Seasonal Changes in Phosphorus in Soils and Vegetation of Vegetated Filter Strips in Cold Climate Agricultural Systems
Abstract
:1. Introduction
2. Materials and Methods
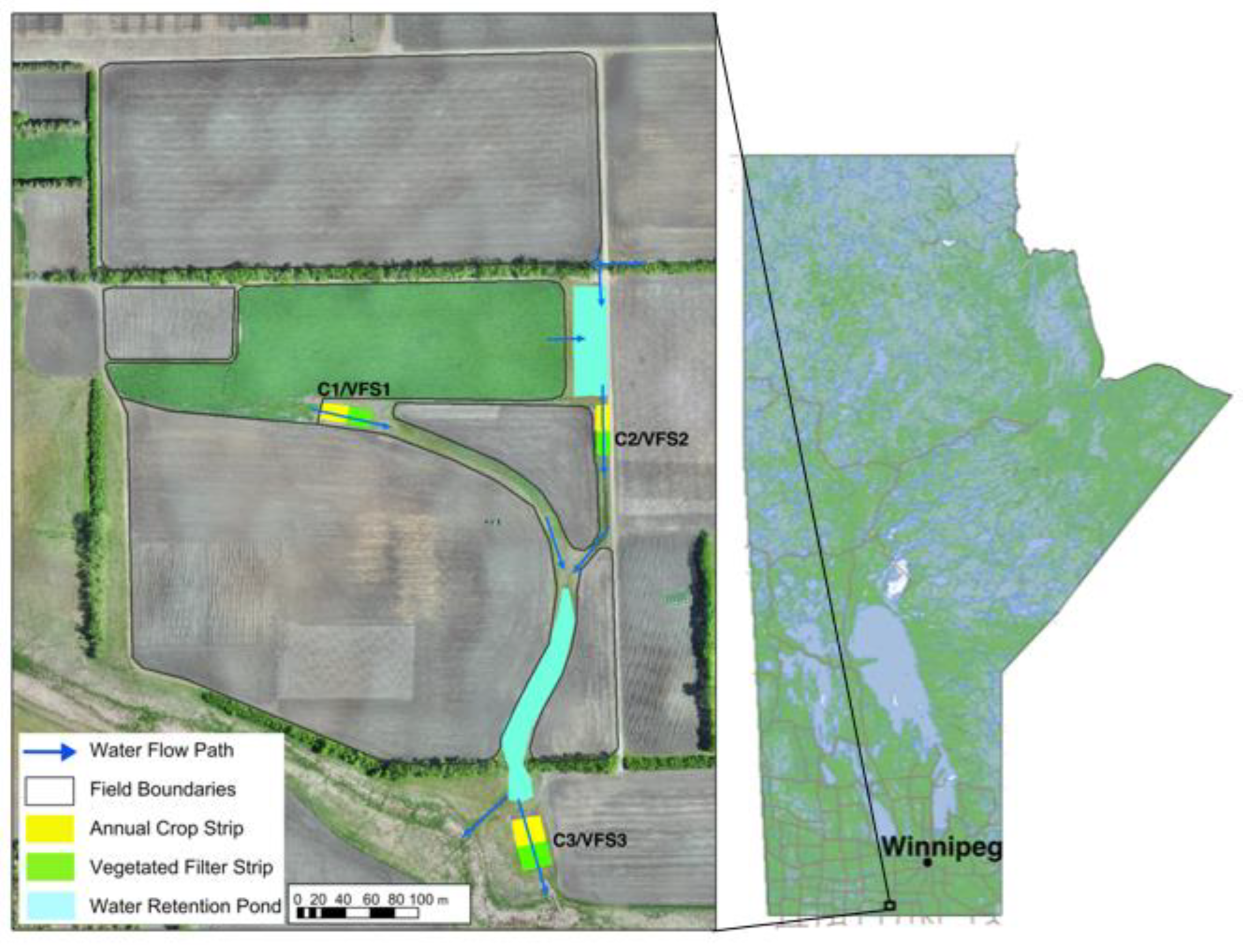
2.1. Study Location
2.2. Climate Conditions
2.3. Soil Sampling
2.4. Vegetation Sampling
2.5. Soil Analysis
2.6. Vegetation Analysis
2.7. Statistical Analysis
3. Results
3.1. Soil Phosphorus
3.1.1. Soluble Phosphorus in Soils
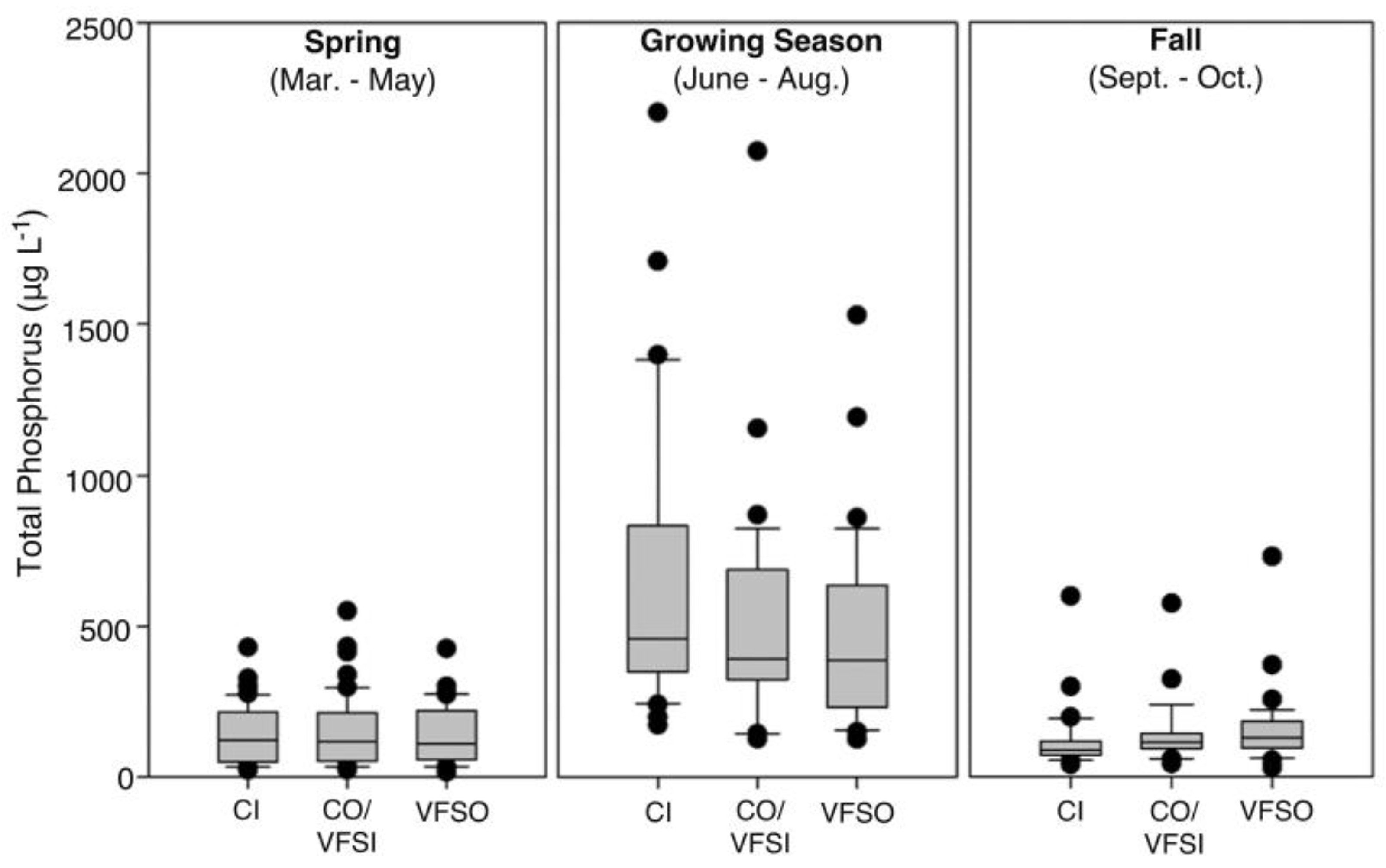
3.1.2. Total Phosphorus in Soils
3.2. Vegetation Phosphorus
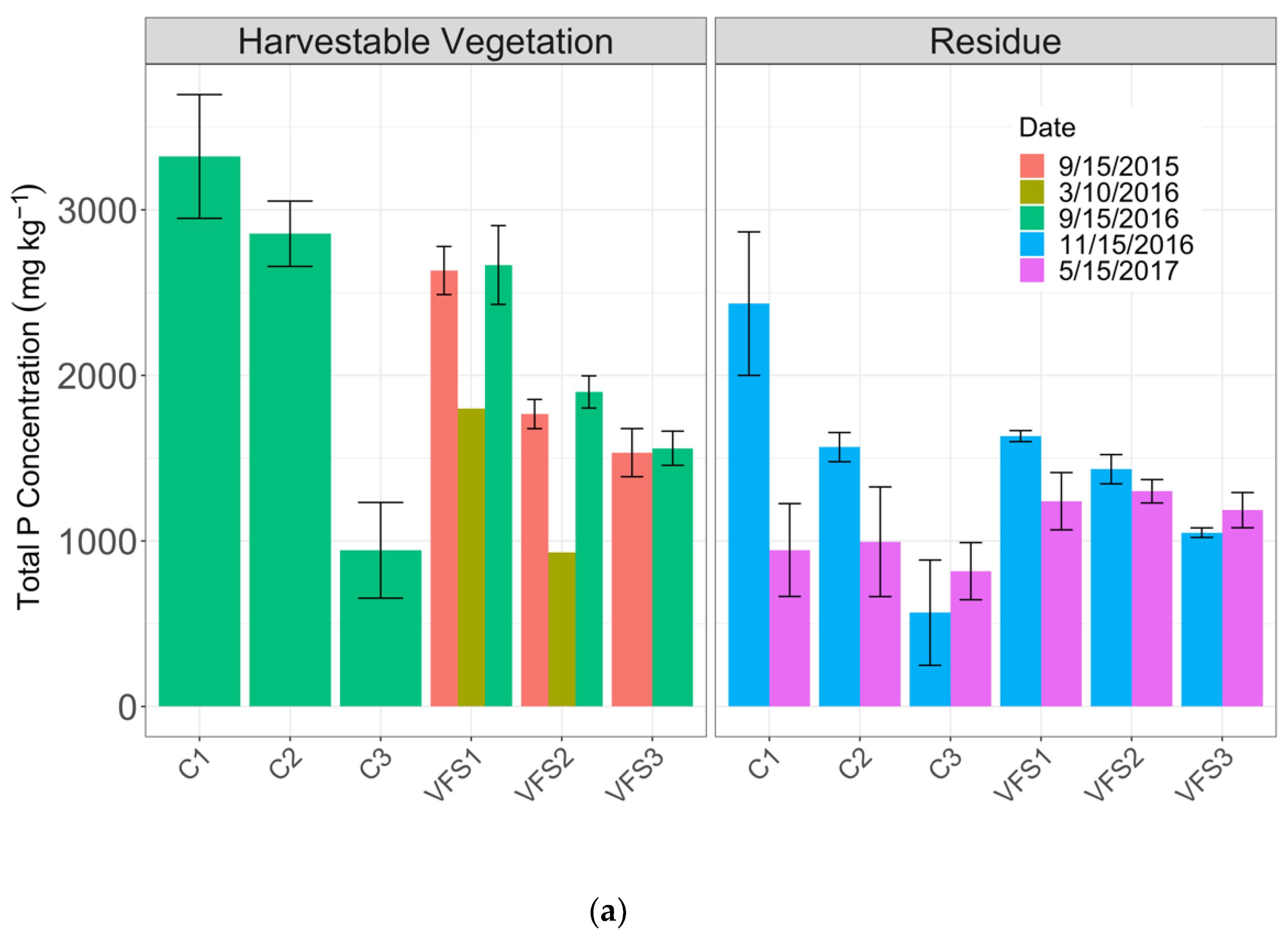
3.2.1. Total Phosphorus Content in Vegetation and Residue
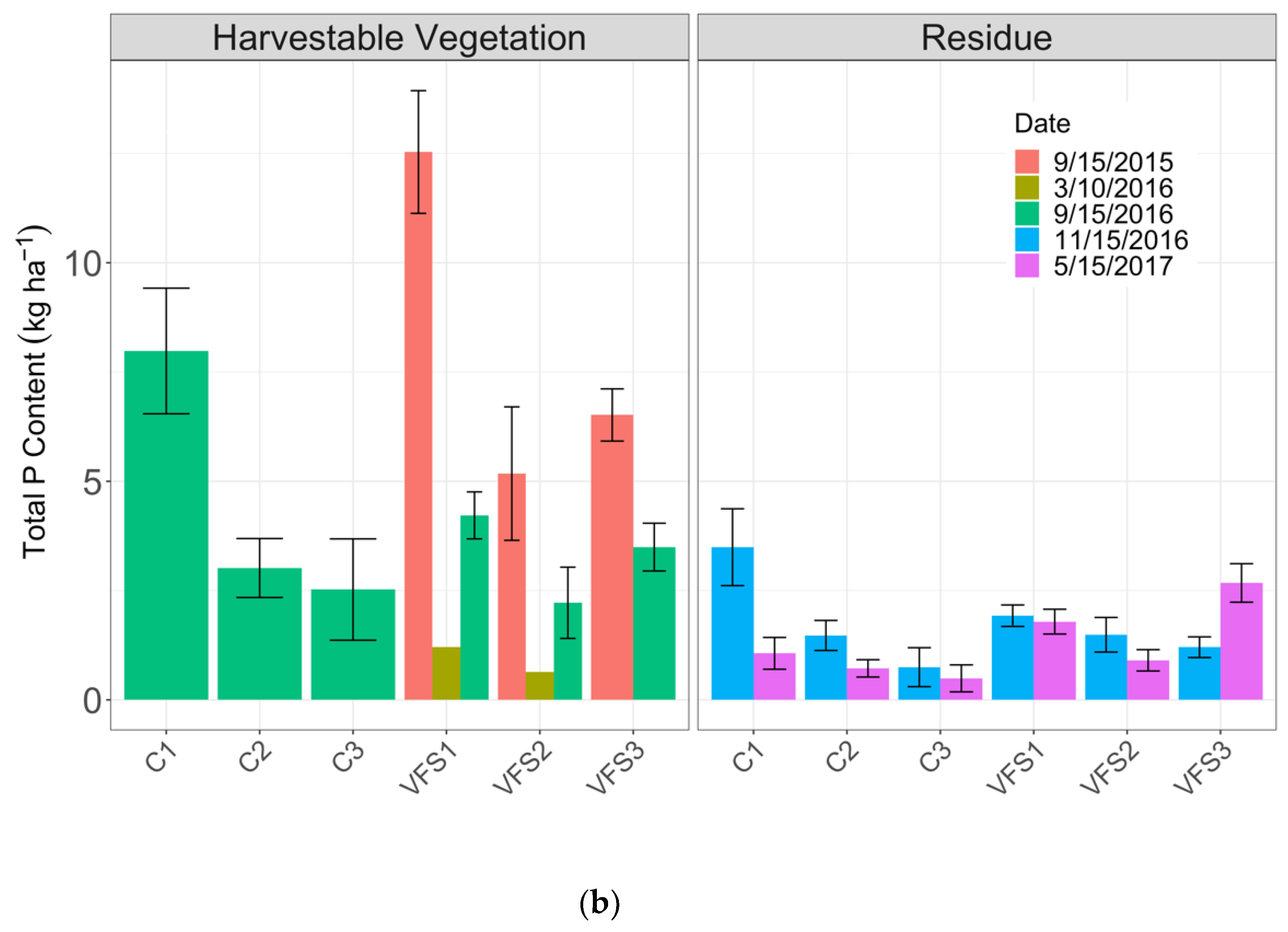
3.2.2. Total Phosphorus Concentrations by Area
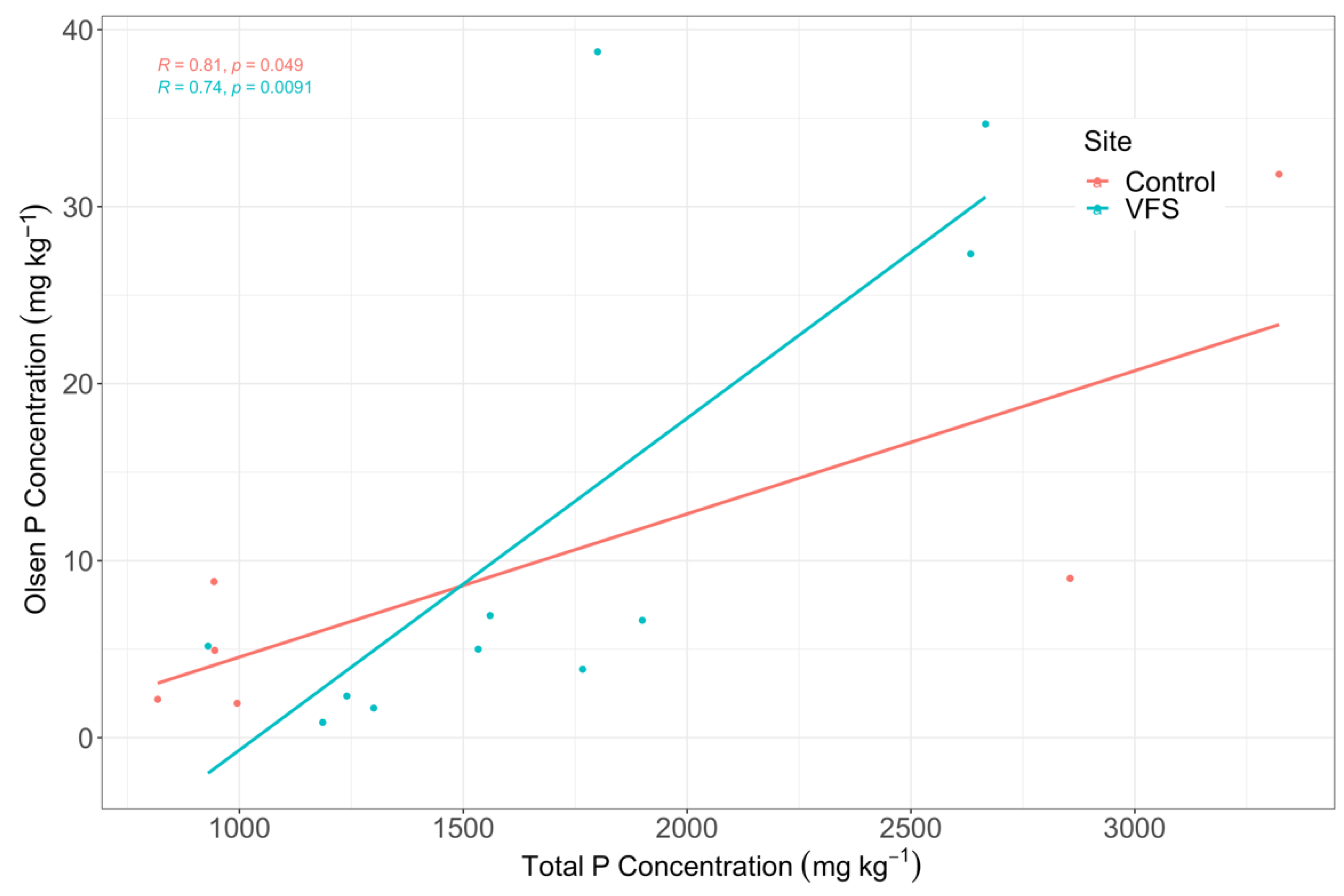
3.2.3. Correlations between Plant and Soil Phosphorus
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frossard, E.; Condron, L.M.; Oberson, A. Processes governing phosphorus availability in temperate soils. J. Environ. Qual. 2000, 29, 15–23. [Google Scholar] [CrossRef] [Green Version]
- Hansen, N.C.; Daniel, T.C.; Sharpley, A.N.; Lemunyon, J.L. The fate and transport of phosphorus in agricultural systems. J. Soil Water Conserv. 2000, 57, 408–417. [Google Scholar]
- Holman, I.P.; Whelan, M.J.; Howden, N.J.K.; Bellamy, P.H.; Willby, N.J.; Rivas-Casado, M.; McConvey, P. Phosphorus in groundwater—An overlooked contributor to eutrophication? Hydrol. Process. 2008, 22, 5127. [Google Scholar] [CrossRef]
- Sharpley, A.N.; Robinson, J.S.; Smith, S.J. Assessing environmental sustainability of agricultural systems by simulation of nitrogen and phosphorus loss in runoff. Eur. J. Agron. 1995, 4, 453–464. [Google Scholar] [CrossRef]
- Hansen, N.C.; Gupta, S.C.; Moncrief, J.F. Snowmelt runoff, sediment, and phosphorus losses under three different tillage systems. Soil Till. Res. 2000, 57, 93–100. [Google Scholar] [CrossRef]
- Woltemade, C.J. Ability of restored wetlands to reduce nitrogen and phosphorus concentrations in agricultural drainage water. J. Soil Water Conserv. 2000, 55, 303–309. [Google Scholar]
- Braskerud, B.C. Factors affecting phosphorus retention in small constructed wetlands treating agricultural non-point source pollution. Ecol. Eng. 2002, 19, 41–61. [Google Scholar] [CrossRef]
- Sheppard, S.C.; Sheppard, M.I.; Long, J.; Sanipelli, B.; Tait, J. Runoff phosphorus retention in vegetated field margins on flat landscapes. Can. J. Soil Sci. 2006, 86, 871–884. [Google Scholar] [CrossRef] [Green Version]
- Uusi-Kämppä, J.; Jauhiainen, L. Long-term monitoring of buffer zone efficiency under different cultivation techniques in boreal conditions. Agric. Ecosyst. Environ. 2010, 137, 75–85. [Google Scholar] [CrossRef]
- Clausen, J.C.; Guillard, K.; Sigmund, C.M.; Martin Dors, K. Water quality changes from riparian buffer restoration in Connecticut. J. Environ. Qual. 2000, 29, 1751–1761. [Google Scholar] [CrossRef] [Green Version]
- Al-wadaey, A.; Wortmann, C.S.; Franti, T.G.; Shapiro, C.A.; Eisenhauer, D.E. Effectiveness of grass filters in reducing phosphorus and sediment runoff. Water Air Soil Poll. 2012, 223, 5865–5875. [Google Scholar] [CrossRef] [Green Version]
- Kieta, K.A.; Owens, P.N.; Lobb, D.A.; Vanrobaeys, J.A.; Flaten, D.N. Phosphorus dynamics in vegetated buffer strips in cold climates: A review. Environ. Rev. 2018, 26, 255–272. [Google Scholar] [CrossRef]
- Glozier, N.E.; Elliott, J.A.; Holliday, B.; Yarotski, J.; Harker, B. Water Quality Characteristics and Trends in a Small Agricultural Watershed: South Tobacco Creek, Manitoba; Environment Canada: Saskatoon, SK, Canada, 2006; p. 86. [Google Scholar]
- Little, J.L.; Nolan, S.C.; Casson, J.P.; Olson, B.M. Relationships between soil and runoff phosphorus in small Alberta watersheds. J. Environ. Qual. 2007, 36, 1289–1300. [Google Scholar] [CrossRef] [Green Version]
- Habibiandehkordi, R.; Lobb, D.A.; Sheppard, S.C.; Flaten, D.N.; Owens, P.N. Uncertainties in vegetated buffer strip function in controlling phosphorus export from agricultural land in the Canadian Prairies. Environ. Sci. Pollut. Res. 2017, 24, 18372–18382. [Google Scholar] [CrossRef] [PubMed]
- Vanrobaeys, J.A.; Owens, P.N.; Lobb, D.A.; Kieta, K.A.; Campbell, J.M. Seasonal efficacy of vegetated filter strips for phosphorus reduction in surface runoff. J. Environ. Qual. 2019, 48, 880–888. [Google Scholar] [CrossRef] [Green Version]
- Bechmann, M.E.; Kleinman, P.; Sharpley, A.N.; Saporito, L.S. Freeze–thaw effects on phosphorus loss in runoff from manured and catch-cropped soils. J. Environ. Qual. 2005, 34, 2301–2309. [Google Scholar] [CrossRef] [Green Version]
- Levitt, J. Responses of Plants to Environmental Stresses; Academic Press: New York, NY, USA, 1980; 709p. [Google Scholar]
- Webb, M.S.; Uemura, M.; Steponkus, P.L. A comparison of freezing injury in oat and rye: Two cereals at the extremes of freezing tolerance. Plant Physiol. 1994, 104, 467–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Khalaf, R.; Ulén, B.; Bergkvist, G. Potential phosphorus release from catch crop shoots and roots after freezing-thawing. Plant Soil 2013, 371, 543–557. [Google Scholar] [CrossRef] [Green Version]
- Kleinman, P.; Bryant, R.B.; Reid, W.S.; Sharpley, A.N.; Pimentel, D. Using soil phosphorus behavior to identify environmental thresholds. Soil Sci. 2000, 165, 943–950. [Google Scholar] [CrossRef]
- Joergensen, R.G.; Kübler, H.; Meyer, B.; Wolters, V. Microbial biomass phosphorus in soils of beech forests. Biol. Fertil. Soils 1995, 19, 215–219. [Google Scholar] [CrossRef]
- Roberts, W.M.; Matthews, R.A.; Blackwell, M.S.A.; Peukert, S.; Collins, A.L.; Stutter, M.I.; Haygarth, P.M. Microbial biomass phosphorus contributions to phosphorus solubility in riparian vegetated buffer strip soils. Biol. Fertil. Soils 2013, 49, 1237–1241. [Google Scholar] [CrossRef]
- Haygarth, P.M.; Condrom, L.M.; Heathwaite, A.L.; Turner, B.L.; Harris, G.P. The phosphorus transfer continuum: Linking source to impact with an interdisciplinary and multi-scaled approach. Sci. Total Environ. 2005, 344, 5–14. [Google Scholar] [CrossRef]
- Smith, R.E.; Michalyna, W.; Wilson, G. Soils of the Morden-Winkler Area. Report No.18 Canada-Manitoba Soil Survey; Manitoba Department of Agricutlure: Winnipeg, MB, Canada, 1973. [Google Scholar]
- Flaten, D.N.; Grant, C. Review of 4R Fertilizer Management in the Northern Great Plains. In Proceedings of the AGVISE Soil Fertility Seminar, Portage la Prairie, MB, Canada, 12 March 2019. [Google Scholar]
- Environment Canada. Morden CDA CS, Manitoba Station Data: Canada’s National Climate Archive. Available online: http://climate.weather.gc.ca/ (accessed on 11 October 2019).
- R Core Team. R: A Language and Environment for Statistical Computing [Internet]; R Foundation for Statistical Computing: Vienna, Austria, 2017; Available online: http://www.R-project.org/ (accessed on 20 December 2021).
- RStudio Team. RStudio: Integrated Development for R; RStudio, Inc.: Boston, MA, USA, 2015; Available online: http://www.rstudio.com/ (accessed on 20 December 2021).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Simard, R.R.; Beauchemin, S.; Haygarth, P.M. Potential for preferential pathways of phosphorus transport. J. Environ. Qual. 2000, 29, 97–105. [Google Scholar] [CrossRef]
- Young, E.O.; Ross, D.S.; Jaisi, D.P.; Vidon, P.G. Phosphorus transport along the cropland–riparian–stream continuum in cold climate agroecosystems: A review. Soil Syst. 2021, 5, 15. [Google Scholar] [CrossRef]
- Kieta, K.A. An Assessment of Vegetation Characteristics and Hydrologic Flow Pathways on the Effectiveness of Vegetated Buffer Strips for Phosphorus Reduction in an Agricultural Watershed. Mater’s Thesis, University of Northern British Columbia, Prince George, BC, Canada, 2017. [Google Scholar]
- Thomashow, M.F. Role of cold-responsive genes in plant freezing tolerance. Plant Physiol. 1998, 118, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Miller, M.H.; Beauchamp, E.G.; Lauzon, J.D. Leaching of nitrogen and phosphorus from the biomass of three cover crop species. J. Environ. Qual. 1994, 23, 267–272. [Google Scholar] [CrossRef]
- Roberson, T.; Bundy, L.G.; Andraski, T.W. Freezing and drying effects on potential plant contributions to phosphorus in runoff. J. Environ. Qual. 2007, 36, 532–539. [Google Scholar] [CrossRef]
- Liu, K.; Elliott, J.A.; Lobb, D.A.; Flaten, D.N.; Yarotski, J. Nutrient and sediment losses in snowmelt runoff from perennial forage and annual cropland in the Canadian Prairies. J. Environ. Qual. 2014, 43, 1644–1655. [Google Scholar] [CrossRef] [PubMed]
- Elliott, J.A. Evaluating the potential contribution of vegetation as a nutrient source in snowmelt runoff. Can. J. Soil Sci. 2013, 93, 435–443. [Google Scholar] [CrossRef]
- Øgaard, A.F. Freezing and thawing effects on phosphorus release from grass and cover crop species. Acta. Agric. Scand. 2015, 65, 529–536. [Google Scholar] [CrossRef]
- Kieta, K.A.; Owens, P.N. Phosphorus release from shoots of Phleum pretense L. after repeated freeze-thaw cycles and harvests. Ecol. Eng. 2019, 127, 204–211. [Google Scholar] [CrossRef]
- Räty, M.; Uusi-Kämppä, J.; Yli-Halla, M.; Rasa, K.; Pietola, L. Phosphorus and nitrogen cycles in the vegetation of differently managed buffer zones. Nutr. Cycl. Agroecosyst. 2010, 86, 121–132. [Google Scholar] [CrossRef]
- Propheter, J.L.; Staggenborg, S. Performance of annual and perennial biofuel crops: Nutrient removal during the first two years. Agron. J. 2010, 102, 798–805. [Google Scholar] [CrossRef]
- Cade-Menun, B.J.; Bell, G.; Baker-Ismail, S.; Fouli, Y.; Hodder, K.; McMartin, D.W.; Perez-Valdivia, C.; Wu, K. Nutrient loss from Saskatchewan cropland and pasture in spring snowmelt runoff. Can. J. Soil Sci. 2013, 93, 445–458. [Google Scholar] [CrossRef]
- Baulch, H.M.; Elliott, J.A.; Cordeiro, M.R.C.; Flaten, D.N.; Wilson, H.F. Soil and water management: Opportunities to mitigate nutrient losses to surface waters in the Northern Great Plains. Environ. Rev. 2019, 27, 447–477. [Google Scholar] [CrossRef]
- Liu, J.; Lobb, D.A. An overview of crop residue management impacts on crop water use and runoff in the Canadian Prairies. Water 2021, 13, 2929. [Google Scholar] [CrossRef]


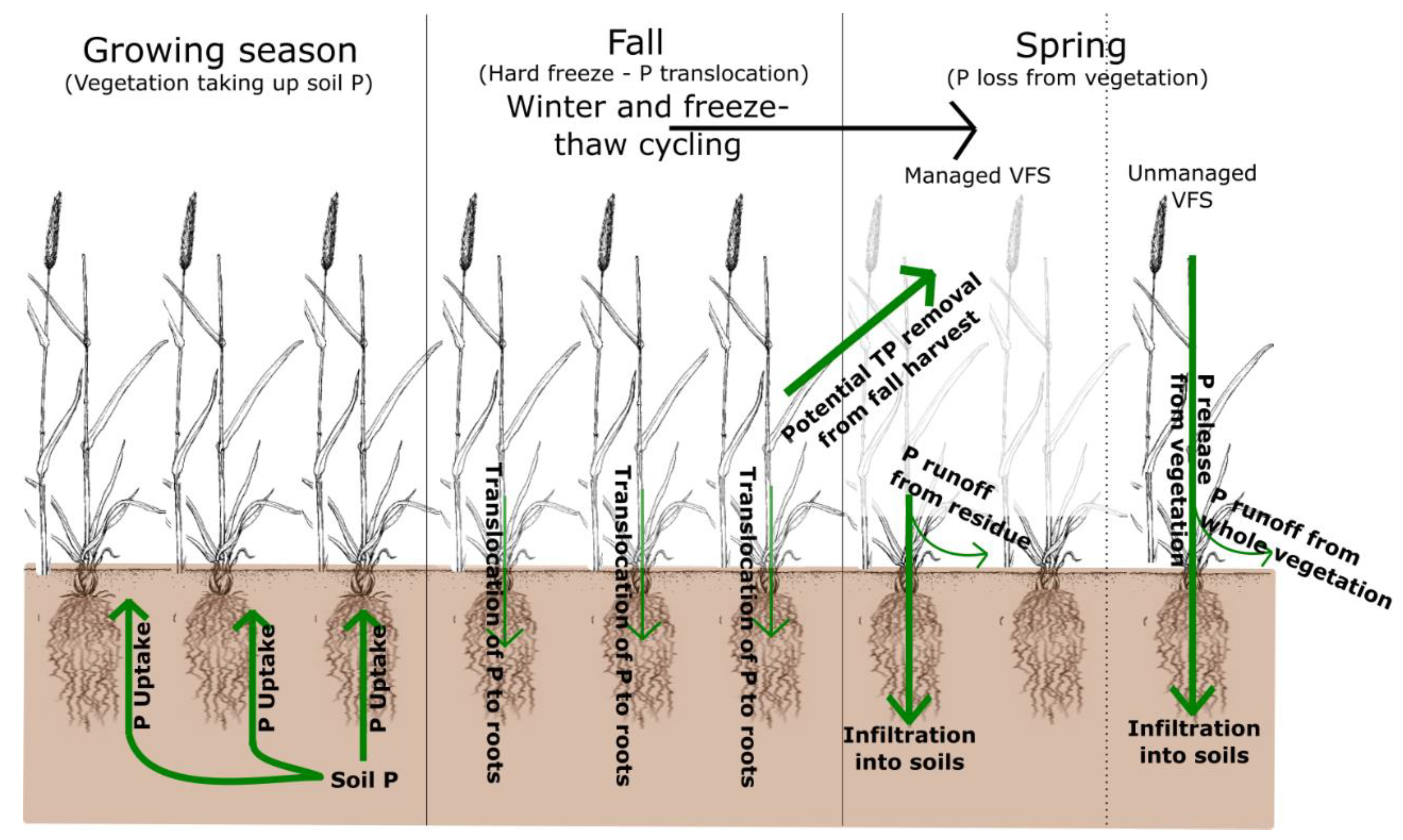
| Fall 2015 | Spring 2016 | Fall 2016 | Spring 2017 | ||||
|---|---|---|---|---|---|---|---|
| Sept 2015 | Oct 2015 | March 2016 | May 2016 | Sept/Oct 2016 | Nov 2016 | May 2017 | |
| Soils | S(24) | S(24) | S(36) | S(36) | |||
| Harvestable Vegetation | V(9) | V(2) | V,C(36) | ||||
| Residue | V,C(18) | V,C(18) | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kieta, K.A.; Owens, P.N.; Vanrobaeys, J.A.; Lobb, D.A. Seasonal Changes in Phosphorus in Soils and Vegetation of Vegetated Filter Strips in Cold Climate Agricultural Systems. Agriculture 2022, 12, 233. https://doi.org/10.3390/agriculture12020233
Kieta KA, Owens PN, Vanrobaeys JA, Lobb DA. Seasonal Changes in Phosphorus in Soils and Vegetation of Vegetated Filter Strips in Cold Climate Agricultural Systems. Agriculture. 2022; 12(2):233. https://doi.org/10.3390/agriculture12020233
Chicago/Turabian StyleKieta, Kristen A., Philip N. Owens, Jason A. Vanrobaeys, and David A. Lobb. 2022. "Seasonal Changes in Phosphorus in Soils and Vegetation of Vegetated Filter Strips in Cold Climate Agricultural Systems" Agriculture 12, no. 2: 233. https://doi.org/10.3390/agriculture12020233
APA StyleKieta, K. A., Owens, P. N., Vanrobaeys, J. A., & Lobb, D. A. (2022). Seasonal Changes in Phosphorus in Soils and Vegetation of Vegetated Filter Strips in Cold Climate Agricultural Systems. Agriculture, 12(2), 233. https://doi.org/10.3390/agriculture12020233







