult. 2015, 120, 701–715. [Google Scholar] [CrossRef]
- Chen, M.; Chen, J.; Fang, J.; Guo, Z.; Lu, S. Down-regulation of S-adenosylmethionine decarboxylase genes results in reduced plant length, pollen viability, and abiotic stress tolerance. Plant Cell Tissue Organ. Cult. 2014, 116, 311–322. [Google Scholar] [CrossRef]
- Tang, Y.F.; Zhou, Q.; Zhao, Y.M.; Peng, Y.Z. Efficient removal of methyl violet from aqueous solution by a low-cost adsor-bent—C. camphora fallen leaves powder. J. Disper. Sci. Technol. 2017, 38, 1135–1141. [Google Scholar] [CrossRef]
- Wang, H.; Zou, Z.; Wang, S.; Gong, M. Deep sequencing-based transcriptome analysis of the oil-bearing plant Physic Nut (Jatropha curcas L.) under cold stress. Plant Omics 2014, 7, 178–187. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pompelli, M.F.; Jarma-Orozco, A.; Rodríguez-Páez, L.A. Salinity in Jatropha curcas: A Review of Physiological, Biochemical, and Molecular Factors Involved. Agriculture 2022, 12, 594. https://doi.org/10.3390/agriculture12050594
Pompelli MF, Jarma-Orozco A, Rodríguez-Páez LA. Salinity in Jatropha curcas: A Review of Physiological, Biochemical, and Molecular Factors Involved. Agriculture. 2022; 12(5):594. https://doi.org/10.3390/agriculture12050594
Chicago/Turabian StylePompelli, Marcelo F., Alfredo Jarma-Orozco, and Luis Alfonso Rodríguez-Páez. 2022. "Salinity in Jatropha curcas: A Review of Physiological, Biochemical, and Molecular Factors Involved" Agriculture 12, no. 5: 594. https://doi.org/10.3390/agriculture12050594
APA StylePompelli, M. F., Jarma-Orozco, A., & Rodríguez-Páez, L. A. (2022). Salinity in Jatropha curcas: A Review of Physiological, Biochemical, and Molecular Factors Involved. Agriculture, 12(5), 594. https://doi.org/10.3390/agriculture12050594







