Simulated Bioavailability of Heavy Metals (Cd, Cr, Cu, Pb, Zn) in Contaminated Soil Amended with Natural Zeolite Using Diffusive Gradients in Thin-Films (DGT) Technique
Abstract
:1. Introduction
2. Materials and Methods
2.1. DGT Assembly and Reagents
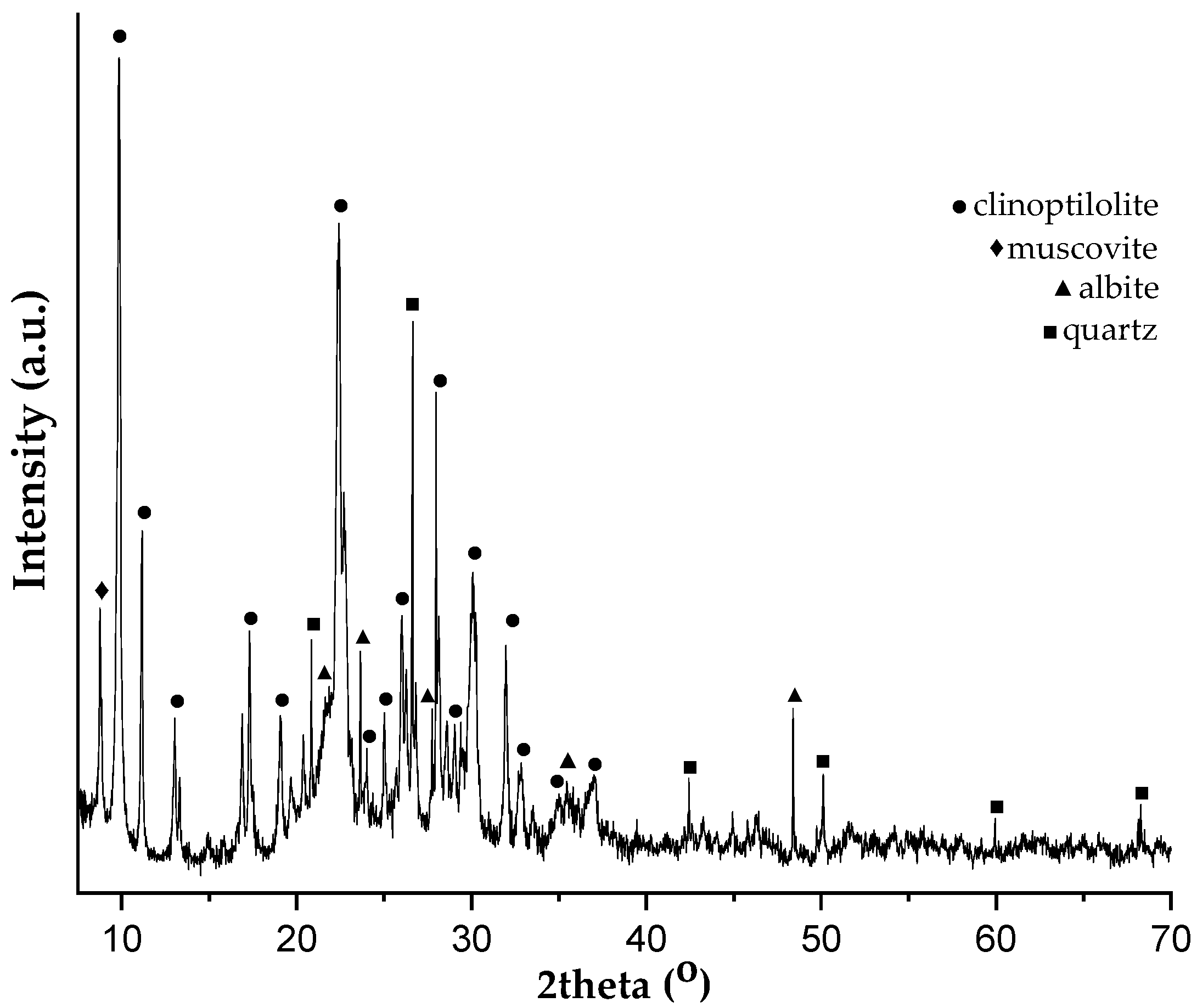
2.2. Soil and Zeolitic Tuff Samples Characterization
2.3. DGT Experiments
- Amendment of 485 g and, respectively, 470 g soil with 15 g and 30 g natural zeolite (NZ), carefully mixed in 1000 mL plastic containers. The obtained mixtures containing 3% and 6% zeolite (NZS3 and NZS6, respectively) and a control pot containing only soil (CS) were moistened until approx. 80–90% of maximum water holding capacity (MWHC) with distilled water and stored for three months. To maintain the soil moisture at a similar level, the pots were covered with a parafilm, watered and homogenized weekly with distilled water. Each pot experiment was maintained at 20 ± 2 °C and was performed in triplicate.
- Determination of DGT metal concentration in initial soil (CS) and their mixtures (NZS3 and NZS6). Samples were watered to 80–90% MWHC, then the assembled DGT devices were gently placed on the mixture surface of each sample for 24 h at 20 ± 2 °C, for equilibration.
- DGT retrieval and elution: after 24 h deployment, the DGT devices were retrieved and carefully rinsed with distilled water. The binding gels were placed in polyethylene vials containing 1 mL of 1 M HNO3 and kept 24 h for elution. The metal concentrations in the eluent were measured by GFAAS.
- DGT calculation, as described by Zhang and co-workers [38]. The metal concentrations accumulated by the DGT devices were calculated according to Equations (1) and (2):
- Determination of DGT metal concentration in samples from pot experiments after storage periods of 0, 1, 2 and 3 months (NZS3-I, NZS6-I; NZS3-1, NZS6-1; NZS3-2, NZS6-2; NZS3-3, NZS6-3). Assembled DGT devices were placed on the mixture surface of each pot experiment for 24 h at 20 ± 2 °C, for equilibration. DGT retrieval, elution, metal determination and calculation steps were similar with those used and described for the initial samples.
2.4. Soil Solution Metal Concentration
3. Results
3.1. Soil and Zeolite Physico-Chemical Characteristics
3.2. pH during the Storage Period
3.3. Heavy Metal Concentrations in Soil Solution
3.4. DGT Measurement
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hamid, Y.; Tang, L.; Sohail, M.I.; Cao, X.; Hussain, B.; Aziz, Z.M.; Usman, M.; He, Z.; Yang, X. An explanation of soil amendments to reduce cadmium phytoavailability and transfer to food chain. Sci. Total Environ. 2019, 660, 80–96. [Google Scholar] [CrossRef] [PubMed]
- Adriano, D.C.; Wenzel, W.W.; Vangronsveld, J.; Bolan, N.S. Role of assisted natural remediation in environmental cleanup. Geoderma 2004, 122, 121–142. [Google Scholar] [CrossRef]
- Garau, G.; Castaldi, P.; Santona, L.; Deiana, P.; Melis, P. Influence of red mud, zeolite and lime on heavy metal immobilization, culturable heterotrophic microbial populations and enzyme activities in a contaminated soil. Geoderma 2007, 142, 47–57. [Google Scholar] [CrossRef]
- Senila, M.; Covaci, E.; Cadar, O.; Ponta, M.; Frentiu, M.; Frentiu, T. Mercury speciation in fish tissue by eco-scale thermal decomposition atomic absorption spectrometry: Method validation and risk exposure to methylmercury. Chem. Pap. 2018, 72, 441–448. [Google Scholar] [CrossRef]
- Massa, N.; Andreucci, F.; Poli, M.; Aceto, M.; Barbato, R.; Berta, G. Screening for heavy metal accumulators amongst autochthonous plants in a polluted site in Italy. Ecotoxicol. Environ. Saf. 2010, 73, 1988–1997. [Google Scholar] [CrossRef]
- Covaci, E.; Senila, M.; Ponta, M.; Darvasi, E.; Petreus, D.; Frentiu, M.; Frentiu, T. Methylmercury determination in seafood by photochemical vapor generation capacitively coupled plasma microtorch optical emission spectrometry. Talanta 2017, 170, 464–472. [Google Scholar] [CrossRef]
- Kushwaha, A.; Goswami, L.; Lee, J.; Sonne, C.; Brown, R.J.C.; Kim, K.H. Selenium in soil-microbe-plant systems: Sources, distribution, toxicity, tolerance, and toxification. Crit. Rev. Environ. Sci. Technol. 2021, 1883187, 1–38. [Google Scholar] [CrossRef]
- Devi, G.; Goswami, L.; Kushwaha, A.; Sathe, S.S.; Sen, B.; Sarma, H.P. Fluoride distribution and groundwater hydrogeochemistry for drinking, domestic and irrigation in an area interfaced near Brahmaputra floodplain of North-Eastern India. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100500. [Google Scholar] [CrossRef]
- Kushwaha, A.; Hans, N.; Kumar, S.; Radha, R. A critical review on speciation, mobilization and toxicity of lead in soil microbeplant system and bioremediation strategies. Ecotoxicol. Environ. Saf. 2018, 147, 1035–1045. [Google Scholar] [CrossRef]
- Eslami, H.; Esmaeili, A.; Razaeian, M.; Salari, M.; Hosseini, A.N.; Mobini, M.; Barani, A. Potentially toxic metal concentration, spatial distribution, and health risk assessment in drinking groundwater resources of southeast Iran. Geosci. Front. 2022, 13, 101276. [Google Scholar] [CrossRef]
- Wieczorek, K.; Turek, A.; Szczesio, M.; Wolf, W.M. Comprehensive evaluation of metal pollution in urban soils of a post-industrial city—A case of Lodz, Poland. Molecules 2019, 25, 4350. [Google Scholar] [CrossRef] [PubMed]
- Morales Arteaga, J.F.; Gluhar, S.; Kaurin, A.; Lestan, D. Simultaneous removal of arsenic and toxic metals from contaminated soil: Laboratory development and pilot scale demonstration. Environ. Pollut. 2022, 294, 118656. [Google Scholar] [CrossRef] [PubMed]
- Relic, D.; Sakan, S.; Andelkovic, I.; Popovic, A.; Dordevic, D. Pollution and Health Risk Assessments of Potentially Toxic Elements in Soil and Sediment Samples in a Petrochemical Industry and Surrounding Area. Molecules 2019, 24, 2139. [Google Scholar] [CrossRef] [Green Version]
- Order 756. In Order of the Ministry of Water, Forestry and Environmental Protection for the Approval of the Regulation Regarding the Assessment of Environmental Pollution, No. 756 of November 3, 1997, Official Gazette No. 303 bis of November 6; Official Gazette of Romania: Bucharest, Romania, 1997. (In Romanian)
- Order 344. In Order of the Ministry of Water, Forestry and Environmental Protection for the Approval of the Technical Norms on the Protection of the Environment and Especially of the Soils, When the Sewage Sludges Are Used in Agriculture, No. 344/708 of August 16, 2004, Official Gazette No. 959 of November 11; Official Gazette of Romania: Bucharest, Romania, 2004. (In Romanian)
- Berar Sur, I.M.; Micle, V.; Avram, S.; Senila, M.; Oros, V. Bioleaching of some heavy metals from polluted soils. Environ. Eng. Manag. J. 2012, 11, 1389–1393. [Google Scholar]
- Cui, H.; Li, H.; Zhang, S.; Yi, Q.; Zhou, J.; Fang, G.; Zhou, J. Bioavailability and mobility of copper and cadmium in polluted soil after phytostabilization using different plants aided by limestone. Chemosphere 2020, 242, 125252. [Google Scholar] [CrossRef]
- Mahar, A.; Wang, P.; Ali, A.; Awasthi, M.K.; Lahori, A.H.; Wang, Q.; Li, R.; Zhang, Z. Challenges and opportunities in the phytoremediation of heavy metals contaminated soils: A review. Ecotoxicol. Environ. Saf. 2016, 126, 111–121. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, S.; Yang, H.; Huang, Y.; Zheng, L.; Yuan, J.; Zhou, S. Comparative study on effects of four energy plants growth on chemical fractions of heavy metals and activity of soil enzymes in copper mine tailings. Int. J. Phytorem. 2018, 20, 616–623. [Google Scholar] [CrossRef]
- Shi, P.; Schulin, R. Erosion-induced losses of carbon, nitrogen, phosphorus and heavy metals from agricultural soils of contrasting organic matter management. Sci. Total Environ. 2018, 618, 210–218. [Google Scholar] [CrossRef]
- Egene, C.E.; Van Poucke, R.; Ok, Y.S.; Meers, E.; Tack, F.M.G. Impact of organic amendments (biochar, compost and peat) on Cd and Zn mobility and solubility in contaminated soil of the Campine region after three years. Sci. Total Environ. 2018, 626, 195–202. [Google Scholar] [CrossRef]
- Campillo-Cora, C.; Rodriguez-Gonzalez, L.; Arias-Estevez, M.; Fernandez-Calvino, D.; Soto-Gómez, D. Influence of soil properties and initial concentration on the fractionation of nickel, zinc, copper and lead in soils derived from different parent materials. Agronomy 2021, 11, 301. [Google Scholar] [CrossRef]
- Burges, A.; Alkorta, I.; Epelde, L.; Garbisu, C. From phytoremediation of soil contaminants to phytomanagement of ecosystem services in metal contaminated sites. Int. J. Phytorem. 2018, 20, 384–397. [Google Scholar] [CrossRef] [PubMed]
- Vrinceanu, N.O.; Motelica, D.M.; Dumitru, M.; Calciu, I.; Tanase, V.; Preda, M. Assessment of using bentonite, dolomite, natural zeolite and manure for the immobilization of heavy metals in a contaminated soil: The Copșa Mică case study (Romania). Catena 2019, 176, 336–342. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Shaheen, S.M.; Chen, S.S.; Tsang, D.C.W.; Hashimoto, Y.; Hou, D.; Bolan, N.S.; Rinklebe, J.; Ok, Y.S. Soil amendments for immobilization of potentially toxic elements in contaminated soils: A critical review. Environ. Int. 2020, 134, 105046. [Google Scholar] [CrossRef]
- Wang, W.; Lu, T.; Liu, L.; Yang, X.; Sun, X.; Qiu, G.; Hua, D.; Zhou, D. Zeolite-supported manganese oxides decrease the Cd uptake of wheat plants in Cd-contaminated weakly alkaline arable soils. J. Hazard. Mater. 2021, 419, 126464. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.; Feng, X.; Zou, W.; Wang, R.; Yang, D.; Wei, W.; Li, S.; Chen, H. Converting loess into zeolite for heavy metal polluted soil remediation based on “soil for soil-remediation” strategy. J. Hazard. Mater. 2021, 412, 125199. [Google Scholar] [CrossRef] [PubMed]
- Belviso, C. Zeolite for Potential Toxic Metal Uptake from Contaminated Soil: A Brief Review. Processes 2020, 8, 820. [Google Scholar] [CrossRef]
- Neag, E.; Török, A.I.; Tanaselia, C.; Aschilean, I.; Senila, M. Kinetics and Equilibrium Studies for the Removal of Mn and Fe from Binary Metal Solution Systems Using a Romanian Thermally Activated Natural Zeolite. Water 2020, 12, 1614. [Google Scholar] [CrossRef]
- Cadar, O.; Dinca, Z.; Senila, M.; Torok, A.I.; Todor, F.; Levei, E.A. Immobilization of Potentially Toxic Elements in Contaminated Soils Using Thermally Treated Natural Zeolite. Materials 2021, 14, 3777. [Google Scholar] [CrossRef]
- Subova, E.; Sasakova, N.; Zigo, F.; Mindzakova, I.; Vargova, M.; Kachnic, J.; Lakticova, K.V. Amendment of Livestock Manure with Natural Zeolite-Clinoptilolite and Its Effect on Decomposition Processes during Composting. Agriculture 2021, 11, 980. [Google Scholar] [CrossRef]
- Noviello, M.; Gattullo, C.E.; Faccia, M.; Paradiso, V.M.; Gambacorta, G. Application of natural and synthetic zeolites in the oenological field. Food Res. Int. 2021, 150, 110737. [Google Scholar] [CrossRef]
- Lee, D.S.; Limb, S.S.; Park, H.J.; Yang, H.I.; Park, S.I.; Kwak, J.H.; Choi, W.J. Fly ash and zeolite decrease metal uptake but do not improve rice growth in paddy soils contaminated with Cu and Zn. Environ. Int. 2019, 129, 551–564. [Google Scholar] [CrossRef] [PubMed]
- Lahori, A.H.; Mierzwa-Hersztek, M.; Demiraj, E.; Sajjad, R.U.; Ali, I.; Shehnaz, I.; Aziz, A.; Zuberi, M.H.; Pirzada, A.M.; Hassan, K.; et al. Direct and residual impacts of zeolite on the remediation of harmful elements in multiple contaminated soils using cabbage in rotation with corn. Chemosphere 2020, 250, 126317. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Nasir, M.; Zhang, Y.; Gao, J.; Lv, Y.; Lv, J. Comparison of DGT with traditional extraction methods for assessing arsenic bioavailability to Brassica chinensis in different soils. Chemosphere 2018, 191, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Zhao, X.; Jin, J.; Gao, B.; Yang, Y.; Sun, K.; Li, F. Using sequential extraction and DGT techniques to assess the efficacy of plant- and manure-derived hydrochar and pyrochar for alleviating the bioavailability of Cd in soils. Sci. Total Environ. 2019, 678, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Hlodak, M.; Matus, P.; Urik, M.; Korenkova, L.; Mikusova, P.; Senila, M.; Divis, P. Evaluation of Various Inorganic and Biological Extraction Techniques Suitability for Soil Mercury Phytoavailable Fraction Assessment. Water Air Soil Pollut. 2015, 226, 198. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, F.J.; Sun, B.; Davison, W.; McGrath, S. A new method to measure effective soil solution concentration predicts copper availability to plants. Environ. Sci. Technol. 2001, 35, 2602–2607. [Google Scholar] [CrossRef]
- Bravin, M.N.; Michaud, A.M.; Larabi, B.; Hinsinger, P. RHIZOtest: A plant-based biotest to account for rhizosphere processes when assessing copper bioavailability. Environ. Pollut. 2010, 158, 3330–3337. [Google Scholar] [CrossRef]
- Marrugo-Madrid, S.; Turull, M.; Zhang, H.; Diez, S. Difusive gradients in thin flms for the measurement of labile metal species in water and soils: A review. Environ. Chem. Lett. 2021, 19, 3761–3788. [Google Scholar] [CrossRef]
- Senila, M.; Levei, E.; Cadar, O.; Senila, L.R.; Roman, M.; Puskas, F.; Sima, M. Assessment of availability and human health risk posed by arsenic contaminated well waters from Timis-Bega area, Romania. J. Anal. Methods Chem. 2017, 2017, 3037651. [Google Scholar] [CrossRef] [Green Version]
- Guan, D.X.; Williams, P.N.; Xu, H.C.; Li, G.; Luo, J.; Ma, L.Q. High-resolution measurement and mapping of tungstate in waters, soils and sediments using the low-disturbance DGT sampling technique. J. Hazard. Mater. 2016, 316, 69–76. [Google Scholar] [CrossRef] [Green Version]
- Senila, M. Real and simulated bioavailability of lead in contaminated and uncontaminated soils. J. Environ. Health Sci. Eng. 2014, 12, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Q.; Zhao, W.; Guan, D.X.; Teng, H.H.; Ji, J.; Ma, L.Q. Comparing CaCl2, EDTA and DGT methods to predict Cd and Ni accumulation in rice grains from contaminated soils. Environ. Pollut. 2020, 260, 114042. [Google Scholar] [CrossRef] [PubMed]
- Ngo, L.K.; Price, H.L.; Bennett, W.W.; Teasdale, P.R.; Jolley, D.F. DGT and selective extractions reveal differences in arsenic and antimony uptake by the white icicle radish (Raphanus sativus). Environ. Pollut. 2020, 259, 113815. [Google Scholar] [CrossRef] [PubMed]
- Senila, M.; Levei, E.A.; Senila, L.R. Assessment of metals bioavailability to vegetables under field conditions using DGT, single extractions and multivariate statistics. Chem. Cent. J. 2012, 6, 119. [Google Scholar] [CrossRef] [Green Version]
- Luo, H.; Du, P.; Shi, J.; Yang, B.; Liang, T.; Wang, P.; Chen, J.; Zhang, I.; He, Y.; Jia, X.; et al. DGT methodology is more sensitive than conventional extraction strategies in assessing amendment-induced soil cadmium availability to rice. Sci. Total Environ. 2021, 760, 143949. [Google Scholar] [CrossRef] [PubMed]
- Turull, M.; Fontas, C.; Diez, S. Effect of different amendments on trace metal bioavailability in agricultural soils and metal uptake on lettuce evaluated by Diffusive Gradients in Thin Films. Environ. Technol. Innov. 2021, 21, 101319. [Google Scholar] [CrossRef]
- Zhang, C.; Zhai, F.; Shan, B. Effectively reducing the bioavailability of heavy metals in disturbed sediment with biochar: In-situ remediation. Environ. Res. 2021, 6, 100141. [Google Scholar] [CrossRef]
- ISO 23470: 2018; Soil Quality—Determination of Effective Cation Exchange Capacity (CEC) and Exchangeable Cations Using a Hexamminecobalt(III)Chloride Solution. International Organization for Standardization: Geneva, Switzerland, 2018.
- ISO 11466: 1995; Soil Quality—Extraction of Trace Elements Soluble in Aqua Regia. International Organization for Standardization: Geneva, Switzerland, 1995.
- Muhammad, I.; Puschenreiter, M.; Wenzel, W.W. Cadmium and Zn availability as affected by pH manipulation and its assessment by soil extraction, DGT and indicator plants. Sci. Total Environ. 2012, 46, 490–500. [Google Scholar] [CrossRef]
- Cadar, O.; Mocan, T.; Roman, C.; Senila, M. Analytical performance and validation of a reliable method based on graphite furnace atomic absorption spectrometry for the determination of gold nanoparticles in biological tissues. Nanomaterials 2021, 11, 3370. [Google Scholar] [CrossRef]
- Senila, M.; Cadar, O.; Miu, I. Development and validation of a spectrometric method for Cd and Pb determination in zeolites and safety evaluation. Molecules 2020, 25, 2591. [Google Scholar] [CrossRef]
- Kraljevic Pavelic, S.; Simovic Medica, J.; Gumbarevic, D.; Filosevic, A.; Przulj, N.; Pavelic, K. Critical review on zeolite clinoptilolite safety and medical applications in vivo. Front. Pharmacol. 2018, 9, 1350. [Google Scholar] [CrossRef] [PubMed]
- Zamzow, M.J.; Eichbaum, B.R.; Sandgren, K.R.; Shanks, D.E. Removal of heavy metals and other cations from wastewater using zeolites. Sep. Sci. Technol. 1990, 25, 1555–1569. [Google Scholar] [CrossRef]
- Yao, Y.; Sun, Q.; Wang, C.; Wang, P.F.; Ding, S.M. Evaluation of organic amendment on the effect of cadmium bioavailability in contaminated soils using the DGT technique and traditional methods. Environ. Sci. Pollut. Res. 2017, 24, 7959–7968. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, L.; Wu, J.; Xu, Y.; Wang, F.; Tang, X.; Xu, J.; Ok, Y.S.; Meng, J.; Liu, X. Zeolite-supported nanoscale zero-valent iron for immobilization of cadmium, lead, and arsenic in farmland soils: Encapsulation mechanisms and indigenous microbial responses. Environ. Pollut. 2020, 260, 114098. [Google Scholar] [CrossRef]
- Tica, D.; Udovic, M.; Lestan, D. Immobilization of potentially toxic metals using different soil amendments. Chemosphere 2011, 85, 577–583. [Google Scholar] [CrossRef]
- Li, H.; Shi, W.; Shao, H.; Shao, M. The remediation of the lead-polluted garden soil by natural zeolite. J. Hazard. Mater. 2009, 169, 1106–1111. [Google Scholar] [CrossRef]
- Tahervand, S.; Jalali, M. Sorption and desorption of potentially toxic metals (Cd, Cu, Ni and Zn) by soil amended with bentonite, calcite and zeolite as a function of pH. J. Geochem. Explor. 2017, 181, 148–159. [Google Scholar] [CrossRef]
- Putwattana, N.; Kruatrachue, M.; Kumsopa, A.; Pokethitiyook, P. Evaluation of organic and inorganic amendments on maize growth and uptake of Cd and Zn from contaminated paddy soils. Int. J. Phytorem. 2015, 17, 165–174. [Google Scholar] [CrossRef]
- Shi, W.; Li, H.; Du, S.; Wang, K.; Shao, B. Immobilization of lead by application of zeolite: Leaching column and rhizobox incubation studies. Appl. Clay Sci. 2013, 85, 103–108. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Biswas, B.; Qi, F.; Biswas, J.K.; Wijayawardena, A.; Khan, M.A.I.; Naidu, R. The fate of chemical pollutants with soil properties and processes in the climate change paradigm—A review. Soil Syst. 2018, 2, 51. [Google Scholar] [CrossRef] [Green Version]

| NZ | CS | NZS3 | NZS6 | Intervention Threshold Soil * | Maximum Threshold Zeolite ** | |
|---|---|---|---|---|---|---|
| pH | 9.55 ± 0.20 | 8.58 ± 0.12 | 8.77 ± 0.15 | 8.78 ± 0.15 | - | - |
| Cd (mg kg−1) | <1.0 | 34.7 ± 1.7 | 33.6 ± 1.8 | 31.4 ± 1.2 | 5 | 10 |
| Cr (mg kg−1) | 4.25 ± 0.23 | 15.2 ± 0.7 | 14.7 ± 0.5 | 14.7 ± 0.6 | 300 | 500 |
| Cu (mg kg−1) | 1.16 ± 0.12 | 553 ± 24 | 522 ± 20 | 516 ± 15 | 200 | 500 |
| Pb (mg kg−1) | 6.33 ± 0.43 | 392 ± 25 | 378 ± 18 | 366 ± 19 | 100 | 300 |
| Zn (mg kg−1) | 4.40 ± 0.38 | 2800 ± 110 | 2779 ± 87 | 2742 ± 65 | 600 | 2000 |
| CEC (meq/100 g) | 129 ± 6.5 | 60.2 ± 4.1 | 62.4 ± 3.5 | 61.5 ± 3.1 | - | - |
| CT (%) | <0.01 | 2.65 ± 0.14 | 2.58 ± 0.21 | 2.52 ± 0.17 | - | - |
| NT (%) | <0.01 | 1.10 ± 0.06 | 1.04 ± 0.06 | 1.05 ± 0.10 | - | - |
| SiO2 (%) | 71.79 ± 1.12 | - | - | - | - | - |
| Al2O3 (%) | 11.19 ± 0.35 | - | - | - | - | - |
| CaO (%) | 2.64 ± 0.04 | - | - | - | - | - |
| MgO (%) | 0.66 ± 0.02 | - | - | - | - | - |
| K2O (%) | 2.50 ± 0.10 | - | - | - | - | - |
| Na2O (%) | 0.52 ± 0.02 | - | - | - | - | - |
| Fe2O3 (%) | 1.55 ± 0.03 | - | - | - | - | - |
| MnO (%) | 0.03 ± 0.003 | - | - | - | - | - |
| Others (%) | 9.12 | - | - | - | - | - |
| CS | t calc * | NZS3 | t calc | NZS6 | t calc | |
|---|---|---|---|---|---|---|
| Initial | ||||||
| pH | 8.58 ± 0.12 | - | 8.77 ± 0.15 | 1.316 | 8.78 ± 0.15 | 1.386 |
| Csol Cd | 2.46 ± 0.25 | - | 2.23 ± 0.34 | 0.944 | 2.13 ± 0.22 | 1.716 |
| Csol Cr | 2.43 ± 0.28 | - | 2.22 ± 0.26 | 0.952 | 2.38 ± 0.25 | 0.231 |
| Csol Cu | 30.1 ± 2.63 | - | 25.5 ± 3.16 | 1.938 | 24.9 ± 2.98 | 2.266 |
| Csol Pb | 3.23 ± 0.43 | - | 3.04 ± 0.40 | 0.560 | 3.14 ± 0.32 | 0.291 |
| Csol Zn | 279 ± 27.4 | - | 241 ± 24.0 | 1.807 | 207 ± 21.4 | 3.587 |
| 1 month storage | ||||||
| pH | 8.47 ± 0.14 | 0.780 | 8.63 ± 0.11 | 0.379 | 8.60 ± 0.14 | 0.142 |
| Csol Cd | 2.30 ± 0.16 | 0.934 | 1.83 ± 0.25 | 3.086 | 1.76 ± 0.32 | 2.986 |
| Csol Cr | 2.30 ± 0.33 | 0.520 | 2.12 ± 0.15 | 1.690 | 2.28 ± 0.26 | 0.680 |
| Csol Cu | 27.2 ± 2.10 | 1.492 | 31.0 ± 2.00 | 0.472 | 28.1 ± 3.25 | 0.824 |
| Csol Pb | 3.01 ± 0.22 | 0.789 | 2.09 ± 0.16 | 4.304 | 1.99 ± 0.19 | 4.569 |
| Csol Zn | 288 ± 31.4 | 0.374 | 232 ± 25.5 | 2.175 | 216 ± 33.3 | 2.514 |
| 2 months storage | ||||||
| pH | 8.48 ± 0.20 | 0.612 | 8.74 ± 0.19 | 1.005 | 8.81 ± 0.21 | 1.374 |
| Csol Cd | 2.19 ± 0.28 | 1.246 | 1.70 ± 0.20 | 4.112 | 1.53 ± 0.18 | 5.229 |
| Csol Cr | 2.40 ± 0.40 | 0.106 | 1.95 ± 0.21 | 2.375 | 1.72 ± 0.14 | 3.928 |
| Csol Cu | 32.4 ± 4.48 | 0.767 | 27.3 ± 1.08 | 1.706 | 26.8 ± 2.35 | 1.630 |
| Csol Pb | 2.90 ± 0.38 | 0.996 | 1.83 ± 0.13 | 5.398 | 1.73 ± 0.16 | 5.663 |
| Csol Zn | 264 ± 22.8 | 0.729 | 227 ± 35.0 | 2.026 | 237 ± 27.0 | 1.869 |
| 3 months storage | ||||||
| pH | 8.63 ± 0.18 | 0.322 | 8.82 ± 0.20 | 1.470 | 8.63 ± 0.18 | 0.322 |
| Csol Cd | 2.23 ± 0.21 | 1.220 | 1.65 ± 0.08 | 5.345 | 1.20 ± 0.14 | 7.617 |
| Csol Cr | 2.32 ± 0.15 | 0.600 | 2.01 ± 0.12 | 2.388 | 1.77 ± 0.32 | 2.688 |
| Csol Cu | 28.8 ± 2.04 | 0.676 | 29.1 ± 1.15 | 0.634 | 27.3 ± 3.40 | 1.116 |
| Csol Pb | 3.11 ± 0.25 | 0.418 | 2.08 ± 0.15 | 4.374 | 1.66 ± 0.19 | 5.784 |
| Csol Zn | 280 ± 11.0 | 0.059 | 289 ± 9.70 | 0.596 | 304 ± 16.5 | 1.375 |
| CS | t calc * | NZS3 | t calc | NZS6 | t calc | |
|---|---|---|---|---|---|---|
| Initial | ||||||
| Csol Cd | 1.01 ± 0.20 | - | 0.90 ± 0.15 | 0.759 | 0.88 ± 0.13 | 0.960 |
| Csol Cr | 0.92 ± 0.17 | - | 0.84 ± 0.12 | 0.645 | 0.81 ± 0.14 | 0.896 |
| Csol Cu | 15.5 ± 2.35 | - | 14.5 ± 1.41 | 0.647 | 15.1 ± 1.05 | 0.298 |
| Csol Pb | 1.25 ± 0.29 | - | 1.26 ± 0.21 | 0.082 | 1.27 ± 0.24 | 0.092 |
| Csol Zn | 155 ± 21.2 | - | 148 ± 17.2 | 0.445 | 131 ± 20.3 | 1.397 |
| 1 month storage | ||||||
| Csol Cd | 1.13 ± 0.19 | 0.759 | 0.89 ± 0.14 | 0.831 | 0.66 ± 0.12 | 2.745 |
| Csol Cr | 1.08 ± 0.14 | 1.255 | 0.96 ± 0.12 | 0.304 | 0.77 ± 0.16 | 1.417 |
| Csol Cu | 14.0 ± 1.76 | 0.871 | 13.2 ± 1.31 | 1.521 | 11.0 ± 0.16 | 3.159 |
| Csol Pb | 1.20 ± 0.26 | 0.217 | 1.17 ± 0.25 | 0.337 | 0.95 ± 0.19 | 1.650 |
| Csol Zn | 145 ± 19.7 | 0.624 | 131 ± 15.5 | 1.297 | 146 ± 12.3 | 0.657 |
| 2 months storage | ||||||
| Csol Cd | 0.96 ± 0.18 | 0.332 | 0.41 ± 0.10 | 4.830 | 0.40 ± 0.09 | 4.891 |
| Csol Cr | 0.73 ± 0.09 | 1.502 | 0.83 ± 0.10 | 0.801 | 0.79 ± 0.07 | 1.219 |
| Csol Cu | 19.2 ± 2.33 | 1.544 | 12.6 ± 1.08 | 1.919 | 10.0 ± 0.75 | 3.945 |
| Csol Pb | 1.37 ± 0.22 | 0.610 | 0.58 ± 0.11 | 3.605 | 0.39 ± 0.09 | 4.606 |
| Csol Zn | 146 ± 23.3 | 0.589 | 135 ± 19.0 | 1.376 | 112 ± 13.6 | 3.202 |
| 3 months storage | ||||||
| Csol Cd | 0.93 ± 0.21 | 0.548 | 0.28 ± 0.08 | 5.788 | 0.29 ± 0.09 | 5.837 |
| Csol Cr | 1.05 ± 0.15 | 0.934 | 0.88 ± 0.12 | 0.328 | 0.68 ± 0.16 | 2.081 |
| Csol Cu | 13.7 ± 2.04 | 0.987 | 11.2 ± 1.12 | 3.036 | 11.4 ± 0.91 | 2.858 |
| Csol Pb | 1.15 ± 0.25 | 0.469 | 0.58 ± 0.10 | 3.928 | 0.48 ± 0.09 | 4.456 |
| Csol Zn | 117 ± 11.0 | 2.332 | 126 ± 9.70 | 2.296 | 152 ± 8.88 | 0.223 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senila, M.; Cadar, O.; Senila, L.; Angyus, B.S. Simulated Bioavailability of Heavy Metals (Cd, Cr, Cu, Pb, Zn) in Contaminated Soil Amended with Natural Zeolite Using Diffusive Gradients in Thin-Films (DGT) Technique. Agriculture 2022, 12, 321. https://doi.org/10.3390/agriculture12030321
Senila M, Cadar O, Senila L, Angyus BS. Simulated Bioavailability of Heavy Metals (Cd, Cr, Cu, Pb, Zn) in Contaminated Soil Amended with Natural Zeolite Using Diffusive Gradients in Thin-Films (DGT) Technique. Agriculture. 2022; 12(3):321. https://doi.org/10.3390/agriculture12030321
Chicago/Turabian StyleSenila, Marin, Oana Cadar, Lacrimioara Senila, and Bogdan Simion Angyus. 2022. "Simulated Bioavailability of Heavy Metals (Cd, Cr, Cu, Pb, Zn) in Contaminated Soil Amended with Natural Zeolite Using Diffusive Gradients in Thin-Films (DGT) Technique" Agriculture 12, no. 3: 321. https://doi.org/10.3390/agriculture12030321
APA StyleSenila, M., Cadar, O., Senila, L., & Angyus, B. S. (2022). Simulated Bioavailability of Heavy Metals (Cd, Cr, Cu, Pb, Zn) in Contaminated Soil Amended with Natural Zeolite Using Diffusive Gradients in Thin-Films (DGT) Technique. Agriculture, 12(3), 321. https://doi.org/10.3390/agriculture12030321








