Vaginal and Uterine Microbiomes during Puerperium in Dairy Cows
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Farm and Management
2.2. Study Population and Design
2.3. Clinical and Gynecological Examination
2.4. Antimicrobial Treatment
2.5. Bacteriological Samples
2.6. Bacteriological Cultures
2.7. Identification (MALDI-TOF MS)
2.8. Statistical Analysis
3. Results
3.1. Clinical Results
3.1.1. Antibiotic Treatment
3.1.2. Vaginal Discharge
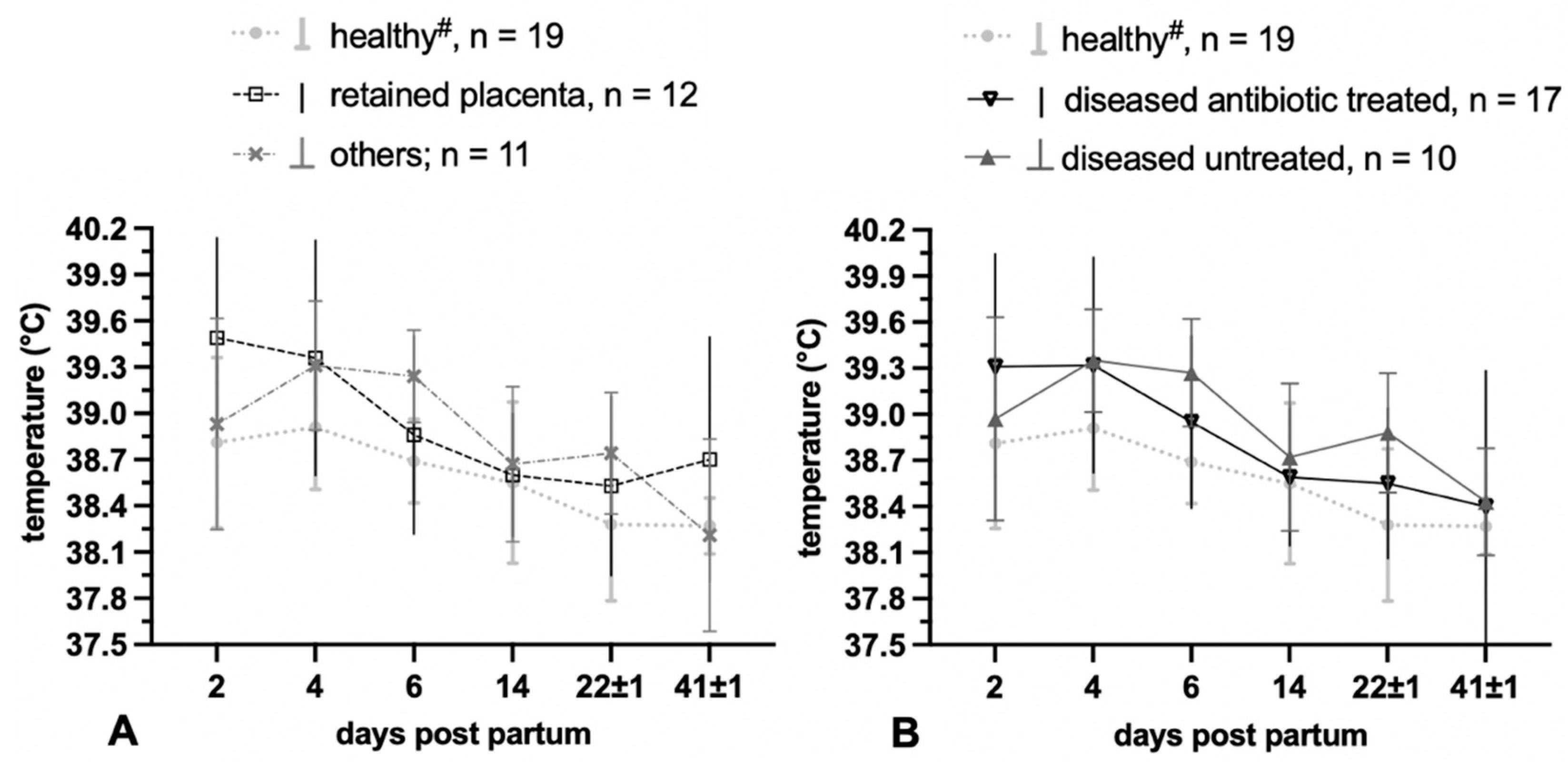
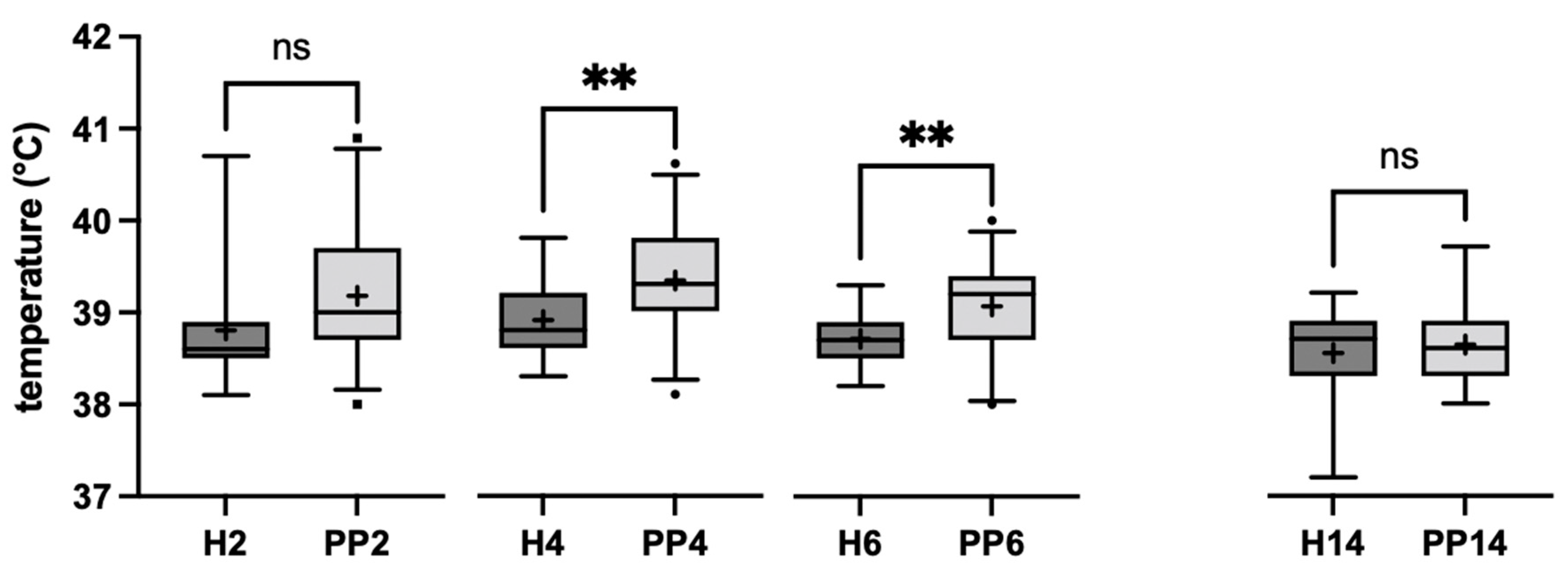
3.1.3. Temperature Measurement
3.2. Microbiological Results
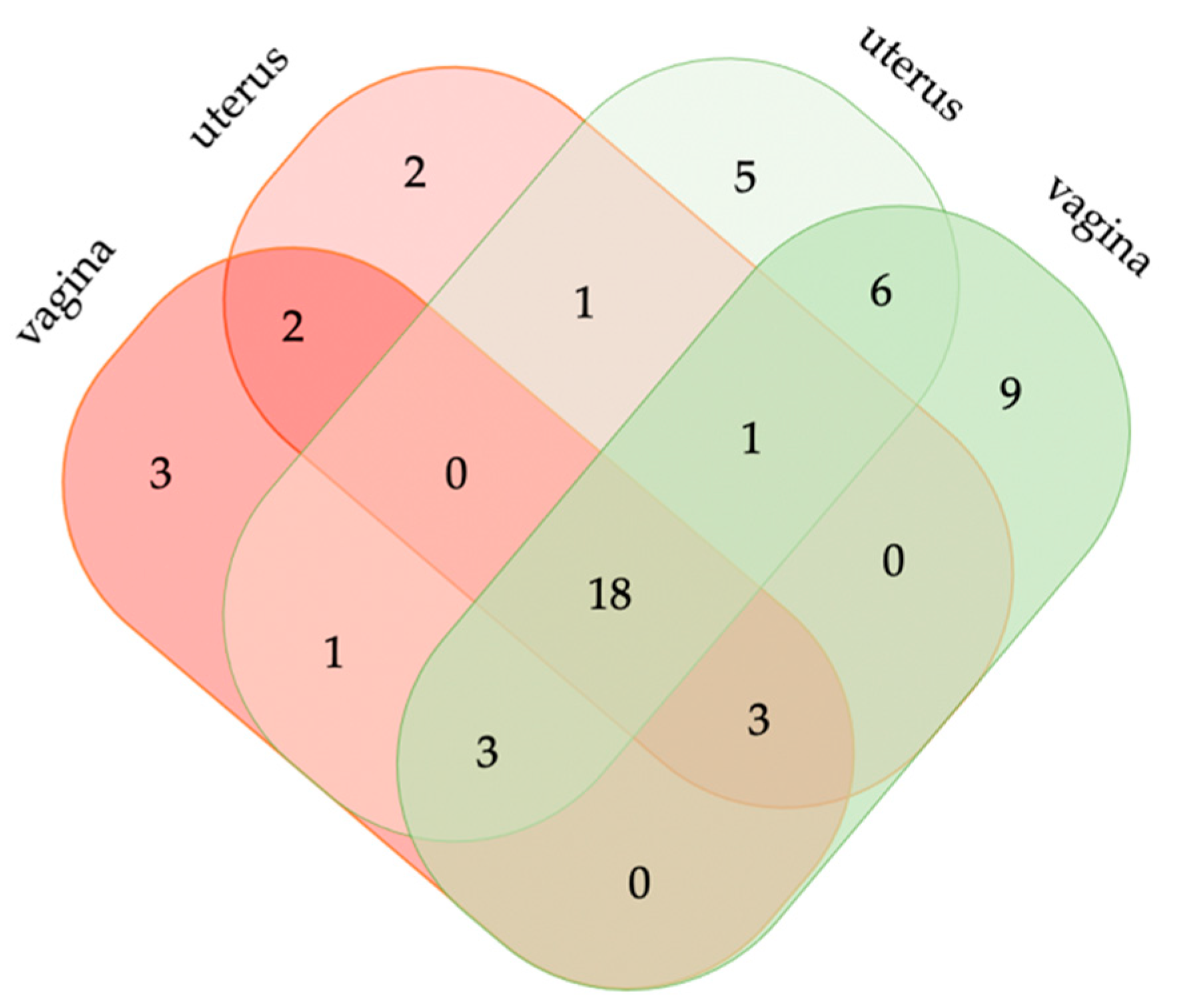
3.2.1. Postpartum Vaginal and Uterine Bacterial Diversity
3.2.2. Vaginal Bacterial Diversity in Healthy and Diseased Animals
3.2.3. Uterine Bacterial Diversity in Healthy and Diseased Animals
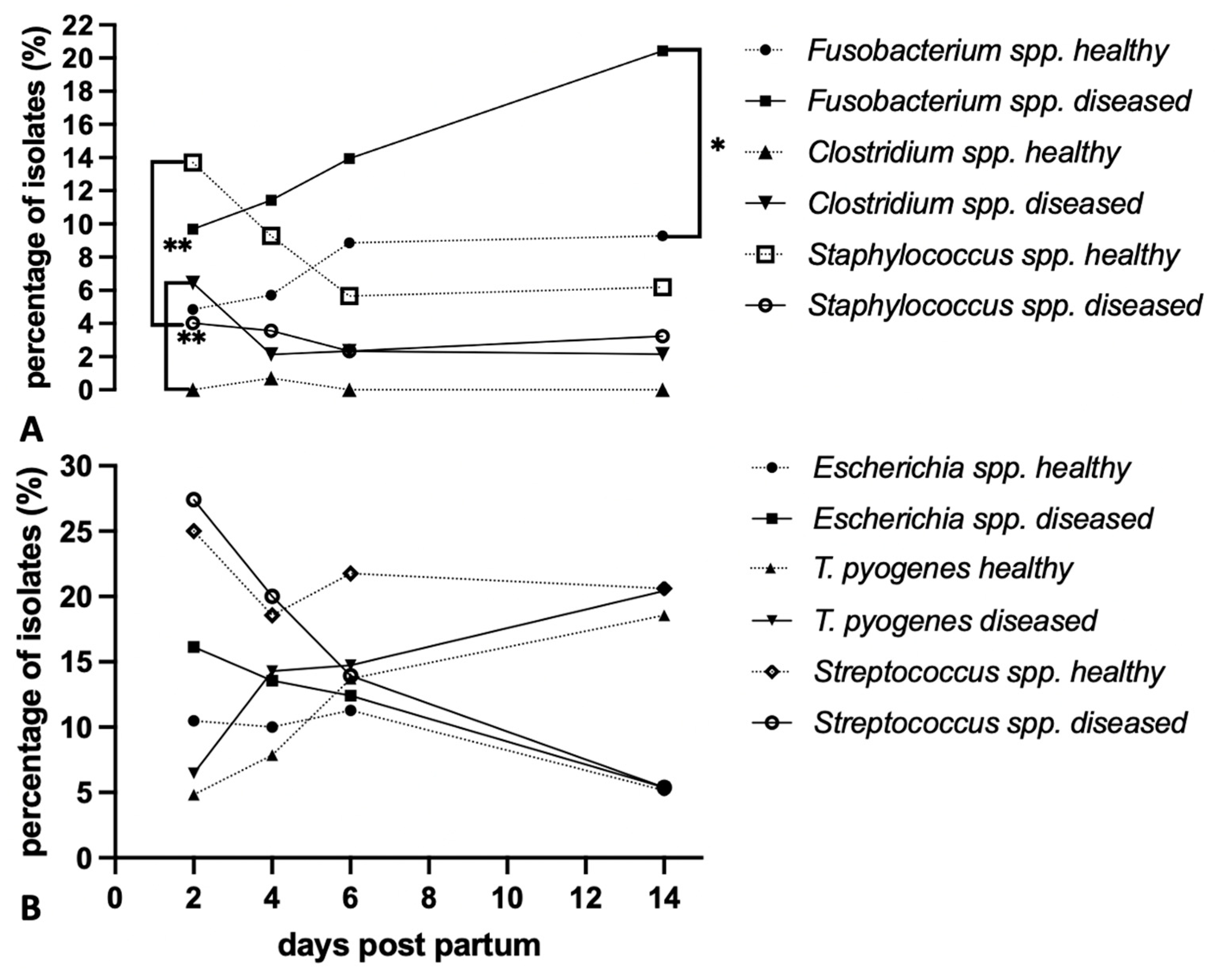
3.2.4. Microbiota Community Structure and Dynamics in Healthy and Diseased Cattle
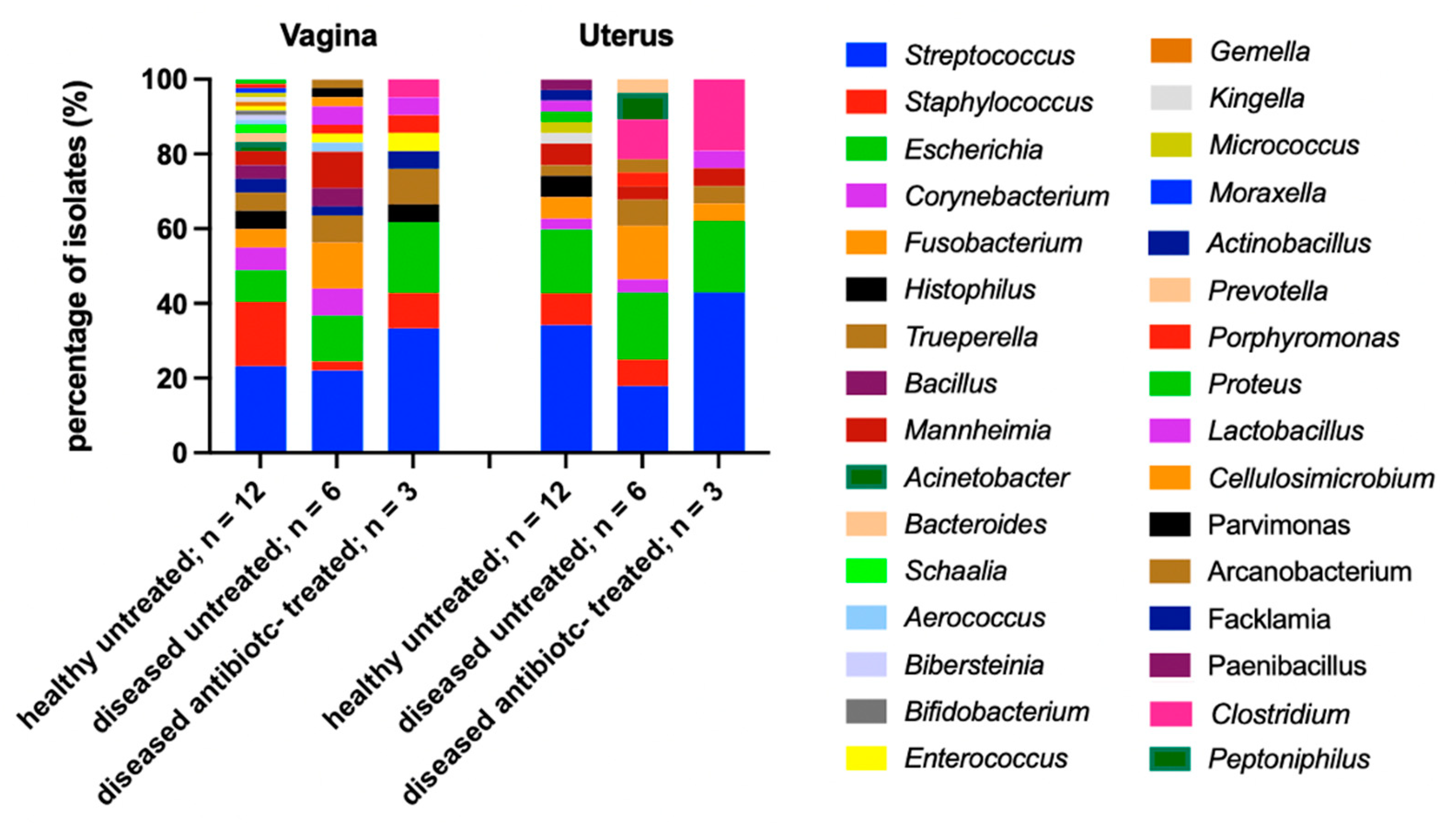
3.2.5. Vaginal and Uterine Microbiome of Antibiotic-Treated and Untreated Cows
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gilbert, R.O. Management of Reproductive Disease in Dairy Cows. Vet. Clin. N. Am. Food Anim. Pract. 2016, 32, 387–410. [Google Scholar] [CrossRef] [PubMed]
- Overton, M. Economics of postpartum uterine health. In Proceedings of the Dairy Cattle Reproduction Council Convention, Omaha, NE, USA, 7–8 November 2008; pp. 39–43. [Google Scholar]
- Wagener, K.; Prunner, I.; Pothmann, H.; Drillich, M.; Ehling-Schulz, M. Diversity and health status specific fluctuations of intrauterine microbial communities in postpartum dairy cows. Vet. Microbiol. 2015, 175, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Neubrand, L.; Wagener, K.; Drillich, M. Bovine uterine diseases: Aspects of microbiology, molecular biology, and immunology. Tierarztl. Praxis. Ausg. G Grosstiere/Nutztiere 2020, 48, 253–261. [Google Scholar] [CrossRef]
- Stojkov, J.; von Keyserlingk, M.A.; Marchant-Forde, J.N.; Weary, D.M. Assessment of visceral pain associated with metritis in dairy cows. J. Dairy Sci. 2015, 98, 5352–5361. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C. Postpartum Uterine Infection. In Bovine Reproduction; Hopper, R.M., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2015; p. 440. [Google Scholar]
- Frazer, G.S. A rational basis for therapy in the sick postpartum cow. Vet. Clin. N. Am. Food Anim. Pract. 2005, 21, 523–568. [Google Scholar] [CrossRef] [PubMed]
- Dadarwal, D.; Palmer, C.; Griebel, P. Mucosal immunity of the postpartum bovine genital tract. Theriogenology 2017, 104, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Miranda-CasoLuengo, R.; Lu, J.; Williams, E.J.; Miranda-CasoLuengo, A.A.; Carrington, S.D.; Evans, A.C.O.; Meijer, W.G. Delayed differentiation of vaginal and uterine microbiomes in dairy cows developing postpartum endometritis. PLoS ONE 2019, 14, e0200974. [Google Scholar] [CrossRef] [Green Version]
- Foldi, J.; Kulcsar, M.; Pecsi, A.; Huyghe, B.; de Sa, C.; Lohuis, J.A.; Cox, P.; Huszenicza, G. Bacterial complications of postpartum uterine involution in cattle. Anim. Reprod. Sci. 2006, 96, 265–281. [Google Scholar] [CrossRef]
- Sheldon, I.M.; Dobson, H. Postpartum uterine health in cattle. Anim. Reprod. Sci. 2004, 82–83, 295–306. [Google Scholar] [CrossRef]
- Sheldon, I.M.; Noakes, D.E.; Rycroft, A.N.; Pfeiffer, D.U.; Dobson, H. Influence of uterine bacterial contamination after parturition on ovarian dominant follicle selection and follicle growth and function in cattle. Reproduction 2002, 123, 837–845. [Google Scholar] [CrossRef]
- Williams, E.J.; Fischer, D.P.; Pfeiffer, D.U.; England GC, W.; Noakes, D.E.; Dobson, H.; Sheldon, I.M. Clinical evaluation of postpartum vaginal mucus reflects uterine bacterial infection and the immune response in cattle. Theriogenology 2005, 63, 102–117. [Google Scholar] [CrossRef] [PubMed]
- Andriamanga, S.; Steffan, J.; Thibier, M. Metritis in dairy herds: An epidemiological approach with special reference to ovarian cyclicity. Ann. Rech. Vet. 1984, 15, 503–508. [Google Scholar] [PubMed]
- Kimura, K.; Goff, J.P.; Kehrli, M.E., Jr.; Reinhardt, T.A. Decreased Neutrophil Function as a Cause of Retained Placenta in Dairy Cattle. J. Dairy Sci. 2002, 85, 544–550. [Google Scholar] [CrossRef]
- Peter, A.T. Retained Fetal Membranes. In Bovine Reproduction, 2nd ed.; Hopper, R.M., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2021. [Google Scholar]
- Pohl, A.; Bertulat, S.; Borchardt, S.; Burfeind, O.; Heuwieser, W. Randomized, controlled clinical trial on the efficacy of nonsteroidal antiinflammatory drugs for the treatment of acute puerperal metritis in dairy cows. J. Dairy Sci. 2016, 99, 8241–8249. [Google Scholar] [CrossRef] [Green Version]
- Sheldon, I.M.; Williams, E.J.; Miller, A.N.; Nash, D.M.; Herath, S. Uterine diseases in cattle after parturition. Vet. J. 2008, 176, 115–121. [Google Scholar] [CrossRef]
- Sheldon, I.M.; Cronin, J.; Goetze, L.; Donofrio, G.; Schuberth, H.J. Defining postpartum uterine disease and the mechanisms of infection and immunity in the female reproductive tract in cattle. Biol. Reprod. 2009, 81, 1025–1032. [Google Scholar] [CrossRef]
- Benzaquen, M.E.; Risco, C.A.; Archbald, L.F.; Melendez, P.; Thatcher, M.J.; Thatcher, W.W. Rectal temperature, calving-related factors, and the incidence of puerperal metritis in postpartum dairy cows. J. Dairy Sci. 2007, 90, 2804–2814. [Google Scholar] [CrossRef] [Green Version]
- Chenault, J.R.; McAllister, J.F.; Chester, S.T.; Dame, K.J.; Kausche, F.M.; Robb, E.J. Efficacy of ceftiofur hydrochloride sterile suspension administered parenterally for the treatment of acute postpartum metritis in dairy cows. J. Am. Vet. Med. Assoc. JAVMA 2004, 224, 1634–1639. [Google Scholar] [CrossRef]
- Leutert, C.; von Krueger, X.; Plontzke, J.; Heuwieser, W. Evaluation of vaginoscopy for the diagnosis of clinical endometritis in dairy cows. J. Dairy Sci. 2012, 95, 206–212. [Google Scholar] [CrossRef] [Green Version]
- Sheldon, I.M.; Rycroft, A.N.; Zhou, C. Associationbetweenpostpartumpyrexia and uterinebacterial infectionin dairy cattle. Vet. Rec. 2004, 154, 289–293. [Google Scholar] [CrossRef]
- LeBlanc, S.J.; Duffield, T.F.; Leslie, K.E.; Bateman, K.G.; Keefe, G.P.; Walton, J.S.; Johnson, W.H. Defining and Diagnosing Postpartum Clinical Endometritis and its Impact on Reproductive Performance in Dairy Cows. J. Dairy Sci. 2002, 85, 2223–2236. [Google Scholar] [CrossRef]
- Jeremejeva, J.; Orro, T.; Waldmann, A.; Kask, K. Treatment of dairy cows with PGF2alpha or NSAID, in combination with antibiotics, in cases of postpartum uterine inflammation. Acta Vet. Scand. 2012, 54, 45. [Google Scholar] [CrossRef] [Green Version]
- Senger, P.L. The puerperium and Lactation. In Pathways to Pregnancy and Parturition, 2nd ed.; Current Conceptions: Redmond, OR, USA, 2003; p. 329. [Google Scholar]
- Youngquist, R.S.; Shore, M.D. Postpartum uterine infection. In Current Therapy of Large Animal; Youngquist, R.S., Ed.; Saunders Elsevier: St. Louis, MO, USA, 1997; pp. 335–340. [Google Scholar]
- Sheldon, I.M.; Lewis, G.S.; LeBlanc, S.; Gilbert, R.O. Defining postpartum uterine disease in cattle. Theriogenology 2006, 65, 1516–1530. [Google Scholar] [CrossRef] [PubMed]
- Bicalho, M.L.; Machado, V.S.; Oikonomou, G.; Gilbert, R.O.; Bicalho, R.C. Association between virulence factors of Escherichia coli, Fusobacterium necrophorum, and Arcanobacterium pyogenes and uterine diseases of dairy cows. Vet. Microbiol. 2012, 157, 125–131. [Google Scholar] [CrossRef]
- Karstrup, C.C.; Agerholm, J.S.; Jensen, T.K.; Swaro, L.R.V.; Klitgaard, K.; Rasmussen, E.L.; Krogh, K.M.; Pedersen, H.G. Presence and localization of bacteria in the bovine endometrium postpartum using fluorescence in situ hybridization. Theriogenology 2017, 92, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Cunha, F.; Jeon, S.J.; Daetz, R.; Vieira-Neto, A.; Laporta, J.; Jeong, K.C.; Barbet, A.F.; Risco, C.A.; Galvão, K.N. Quantifying known and emerging uterine pathogens, and evaluating their association with metritis and fever in dairy cows. Theriogenology 2018, 114, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Madoz, L.V.; Giuliodori, M.J.; Migliorisi, A.L.; Jaureguiberry, M.; de la Sota, R.L. Endometrial cytology, biopsy, and bacteriology for the diagnosis of subclinical endometritis in grazing dairy cows. J. Dairy Sci. 2014, 97, 195–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huszenicza, G.; Fodor, M.; Gacs, M.; Kulcsar, M.; Dohmen, M.J.V.; Vamos, M.; Porkolab, L.; Kegl, T.; Bartyik, J.; Lohuis, J.A.C.M.; et al. Uterine bacteriology, resumption of cyclic ovarian activity and fertility in postpartum cows kept in large-scale dairy herds. Reprod. Domest. Anim. 1999, 34, 237–245. [Google Scholar] [CrossRef]
- Moore, S.G.; Ericsson, A.C.; Behura, S.K.; Lamberson, W.R.; Evans, T.J.; McCabe, M.S.; Poock, S.E.; Lucy, M.C. Concurrent and long-term associations between the endometrial microbiota and endometrial transcriptome in postpartum dairy cows. BMC Genom. 2019, 20, 405. [Google Scholar] [CrossRef]
- Al-Katib, W.A.; Dennis, S.M. Epididymal and testicular lesions in rams following experimental infection with Actinobacillus seminis. N. Z. Vet. J. 2007, 55, 125–129. [Google Scholar] [CrossRef]
- Moore, S.G.; Ericsson, A.C.; Poock, S.E.; Melendez, P.; Lucy, M.C. Hot topic: 16S rRNA gene sequencing reveals the microbiome of the virgin and pregnant bovine uterus. J. Dairy Sci. 2017, 100, 4953–4960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.L.; Liu, M.C.; Xu, J.; An, L.G.; Wang, J.F.; Zhu, Y.H. Uterine Microbiota of Dairy Cows With Clinical and Subclinical Endometritis. Front. Microbiol. 2018, 9, 2691. [Google Scholar] [CrossRef] [PubMed]
- Jánosi, K.; Stipkovits, L.; Glávits, R.; Molnár, T.; Makrai, L.; Gyuranecz, M.; Varga, J.; Fodor, L. Aerosol infection of calves with Histophilus somni. Acta Vet. Hung. 2009, 57, 347–356. [Google Scholar] [CrossRef]
- Miller, R.B.; Barnum, D.A.; McEntee, K.E. Haemophilus somnus in the reproductive tracts of slaughtered cows: Location and frequency of isolations and lesions. Vet. Pathol. 1983, 20, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Moreno, C.; Fontana, C.; Cocconcelli, P.S.; Callegari, M.L.; Otero, M.C. Vaginal microbial communities from synchronized heifers and cows with reproductive disorders. J. Appl. Microbiol. 2016, 121, 1232–1241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Burgt, G.; Clark, W.; Knight, R.; Colles, K. Cattle fertility problems and Histophilus somni. Vet. Rec. 2007, 160, 600. [Google Scholar] [CrossRef] [PubMed]
- Francino, M.P. Antibiotics and the Human Gut Microbiome: Dysbioses and Accumulation of Resistances. Front. Microbiol. 2016, 6, 1543. [Google Scholar] [CrossRef] [Green Version]
- Karpinets, T.V.; Solley, T.N.; Mikkelson, M.D.; Dorta-Estremera, S.; Nookala, S.S.; Medrano, A.Y.D.; Petrosino, P.F.; Mezzari, M.P.; Zhang, J.; Futreal, P.A.; et al. Effect of Antibiotics on Gut and Vaginal Microbiomes Associated with Cervical Cancer Development in Mice. Cancer Prev. Res. 2020, 13, 997–1006. [Google Scholar] [CrossRef]
- Neuvonen, P.J. Interactions with the Absorption of Tetracyclines. Drugs 1976, 11, 45–54. [Google Scholar] [CrossRef]

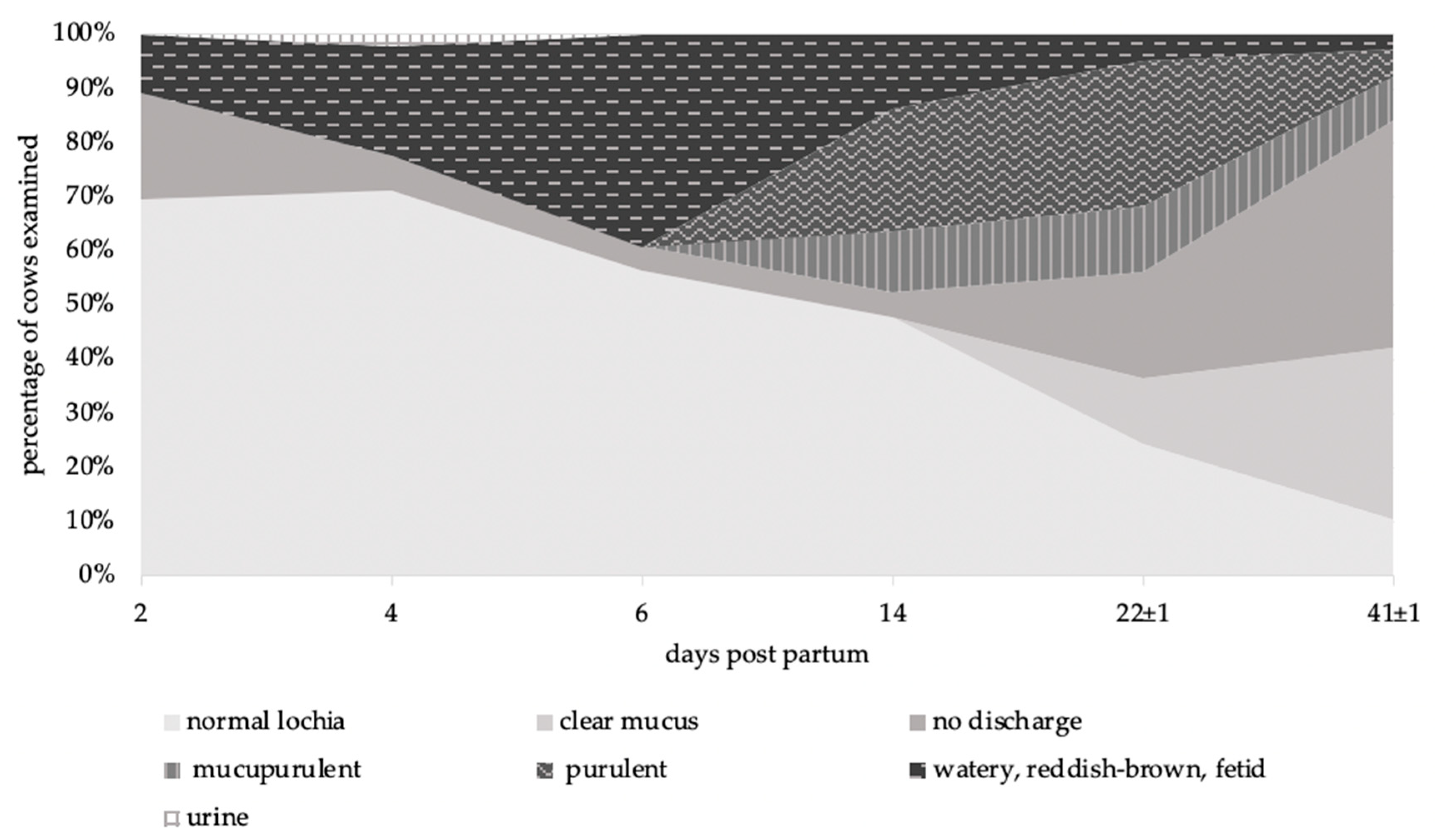
| Description | Score |
|---|---|
| Mucus Character | |
| Clear mucus | 0 |
| Normal lochia (reddish- brown, yellowish, non-smelling, mucous discharge) | 1 |
| Mucupurulent (discharge containing ≤ 50% white or off- white mucupurulent material) | 2 |
| Purulent (discharge containing > 50% purulent material, usually white, yellow or occasionally sanguineous) | 3 |
| Watery, reddish-brown, fetid | 4 |
| No discharge | 5 |
| Urine | 6 |
| Total | Previous Illnesses | Obstetrics | Birth of Twins | Stillbirth | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| % | n | % | n | % | n | % | n | % | n | ||
| Diagnosis | |||||||||||
| Healthy | 41.3 | 19 | 15.8 | 3 # | 15.8 | 3 | 5.3 | 1 | |||
| Pathological puerperium | 58.7 | 27 | 11.1 | 3 ## | 18.5 | 5 ### | 11.1 | 3 | 3.7 | 1 | |
| thereof | |||||||||||
| retained fetal membranes | 26.1 | 12 | 8.3 | 1 | 16.7 | 2 | 25.0 | 3 | 8.3 | 1 | |
| puerperal metritis | 17.4 | 8 | 12.5 | 1 | 12.5 | 1 | 12.5 | 1 | |||
| grade 1 | 15.2 | 7 | 14.3 | 1 | 14.3 | 1 | |||||
| grade 2 | 2.2 | 1 | 100.0 | 1 | |||||||
| clinical metritis | 52.2 | 24 | 12.5 | 3 | 20.8 | 5 | 12.5 | 3 | 4.2 | 1 | |
| clinical endometritis | 39.1 | 18 | 11.1 | 2 | 22.2 | 4 | 16.7 | 3 | |||
| urovagina | 2.2 | 1 | |||||||||
| No Treatment | TC | TC + BP | BP | BP + P | TC + BP + P | P | |
|---|---|---|---|---|---|---|---|
| % (n) | % (n) | % (n) | % (n) | % (n) | % (n) | % (n) | |
| Diagnosis | |||||||
| Healthy | 94.7 (18) | 5.3 (1) | |||||
| Pathological puerperium | 37.0 (10) | 29.6 (8) | 11.1 (3) | 11.1 (3) # | 3.7 (1) | 3.7 (1) | 3.7 (1) |
| thereof | |||||||
| retained fetal membranes | 16.7 (2) | 58.3 (7) | 16.7 (2) | 8.3 (1) | |||
| puerperal metritis | |||||||
| grade 1 | 42.9 (3) | 28.6 (2) | 14.3 (1) | 14.3 (1) | |||
| grade 2 | 100.0 (1) | ||||||
| clinical metritis | 33.3 (8) | 33.3 (8) | 12.5 (3) | 12.5 (3) # | 4.2 (1) | 4.2 (1) | |
| clinical endometritis | 33.3 (6) | 33.3 (6) | 11.1 (2) | 16.7 (3) # | 5.6 (1) | ||
| urovagina | 100 (1) |
| Bacterial Findings | Localization | ||
|---|---|---|---|
| p.p. Vagina | p.p. Uterus | ||
| % (n) | % (n) | (ρ) | |
| Streptococcus | 20.3 (114) | 18.6 (76) | n.s |
| Trueperella | 11.2 (63) | 13.5 (55) | 0.61 * |
| Escherichia | 9.6 (54) | 12.7 (52) | 0.50 * |
| Fusobacterium | 9.1 (51) | 11.7 (48) | 0.65 ** |
| Staphylococcus | 7.3 (41) | 4.4 (18) | n.s. |
| Bacillus | 4.5 (25) * | 1.7 (7) | n.s. |
| Corynebacterium | 3.9 (22) ** | 1.0 (4) | n.s. |
| Actinobacillus | 2.7 (15) | 3.7 (15) | n.s. |
| Mannheimia | 2.7 (15) | 2.9 (12) | n.s. |
| Bacteroides | 2.7 (15) | 2.0 (8) | 0.81 *** |
| Histophilus | 2.5 (14) | 2.0 (8) | n.s. |
| Atopobium | 2.1 (12) | 1.2 (5) | n.s. |
| Enterococcus | 2.0 (11) | 1.0 (4) | n.s. |
| Acinetobacter | 1.8 (10) | 0.2 (1) | n.s. |
| Helcococcus | 1.8 (10) | 2.2 (9) | 0.61 * |
| Porphyromonas | 1.8 (10) | 1.5 (6) | n.s. |
| Prevotella | 1.8 (10) | 2.4 (10) | 0.54 ** |
| Lactobacillus | 1.1 (6) | 1.0 (4) | n.s. |
| Peptoniphilus | 1.1 (6) | 2.4 (10) | n.s. |
| Proteus | 1.1 (6) | 1.5 (6) | 0.73 *** |
| Aerococcus | 0.7 (4) | 0.7 (3) | n.s. |
| Clostridium | 0.7 (4) | 3.2 (13) ** | n.s. |
| Micrococcus | 0.7 (4) | 0.7 (3) | n.s. |
| Bibersteinia | 0.7 (4) | 0.5 (2) | n.s. |
| Glutamicibacter | 0.5 (3) | 0.0 (0) | n.s. |
| Peptostreptococcus | 0.5 (3) | 0.7 (3) | n.s. |
| Schaalia | 0.5 (3) | 1.0 (4) | n.s. |
| Shewanella | 0.5 (3) | 1.0 (4) | n.s. |
| Others | 4.1(23) | 4.6 (19) | n.d. |
| Total | 100 (561) | 100 (409) |
| Bacterial Findings | Localization and Health Status | |||
|---|---|---|---|---|
| Healthy Vagina | Healthy Uterus | Diseased Vagina | Diseased Uterus | |
| % (n) | % (n) | % (n) | (n) | |
| Streptococcus | 23.4 (69) | 18.4 (35) | 16.9 (45) | 18.2 (40) |
| Staphylococcus *** | 10.5 (31) | 6.3 (12) | 3.8 (10) | 2.7 (6) |
| Trueperella | 9.5 (28) | 12.6 (24) | 13.2 (35) | 14.1 (31) |
| Escherichia | 8.5 (25) | 11.1 (21) | 11.0 (29) | 14.1 (31) |
| Fusobacterium ** | 6.4 (19) | 7.9 (15) | 12.0 (32) | 15.0 (33) |
| Bacillus | 5.1 (15) | 1.6 (3) | 3.8 (10) | 1.8 (4) |
| Actinobacillus ** | 3.4 (10) | 6.8 (13) | 1.9 (5) | 0.9 (2) |
| Corynebacterium | 3.4 (10) | 1.6 (3) | 4.5 (12) | 0.0 (0) |
| Histophilus * | 3.4 (10) | 4.2 (8) | 1.5 (4) | 0.9 (2) |
| Acinetobacter ** | 3.1 (9) | 0.5 (1) | 0.4 (1) | 0.0 (0) |
| Mannheimia | 2.7 (8) | 3.7 (7) | 2.6 (7) | 2.3 (5) |
| Prevotella | 2.0 (6) | 3.7 (7) | 1.5 (4) | 1.4 (3) |
| Peptoniphilus | 1.7 (5) | 3.2 (6) | 0.4 (1) | 1.8 (4) |
| Bacteroides * | 1.4 (4) | 1.1 (2) | 4.1 (11) | 2.7 (6) |
| Porphyromonas | 1.4 (4) | 0.0 (0) | 2.3 (6) | 2.7 (6) |
| Atopobium * | 1.0 (3) | 0.5 (1) | 3.4 (9) | 1.8 (4) |
| Enterococcus * | 0.7 (2) | 0.5 (1) | 3.4 (9) | 1.4 (3) |
| Proteus | 0.7 (2) | 0.5 (1) | 1.5 (4) | 2.3 (5) |
| Helcococcus * | 0.3 (1) | 1.6 (3) | 3.4 (9) | 2.7 (6) |
| Clostridium *** | 0.3 (1) | 0.0 (0) | 1.1 (3) | 5.9 (13) |
| Others | 11.2 (33) | 14.2 (27) | 7.5 (20) | 7.3 (16) |
| Total | 100 (295) | 100 (190) | 100 (266) | 100 (220) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kronfeld, H.; Kemper, N.; Hölzel, C.S. Vaginal and Uterine Microbiomes during Puerperium in Dairy Cows. Agriculture 2022, 12, 405. https://doi.org/10.3390/agriculture12030405
Kronfeld H, Kemper N, Hölzel CS. Vaginal and Uterine Microbiomes during Puerperium in Dairy Cows. Agriculture. 2022; 12(3):405. https://doi.org/10.3390/agriculture12030405
Chicago/Turabian StyleKronfeld, Hanna, Nicole Kemper, and Christina S. Hölzel. 2022. "Vaginal and Uterine Microbiomes during Puerperium in Dairy Cows" Agriculture 12, no. 3: 405. https://doi.org/10.3390/agriculture12030405
APA StyleKronfeld, H., Kemper, N., & Hölzel, C. S. (2022). Vaginal and Uterine Microbiomes during Puerperium in Dairy Cows. Agriculture, 12(3), 405. https://doi.org/10.3390/agriculture12030405







