Utilization of Sewage Sludge-Derived Pyrogenic Material as a Promising Soil Amendment
Abstract
:1. Introduction
2. Materials and Methods
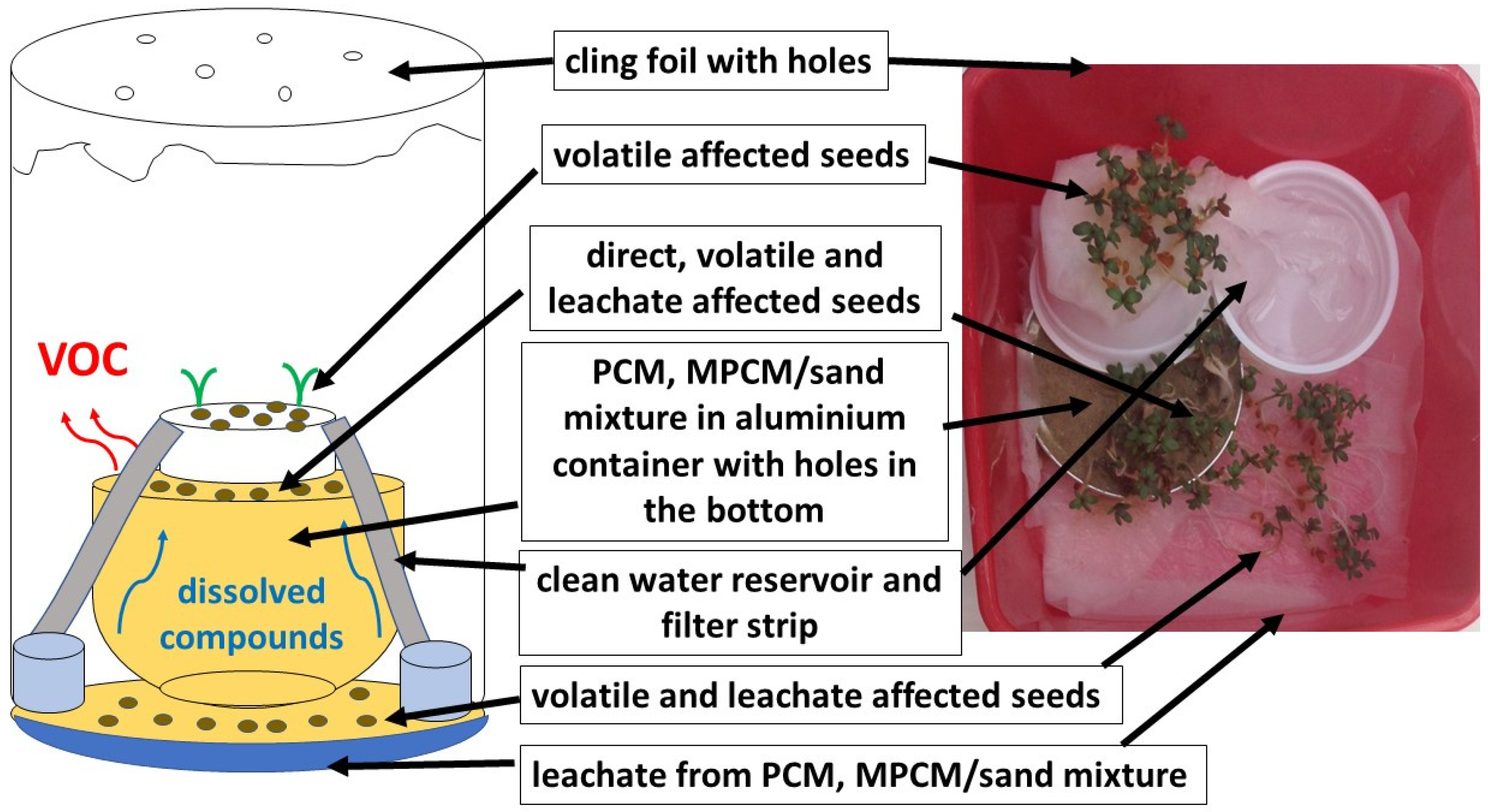
2.1. Simulation Experiment
2.2. Sewage Sludge Pretreatment and Pyrolysis Treatment
2.3. Physicochemical Characterization
2.4. Germination Test
2.5. Neubauer Test
3. Results
3.1. Chemicals Pyrolysis
3.2. PCM and MPCM Characterization
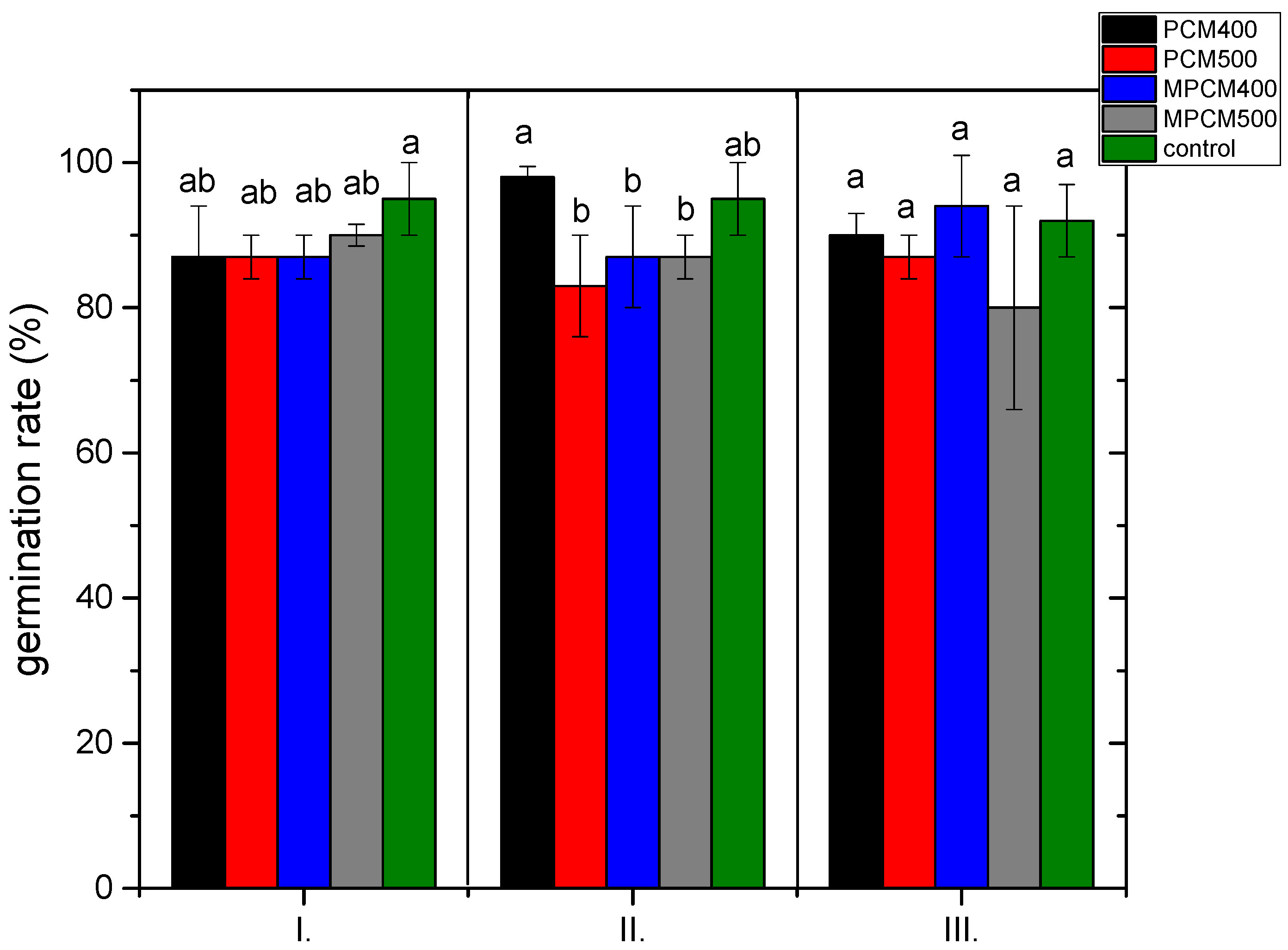
3.3. Assessment of Phytotoxicity
3.4. P Bioavailability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Schmidt, M.W.I.; Torn, M.S.; Abiven, S.; Dittmar, T.; Guggenberger, G.; Janssens, I.A.; Kleber, M.; Kogel-Knabner, I.; Lehmann, J.; Manning, D.A.C.; et al. Persistence of soil organic matter as an ecosystem property. Nature 2011, 478, 49–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Foll, S. Initiative “4 per 1000”: Soils for Food Security and Climate; Scientific and Technical Committee: Montpellier, France, 2019. [Google Scholar]
- Jones, A.; Panagos, S.; Barcelo, S.; Bouraoui, F.; Bosco, C.; Dewitte, O.; Gardi, C.; Erhard, M.; Hiederer, H.R.; Jeffery, S.; et al. The State of Soil in Europe. In JRC Reference Reports; Publications Office of the European Union: Luxembourg, 2021; Volume 78. [Google Scholar]
- Schroeder, J.I.; Delhaize, E.; Frommer, W.B.; Guerinot, M.L.; Harrison, M.J.; Herrera-Estrella, L.; Horie, T.; Kochian, L.V.; Munns, R.; Nishizawa, N.K.; et al. Using membrane transporters to improve crops for sustainable food production. Nature 2013, 497, 60–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kloss, S.; Zehetner, F.; Wimmer, B.; Buecker, J.; Rempt, F.; Soja, G. Biochar application to temperate soils: Effects on soil fertility and crop growth under greenhouse conditions. J. Plant Nutr. Soil Sci. 2014, 177, 3–15. [Google Scholar] [CrossRef]
- Frišták, V.; Pipíška, M.; Soja, G. Pyrolysis treatment of sewage sludge: A promising way to produce phosphorus fertilizer. J. Clean. Prod. 2018, 172, 1772–1778. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar effects on soil biota—A review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Xiao, Y.; Raheem, A.; Ding, L.; Chen, W.H.; Chen, X.; Wang, F.; Lin, S.L. Pretreatment, modification and applications of sewage sludge-derived biochar for resource recovery—A review. Chemosphere 2022, 287, 131969. [Google Scholar] [CrossRef]
- Li, R.; Zhang, Z.; Li, Y.; Teng, W.; Wang, W.; Yang, T. Transformation of apatite phosphorus and non-apatie inorganic phosphorus during incineration of sewage sludge. Chemosphere 2015, 141, 57–61. [Google Scholar] [CrossRef]
- Kleemann, R.; Chenoweth, J.; Clift, R.; Morse, S.; Pearce, P.; Saroj, D. Comparison of phosphorus recovery from incinerated sewage sludge ash (ISSA) and pyrolyzed sewage sludge char (PSSC). Waste Manag. 2017, 60, 201–210. [Google Scholar] [CrossRef] [Green Version]
- Sun, D.; Hale, L.; Kar, G.; Soolanayakanahally, R.; Adl, S. Phosphorus recovery and reuse by pyrolysis: Applications for agriculture and environment. Chemosphere 2018, 194, 682–691. [Google Scholar] [CrossRef]
- Qian, T.; Yang, Q.; Jun, D.C.F.; Dong, F.; Zhou, Y. Transformation of phosphorus in sewage sludge biochar mediated by a phosphate-solubilizing microorganism. Chem. Eng. J. 2019, 359, 1573–1580. [Google Scholar] [CrossRef]
- Liu, Q.; Fang, Z.; Liu, Y.; Liu, Y.; Xu, Y.; Ruan, X.; Zhang, X.; Cao, W. Phosphorus speciation and bioavailability of sewage sludge derived biochar amended with CaO. Waste Manag. 2019, 2019. 87, 71–77. [Google Scholar] [CrossRef]
- Xia, Y.; Tang, Y.; Shib, K.; Li, B. Enhanced phosphorus availability and heavy metal removal by chlorination during sewage sludge pyrolysis. J. Hazard. Mater. 2020, 382, 121110. [Google Scholar] [CrossRef] [PubMed]
- Fachini, J.; de Figueiredo, C.C.; Frazao, J.J.; Rosa, S.D.; da Silva, J.; do Vale, A.T. Novel K-enriched organomineral fertilizer from sewage sludge-biochar: Chemical, physical and mineralogical characterization. Waste Manag. 2021, 135, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Wzorek, Z.; Gorazda, K.; Minda, D.; Kulczycka, J. Influence of thermal processing of FePO4·2H2O with Na2CO3 on solubility of phosphorus compounds in mixtures after sintering. Manag. Environ. Qual. 2005, 16, 639–646. [Google Scholar] [CrossRef]
- ÖNORM L 1087. In Chemische Bodenuntersuchungen—Bestimmung von “Pflanzenverfügbarem” Phosphor und Kalium nach der Calcium-Acetat-Lactat (CAL)-Methode; Ausgabe: Wien, Austria, 2006.
- Frišták, V.; Bošanská, D.; Pipíška, M.; Duriška, L.; Bell, S.M.; Soja, G. Physicochemical Characterization of Cherry Pits-Derived Biochar. Materials 2022, 15, 408. [Google Scholar] [CrossRef] [PubMed]
- Enders, A.; Lehmann, J. Comparison of wet-digestion and dry-ashing methodsfor total elemental analysis of biochar. Commun. Soil Sci. Plant Anal. 2012, 43, 1042–1052. [Google Scholar] [CrossRef]
- Frišták, V.; Laughinghouse, H.D.; Packová, A.; Graser, M.; Soja, G. Monitoring of methylated naphthalenes in sludge-derived pyrogenic carbonaceous materials. Chemosphere 2019, 217, 456–462. [Google Scholar] [CrossRef]
- Hilber, I.; Blum, F.; Leifeld, J.; Schmidt, H.P.; Bücheli, T.D. Quantitative determination of PAHs in biochar: A prerequisite to ensure its quality and safe application. J. Agric. Food Chem. 2012, 60, 3042–3305. [Google Scholar] [CrossRef]
- Buss, W.; Mašek, O. Mobile organic compounds in biochar—A potential source of contamination—Phytotoxic effects on cress seed (Lepidum sativum) germination. J. Environ. Manag. 2014, 137, 111–119. [Google Scholar] [CrossRef] [Green Version]
- Neubauer, H.; Schneider, W. The nutrient uptake of seedlings and its application for the estimation of the nutrient content in soils. Z. Pflanzenernähr. Düng. Bodenk. 1923, A2, 329–362. [Google Scholar]
- Klimuk, E.; Łebkowska, M. Biotechnologia w Ochronie Srodowiska; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2003; ISBN 8301140674. [Google Scholar]
- EBC. European Biochar Certificate—Guidelines for a Sustainable Production of Biochar; Version 10.1 from 10th January 2022; European Biochar Foundation (EBC): Arbaz, Switzerland, 2022. [Google Scholar]
- Dai, Q.; Jiang, X.; Jiang, Y.; Jin, Y.; Wang, F.; Chi, Y.; Yan, J.; Xu, A. Temperature influence and distribution in three phases of PAHs in wet sewage sludge pyrolysis using conventional and microwave heating. Energy Fuels 2014, 28, 3317–3325. [Google Scholar] [CrossRef]
- Bűcheli, T.; Hilber, I.; Schmidt, H.P. Polycyclic Aromatic Hydrocarbons and Polychlorinated Aromatic Compounds in Biochar. In Biochar for Environmental Management. Science and Technology and Implementation; Routledge: Abingdon-on-Thames, UK, 2015; pp. 595–624. [Google Scholar]
- Zielińska, A.; Oleszczuk, P. The conversion of sewage sludge into biochar educes polycyclic aromatic hydrocarbon content and ecotoxicity but increases trace metal content. Biomass Bioenerg. 2015, 75, 235–244. [Google Scholar] [CrossRef]
- Oleszczuk, P. The tenax fraction of PAHs relates to effects in sewage sludges. Ecotoxicol. Environ. Saf. 2009, 72, 1320–1325. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, W.; Wang, S.; Zhuang, L.; Yang, Y.; Qiu, R. Characterization of sewage sludge-derived biochars from different feedstocks and pyrolysis temperatures. J. Anal. Appl. Pyrolysis 2013, 102, 137–143. [Google Scholar] [CrossRef]
- Karayildirim, T.; Yanik, J.; Yuksel, M.; Bock-Horn, H. Characterization of products from pyrolysis of waste sludges. Fuel 2006, 85, 1498–1508. [Google Scholar] [CrossRef]
- Kwon, E.E.; Lee, T.; Ok, Y.S.; Tsang, D.C.W.; Park, C.; Lee, J. Effects of calcium carbonate on pyrolysis of sewage sludge. Energy 2018, 153, 726–731. [Google Scholar] [CrossRef]
- Pipíška, M.; Ballová, S.; Frišták, V.; Ďuriška, L.; Horník, M.; Demčák, Š.; Holub, M.; Soja, G. Assessment of pyrogenic carbonaceous materials for effective removal of radiocesium. Key Eng. Mater. 2020, 838, 103–110. [Google Scholar] [CrossRef]
- Ronsse, F.; van Hecke, S.; Dickinson, D.; Prins, W. Production and characterization of slow pyrolysis biochar: Influence of feedstock type and pyrolysis conditions. GCB Bioenergy 2013, 5, 104–115. [Google Scholar] [CrossRef]
- Zwetsloot, M.J.; Lehmann, J.; Solomon, D. Recycling slaughterhouse waste into fertilizer: How do pyrolysis temperature and biomass additions affect phosphorus availability and chemistry? J. Sci. Food Agric. 2015, 95, 281–288. [Google Scholar] [CrossRef]
- Uchimiya, M.; Hiradate, S. Pyrolysis temperature dependent changes in dissolved phosphorus speciation of plant and manure biochars. J. Agric. Food Chem. 2014, 62, 1802–1809. [Google Scholar] [CrossRef]
- Weber, B.; Stadlbauer, E.A.; Schlich, E.; Eichenauer, S.; Kern, J.; Steffens, D. Phosphorus bioavailability of biochars produced by thermo-chemical conversion. J. Plant Nutr. Soil Sci. 2014, 177, 84–90. [Google Scholar] [CrossRef]



| Sample | Pyrolysis Temperature (°C) | Molar Ratio | Pextractable (mg/g) |
|---|---|---|---|
| FePO4·2H2O + Na2CO3 | 400 | 1 mol/1 mol | 9.078 ± 0.05 e |
| FePO4·2H2O + Na2CO3 | 500 | 1 mol/1 mol | 58.670 ± 0.81 a |
| Al(H2PO4)3 + Na2CO3 | 400 | 1 mol/1 mol | 4.406 ± 0.15 g |
| Al(H2PO4)3 + Na2CO3 | 500 | 1 mol/1 mol | 5.585 ± 0.15 fg |
| FePO4·2H2O | 400 | 1 mol/1 mol | 0.073 ± 0.01 i |
| FePO4·2H2O | 500 | 1 mol/1 mol | 0.167 ± 0.01 i |
| Al(H2PO4)3 | 400 | 1 mol/1 mol | 0.960 ± 0.03 h |
| Al(H2PO4)3 | 500 | 1 mol/1 mol | <LOD |
| FePO4·2H2O + Na2CO3 | 400 | 1 mol/2 mol | 5.763 ± 0.06 fg |
| FePO4·2H2O + Na2CO3 | 500 | 1 mol/2 mol | 31.881 ± 0.37 b |
| Al(H2PO4)3 + Na2CO3 | 400 | 1 mol/2 mol | 13.791 ± 0.15 d |
| Al(H2PO4)3 + Na2CO3 | 500 | 1 mol/2 mol | 26.242 ± 0.84 c |
| Sample | Yield (%) | pH | EC (mS/cm) | Ash Content (%) | Ctot (%) | Ntot (%) | Htot (%) | Cd (mg/kg) | Fe (mg/kg) | P (mg/g) | Pb (mg/kg) | Zn (mg/g) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PCM400 | 45.1 | 8.81 ± 0.15 | 0.29 ± 0.05 | 38.7 ± 0.50 | 33.37 * | 4.19 * | 2.14 * | 0.19 ± 0.01 | 20.55 ± 0.67 | 55.5 ± 0.5 | <LOD | 0.34 ± 0.01 |
| PCM500 | 38.5 | 9.58 ± 0.10 | 0.19 ± 0.02 | 43.9 ± 0.70 | 30.94 * | 3.07 * | 1.85 * | 0.10 ± 0.01 | 23.06 ± 0.07 | 58.7 ± 1.9 | <LOD | 0.37 ± 0.01 |
| MPCM400 | 48.1 | 10.38 ± 0.15 | 0.54 ± 0.05 | 39.8 ± 0.30 | 29.92 * | 2.93 * | 2.54 * | 0.19 ± 0.01 | 21.34 ± 0.74 | 51.7 ± 2.5 | <LOD | 0.32 ± 0.01 |
| MPCM500 | 46.3 | 10.52 ± 0.12 | 0.57 ± 0.01 | 44.5 ± 0.60 | 27.64 * | 2.04 * | 2.06 * | 0.10 ± 0.01 | 20.82 ± 0.30 | 56.1 ± 0.8 | <LOD | 0.34 ± 0.02 |
| Polycyclic Aromatic Hydrocarbon (PAH) | PCM400 (mg/kg) | PCM500 (mg/kg) | MPCM400 (mg/kg) | MPCM500 (mg/kg) |
|---|---|---|---|---|
| Naphthalene | 2.27 ± 0.16 | 9.40 ± 0.05 | 6.71 ± 0.31 | 9.29 ± 0.41 |
| Acenaphtene | ND | ND | ND | ND |
| Acenaphthylene | 0.72 ± 0.03 | 4.41 ± 0.2 | 0.43 ± 0.02 | 5.81 ± 0.2 |
| Phenanthrene | 0.37 ± 0.02 | 1.05 ± 0.05 | 0.14 ± 0.01 | 1.06 ± 0.47 |
| Fluorene | ND | 2.59 ± 0.11 | ND | 1.85 ± 0.06 |
| Anthracene | 0.49 ± 0.01 | 2.08 ± 0.2 | 0.27 ± 0.01 | 2.06 ± 0.2 |
| Benz[a]anthracene | ND | 0.52 ± 0.01 | ND | 0.65 ± 0.01 |
| Chrysene | ND | 0.82 ± 0.01 | ND | 1.14 ± 0.05 |
| Pyrene | ND | 0.36 ± 0.02 | ND | 0.33 ± 0.02 |
| Fluoranthene | 0.53 ± 0.02 | 0.25 ± 0.01 | ND | 0.37 ± 0.01 |
| Benzo[b]fluoranthene | ND | 0.23 ± 0.01 | ND | 0.26 ± 0.01 |
| Benzo[k]fluoranthene | ND | ND | ND | ND |
| Benzo[a]pyrene | ND | 0.16 ± 0.01 | ND | 0.30 ± 0.01 |
| Indeno[1,2,3-cd]pyrene | ND | ND | ND | ND |
| Benzo[ghi]perylene | ND | ND | ND | ND |
| Dibenz[ah]anthracene | ND | ND | ND | ND |
| ∑16 PAHs | 4.38 | 21.87 | 7.55 | 23.12 |
| Cd (mg/kg) | Fe (mg/kg) | Pb (mg/kg) | Zn (mg/kg) | |
|---|---|---|---|---|
| PCM400 | <LOD | 0.041 ± 0.003 c | <LOD | 0.020 ± 0.003 a |
| PCM500 | <LOD | <LOD | <LOD | 0.020 ± 0.003 a |
| MPCM400 | 0.027 ± 0.002 b | 0.050 ± 0.002 b | <LOD | 0.020 ± 0.002 a |
| MPCM500 | 0.026 ± 0.002 b | 0.060 ± 0.003 a | <LOD | 0.022 ± 0.003 a |
| IF | 0.050 ± 0.008 a | 0.040 ± 0.002 c | <LOD | 0.025 ± 0.004 a |
| CONTROL | 0.031 ± 0.011 ab | 0.040 ± 0.005 c | <LOD | 0.020 ± 0.002 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frišták, V.; Pipíška, M.; Koperová, D.; Jagerhofer, R.; Soja, G.; Bell, S.M. Utilization of Sewage Sludge-Derived Pyrogenic Material as a Promising Soil Amendment. Agriculture 2022, 12, 360. https://doi.org/10.3390/agriculture12030360
Frišták V, Pipíška M, Koperová D, Jagerhofer R, Soja G, Bell SM. Utilization of Sewage Sludge-Derived Pyrogenic Material as a Promising Soil Amendment. Agriculture. 2022; 12(3):360. https://doi.org/10.3390/agriculture12030360
Chicago/Turabian StyleFrišták, Vladimír, Martin Pipíška, Dominika Koperová, Reinhard Jagerhofer, Gerhard Soja, and Stephen M. Bell. 2022. "Utilization of Sewage Sludge-Derived Pyrogenic Material as a Promising Soil Amendment" Agriculture 12, no. 3: 360. https://doi.org/10.3390/agriculture12030360
APA StyleFrišták, V., Pipíška, M., Koperová, D., Jagerhofer, R., Soja, G., & Bell, S. M. (2022). Utilization of Sewage Sludge-Derived Pyrogenic Material as a Promising Soil Amendment. Agriculture, 12(3), 360. https://doi.org/10.3390/agriculture12030360









