Manure Maturation with Biochar: Effects on Plant Biomass, Manure Quality and Soil Microbiological Characteristics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Manure Modification
2.2. Pot Experiment
2.3. Plant Biomass
2.4. Soil Sampling, Chemical and Biological Analyses
2.5. Statistical Analysis
3. Results
3.1. Effect of Amendments on Physical and Chemical Properties of Manure after Maturation
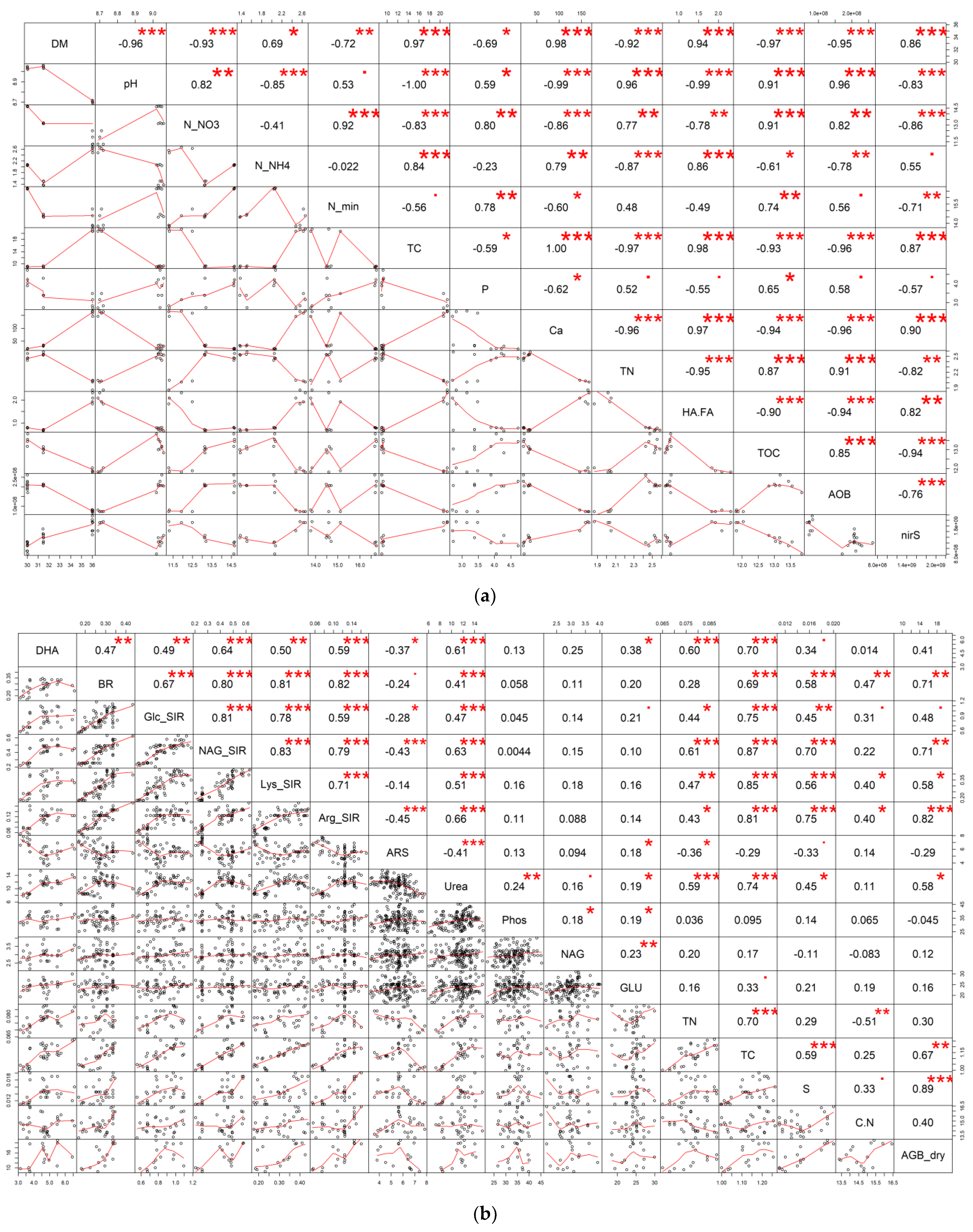
3.2. Effect of Amendments on Microbial Properties of Manure after Maturation
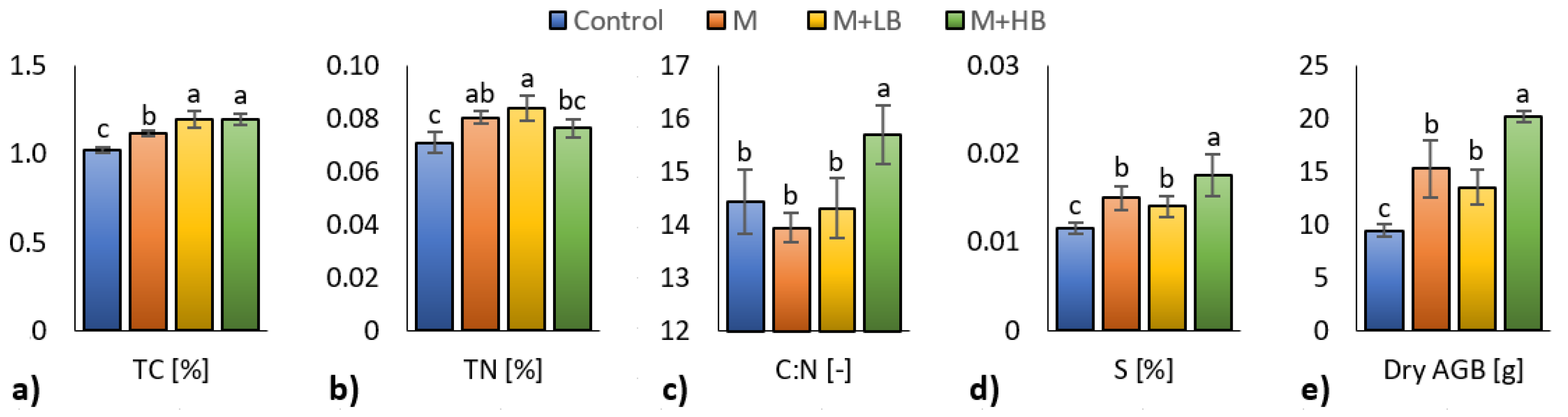
3.3. Effect of Matured Manure Types on Soil Chemical Properties and Plant Biomass
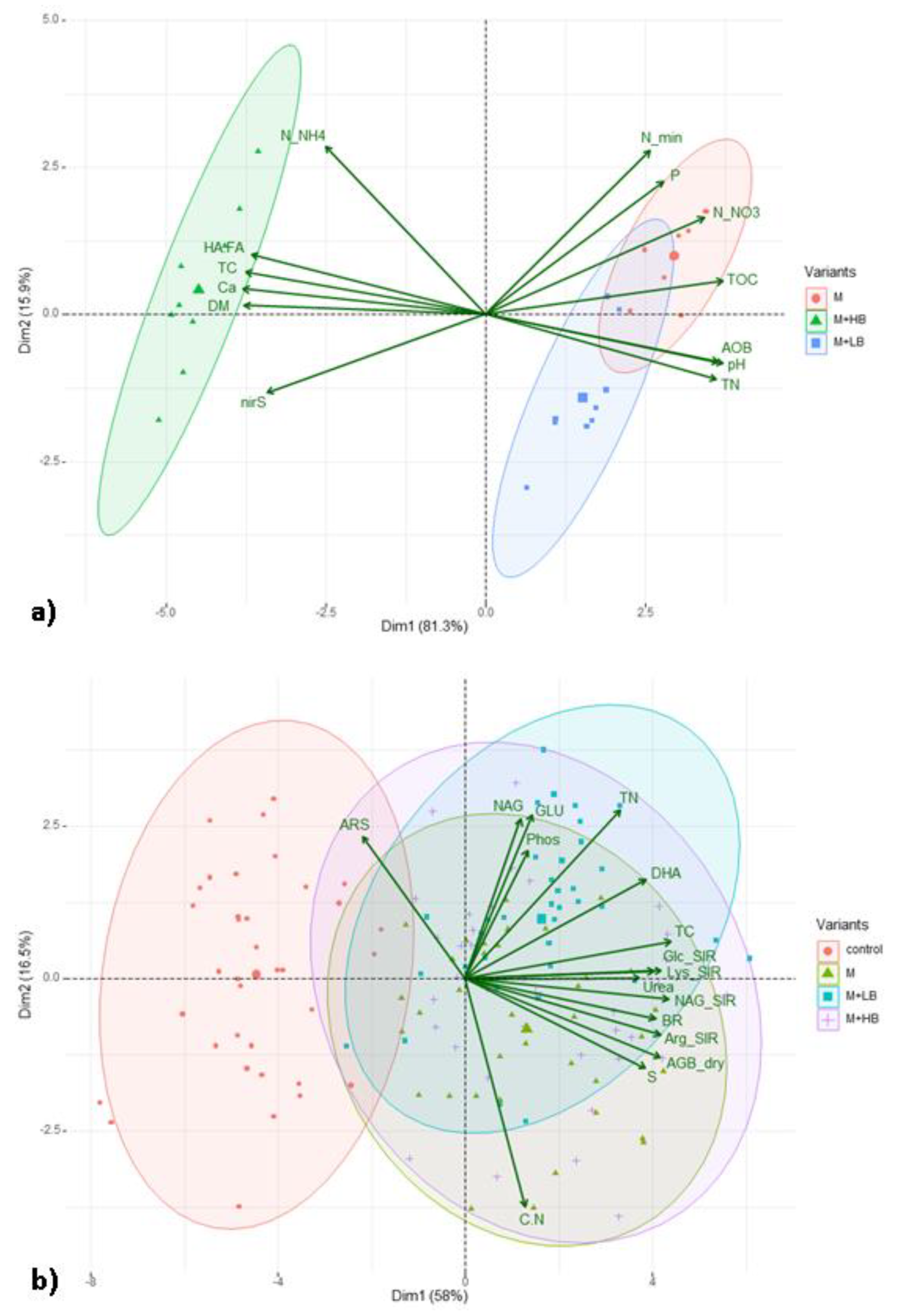
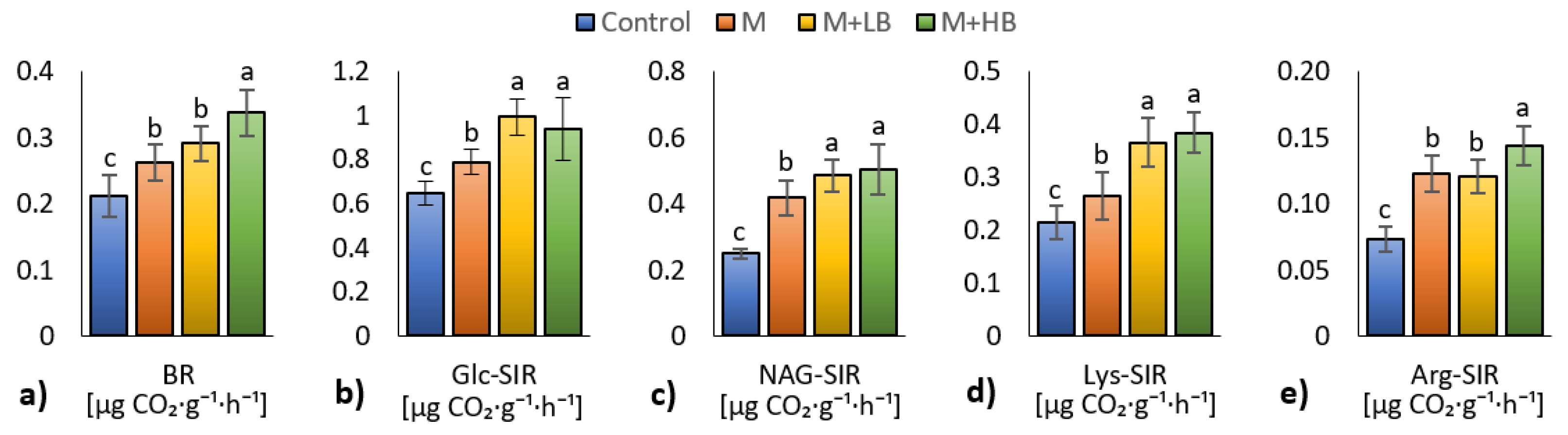
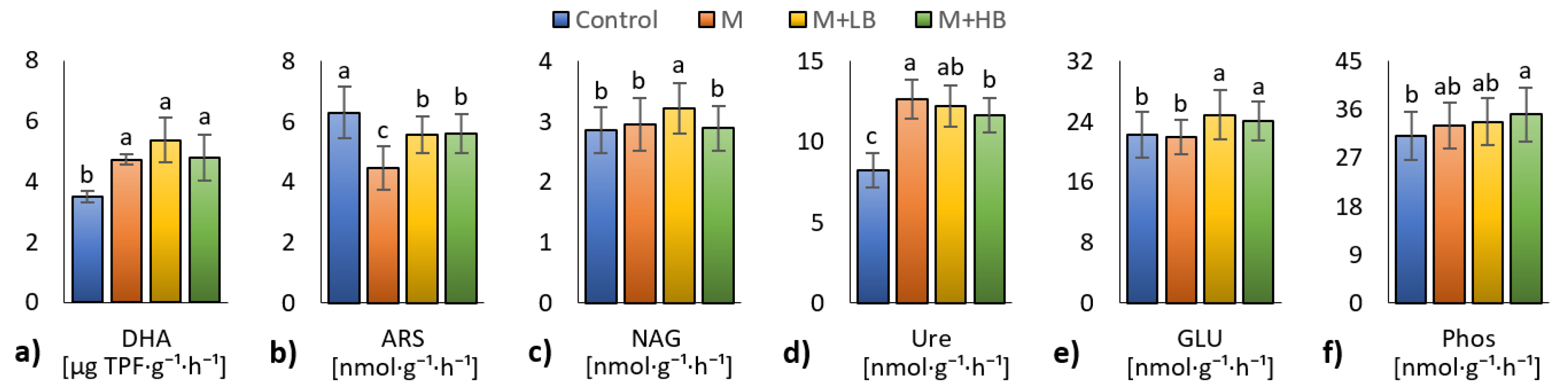
3.4. Effect of Matured Manure Types on Soil Microbial Properties
4. Discussion
4.1. Effect of Amendments on Physical and Chemical Properties of Manure after Maturation
4.2. Effect of Amendments on Microbial Properties of Manure after Maturation
4.3. Effect of Matured Manure Types on Soil Chemical Properties and Plant Biomass
4.4. Effect of Matured Manure Types on Soil Microbial Properties
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Li, S.; Harris, S.; Anandhi, A.; Chen, G. Predicting biochar properties and functions based on feedstock and pyrolysis temperature: A review and data syntheses. J. Clean. Prod. 2019, 215, 890–902. [Google Scholar] [CrossRef]
- Verheijen, F.G.A.; Jeffery, S.; Bastos, A.C.; van der Velde, M.; Diafas, I. Biochar Application to Soils—A Critical Scientific Review of Effects on Soil Properties, Processes and Functions; Office for the Official Publications of the European Communities: Luxembourg, 2009; p. 149. [Google Scholar]
- Ennis, C.J.; Evans, A.G.; Islam, M.; Ralebitso-Senior, T.K.; Senior, E. Biochar: Carbon sequestration, land remediation, and impacts on soil microbiology. Crit. Rev. Environ. Sci. Technol. 2012, 42, 2311–2364. [Google Scholar] [CrossRef] [Green Version]
- Stewart, C.E.; Zheng, J.; Botte, J.; Cotrufo, M.F. Co-generated fast pyrolysis biochar mitigates green-house gas emissions and increases carbon sequestration in temperate soils. GCB Bioenergy 2013, 5, 153–164. [Google Scholar] [CrossRef]
- Zheng, W.; Guo, M.; Chow, T.; Bennett, D.N.; Rajagopalan, N. Sorption properties of greenwaste biochar for two triazine pesticides. J. Hazard. Mater. 2010, 181, 121–126. [Google Scholar] [CrossRef] [Green Version]
- Teixidó, M.; Pignatello, J.J.; Beltrán, J.L.; Granados, M.; Peccia, J. Speciation of the Ionizable Antibiotic Sulfamethazine on Black Carbon (Biochar). Environ. Sci. Technol. 2011, 45, 10020–10027. [Google Scholar] [CrossRef]
- Xu, X.; Cao, X.; Zhao, L. Comparison of rice husk- and dairy manure-derived biochars for simultaneously removing heavy metals from aqueous solutions: Role of mineral components in biochars. Chemosphere 2013, 92, 955–961. [Google Scholar] [CrossRef]
- Gao, F.; Xue, Y.; Deng, P.; Cheng, X.; Yang, K. Removal of aqueous ammonium by biochars derived from agricultural residuals at different pyrolysis temperatures. Chem. Speciat. Bioavailab. 2015, 27, 92–97. [Google Scholar] [CrossRef] [Green Version]
- Shang, G.; Li, Q.; Liu, L.; Chen, P.; Huang, X. Adsorption of hydrogen sulfide by biochars derived from pyrolysis of different agricultural/forestry wastes. J. Air Waste Manag. Assoc. 2016, 66, 8–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glaser, B.; Haumaier, L.; Guggenberger, G.; Zech, W. The ‘Terra Preta’ phenomenon: A model for sustainable agriculture in the humid tropics. Naturwissenschaften 2001, 88, 37–41. [Google Scholar] [CrossRef]
- Taketani, R.G.; Lima, A.B.; da Conceicao Jesus, E.; Teixeira, W.G.; Tiedje, J.M.; Tsai, S.M. Bacterial community composition of anthropogenic biochar and Amazonian anthrosols assessed by 16s rRNA gene 454 pyrosequencing. Antonie Van Leeuwenhoek 2013, 104, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Biederman, L.A.; Harpole, W.S. Biochar and its effects on plant productivity and nutrient cycling: A meta-analysis. GCB Bioenergy 2013, 5, 202–214. [Google Scholar] [CrossRef]
- Wang, L.; Butterly, C.R.; Wang, Y.; Herath, H.M.S.K.; Xi, Y.G.; Xiao, X.J. Effect of crop residue biochar on soil acidity amelioration in strongly acidic tea garden soils. Soil Use Manag. 2014, 30, 119–128. [Google Scholar] [CrossRef]
- Elbl, J.; Mikajlo, I.; Brtnicky, M.; Kynicky, J. Biochar and organic-waste compost as soil amendments to arable soil: Potential influence on soil reaction, salinity and phytotoxicity. In Proceedings of the 22nd International PhD Students Conference, Brno, Czech Republic, 11–12 November 2015; pp. 212–217. [Google Scholar]
- Naveed, M.; Ditta, A.; Ahmad, M.; Mustafa, A.; Ahmad, Z.; Conde-Cid, M.; Tahir, S.; Shah, S.A.A.; Abrar, M.M.; Fahad, S. Processed animal manure improves morpho-physiological and biochemical characteristics of Brassica napus L. under nickel and salinity stress. Environ. Sci. Pollut. Res. 2021, 28, 45629–45645. [Google Scholar] [CrossRef]
- Schulz, H.; Dunst, G.; Glaser, B. Positive effects of composted biochar on plant growth and soil fertility. Agron. Sustain. Dev. 2013, 33, 817–827. [Google Scholar] [CrossRef] [Green Version]
- Agegnehu, G.; Bass, A.M.; Nelson, P.N.; Muirhead, B.; Wright, G.; Bird, M.I. Biochar and biochar-compost as soil amendments: Effects on peanut yield, soil properties and greenhouse gas emissions in tropical North Queensland, Australia. Agric. Ecosyst. Environ. 2015, 213, 72–85. [Google Scholar] [CrossRef]
- Dias, B.O.; Silva, C.A.; Higashikawa, F.S.; Roig, A.; Sánchez-Monedero, M.A. Use of biochar as bulking agent for the composting of poultry manure: Effect on organic matter degradation and humification. Bioresour. Technol. 2010, 101, 1239–1246. [Google Scholar] [CrossRef]
- Chen, W.; Liao, X.D.; Wu, Y.B.; Liang, J.B.; Mi, J.D.; Huang, J.J.; Zhang, H.; Wu, Y.; Qiao, Z.F.; Li, X.; et al. Effects of different types of biochar on methane and ammonia mitigation during layer manure composting. Waste Manag. 2017, 61, 506–515. [Google Scholar] [CrossRef]
- Maurer, D.L.; Koziel, J.A.; Kalus, K.; Andersen, D.S.; Opalinski, S. Pilot-Scale Testing of Non-Activated Biochar for Swine Manure Treatment and Mitigation of Ammonia, Hydrogen Sulfide, Odorous Volatile Organic Compounds (VOCs), and Greenhouse Gas Emissions. Sustainability 2017, 9, 929. [Google Scholar] [CrossRef] [Green Version]
- Janczak, D.; Malińska, K.; Czekała, W.; Cáceres, R.; Lewicki, A.; Dach, J. Biochar to reduce ammonia emissions in gaseous and liquid phase during composting of poultry manure with wheat straw. Waste Manag. 2017, 66, 36–45. [Google Scholar] [CrossRef]
- Hagemann, N.; Subdiaga, E.; Orsetti, S.; de la Rosa, J.M.; Knicker, H.; Schmidt, H.-P.; Kappler, A.; Behrens, S. Effect of biochar amendment on compost organic matter composition following aerobic composting of manure. Sci. Total Environ. 2018, 613–614, 20–29. [Google Scholar] [CrossRef]
- Czekała, W.; Malińska, K.; Cáceres, R.; Janczak, D.; Dach, J.; Lewicki, A. Co-composting of poultry manure mixtures amended with biochar—The effect of biochar on temperature and C-CO2 emission. Bioresour. Technol. 2016, 200, 921–927. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Yin, H.; Sun, X.; Han, L.; Huang, G. Effect of different particle-size biochar on methane emissions during pig manure/wheat straw aerobic composting: Insights into pore characterization and microbial mechanisms. Bioresour. Technol. 2018, 268, 633–637. [Google Scholar] [CrossRef] [PubMed]
- Jindo, K.; Sanchez-Monedero, M.A.; Hernandez, T.; Garcia, C.; Furukawa, T.; Matsumoto, K.; Sonoki, T.; Bastida, F. Biochar influences the microbial community structure during manure composting with agricultural wastes. Sci. Total Environ. 2012, 416, 476–481. [Google Scholar] [CrossRef]
- Bird, M.I. Test procedures for biochar analysis in soils. In Biochar for Environmental Management: Science Technology and Implementation; Lehmann, J., Joseph, S., Eds.; Routledge: Abingdon, UK, 2015; pp. 677–714. [Google Scholar]
- Al-Wabel, M.I.; Hussain, Q.; Usman, A.R.A.; Ahmad, M.; Abduljabbar, A.; Sallam, A.S.; Ok, Y.S. Impact of biochar properties on soil conditions and agricultural sustainability: A review. Land Degrad. Dev. 2018, 29, 2124–2161. [Google Scholar] [CrossRef]
- Agegnehu, G.; Bird, M.I.; Nelson, P.N.; Bass, A.M. The ameliorating effects of biochar and compost on soil quality and plant growth on a Ferralsol. Soil Res. 2015, 53, 1–12. [Google Scholar] [CrossRef]
- Wei, S.; Zhu, M.; Fan, X.; Song, J.; Peng, P.; Li, K.; Jia, W.; Song, H. Influence of pyrolysis temperature and feedstock on carbon fractions of biochar produced from pyrolysis of rice straw, pine wood, pig manure and sewage sludge. Chemosphere 2019, 218, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Malinowski, M.; Wolny-Koładka, K.; Vaverková, M.D. Effect of biochar addition on the OFMSW composting process under real conditions. Waste Manag. 2019, 84, 364–372. [Google Scholar] [CrossRef]
- Li, J.; Bao, H.; Xing, W.; Yang, J.; Liu, R.; Wang, X.; Lv, L.; Tong, X.; Wu, F. Succession of fungal dynamics and their influence on physicochemical parameters during pig manure composting employing with pine leaf biochar. Bioresour. Technol. 2020, 297, 122377. [Google Scholar] [CrossRef]
- Qayyum, M.F.; Liaquat, F.; Rehman, R.A.; Gul, M.; Ul Hye, M.Z.; Rizwan, M.; Rehaman, M.Z.U. Effects of co-composting of farm manure and biochar on plant growth and carbon mineralization in an alkaline soil. Environ. Sci. Pollut. Res. 2017, 24, 26060–26068. [Google Scholar] [CrossRef]
- Khan, N.; Clark, I.; Sanchez-Monedero, M.A.; Shea, S.; Meier, S.; Bolan, N. Maturity indices in co-composting of chicken manure and sawdust with biochar. Bioresour. Technol. 2014, 168, 245–251. [Google Scholar] [CrossRef]
- Agyarko-Mintah, E.; Cowie, A.; Van Zwieten, L.; Singh, B.P.; Smillie, R.; Harden, S.; Fornasier, F. Biochar lowers ammonia emission and improves nitrogen retention in poultry litter composting. Waste Manag. 2017, 61, 129–137. [Google Scholar] [CrossRef]
- Yuan, Y.; Chen, H.; Yuan, W.; Williams, D.; Walker, J.T.; Shi, W. Is biochar-manure co-compost a better solution for soil health improvement and N2O emissions mitigation? Soil Biol. Biochem. 2017, 113, 14–25. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Villamil, M.B.; Davidson, P.C.; Akdeniz, N. A quantitative understanding of the role of co-composted biochar in plant growth using meta-analysis. Sci. Total Environ. 2019, 685, 741–752. [Google Scholar] [CrossRef]
- Brtnicky, M.; Hammerschmiedt, T.; Elbl, J.; Kintl, A.; Skulcova, L.; Radziemska, M.; Latal, O.; Baltazar, T.; Kobzova, E.; Holatko, J. The potential of biochar made from agricultural residues to increase soil fertility and microbial activity: Impacts on soils with varying sand content. Agronomy 2021, 11, 1174. [Google Scholar] [CrossRef]
- ISO_10390; Soil Quality—Determination of pH. International Organization for Standardization: Geneva, Switzerland, 2005.
- ISO_11465; Soil Quality—Determination of Dry Matter and Water Content on a Mass Basis—Gravimetric Method. International Organization for Standardization: Geneva, Switzerland, 1993.
- Egnér, H.; Riehm, H.; Domingo, W.R. Untersuchungen uber die chemische bodenanalyse als grundlage fur die beurteilung des nährstoffzustandes der böden. Ii. Chemische extraktionsmethoden zur phosphor- und kaliumbestimmung. Kungliga Lantbrukshögskolans Annaler 1960, 26, 199–215. [Google Scholar]
- ISO_14869-3; Soil Quality—Dissolution for The Determination of Total Element Content—Part 3: Dissolution with Hydrofluoric, Hydrochloric and Nitric Acids Using Pressurised Microwave Technique. International Organization for Standardization: Geneva, Switzerland, 2017.
- ISO_10694; Soil Quality—Determination of Organic and Total Carbon after Dry Combustion (Elementary Analysis). International Organization for Standardization: Geneva, Switzerland, 1995.
- ISO_11261; Soil Quality—Determination of Total Nitrogen—Modified Kjeldahl Method. International Organization for Standardization: Geneva, Switzerland, 1995.
- ISO_19822; Fertilizers and Soil Conditioners—Determination of Humic and Hydrophobic Fulvic Acids Concentrations in Fertilizer Materials. International Organization for Standardization: Geneva, Switzerland, 2018.
- ISO_15476; Fertilizers—Determination of Nitric and Ammoniacal Nitrogen According to Devarda. International Organization for Standardization: Geneva, Switzerland, 2009.
- Rotthauwe, J.H.; Witzel, K.P.; Liesack, W. The ammonia monooxygenase structural gene amoa as a functional marker: Molecular fine-scale analysis of natural ammonia-oxidizing populations. Appl. Environ. Microbiol. 1997, 63, 4704–4712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kandeler, E.; Deiglmayr, K.; Tscherko, D.; Bru, D.; Philippot, L. Abundance of narG, nirS, nirK, and nosZ Genes of Denitrifying Bacteria during Primary Successions of a Glacier Foreland. Appl. Environ. Microbiol. 2006, 72, 5957–5962. [Google Scholar] [CrossRef] [Green Version]
- ISO_20130; Soil Quality—Measurement of Enzyme Activity Patterns in Soil Samples Using Colorimetric Substrates in Micro-Well Plates. International Organization for Standardization: Geneva, Switzerland, 2018.
- Tabatabai, M.A. Part 2—Methods of soil analysis. In Chemical and Microbiological Properties; American Society of Agronomy: Madison, WI, USA; Soil Science Society of America: Madison, WI, USA, 1982. [Google Scholar] [CrossRef] [Green Version]
- Campbell, C.D.; Chapman, S.J.; Cameron, C.M.; Davidson, M.S.; Potts, J.M. A Rapid Microtiter Plate Method to Measure Carbon Dioxide Evolved from Carbon Substrate Amendments so as To Determine the Physiological Profiles of Soil Microbial Communities by Using Whole Soil. Appl. Environ. Microbiol. 2003, 69, 3593–3599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammerschmiedt, T.; Holatko, J.; Pecina, V.; Huska, D.; Latal, O.; Kintl, A.; Radziemska, M.; Muhammad, S.; Gusiatin, Z.M.; Kolackova, M.; et al. Assessing the potential of biochar aged by humic substances to enhance plant growth and soil biological activity. Chem. Biol. Technol. Agric. 2021, 8, 46. [Google Scholar] [CrossRef]
- Lungu, O.I.; Dynoodt, R.F. Acidification from Long-Term Use of Urea and Its Effect on Selected Soil Properties. Afr. J. Food Agric. Nutr. Dev. 2008, 8, 63–76. [Google Scholar] [CrossRef] [Green Version]
- Jien, S.-H.; Wang, C.-C.; Lee, C.-H.; Lee, T.-Y. Stabilization of organic matter by biochar application in compost-amended soils with contrasting ph values and textures. Sustainability 2015, 7, 13317–13333. [Google Scholar] [CrossRef] [Green Version]
- Lentz, R.D.; Ippolito, J.A.; Spokas, K.A. Biochar and Manure Effects on Net Nitrogen Mineralization and Greenhouse Gas Emissions from Calcareous Soil under Corn. Soil Sci. Soc. Am. J. 2014, 78, 1641–1655. [Google Scholar] [CrossRef]
- Kizito, S.; Wu, S.; Kipkemoi Kirui, W.; Lei, M.; Lu, Q.; Bah, H.; Dong, R. Evaluation of slow pyrolyzed wood and rice husks biochar for adsorption of ammonium nitrogen from piggery manure anaerobic digestate slurry. Sci. Total Environ. 2015, 505, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; He, S.; Liang, Y.; Li, G.; Li, S.; Chen, S.; Nadeem, F.; Hu, J. Effect of phosphate additive on the nitrogen transformation during pig manure composting. Environ. Sci. Pollut. Res. 2017, 24, 17760–17768. [Google Scholar] [CrossRef] [PubMed]
- Baninajarian, S.; Shirvani, M. Use of biochar as a possible means of minimizing phosphate fixation and external P requirement of acidic soil. J. Plant Nutr. 2020, 44, 59–73. [Google Scholar] [CrossRef]
- Haghighatjou, M.; Shirvani, M. Sugarcane Bagasse Biochar: Preparation, Characterization, and Its Effects on Soil Properties and Zinc Sorption-desorption. Commun. Soil Sci. Plant Anal. 2020, 51, 1391–1405. [Google Scholar] [CrossRef]
- Brtnicky, M.; Datta, R.; Holatko, J.; Bielska, L.; Gusiatin, Z.M.; Kucerik, J.; Hammerschmiedt, T.; Danish, S.; Radziemska, M.; Mravcova, L.; et al. A critical review of the possible adverse effects of biochar in the soil environment. Sci. Total Environ. 2021, 796, 148756. [Google Scholar] [CrossRef]
- Pignatello, J.J.; Kwon, S.; Lu, Y. Effect of Natural Organic Substances on the Surface and Adsorptive Properties of Environmental Black Carbon (Char): Attenuation of Surface Activity by Humic and Fulvic Acids. Environ. Sci. Technol. 2006, 40, 7757–7763. [Google Scholar] [CrossRef] [PubMed]
- Veeken, A.; Nierop, K.; de Wilde, V.; Hamelers, H. Characterisation of NaOH-extracted humic acids during composting of a biowaste. Bioresour. Technol. 2000, 72, 33–41. [Google Scholar] [CrossRef]
- Raut, N.; Dörsch, P.; Sitaula, B.K.; Bakken, L.R. Soil acidification by intensified crop production in South Asia results in higher N2O/(N2 + N2O) product ratios of denitrification. Soil Biol. Biochem. 2012, 55, 104–112. [Google Scholar] [CrossRef]
- Davidsson, T.E.; Stepanauskas, R.; Leonardson, L. Vertical patterns of nitrogen transformations during infiltration in two wetland soils. Appl. Environ. Microbiol. 1997, 63, 3648–3656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Xiong, Z.; Kuzyakov, Y. Biochar stability in soil: Meta-analysis of decomposition and priming effects. GCB Bioenergy 2015, 8, 512–523. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Cao, X.; Zhao, L.; Sun, T. Comparison of sewage sludge- and pig manure-derived biochars for hydrogen sulfide removal. Chemosphere 2014, 111, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Saviozzi, A.; Cardelli, R.; Cipolli, S.; Levi-Minzi, R.; Riffaldi, R. Sulphur mineralization kinetics of cattle manure and green waste compost in soils. Waste Manag. Res. 2006, 24, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Agegnehu, G.; Bass, A.M.; Nelson, P.N.; Bird, M.I. Benefits of biochar, compost and biochar-compost for soil quality, maize yield and greenhouse gas emissions in a tropical agricultural soil. Sci. Total Environ. 2016, 543 Pt A, 295–306. [Google Scholar] [CrossRef]
- Rogovska, N.; Laird, D.; Cruse, R.; Fleming, P.; Parkin, T.; Meek, D. Impact of Biochar on Manure Carbon Stabilization and Greenhouse Gas Emissions. Soil Sci. Soc. Am. J. 2011, 75, 871–879. [Google Scholar] [CrossRef] [Green Version]
- Curci, M.; Pizzigallo, M.D.R.; Crecchio, C.; Mininni, R.; Ruggiero, P. Effects of conventional tillage on biochemical properties of soils. Biol. Fertil. Soils 1997, 25, 1–6. [Google Scholar] [CrossRef]
- Irmak Yilmaz, F. Impact of biochar and animal manure on some biological and chemical properties of soil. Appl. Ecol. Environ. Res. 2019, 17, 8865–8876. [Google Scholar] [CrossRef]
- Whalen, J.K.; Warman, P.R. Arylsulfatase activity in soil and soil extracts using natural and artificial substrates. Biol. Fertil. Soils 1996, 22, 373–378. [Google Scholar] [CrossRef]
- Ekenler, M.; Tabatabai, M. β-glucosaminidase activity of soils: Effect of cropping systems and its relationship to nitrogen mineralization. Biol. Fertil. Soils 2002, 36, 367–376. [Google Scholar] [CrossRef]
- Watson, C.J. Urease Activity and Inhibition—Principles and Practice. In International Fertiliser Society 2000; International Fertiliser Society: London, UK, 2000. [Google Scholar]
- Lammirato, C.; Miltner, A.; Kaestner, M. Effects of wood char and activated carbon on the hydrolysis of cellobiose by β-glucosidase from aspergillus niger. Soil Biol. Biochem. 2011, 43, 1936–1942. [Google Scholar] [CrossRef]
- Hussain, M.; Farooq, M.; Nawaz, A.; Al-Sadi, A.M.; Solaiman, Z.M.; Alghamdi, S.S.; Ammara, U.O.; Yong, S.S.; Kadambot, H.M. Biochar for crop production: Potential benefits and risks. J. Soils Sediments 2016, 17, 685–716. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Chen, L.J.; Zhang, Y.G.; Wu, Z.J.; Ma, X.Z.; Yang, X.Z. Examining the effects of biochar application on soil phosphorus levels and phosphatase activities with visible and fluorescence spectroscopy. Guang Pu Xue Yu Guang Pu Fen Xi 2016, 36, 2325–2329. [Google Scholar] [PubMed]
- Minhas, W.A.; Hussain, M.; Mehboob, N.; Nawaz, A.; Ul-Allah, S.; Rizwan, M.S.; Hassan, Z. Synergetic use of biochar and synthetic nitrogen and phosphorus fertilizers to improves maize productivity and nutrient retention in loamy soil. J. Plant Nutr. 2020, 43, 1356–1368. [Google Scholar] [CrossRef]
- Jindo, K.; Suto, K.; Matsumoto, K.; Garcia, C.; Sonoki, T.; Sanchez-Monedero, M.A. Chemical and biochemical characterisation of biochar-blended composts prepared from poultry manure. Bioresour. Technol. 2012, 110, 396–404. [Google Scholar] [CrossRef] [PubMed]
| Variant | Abbrev. | Manure (M) per Barrel | Biochar (B) per Barrel | Dry Matter Ratio M:B |
|---|---|---|---|---|
| Manure | M | 10 kg | 0 | 0 |
| Manure + biochar low dose | M + LB | 10 kg | 0.4 kg | 12.5:1 |
| Manure + biochar high dose | M + HB | 10 kg | 4.0 kg | 1.25:1 |
| Abbrev. | Property, Method | Unit | Reference |
|---|---|---|---|
| pH | pH determined in CaCl2 | - | [38] |
| DM | dry matter, gravimetry | % | [39] |
| P | available phosphorus, extraction | g·kg⁻1 | [40] |
| Ca | calcium, extractable (Mehlich III) | g·kg⁻1 | [41] |
| TN | total Kjeldahl nitrogen | % | [42] |
| TC | total carbon, dry combustion | % | [43] |
| TOC | total organic carbon, dry combustion | ||
| HA:FA | humic acid:fulvic acid ratio | - | [44] |
| N-min | mineral nitrogen | % | |
| N-NH4 | ammonium nitrogen | mg·kg⁻1 | [45] |
| N-NO3 | nitrogen in nitrate form | mg·kg⁻1 | [45] |
| AOB | ammonium-oxidizing bacteria, qPCR (gene amoA) | copies·g⁻1 | [46] |
| nirS | denitrifying bacteria, qPCR (gene nirS) | copies·g⁻1 | [47] |
| Property [Unit] | M | M + LB | M + HB |
|---|---|---|---|
| Mean ± SD * | Mean ± SD * | Mean ± SD * | |
| pH [-] | 9.04 ± 0.01 a | 9.05 ± 0.01 a | 8.71 ± 0.01 b |
| DM [%] | 30.01 ± 0.02 c | 31.48 ± 0.02 b | 36.01 ± 0.02 a |
| TC [%] | 9.10 ± 0.12 b | 9.13 ± 0.18 b | 21.01 ± 0.34 a |
| TOC [%] | 13.50 ± 0.24 a | 13.01 ± 0.12 b | 11.89 ± 0.09 c |
| TN [%] | 2.48 ± 0.05 a | 2.54 ± 0.02 a | 1.99 ± 0.07 b |
| N-min [%] | 16.70 ± 0.03 a | 14.58 ± 0.09 b | 14.33 ± 0.55 b |
| N-NH₄ [mg·kg⁻1] | 2.06 ± 0.01 b | 1.44 ± 0.07 c | 2.58 ± 0.07 a |
| N-NO₃ [mg·kg⁻1] | 14.64 ± 0.02 a | 13.14 ± 0.03 b | 11.75 ± 0.50 c |
| HA:FA [-] | 0.79 ± 0.01 b | 0.69 ± 0.02 b | 2.04 ± 0.18 a |
| P [g·kg⁻1] | 4.22 ± 0.31 a | 3.45 ± 0.55 ab | 3.04 ± 0.30 b |
| Ca [g·kg⁻1] | 20.93 ± 0.76 c | 32.95 ± 1.69 b | 159.34 ± 8.03 a |
| Property [Unit] | M | M + LB | M + HB |
|---|---|---|---|
| Mean ± SD * | Mean ± SD * | Mean ± SD * | |
| AOB [copies·g⁻1] | 2.11 × 108 ± 2.48 × 107 a | 2.09 × 108 ± 3.43 × 106 a | 7.46 × 107 ± 5.26 × 106 b |
| nirS [copies × g⁻1] | 1.07 × 109 ± 1.50 × 108 c | 1.40 × 109 ± 1.59 × 108 b | 1.85 × 109 ± 2.06 × 108 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hammerschmiedt, T.; Holatko, J.; Kucerik, J.; Mustafa, A.; Radziemska, M.; Kintl, A.; Malicek, O.; Baltazar, T.; Latal, O.; Brtnicky, M. Manure Maturation with Biochar: Effects on Plant Biomass, Manure Quality and Soil Microbiological Characteristics. Agriculture 2022, 12, 314. https://doi.org/10.3390/agriculture12030314
Hammerschmiedt T, Holatko J, Kucerik J, Mustafa A, Radziemska M, Kintl A, Malicek O, Baltazar T, Latal O, Brtnicky M. Manure Maturation with Biochar: Effects on Plant Biomass, Manure Quality and Soil Microbiological Characteristics. Agriculture. 2022; 12(3):314. https://doi.org/10.3390/agriculture12030314
Chicago/Turabian StyleHammerschmiedt, Tereza, Jiri Holatko, Jiri Kucerik, Adnan Mustafa, Maja Radziemska, Antonin Kintl, Ondrej Malicek, Tivadar Baltazar, Oldrich Latal, and Martin Brtnicky. 2022. "Manure Maturation with Biochar: Effects on Plant Biomass, Manure Quality and Soil Microbiological Characteristics" Agriculture 12, no. 3: 314. https://doi.org/10.3390/agriculture12030314
APA StyleHammerschmiedt, T., Holatko, J., Kucerik, J., Mustafa, A., Radziemska, M., Kintl, A., Malicek, O., Baltazar, T., Latal, O., & Brtnicky, M. (2022). Manure Maturation with Biochar: Effects on Plant Biomass, Manure Quality and Soil Microbiological Characteristics. Agriculture, 12(3), 314. https://doi.org/10.3390/agriculture12030314









