Short-Term Resilience of Soil Microbial Communities and Functions Following Severe Environmental Changes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soils and Soil Treatments
2.2. Soil Respiration, N Ammonification, Microbial Biomass and Enzymatic Activities
2.3. Community-Level Physiological Profile (CLPP) Fingerprinting
2.4. Soil DNA Extraction and PCR-DGGE Fingerprinting
2.5. Data Analysis
3. Results
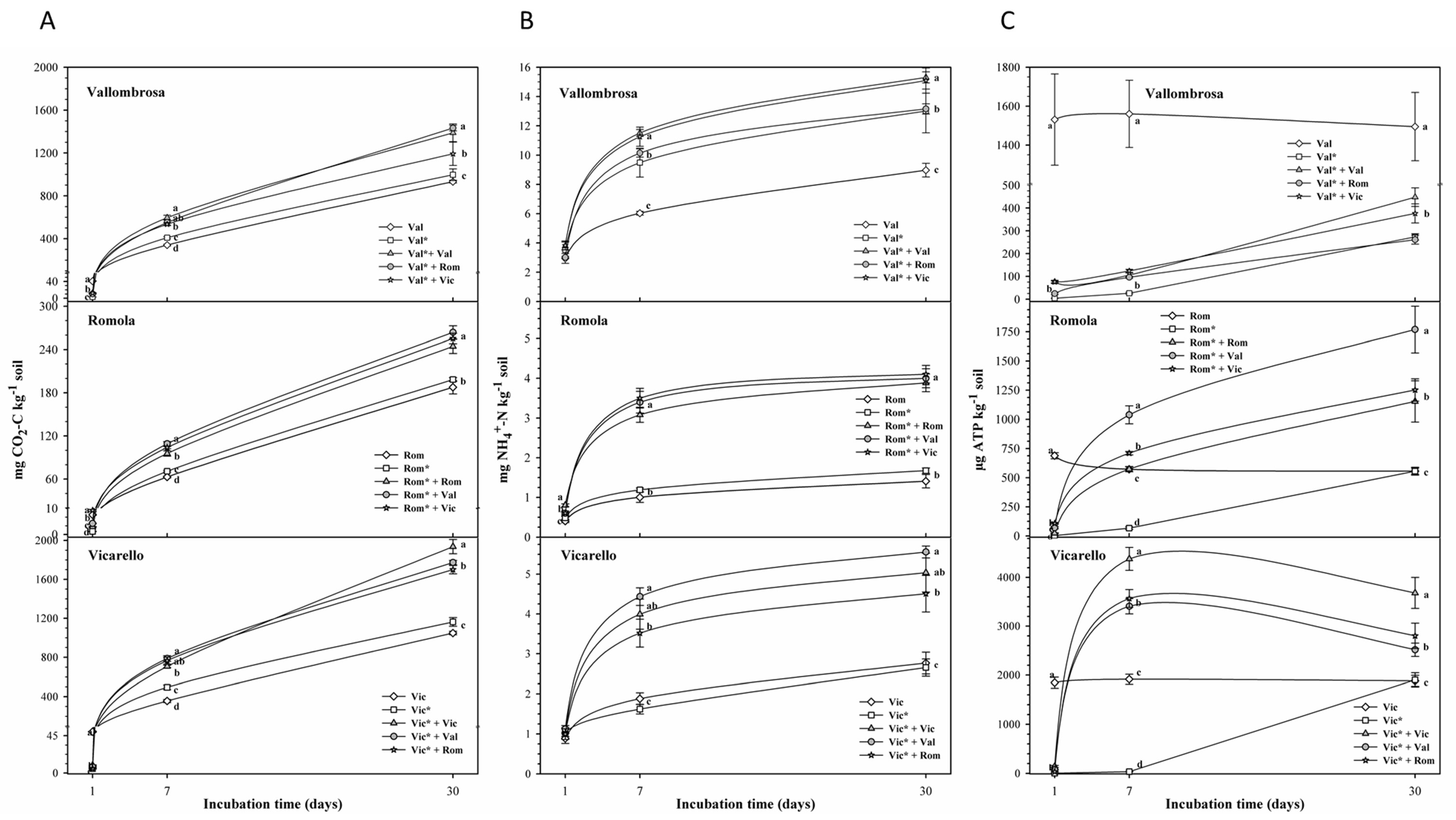
3.1. Soil Respiration, N Ammonification, ATP and Microbial Biomass
3.2. Soil Enzymatic Activities
3.2.1. Enzymatic Activities in the Vallombrosa Soil
3.2.2. Enzymatic Activities in the Romola Soil
3.2.3. Enzymatic Activities in the Vicarello Soil
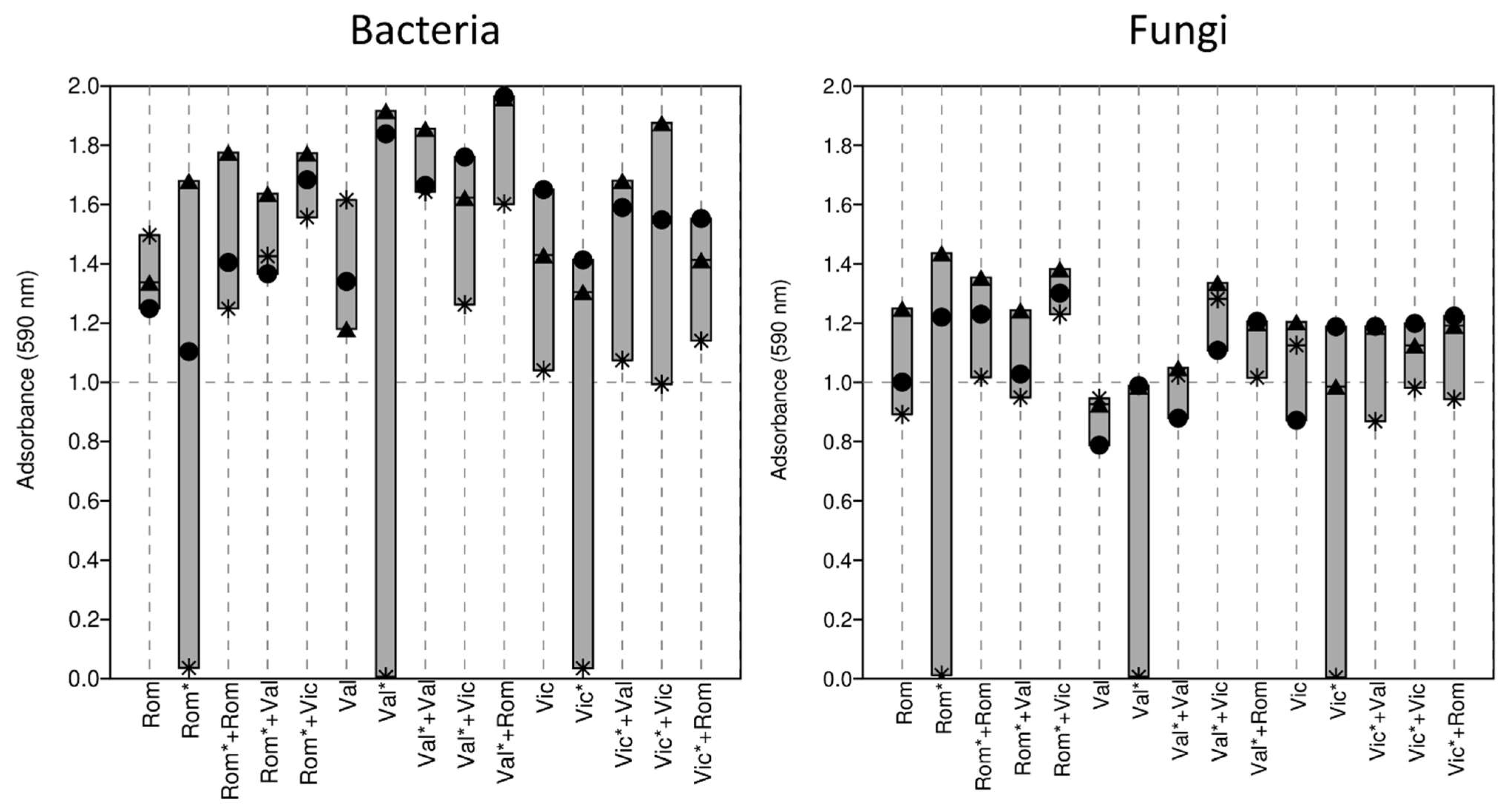
3.3. Soil Community-Level Physiological Profile
3.3.1. Community-Level Physiological Profile of the Vallombrosa Soil
3.3.2. Community-Level Physiological Profile of the Romola Soil
3.3.3. Community-Level Physiological Profile of the Vicarello Soil
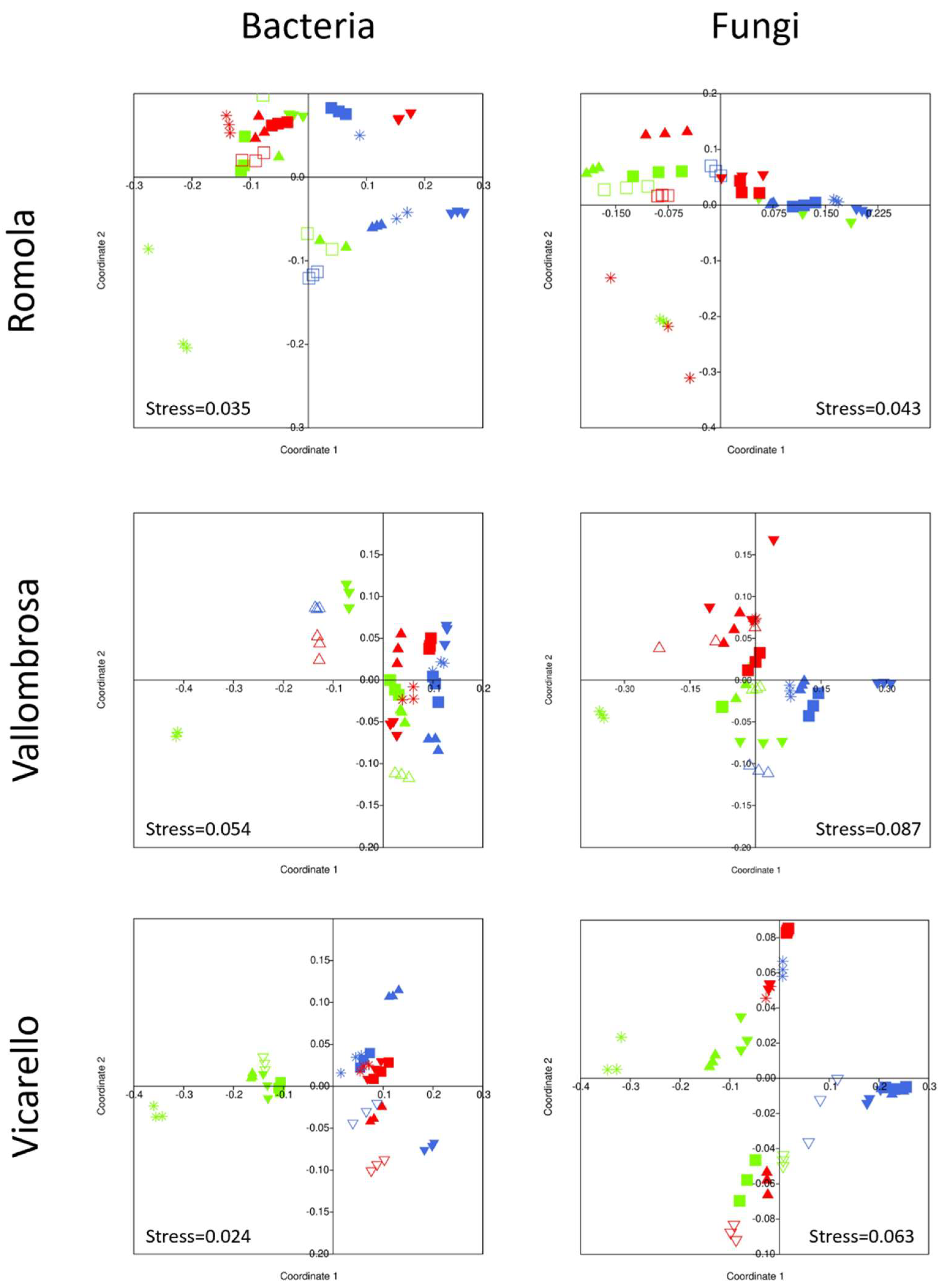
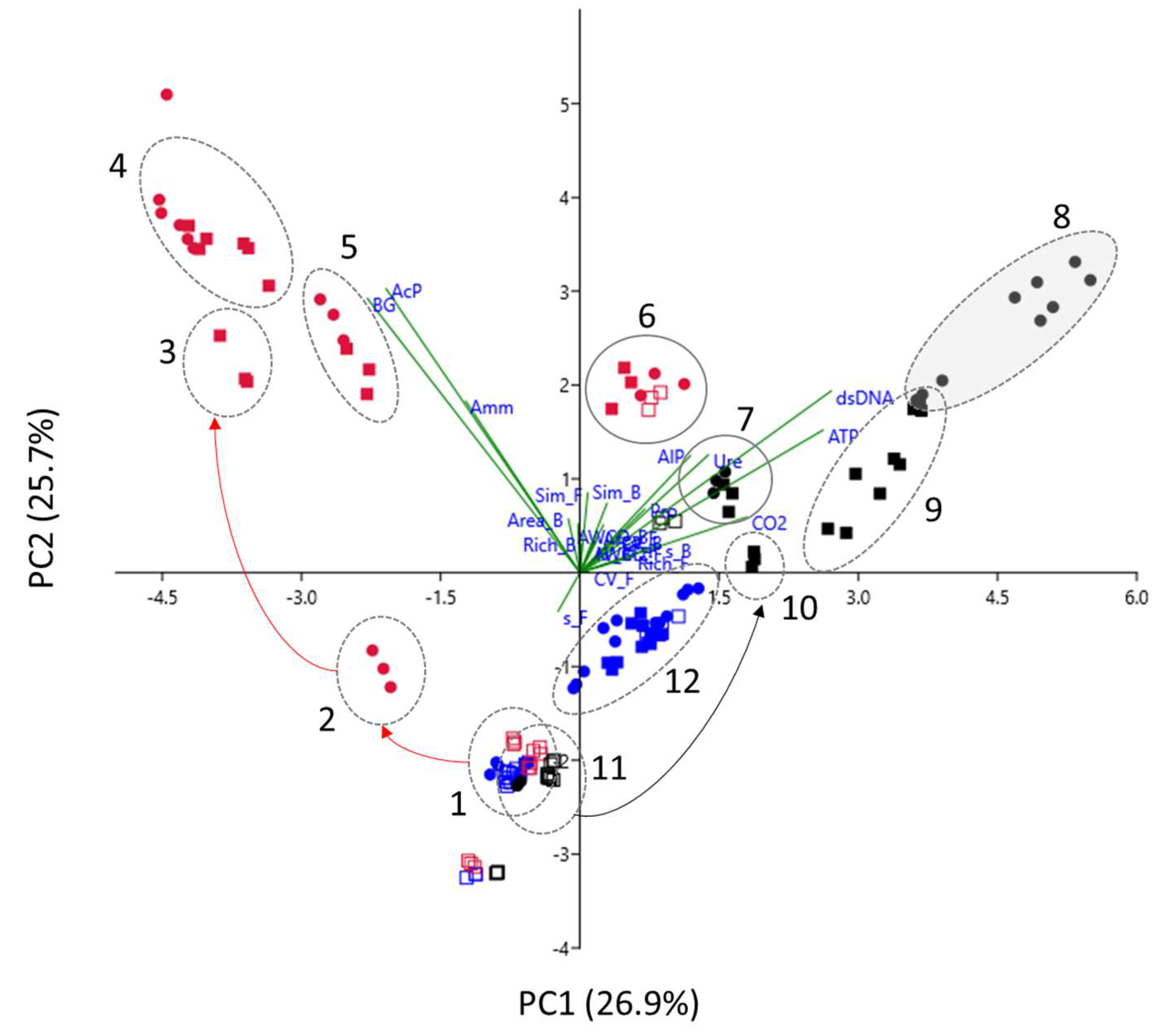
3.3.4. Multivariate Analysis of CLPP Data
3.4. Bacterial and Fungal Community Structure
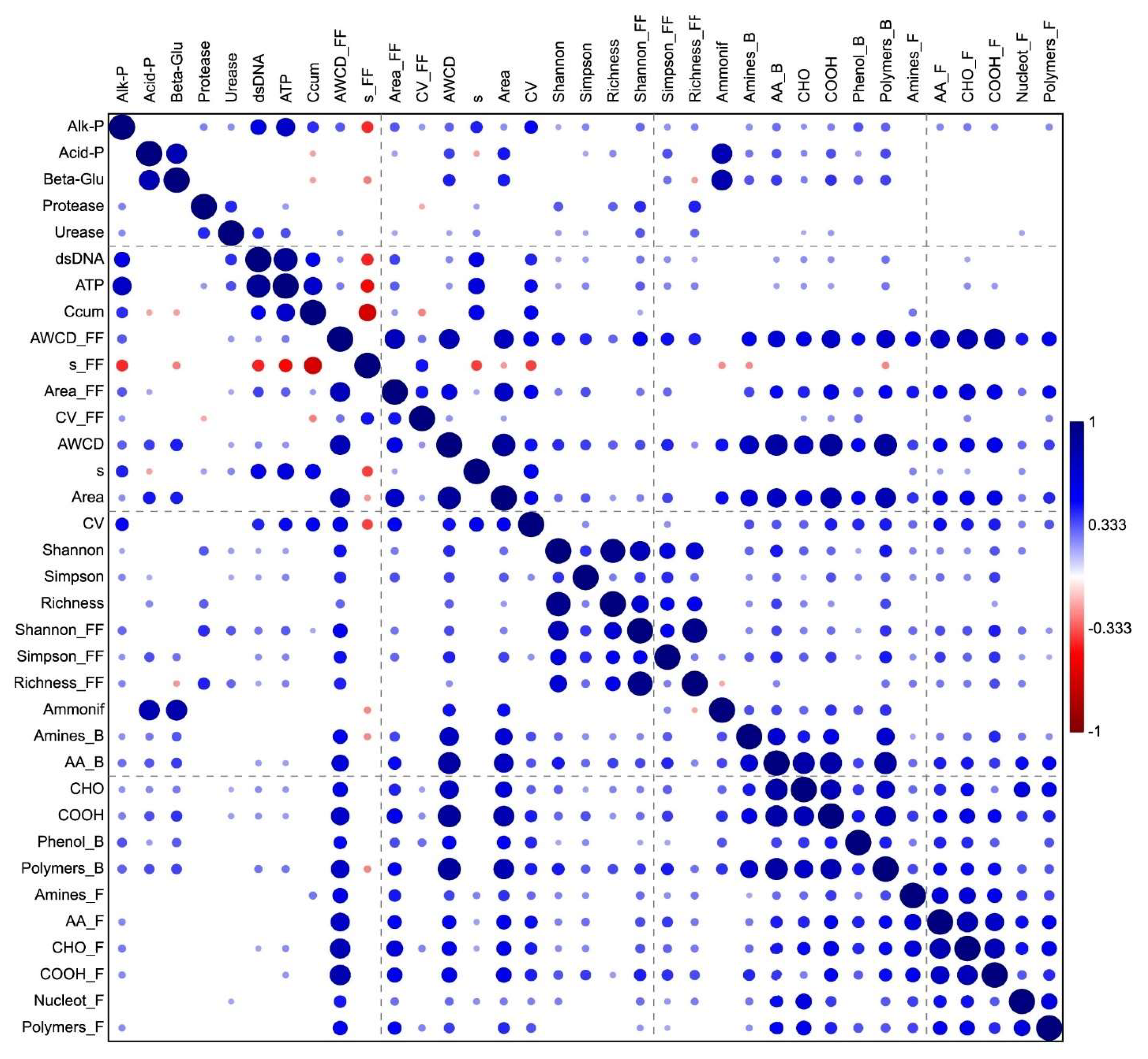
3.5. Linking Enzyme, Biochemical and Metabolic Data
4. Discussion
4.1. Soil Respiration, N Ammonification, ATP and Microbial Biomass
4.2. Soil Enzymatic Activities
4.3. Soil Community-Level Physiological Profile
4.4. Bacterial and Fungal Community Structure
4.5. The Effect of Inocula and Substrates
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Seybold, C.A.; Herrick, J.E.; Brejda, J.J. Soil resilience: A fundamental component of soil quality. Soil Sci. 1999, 164, 224–234. [Google Scholar] [CrossRef]
- Strickland, M.S.; Lauber, C.; Fierer, N.; Bradford, M.A. Testing the functional significance of microbial community composition. Ecology 2009, 90, 441–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mocali, S.; Benedetti, A. Exploring research frontiers in microbiology: The challenge of metagenomics in soil microbiology. Res. Microbiol. 2010, 161, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Lehman, R.M.; Acosta-Martinez, V.; Buyer, J.S.; Cambardella, C.A.; Collins, H.P.; Ducey, T.F.; Halvorson, J.J.; Jin, V.L.; Johnson, J.M.F.; Kremer, R.J.; et al. Soil biology for resilient, healthy soil. J. Soil Water Conserv. 2015, 70, 12A–18A. [Google Scholar] [CrossRef]
- Baas-Becking, L.G.M. Geobiologie of Inleiding tot de Milieukunde; Van Stockkum & Zoon: Hague, The Netherlands, 1934. [Google Scholar]
- Rillig, M.C.; Antonovics, J.; Caruso, T.; Lehmann, A.; Powell, J.R.; Veresoglou, S.D.; Verbruggen, E. Interchange of entire communities: Microbial community coalescence. Trends Ecol. Evol. 2015, 30, 470–476. [Google Scholar] [CrossRef] [Green Version]
- Mony, C.; Vandenkoornhuyse, P.; Bohannan, B.J.; Peay, K.; Leibold, M.A. A Landscape of Opportunities for Microbial Ecology Research. Front. Microbiol. 2020, 11, 2964. [Google Scholar] [CrossRef]
- Meyer, A.F.; Lipson, D.A.; Martin, A.P.; Schadt, C.W.; Schmidt, S.K. Molecular and Metabolic Characterization of Cold-Tolerant Alpine Soil Pseudomonas Sensu Stricto. Appl. Environ. Microbiol. 2004, 70, 483–489. [Google Scholar] [CrossRef] [Green Version]
- Chaparro, J.M.; Sheflin, A.M.; Manter, D.K.; Vivanco, J.M. Manipulating the soil microbiome to increase soil health and plant fertility. Biol. Fertil. Soils 2012, 48, 489–499. [Google Scholar] [CrossRef]
- Fenchel, T.; Finlay, B.J. The Ubiquity of Small Species: Patterns of Local and Global Diversity. BioScience 2004, 54, 777–784. [Google Scholar] [CrossRef]
- Griffiths, B.S.; Philippot, L. Insights into the resistance and resilience of the soil microbial community. FEMS Microbiol. Rev. 2013, 37, 112–129. [Google Scholar] [CrossRef] [Green Version]
- Mocali, S.; Landi, S.; Curto, G.; Dallavalle, E.; Infantino, A.; Colzi, C.; D’Errico, G.; Roversi, P.F.; D’Avino, L.; Lazzeri, L. Resilience of soil microbial and nematode communities after biofumigant treatment with defatted seed meals. Ind. Crops Prod. 2015, 75, 79–90. [Google Scholar] [CrossRef]
- Begonia, M.F.; Kremer, R.J. Chemotaxis of deleterious rhizobacteria to birdsfoot trefoil. Appl. Soil Ecol. 1999, 11, 35–42. [Google Scholar] [CrossRef]
- Postma, J.J.; Hok-A-Hin, C.H.; Voshaar, J.H.O. Influence of the inoculum density on the growth and survival of Rhizobium leguminosarumbiovartrifoliiintroduced into sterile and non-sterile loamy sand and silt loam. FEMS Microbiol. Lett. 1990, 73, 49–57. [Google Scholar] [CrossRef]
- Powlson, D.; Jenkinson, D. The effects of biocidal treatments on metabolism in soil—II. Gamma irradiation, autoclaving, air-drying and fumigation. Soil Biol. Biochem. 1976, 8, 179–188. [Google Scholar] [CrossRef]
- Salonius, P.O.; Robinson, J.B.; Chase, F.E. A comparison of autoclaved and gamma-irradiated soils as media for microbial colonization experiments. Plant Soil 1967, 27, 239–248. [Google Scholar] [CrossRef]
- Al-Achi, B.J.; Platsouka, E.; Levy, S.B. Competitive colonization between Pseudomonas species in sterile soils. Curr. Microbiol. 1991, 23, 97–104. [Google Scholar] [CrossRef]
- Wertz, S.; Czarnes, S.; Bartoli, F.; Renault, P.; Commeaux, C.; Guillaumaud, N.; Clays-Josserand, A. Early-stage bacterial colonization between a sterilized remoulded soil clod and natural soil aggregates of the same soil. Soil Biol. Biochem. 2007, 39, 3127–3137. [Google Scholar] [CrossRef]
- Latour, X.; Philippot, L.; Corberand, T.; Lemanceau, P. The establishment of an introduced community of fluorescent pseudomonads in the soil and in the rhizosphere is affected by the soil type. FEMS Microbiol. Ecol. 1999, 30, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Delmont, T.O.; Francioli, D.; Jacquesson, S.; Laoudi, S.; Mathieu, A.; Nesme, J.; Ceccherini, M.T.; Nannipieri, P.; Simonet, P.; Vogel, T.M. Microbial community development and unseen diversity recovery in inoculated sterile soil. Biol. Fertil. Soils 2014, 50, 1069–1076. [Google Scholar] [CrossRef]
- Don, A.; Böhme, I.H.; Dohrmann, A.B.; Poeplau, C.; Tebbe, C.C. Microbial community composition affects soil organic carbon turnover in mineral soils. Biol. Fertil. Soils 2017, 53, 445–456. [Google Scholar] [CrossRef]
- Francioli, D.; Schulz, E.; Purahong, W.; Buscot, F.; Reitz, T. Reinoculation elucidates mechanisms of bacterial community assembly in soil and reveals undetected microbes. Biol. Fertil. Soils 2016, 52, 1073–1083. [Google Scholar] [CrossRef]
- Kapagianni, P.D.; Papadopoulos, D.; Menkissoglu-Spiroudi, U.; Stamou, G.P.; Papatheodorou, E.M. Soil functionality produced by soil mixing: The role of inoculum and substrate. Ecol. Res. 2019, 34, 600–611. [Google Scholar] [CrossRef]
- Burns, R.G.; DeForest, J.L.; Marxsen, J.; Sinsabaugh, R.L.; Stromberger, M.E.; Wallenstein, M.D.; Weintraub, M.N.; Zoppini, A. Soil enzymes in a changing environment: Current knowledge and future directions. Soil Biol. Biochem. 2013, 58, 216–234. [Google Scholar] [CrossRef]
- Wolf, D.C.; Skipper, H.D. Soil Sterilization. In Methods of Soil Analysis, Part 2: Microbiological and Biochemical Properties; John Wiley & Sons: Hoboken, NJ, USA, 2018; pp. 41–51. [Google Scholar] [CrossRef]
- Brown, K.A. Biochemical activities in peat sterilized by gamma-irradiation. Soil Biol. Biochem. 1981, 13, 469–474. [Google Scholar] [CrossRef]
- Klose, S.; Tabatabai, M. Urease activity of microbial biomass in soils. Soil Biol. Biochem. 1999, 31, 205–211. [Google Scholar] [CrossRef]
- Renella, G.; Landi, L.; Nannipieri, P. Hydrolase activities during and after the chloroform fumigation of soil as affected by protease activity. Soil Biol. Biochem. 2002, 34, 51–60. [Google Scholar] [CrossRef]
- Krauße, T.; Schütze, E.; Phieler, R.; Fürst, D.; Merten, D.; Büchel, G.; Kothe, E. Changes in element availability induced by sterilization in heavy metal contaminated substrates: A comprehensive study. J. Hazard. Mater. 2019, 370, 70–79. [Google Scholar] [CrossRef]
- Wei, G.; Li, M.; Shi, W.; Tian, R.; Chang, C.; Wang, Z.; Wang, N.; Zhao, G.; Gao, Z. Similar drivers but different effects lead to distinct ecological patterns of soil bacterial and archaeal communities. Soil Biol. Biochem. 2020, 144, 107759. [Google Scholar] [CrossRef]
- Blackmer, A.M.; Bremner, J.M. Gas Chromatographic Analysis of Soil Atmospheres. Soil Sci. Soc. Am. J. 1977, 41, 908–912. [Google Scholar] [CrossRef]
- Ciardi, C.; Nannipieri, P. A comparison of methods for measuring ATP in soil. Soil Biol. Biochem. 1990, 22, 725–727. [Google Scholar] [CrossRef]
- Dick, R.P.; Breakwell, D.P.; Turco, R.F. Soil Enzyme Activities and Biodiversity Measurements as Integrative Microbiological Indicators. Methods Assess. Soil Qual. 1997, 49, 247–271. [Google Scholar]
- Garland, J.L.; Mills, A.L. Classification and Characterization of Heterotrophic Microbial Communities on the Basis of Patterns of Community-Level Sole-Carbon-Source Utilization. Appl. Environ. Microbiol. 1991, 57, 2351–2359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guckert, J.B.; Carr, G.J.; Johnson, T.D.; Hamm, B.G.; Davidson, D.H.; Kumagai, Y. Community analysis by Biolog: Curve integration for statistical analysis of activated sludge microbial habitats. J. Microbiol. Methods 1996, 27, 183–197. [Google Scholar] [CrossRef]
- Burkhardt, C.; Insam, H.; Hutchinson, T.C.; Reber, H.H. Impact of heavy metals on the degradative capabilities of soil bacterial communities. Biol. Fertil. Soils 1993, 16, 154–156. [Google Scholar] [CrossRef]
- Lindstrom, J.E.; Barry, R.P.; Braddock, J.F. Microbial community analysis: A kinetic approach to constructing potential C source utilization patterns. Soil Biol. Biochem. 1998, 30, 231–239. [Google Scholar] [CrossRef]
- Marstorp, H.; Witter, E. Extractable dsDNA and product formation as measures of microbial growth in soil upon substrate addition. Soil Biol. Biochem. 1999, 31, 1443–1453. [Google Scholar] [CrossRef]
- Heuer, H.; Krsek, M.; Baker, P.; Smalla, K.; Wellington, E.M. Analysis of actinomycete communities by specific amplification of genes encoding 16S rRNA and gel-electrophoretic separation in denaturing gradients. Appl. Environ. Microbiol. 1997, 63, 3233–3241. [Google Scholar] [CrossRef] [Green Version]
- Vainio, E.J.; Hantula, J. Direct analysis of wood-inhabiting fungi using denaturing gradient gel electrophoresis of amplified ribosomal DNA. Mycol. Res. 2000, 104, 927–936. [Google Scholar] [CrossRef]
- Hammer, Ø.; Ryan, P.D.; Hammer, Ø.; Harper, D.A.T. Past: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Insam, H. A new set of substrates proposed for community characterization in environmental samples. In Microbial Communities: Functional Versus Structural Approaches; Insam, H., Rangger, A., Eds.; Springer: Berlin/Heidelberg, Germany, 1997; pp. 259–260. [Google Scholar]
- Pastorelli, R.; Landi, S.; Trabelsi, D.; Piccolo, R.; Mengoni, A.; Bazzicalupo, M.; Pagliai, M. Effects of soil management on structure and activity of denitrifying bacterial communities. Appl. Soil Ecol. 2011, 49, 46–58. [Google Scholar] [CrossRef]
- De Nobili, M.; Contin, M.; Mondini, C.; Brookes, P. Soil microbial biomass is triggered into activity by trace amounts of substrate. Soil Biol. Biochem. 2001, 33, 1163–1170. [Google Scholar] [CrossRef]
- Blasco, M.L.; Cornfield, A.H. Volatilization of Nitrogen as Ammonia from Acid Soils. Nature 1966, 212, 1279–1280. [Google Scholar] [CrossRef]
- Antisari, L.; Ciavatta, C.; Sequi, P. Volatilization of ammonia during the chloroform fumigation of soil for measuring microbial biomass N. Soil Biol. Biochem. 1990, 22, 225–228. [Google Scholar] [CrossRef]
- Kemmitt, S.J.; Lanyon, C.V.; Waite, I.S.; Wen, Q.; Addiscott, T.M.; Bird, N.R.; O’Donnell, A.G.; Brookes, P.C. Mineralization of native soil organic matter is not regulated by the size, activity or composition of the soil microbial biomass—A new perspective. Soil Biol. Biochem. 2008, 40, 61–73. [Google Scholar] [CrossRef]
- Bååth, E. Adaptation of soil bacterial communities to prevailing pH in different soils. FEMS Microbiol. Ecol. 1996, 19, 227–237. [Google Scholar] [CrossRef]
- Jenkinson, D.; Powlson, D. The effects of biocidal treatments on metabolism in soil—V: A method for measuring soil biomass. Soil Biol. Biochem. 1976, 8, 209–213. [Google Scholar] [CrossRef]
- Renella, G.; Landi, L.; Ascher, J.; Ceccherini, M.T.; Pietramellara, G.; Nannipieri, P. Phosphomonoesterase production and persistence and composition of bacterial communities during plant material decomposition in soils with different pH values. Soil Biol. Biochem. 2006, 38, 795–802. [Google Scholar] [CrossRef]
- Knight, T.R.; Dick, R.P. Differentiating microbial and stabilized β-glucosidase activity relative to soil quality. Soil Biol. Biochem. 2004, 36, 2089–2096. [Google Scholar] [CrossRef]
- Allison, S.D.; Vitousek, P.M. Responses of extracellular enzymes to simple and complex nutrient inputs. Soil Biol. Biochem. 2005, 37, 937–944. [Google Scholar] [CrossRef]
- Renella, G.; Szukics, U.; Landi, L.; Nannipieri, P. Quantitative assessment of hydrolase production and persistence in soil. Biol. Fertil. Soils 2007, 44, 321–329. [Google Scholar] [CrossRef]
- Stark, S.; Männistö, M.K.; Eskelinen, A. Nutrient availability and pH jointly constrain microbial extracellular enzyme activities in nutrient-poor tundra soils. Plant Soil 2014, 383, 373–385. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Lauber, C.L.; Weintraub, M.N.; Ahmed, B.; Allison, S.D.; Crenshaw, C.; Contosta, A.R.; Frey, S.; Gallo, M.E.; Gartner, T.B.; et al. Stoichiometry of soil enzyme activity at global scale. Ecol. Lett. 2008, 11, 1252–1264. [Google Scholar] [CrossRef] [PubMed]
- Jenneman, G.E.; McInerney, M.J.; Crocker, M.E.; Knapp, R.M. Effect of Sterilization by Dry Heat or Autoclaving on Bacterial Penetration through Berea Sandstone. Appl. Environ. Microbiol. 1986, 51, 39–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pettersson, M.; Bååth, E. Effects of the properties of the bacterial community on pH adaptation during recolonisation of a humus soil. Soil Biol. Biochem. 2004, 36, 1383–1388. [Google Scholar] [CrossRef]
- Demoling, F.; Figueroa, D.; Bååth, E. Comparison of factors limiting bacterial growth in different soils. Soil Biol. Biochem. 2007, 39, 2485–2495. [Google Scholar] [CrossRef]
- Crowley, D.E.; Alvey, S.A. Regulation of Microbial Processes by Soil pH. In Handbook of Plant Growth pH as the Master Variable; CRC Press: Boca Raton, FL, USA, 2002; pp. 343–370. [Google Scholar] [CrossRef]
- Rousk, J.; Brookes, P.C.; Bååth, E. Contrasting Soil pH Effects on Fungal and Bacterial Growth Suggest Functional Redundancy in Carbon Mineralization. Appl. Environ. Microbiol. 2009, 75, 1589–1596. [Google Scholar] [CrossRef] [Green Version]
- Rousk, J.; Bååth, E.; Brookes, P.C.; Lauber, C.L.; Lozupone, C.; Caporaso, J.G.; Knight, R.; Fierer, N. Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J. 2010, 4, 1340–1351. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, Y.T.; Matsumoto, N. DNA- versus RNA-based denaturing gradient gel electrophoresis profiles of a bacterial community during replenishment after soil fumigation. Soil Biol. Biochem. 2007, 39, 434–444. [Google Scholar] [CrossRef]
- Preston-Mafham, J.; Boddy, L.; Randerson, P.F. Analysis of microbial community functional diversity using sole-carbon-source utilisation profiles a critique. FEMS Microbiol. Ecol. 2002, 42, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Pinzari, F.; Ceci, A.; Abu-Samra, N.; Canfora, L.; Maggi, O.; Persiani, A. Phenotype MicroArray™ system in the study of fungal functional diversity and catabolic versatility. Res. Microbiol. 2016, 167, 710–722. [Google Scholar] [CrossRef]
- Oszust, K.; Frąc, M. First report on the microbial communities of the wild and planted raspberry rhizosphere A statement on the taxa, processes and a new indicator of functional diversity. Ecol. Indic. 2020, 121, 107117. [Google Scholar] [CrossRef]
- Zak, J.; Willig, M.; Moorhead, D.; Wildman, H. Functional diversity of microbial communities: A quantitative approach. Soil Biol. Biochem. 1994, 26, 1101–1108. [Google Scholar] [CrossRef]
- Dobranic, J.K.; Zak, J.C. A microtiter plate procedure for evaluating fungal functional diversity. Mycologia 1999, 91, 756–765. [Google Scholar] [CrossRef]
- Nunan, N.; Leloup, J.; Ruamps, L.S.; Pouteau, V.; Chenu, C. Effects of habitat constraints on soil microbial community function. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Borowik, A.; Wyszkowska, J.; Oszust, K. Functional Diversity of Fungal Communities in Soil Contaminated with Diesel Oil. Front. Microbiol. 2017, 8, 1862. [Google Scholar] [CrossRef]
- Frąc, M.; Oszust, K.; Lipiec, J.; Jezierska-Tys, S.; Nwaichi, E.O. Soil Microbial Functional and Fungal Diversity as Influenced by Municipal Sewage Sludge Accumulation. Int. J. Environ. Res. Public Heal. 2014, 11, 8891–8908. [Google Scholar] [CrossRef]
- Xun, F.; Xie, B.; Liu, S.; Guo, C. Effect of plant growth-promoting bacteria (PGPR) and arbuscular mycorrhizal fungi (AMF) inoculation on oats in saline-alkali soil contaminated by petroleum to enhance phytoremediation. Environ. Sci. Pollut. Res. 2014, 22, 598–608. [Google Scholar] [CrossRef]
- Siciliano, S.D.; Palmer, A.S.; Winsley, T.; Lamb, E.; Bissett, A.; Brown, M.V.; van Dorst, J.; Ji, M.; Ferrari, B.C.; Grogan, P.; et al. Soil fertility is associated with fungal and bacterial richness, whereas pH is associated with community composition in polar soil microbial communities. Soil Biol. Biochem. 2014, 78, 10–20. [Google Scholar] [CrossRef]
- Hayano, K.; Tubaki, K. Origin and properties of β-glucosidase activity of tomato-field soil. Soil Biol. Biochem. 1985, 17, 553–557. [Google Scholar] [CrossRef]
- Hu, Z.; Xu, C.; McDowell, N.G.; Johnson, D.; Wang, M.; Luo, Y.; Zhou, X.; Huang, Z. Linking microbial community composition to C loss rates during wood decomposition. Soil Biol. Biochem. 2017, 104, 108–116. [Google Scholar] [CrossRef] [Green Version]
- Fierer, N.; Jackson, R.B. The diversity and biogeography of soil bacterial communities. Proc. Natl. Acad. Sci. USA 2006, 103, 626–631. [Google Scholar] [CrossRef] [Green Version]
- Gelsomino, A.; Azzellino, A. Multivariate analysis of soils: Microbial biomass, metabolic activity, and bacterial-community structure and their relationships with soil depth and type. J. Plant Nutr. Soil. Sc. 2011, 174, 381–394. [Google Scholar] [CrossRef]
- Gelsomino, A.; Petrovičová, B.; Vecchio, G.; Laudicina, V.A.; Badalucco, L. Chemical, biochemical and microbial diversity through a Pachic Humudept profile in a temperate upland grassland. Agrochimica 2013, 57, 214–232. [Google Scholar]
- Felske, A.; Rheims, H.; Wolterink, A.; Stackebrandt, E.; Akkermans, A.D.L. Ribosome analysis reveals prominent activity of an uncultured member of the class Actinobacteria in grassland soils. Microbiology 1997, 143, 2983–2989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elshahed, M.S.; Youssef, N.H.; Spain, A.M.; Sheik, C.; Najar, F.Z.; Sukharnikov, L.O.; Roe, B.A.; Davis, J.P.; Schloss, P.D.; Bailey, V.L.; et al. Novelty and Uniqueness Patterns of Rare Members of the Soil Biosphere. Appl. Environ. Microbiol. 2008, 74, 5422–5428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Epstein, S.S. Microbial awakenings. Nature 2009, 457, 1083. [Google Scholar] [CrossRef]
- Lazzaro, L.; Giuliani, C.; Fabiani, A.; Agnelli, A.E.; Pastorelli, R.; Lagomarsino, A.; Benesperi, R.; Calamassi, R.; Foggi, B. Soil and plant changing after invasion: The case of Acacia dealbata in a Mediterranean ecosystem. Sci. Total Environ. 2014, 497–498, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Shade, A.; Hogan, C.S.; Klimowicz, A.K.; Linske, M.; McManus, P.S.; Handelsman, J. Culturing captures members of the soil rare biosphere. Environ. Microbiol. 2012, 14, 2247–2252. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, X.; Dungait, J.A.J.; Quine, T.A.; Razavi, B.S. Rare microbial taxa rather than phoD gene abundance determine hotspots of alkaline phosphomonoesterase activity in the karst rhizosphere soil. Biol. Fertil. Soils 2020, 57, 257–268. [Google Scholar] [CrossRef]
- Meola, M.; Lazzaro, A.; Zeyer, J. Diversity, resistance and resilience of the bacterial communities at two alpine glacier forefields after a reciprocal soil transplantation. Environ. Microbiol. 2014, 16, 1918–1934. [Google Scholar] [CrossRef]
- de Boer, W.; Folman, L.B.; Summerbell, R.C.; Boddy, L. Living in a fungal world: Impact of fungi on soil bacterial niche development. FEMS Microbiol. Rev. 2005, 29, 795–811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Q.-L.; Ding, J.; Zhu, D.; Hu, H.-W.; Delgado-Baquerizo, M.; Ma, Y.-B.; He, J.-Z.; Zhu, Y.-G. Rare microbial taxa as the major drivers of ecosystem multifunctionality in long-term fertilized soils. Soil Biol. Biochem. 2019, 141, 107686. [Google Scholar] [CrossRef]

| Soil | Sand | Silt | Clay | pH | TOC | TN | CEC | TCa | ACa | NH4+-N | NO3−-N | TOP | Olsen-P |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | g kg−1 | cmolc kg−1 | g kg−1 | mg kg−1 | |||||||||
| Val | 48.9 | 33.0 | 18.1 | 5.0 ± 0.2 | 36.6 ± 1.5 | 2.2 ± 0.3 | 26.6 ± 0.8 | 0 | 0 | 29.8 ± 3.6 | 25.7 ± 5.1 | 34.1 ± 2.8 | 7.4 ± 2.9 |
| Rom | 90.7 | 3.6 | 5.7 | 6.7 ± 0.1 | 10.5 ± 0.3 | 0.98 ± 0.2 | 16.9 ± 0.5 | 0 | 0 | 13.0 ± 2.5 | 22.3 ± 1.4 | 20.9 ± 1.9 | 8.7 ± 3.3 |
| Vic | 20.5 | 37.3 | 42.2 | 8.0 ± 0.1 | 22.9 ± 0.3 | 2.2 ± 0.2 | 25.3 ± 0.7 | 128 ± 5 | 83 ± 3 | 21.7 ± 1.6 | 14.7 ± 2.0 | 22.0 ± 1.8 | 6.2 ± 0.3 |
| Enzymatic Activity | Time (days) | Soil Treatments | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vallombrosa | Romola | Vicarello | ||||||||||||||
| Val | Val* | Val*+ Val | Val*+ Rom | Val*+ Vic | Rom | Rom* | Rom*+ Rom | Rom*+ Val | Rom*+ Vic | Vic | Vic* | Vic*+ Vic | Vic*+ Val | Vic*+ Rom | ||
| Alkaline phosphomonoesterase (mg p-NP a kg−1 h−1) | 1 | 3849 a | BDL | 668 b | BDL | 541 b | 7031 a | BDL | 89 b | 1110 c | 334 d | 17,501 a | BDL | 2323 b | BDL | BDL |
| 7 | 3963 a | BDL | 1845 b | 1570 b | 10,589 c | 6704 a | 2113 b | 7248 a | 2921 b | 15,169 c | 18,019 a | BDL | 17,199 a | 14,625 a | 17,082 a | |
| 30 | 3977 a | 9228 b | 8790 b | 6803 c | 11,597 d | 6304 a | 6645 a | 7624 a | 8811 a | 11,059 b | 17,452 a | 10,050 b | 11,201 b | 14,945 c | 6127 d | |
| Acid phosphomonoesterase (mg p-NP a kg−1 h−1) | 1 | 22,732 a | BDL | 2093 b | BDL | BDL | 2092 a | BDL | 90 b | 763 c | 700 c | 5396 a | BDL | 1016 b | 1848 c | BDL |
| 7 | 22,274 a | 9282 b | 37,036 c | 42,979 c | 35,455 c | 2351 a | BDL | 678 b | 2396 a | 2251 a | 5277 a | BDL | 2174 b | 2260 b | 2446 b | |
| 30 | 21,833 a | 21,930 a | 25,507 b | 23,080 ab | 18,315 c | 2238 a | 774 b | 844 b | 1774 a | 1720 a | 5287 ab | 3314 c | 4915 a | 6494 b | 2979 c | |
| β-Glucosidase (mg p-NP a kg−1 h−1) | 1 | 2492 a | BDL | BDL | BDL | BDL | 1641 a | BDL | 15 b | 247 c | 1004 d | 5853 a | BDL | 1116 b | BDL | BDL |
| 7 | 2588 a | 4054 a | 19,568 b | 17,899 b | 8133 c | 1583 a | 1574 a | 1330 a | 991 b | 1768 a | 6448 a | 2805 b | 4136 c | 4246 c | 4123 c | |
| 30 | 2845 a | 17,584 b | 18,044 b | 23,453 c | 11,293 d | 1531 a | 1583 a | 1090 b | 878 b | 1697 a | 4946 a | 776 b | 960 b | 441 b | 355 c | |
| Protease (mg NH4+-N kg−1 h−1) | 1 | 124.2 a | BDL | 19.0 b | 30.1 c | 14.6 b | 117.0 a | BDL | 19.6 b | 29.5 c | 10.6 d | 106.4 a | BDL | BDL | BDL | BDL |
| 7 | 101.5 a | BDL | 16.9 b | 17.7 b | 25.5 c | 121.8 a | BDL | 23.6 b | 21.0 b | 26.6 b | 100.9 a | BDL | BDL | BDL | BDL | |
| 30 | 108.8 a | 26.5 b | 16.3 c | 12.2 c | 17.0 d | 110.7 a | 3.7 b | 18.9 c | 26.0 d | 10.2 e | 91.1 a | 22.5 b | 44.1 c | 28.4 b | 40.5 c | |
| Urease (mg NH4+-N kg−1 h−1) | 1 | 82.9 a | BDL | 11.7 b | 7.7 b | 10.8 b | 101.0 a | BDL | 14.7 b | 3.6 c | 12.9 b | 7.4 a | BDL | BDL | BDL | BDL |
| 7 | 78.4 a | 6.3 b | 22.8 c | 5.9 b | 34.8 d | 96.0 a | BDL | 14.7 b | 2.0 c | 12.8 b | 7.4 a | BDL | 13.3 a | 124.4 b | 64.7 c | |
| 30 | 69.4 a | 14.7 b | 24.3 c | 19.7 bc | 38.9 d | 87.9 a | 3.2 b | 13.9 c | 4.3 b | 14.7 c | 7.5 a | 43.2 b | 8.2 a | 137.8 c | 49.2 b | |
| Substrate Category | Time (days) | Soil Treatments | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vallombrosa | Romola | Vicarello | ||||||||||||||
| Val | Val* | Val*+Val | Val*+Rom | Val*+Vic | Rom | Rom* | Rom*+Rom | Rom*+Val | Rom*+Vic | Vic | Vic* | Vic*+Vic | Vic*+Val | Vic*+Rom | ||
| Amines | 1 | 1.790 a | 0.000 b | 1.613 a | 1.720 a | 1.148 ab | 1.116 a | 0.018 b | 1.217 a | 1.167 a | 1.510 a | 0.926 a | 0.000 b | 0.942 a | 0.860 a | 0.902 a |
| 7 | 1.215 a | 1.521 a | 2.028 b | 1.601 a | 1.585 a | 1.233 a | 1.675 b | 1.560 b | 1.492 b | 1.564 b | 1.438 a | 1.347 a | 1.662 a | 1.756 a | 1.317 a | |
| 30 | 1.600 a | 2.056 b | 1.757 a | 1.915 ab | 1.559 a | 1.277 a | 1.092 a | 1.376 a | 1.195 a | 1.648 b | 1.527 a | 1.626 a | 1.365 a | 1.789 a | 1.560 a | |
| Amino acids | 1 | 2.030 a | 0.000 b | 1.835 a | 1.872 a | 1.499 a | 1.819 a | 0.055 b | 1.614 a | 1.716 a | 1.847 a | 1.362 a | 0.024 b | 1.168 a | 1.382 a | 1.421 a |
| 7 | 1.297 a | 2.035 ab | 2.019 ab | 2.226 b | 1.815 ab | 1.601 a | 1.743 a | 1.931 a | 1.572 a | 1.909 a | 1.847 a | 1.546 a | 2.189 a | 1.850 a | 1.670 a | |
| 30 | 1.553 a | 1.963 ab | 2.146 b | 2.147 b | 2.006 ab | 1.616 a | 1.483 a | 1.530 a | 1.542 a | 1.684 a | 1.790 a | 1.638 a | 1.745 a | 1.751 a | 1.748 a | |
| Carbohydrates | 1 | 1.630 a | 0.005 b | 1.566 a | 1.690 a | 1.266 a | 1.445 a | 0.044 b | 1.203 a | 1.324 a | 1.546 a | 1.016 a | 0.008 b | 0.883 a | 1.007 a | 1.071 a |
| 7 | 1.519 a | 1.950 a | 1.827 a | 1.923 a | 1.396 a | 1.357 a | 1.619 a | 1.837 b | 1.727 ab | 1.675 a | 1.313 a | 1.325 a | 1.986 a | 1.644 a | 1.354 a | |
| 30 | 1.330 a | 1.830 b | 1.534 b | 1.801 b | 1.726 b | 1.346 a | 1.215 a | 1.542 a | 1.438 a | 1.671 b | 1.463 a | 1.559 a | 1.526 a | 1.641 a | 1.782 a | |
| Carboxylic acids | 1 | 1.441 a | 0.000 b | 1.512 a | 1.513 a | 1.079 a | 1.469 a | 0.037 b | 1.146 a | 1.308 a | 1.413 a | 0.757 a | 0.018 b | 0.748 a | 0.992 a | 0.913 a |
| 7 | 0.945 a | 1.852 b | 1.876 b | 2.003 b | 1.510 a | 1.331 a | 1.620 a | 1.665 a | 1.728 a | 1.687 a | 1.254 a | 1.093 a | 1.880 b | 1.577 a | 1.325 a | |
| 30 | 1.124 a | 1.844 b | 1.344 a | 1.904 b | 1.377 a | 1.138 a | 0.702 a | 1.276 a | 1.275 a | 1.557 b | 1.337 a | 1.268 a | 1.335 a | 1.588 a | 1.585 a | |
| Phenolic compounds | 1 | 0.896 a | 0.006 b | 0.847 a | 0.901 a | 0.678 a | 0.957 a | 0.000 b | 0.637 a | 0.797 a | 0.757 a | 0.820 a | 0.063 b | 1.173 a | 0.510 a | 0.888 a |
| 7 | 0.313 a | 1.142 b | 0.995 b | 1.077 b | 1.864 c | 0.658 a | 1.676 b | 1.421 b | 1.576 b | 1.917 b | 0.952 a | 0.593 a | 0.881 a | 1.470 a | 1.451 a | |
| 30 | 0.886 a | 1.605 b | 1.057 a | 2.184 c | 1.836 b | 0.922 a | 0.761 a | 0.966 a | 0.881 a | 1.683 b | 1.432 a | 0.799 b | 1.408 a | 1.171 a | 0.985 a | |
| Polymers | 1 | 2.182 a | 0.015 b | 1.929 a | 1.878 a | 1.621 c | 1.653 a | 0.016 b | 1.632 a | 1.643 a | 1.845 a | 1.385 a | 0.040 b | 1.378 a | 1.516 a | 1.394 a |
| 7 | 1.522 a | 2.267 b | 2.206 b | 2.240 b | 1.848 ab | 1.295 a | 1.848 ab | 1.868 ab | 1.451 a | 2.002 b | 1.806 a | 1.595 a | 2.243 b | 1.985 a | 1.818 a | |
| 30 | 1.550 a | 2.005 b | 2.042 b | 2.133 b | 2.227 b | 1.310 a | 1.107 a | 1.348 a | 1.322 a | 1.933 b | 2.033 a | 1.880 a | 2.086 a | 1.908 a | 2.069 a | |
| Substrate Category | Time (days) | Soil Treatments | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vallombrosa | Romola | Vicarello | ||||||||||||||
| Val | Val* | Val*+Val | Val*+Rom | Val*+Vic | Rom | Rom* | Rom*+Rom | Rom*+Val | Rom*+Vic | Vic | Vic* | Vic*+Vic | Vic*+Val | Vic*+Rom | ||
| Amines | 1 | 1.104 a | 0.025 b | 1.493 a | 1.806 c | 1.064 a | 1.116 a | 0.340 b | 1.388 a | 0.563 ab | 1.614 c | 1.314 a | 0.001 b | 1.074 a | 1.410 a | 1.132 a |
| 7 | 1.841 a | 1.301 a | 1.409 a | 1.347 a | 1.352 a | 1.111 a | 2.013 b | 1.591 a | 1.323 a | 1.197 a | 1.632 a | 1.396 a | 1.286 a | 1.381 a | 1.323 a | |
| 30 | 0.486 a | 0.869 a | 0.593 a | 1.201 b | 1.592 c | 1.466 a | 0.177 b | 1.243 a | 0.769 a | 0.670 a | 1.366 a | 1.683 a | 1.374 a | 1.677 a | 1.762 a | |
| Amino acids | 1 | 0.894 a | 0.006 b | 1.018 a | 1.329 c | 1.154 a | 1.081 a | 0.322 b | 1.001 a | 1.064 a | 1.200 a | 1.233 a | 0.000 b | 0.995 a | 0.885 a | 1.025 a |
| 7 | 0.987 a | 0.994 a | 1.146 a | 1.332 a | 1.187 a | 1.230 a | 1.293 a | 1.361 a | 1.135 a | 1.393 a | 1.198 a | 1.138 a | 0.974 a | 1.214 a | 1.181 a | |
| 30 | 0.657 a | 1.058 a | 0.996 a | 1.164 a | 1.257 b | 1.035 a | 0.294 b | 1.157 a | 0.729 a | 1.236 a | 0.911 a | 1.364 a | 1.263 a | 1.306 a | 1.307 a | |
| Carbohydrates | 1 | 0.900 a | 0.032 a | 0.902 a | 1.181 a | 0.971 a | 0.819 a | 0.235 a | 0.931 a | 0.869 a | 1.172 a | 1.105 a | 0.002 b | 0.927 a | 0.797 a | 0.892 a |
| 7 | 0.853 a | 0.974 a | 0.986 a | 1.327 b | 1.167 a | 1.243 a | 1.417 a | 1.317 a | 1.222 a | 1.371 a | 1.158 a | 1.175 a | 1.155 a | 1.177 a | 1.180 a | |
| 30 | 0.515 a | 0.993 ab | 0.796 a | 1.075 ab | 1.174 b | 0.930 a | 0.165 b | 1.204 a | 1.069 a | 1.273 a | 0.750 a | 1.249 a | 1.168 a | 1.061 a | 1.077 a | |
| Carboxylic acids | 1 | 1.220 a | 0.027 b | 1.200 a | 1.357 a | 1.061 a | 0.851 a | 0.353 b | 1.190 a | 1.083 a | 1.362 b | 1.183 a | 0.000 b | 1.100 a | 1.000 a | 0.996 a |
| 7 | 1.011 a | 1.051 a | 1.132 a | 1.390 a | 1.283 a | 1.284 a | 1.503 a | 1.378 a | 1.336 a | 1.376 a | 1.255 a | 1.131 a | 1.138 a | 1.180 a | 1.214 a | |
| 30 | 0.797 a | 1.001 a | 1.049 a | 1.136 ab | 1.232 b | 1.086 a | 0.251 b | 1.327 a | 1.258 a | 1.532 b | 1.092 a | 1.364 a | 1.260 a | 1.382 a | 1.366 a | |
| Nucleotides | 1 | 1.246 a | 0.024 b | 1.231 a | 1.580 c | 1.080 a | 1.242 a | 0.425 b | 1.045 a | 1.165 a | 1.178 a | 0.824 a | 0.000 b | 1.129 a | 0.992 a | 1.178 a |
| 7 | 0.761 a | 0.751 a | 0.851 a | 0.995 a | 1.041 a | 1.468 a | 1.577 a | 1.448 a | 1.408 a | 1.446 a | 1.428 a | 0.977 a | 1.215 a | 1.182 a | 1.160 a | |
| 30 | 0.802 a | 0.927 a | 1.000 a | 0.933 a | 0.949 a | 1.081 a | 0.586 a | 1.468 a | 0.596 a | 0.812 a | 0.900 a | 1.389 a | 1.308 a | 1.542 a | 1.561 a | |
| Polymers | 1 | 0.900 a | 0.007 b | 0.765 a | 1.233 c | 0.681 a | 0.558 a | 0.119 a | 0.734 a | 0.452 a | 1.076 a | 0.778 a | 0.000 b | 0.563 a | 0.599 a | 0.724 a |
| 7 | 0.652 a | 0.708 a | 0.740 a | 1.357 b | 1.061 a | 1.005 a | 1.313 a | 1.208 a | 1.042 a | 1.424 a | 0.920 a | 1.065 a | 1.131 a | 1.105 a | 1.166 a | |
| 30 | 0.178 a | 0.678 a | 0.459 a | 0.992 a | 1.133 b | 0.714 a | 0.137 b | 1.050 a | 0.835 a | 1.271 a | 0.488 a | 0.831 a | 0.863 a | 0.917 a | 0.923 a | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mocali, S.; Gelsomino, A.; Nannipieri, P.; Pastorelli, R.; Giagnoni, L.; Petrovicova, B.; Renella, G. Short-Term Resilience of Soil Microbial Communities and Functions Following Severe Environmental Changes. Agriculture 2022, 12, 268. https://doi.org/10.3390/agriculture12020268
Mocali S, Gelsomino A, Nannipieri P, Pastorelli R, Giagnoni L, Petrovicova B, Renella G. Short-Term Resilience of Soil Microbial Communities and Functions Following Severe Environmental Changes. Agriculture. 2022; 12(2):268. https://doi.org/10.3390/agriculture12020268
Chicago/Turabian StyleMocali, Stefano, Antonio Gelsomino, Paolo Nannipieri, Roberta Pastorelli, Laura Giagnoni, Beatrix Petrovicova, and Giancarlo Renella. 2022. "Short-Term Resilience of Soil Microbial Communities and Functions Following Severe Environmental Changes" Agriculture 12, no. 2: 268. https://doi.org/10.3390/agriculture12020268
APA StyleMocali, S., Gelsomino, A., Nannipieri, P., Pastorelli, R., Giagnoni, L., Petrovicova, B., & Renella, G. (2022). Short-Term Resilience of Soil Microbial Communities and Functions Following Severe Environmental Changes. Agriculture, 12(2), 268. https://doi.org/10.3390/agriculture12020268









