A Meta-Analysis of the Effects of a Chemical Additive on the Fermentation and Aerobic Stability of Whole-Plant Maize Silage
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
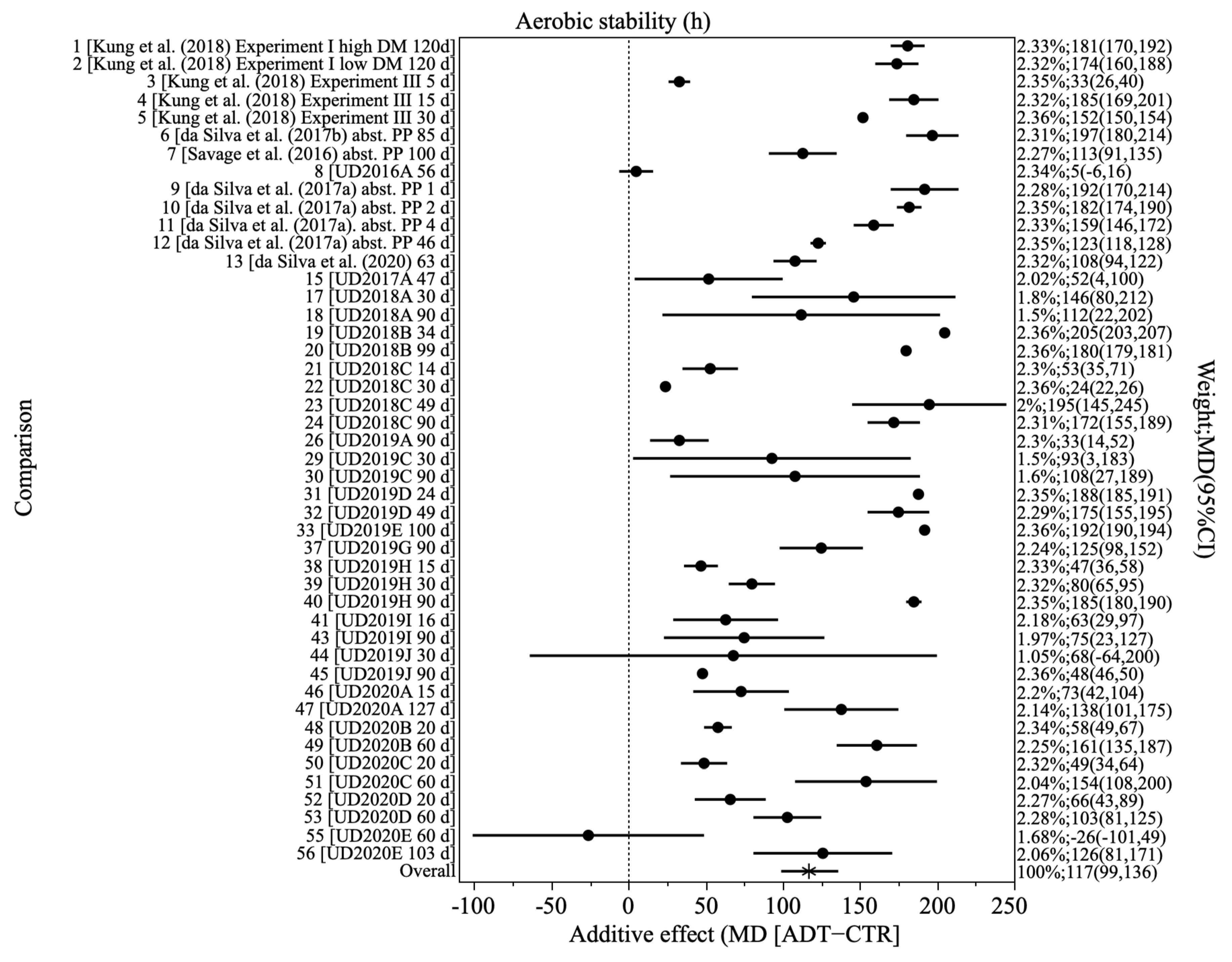
3.1. Effect of Additive Treatment on Maize Silage Characteristics
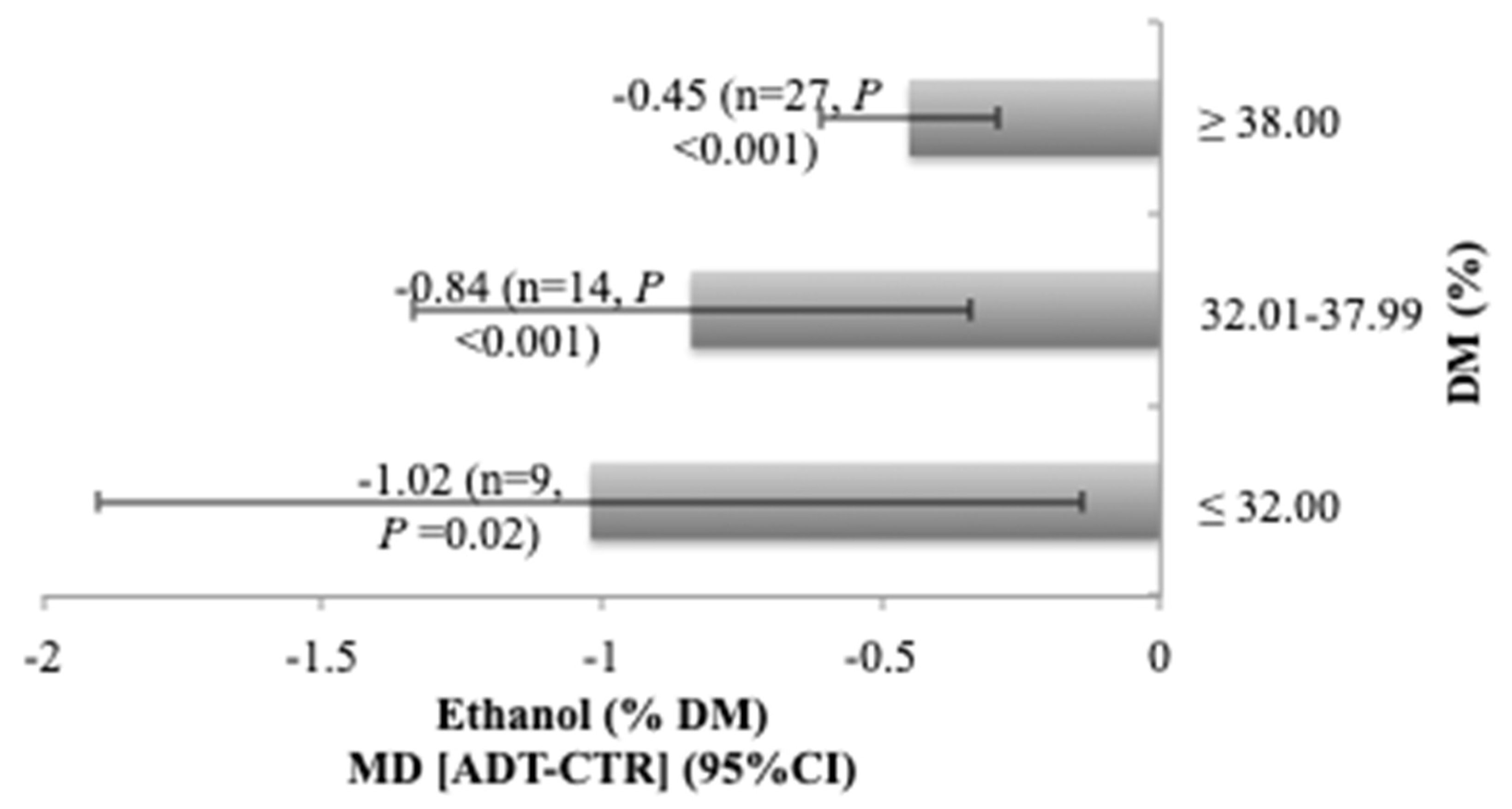
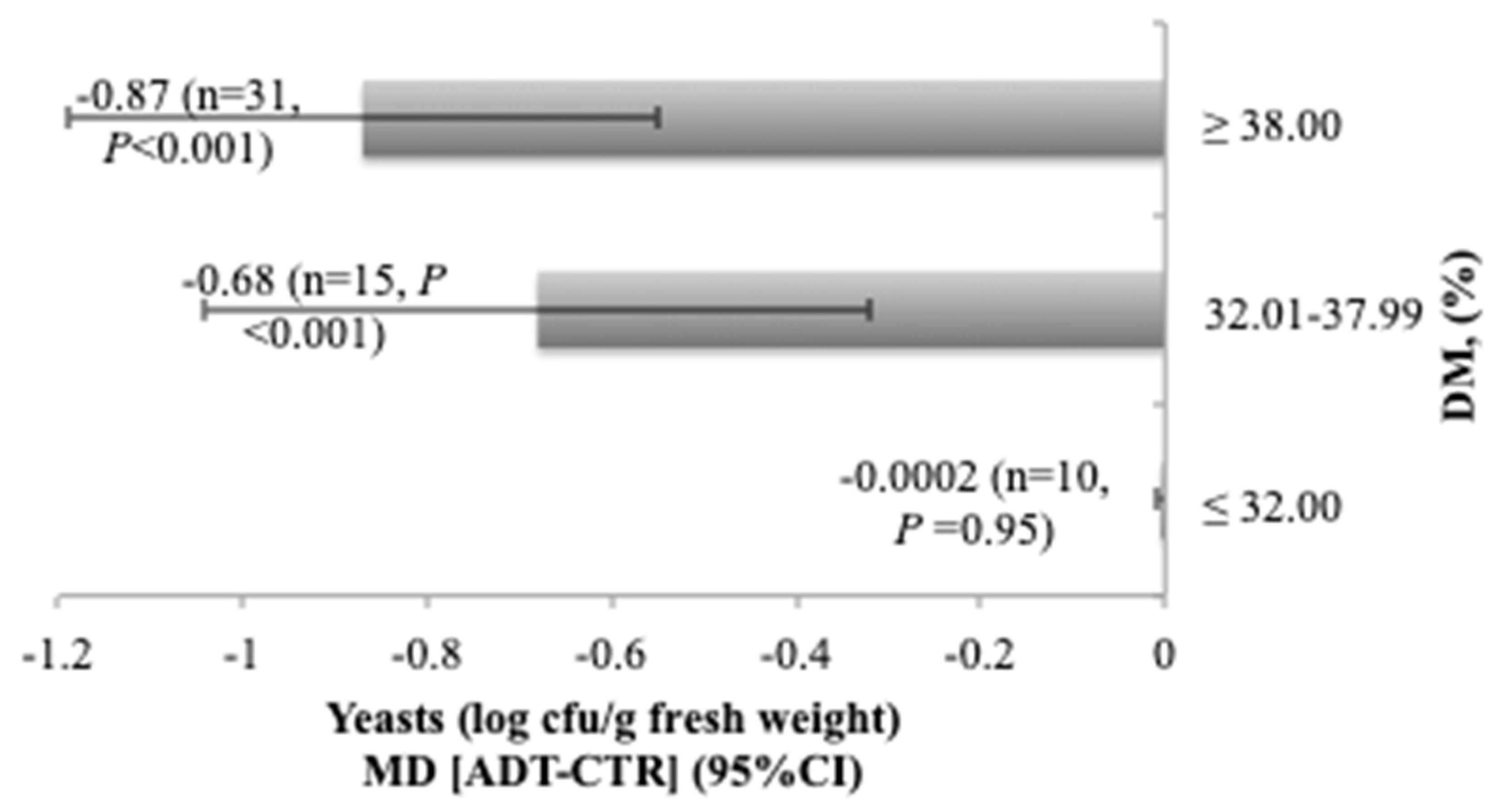
3.2. Influence of DM Content of the Forage on Additive Effects
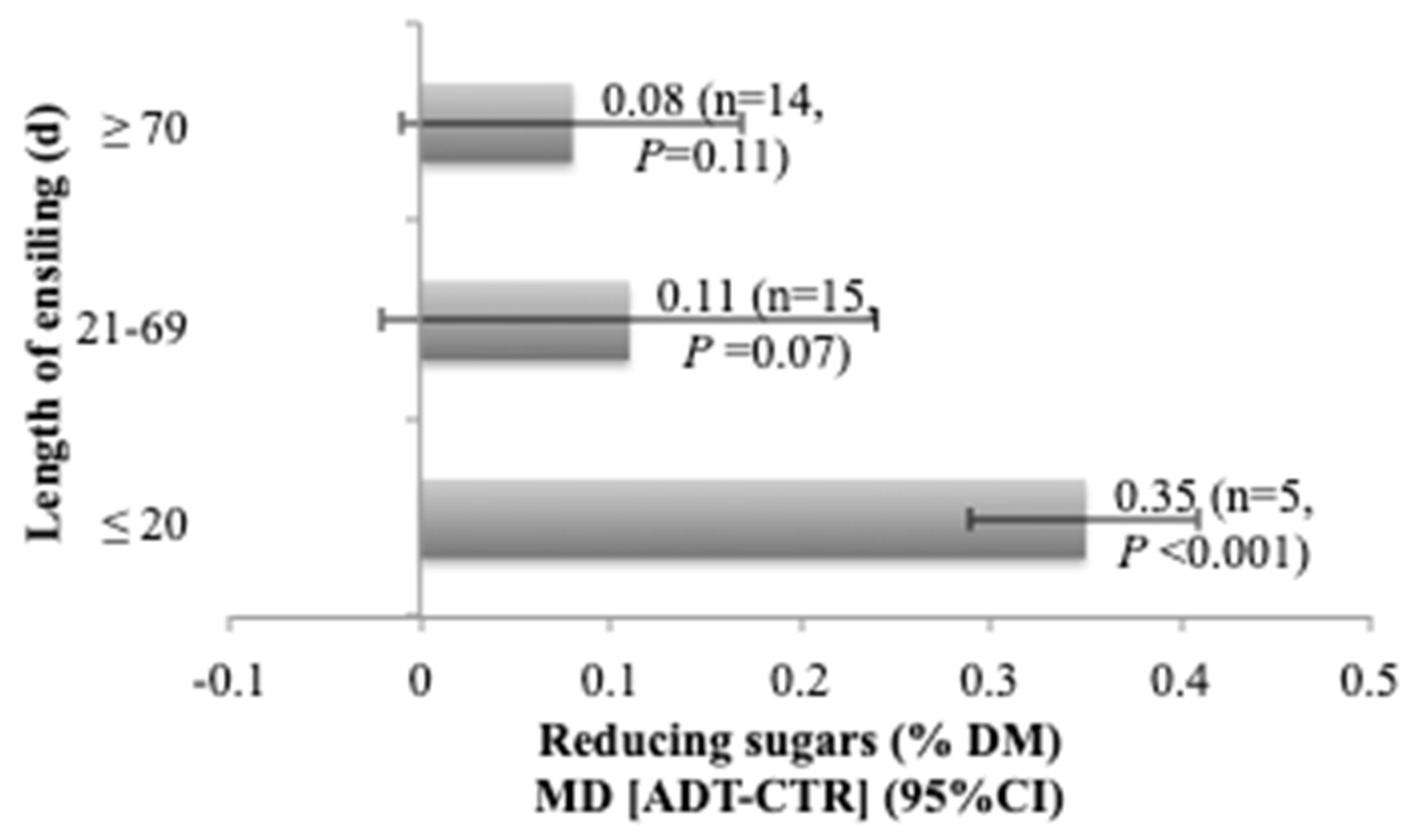
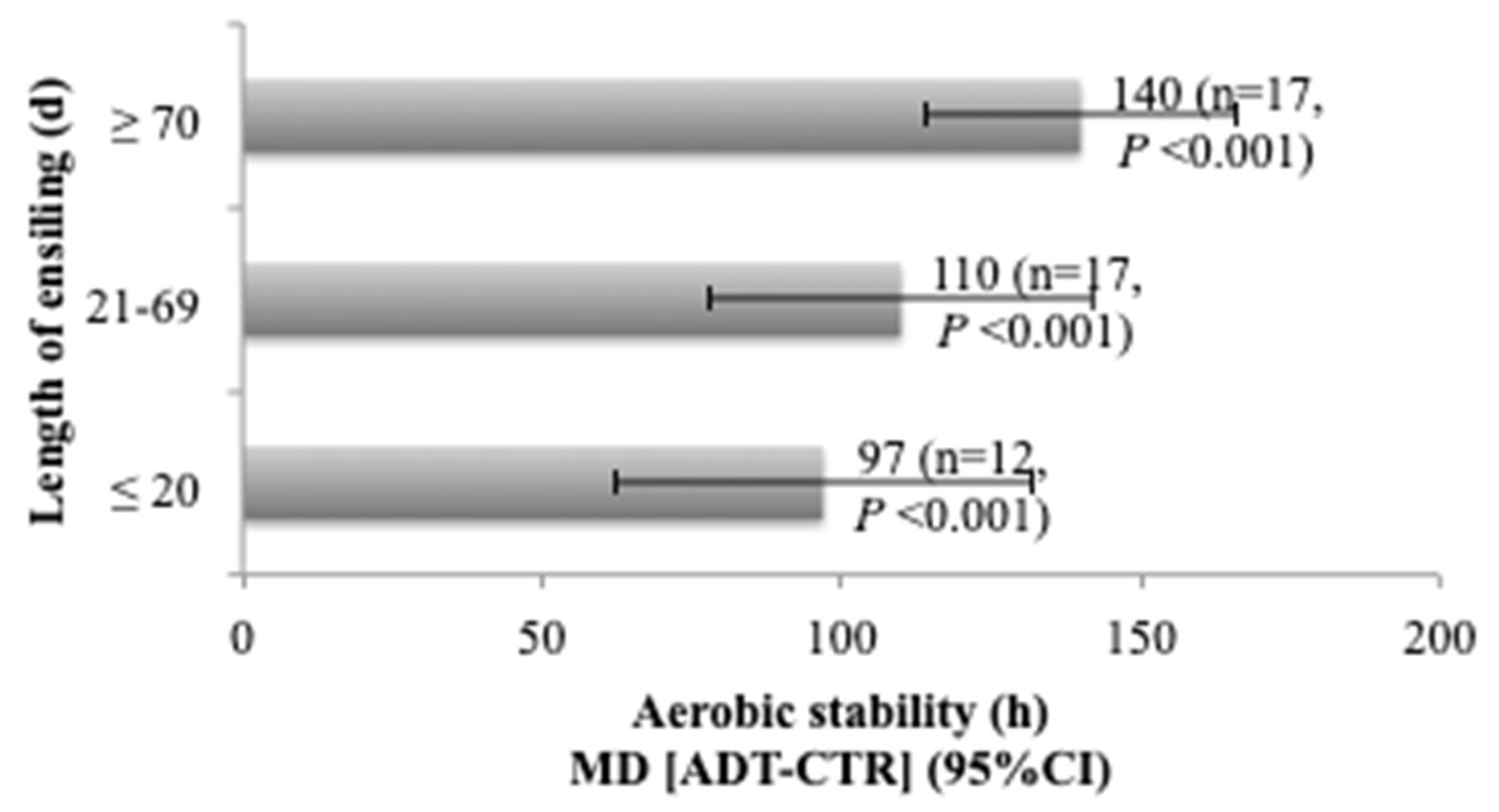
3.3. Influence of Length of Ensiling on Additive Effects
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Muck, R.E.; Nadeau, E.M.G.; McAllister, T.A.; Contreras-Govea, F.E.; Santos, M.C.; Kung, L., Jr. Silage review: Recent advances and future uses of silage additives. J. Dairy Sci. 2018, 101, 3980–4000. [Google Scholar] [CrossRef]
- Auerbach, H.; Nadeau, E.M.G. Chemical additives for silages: When to use it and what are the options? In Proceedings of the VI International Symposium on Forage Quality and Conservation, Piracicaba, Brazil, 7–8 November 2019; Nussio, L.G., da Silva, E.B., Oliveira, K.S., Gritti, V.C., Salvo, P.A.R., Salvati, G.G.S., Dannylo, O.S., Eds.; ESALQ: Piracicaba, Brazil, 2019; pp. 49–88. [Google Scholar]
- Knicky, M.; Spörndly, R. The ensiling capability of a mixture of sodium benzoate, potassium sorbate, and sodium nitrite. J. Dairy Sci. 2011, 94, 824–831. [Google Scholar] [CrossRef] [Green Version]
- da Silva, T.; Smith, M.; Barnard, A.; Kung, L., Jr. The effect of a chemical additive on the fermentation and aerobic stability of high-moisture corn. J. Dairy Sci. 2015, 98, 8904–8912. [Google Scholar] [CrossRef]
- Borreani, G.; Tabacco, E.; Schmidt, R.; Holmes, B.; Muck, R. Silage review: Factors affecting dry matter and quality losses in silages. J. Dairy Sci. 2018, 101, 3952–3979. [Google Scholar] [CrossRef] [Green Version]
- Bujňák, L.; Maskaľová, I.; Vajda, V. Determination of buffering capacity of selected fermented feedstuffs and the effect of dietary acid-base status on ruminal fluid pH. Acta Veter. Brno 2011, 80, 269–273. [Google Scholar] [CrossRef] [Green Version]
- Stanojevic, D.; Comic, L.; Stefanovic, O.; Solujic-Sukdolak, S. Antimicrobial effects of sodium benzoate, sodium nitrite and potassium sorbate and their synergistic action in vitro. Bulg. J. Agric. Sci. 2009, 15, 307–311. [Google Scholar]
- Ferrero, F.; Piano, S.; Tabacco, E.; Borreani, G. Effects of conservation period and Lactobacillus hilgardii inoculum on the fermentation profile and aerobic stability of whole corn and sorghum silages. J. Sci. Food Agric. 2019, 99, 2530–2540. [Google Scholar] [CrossRef]
- Ferrero, F.; Tabacco, E.; Piano, S.; Casale, M.; Borreani, G. Temperature during conservation in laboratory silos affects fermentation profile and aerobic stability of corn silage treated with Lactobacillus buchneri, Lactobacillus hilgardii, and their combination. J. Dairy Sci. 2021, 104, 1696–1713. [Google Scholar] [CrossRef]
- Kung, L., Jr.; Smith, M.L.; Da Silva, E.B.; Windle, M.C.; Da Silva, T.C.; Polukis, S.A. An evaluation of the effectiveness of a chemical additive based on sodium benzoate, potassium sorbate, and sodium nitrite on the fermentation and aerobic stability of corn silage. J. Dairy Sci. 2018, 101, 5949–5960. [Google Scholar] [CrossRef] [Green Version]
- da Silva, É.B.; Savage, R.M.; Polukis, S.A.; Smith, M.L.; Gray, A.M.; Mester, R.N.; Kung, L., Jr. Effectiveness of a chemical additive on improving the aerobic stability of corn silage after short periods of ensiling. J. Dairy Sci. 2017, 100 (Suppl. 2), 263. [Google Scholar]
- da Silva, É.B.; Savage, R.M.; Biddle, A.S.; Polukis, S.A.; Smith, M.L.; Kung, L., Jr. Effects of a chemical additive on the fermentation, microbial communities, and aerobic stability of corn silage with or without air stress during storage. J. Anim. Sci. 2020, 98, 246. [Google Scholar] [CrossRef]
- da Silva, É.B.; Savage, R.M.; Polukis, S.A.; Smith, M.L.; Mester, R.N.; Gray, A.M.; Kung, L., Jr. Effects of a chemical additive on aerobic stability and fungal microbiome of corn silage. J. Dairy Sci. 2017, 100 (Suppl. 2), 61. [Google Scholar]
- Savage, R.M.; Da Silva, E.B.; Smith, M.L.; Polukis, S.A.; Pacer, K.M.; Laubach, A.E.; Gray, A.M.; Kung, L., Jr. The effects of air and heat stress on the aerobic stability of silage treated with a chemical additive. J. Anim. Sci. 2016, 94 (Suppl. 5), 327. [Google Scholar] [CrossRef] [Green Version]
- The Jamovi Project. Jamovi (Version 1.6) [Computer Software]. 2021. Available online: https://www.jamovi.org (accessed on 9 August 2021).
- Viechtbauer, W.; Cheung, M.W.-L. Outlier and influence diagnostics for meta-analysis. Res. Synth. Methods 2010, 1, 112–125. [Google Scholar] [CrossRef]
- Viechtbauer, W. Bias and Efficiency of Meta-Analytic Variance Estimators in the Random-Effects Model. J. Educ. Behav. Stat. 2005, 30, 261–293. [Google Scholar] [CrossRef]
- Cochran, W.G. The Combination of Estimates from Different Experiments. Biometrics 1954, 10, 101–129. [Google Scholar] [CrossRef]
- Begg, C.B.; Mazumdar, M. Operating Characteristics of a Rank Correlation Test for Publication Bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef]
- Rothstein, H.R.; Sutton, A.J.; Borenstein, M. (Eds.) Publication Bias in Meta-Analysis: Prevention, Assessment and Adjustments; John Wiley & Sons: Chichester, UK, 2005. [Google Scholar]
- Queiroz, O.C.M.; Ogunade, I.M.; Weinberg, Z.; Adesogan, A.T. Silage review: Foodborne pathogens in silage and their mitigation by silage additives. J. Dairy Sci. 2018, 101, 4132–4142. [Google Scholar] [CrossRef]
- Spoelstra, S.F. Nitrate in silage. Grass Forage Sci. 1985, 40, 1–11. [Google Scholar] [CrossRef]
- Gomes, A.; Auerbach, H.; Lazzari, G.; Moraes, A.; Nussio, L.; Jobim, C.; Daniel, J. Sodium nitrite-based additives improve the conservation and the nutritive value of guinea grass silage. Anim. Feed Sci. Technol. 2021, 279, 115033. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Meng, Q.; Zhou, Z.; Wu, H. A mixture of potassium sorbate and sodium benzoate improved fermentation quality of whole-plant corn silage by shifting bacterial communities. J. Appl. Microbiol. 2020, 128, 1312–1323. [Google Scholar] [CrossRef]
- Morais, G.; Daniel, J.L.P.; Kleinshmitt, C.; Carvalho, P.A.; Fernandez, J.; Nussio, L.G. Additives for grain silages: A review. Slovak J. Anim. Sci. 2017, 50, 42–54. [Google Scholar]
- Knický, M.; Spörndly, R. Sodium benzoate, potassium sorbate and sodium nitrite as silage additives. J. Sci. Food Agric. 2009, 89, 2659–2667. [Google Scholar] [CrossRef]
- Weiss, K.; Kroschewski, B.; Auerbach, H. Effects of air exposure, temperature and additives on fermentation characteristics, yeast count, aerobic stability and volatile organic compounds in corn silage. J. Dairy Sci. 2016, 99, 8053–8069. [Google Scholar] [CrossRef] [Green Version]
- Kung, L., Jr.; Shaver, R.D.; Grant, R.J.; Schmidt, R.J. Silage review: Interpretation of chemical, microbial, and organoleptic components of silages. J. Dairy Sci. 2018, 101, 4020–4033. [Google Scholar] [CrossRef]
- Pahlow, G.; Muck, R.E.; Driehuis, F.; Oude-Elferink, S.J.W.H.; Spoelstra, S.F. Microbiology of ensiling. In Silage Science and Technology; Buxton, D.R., Muck, R.E., Harrison, J.H., Eds.; American Society of Agronomy: Madison, WI, USA, 2003; pp. 31–94. [Google Scholar]
- Bernardes, T.F.; De Oliveira, I.L.; Lara, M.; Casagrande, D.R.; Ávila, C.L.S.; Pereira, O. Effects of potassium sorbate and sodium benzoate at two application rates on fermentation and aerobic stability of maize silage. Grass Forage Sci. 2015, 70, 491–498. [Google Scholar] [CrossRef]
- da Silva, N.C.; Dos Santos, J.P.; Ávila, C.L.S.; Evangelista, A.R.; Casagrande, D.R.; Bernardes, T.F. Evaluation of the effects of two Lactobacillus buchneristrains and sodium benzoate on the characteristics of corn silage in a hot-climate environment. Grassl. Sci. 2014, 60, 169–177. [Google Scholar] [CrossRef]
- Auerbach, H.; Nadeau, E.M.G. Effects of chemical additives on whole-crop maize silage traits. In Proceedings of the 22nd International Grassland Congress, Sydney, Australia, 15–19 September 2013. [Google Scholar]
- Buxton, D.R.; O’Kiely, O. Preharvest plant factors affecting ensiling. In Silage Science and Technology; Buxton, D.R., Muck, R.E., Harrison, J.H., Eds.; American Society of Agronomy: Madison, WI, USA, 2003; pp. 199–250. [Google Scholar]
- Bal, M.; Coors, J.; Shaver, R. Impact of the Maturity of Corn for Use as Silage in the Diets of Dairy Cows on Intake, Digestion, and Milk Production. J. Dairy Sci. 1997, 80, 2497–2503. [Google Scholar] [CrossRef]
- Hu, W.; Schmidt, R.; McDonell, E.; Klingerman, C.; Kung, L., Jr. The effect of Lactobacillus buchneri 40788 or Lactobacillus plantarum MTD-1 on the fermentation and aerobic stability of corn silages ensiled at two dry matter contents. J. Dairy Sci. 2009, 92, 3907–3914. [Google Scholar] [CrossRef]
- Moon, N.J. Inhibition of the growth of acid tolerant yeasts by acetate, lactate and propionate and their synergistic mixtures. J. Appl. Bacteriol. 1983, 55, 453–460. [Google Scholar] [CrossRef]
- Neal, A.L.; Weinstock, J.O.; Lampen, J.O. Mechanisms of Fatty Acid Toxicity for Yeast. J. Bacteriol. 1965, 90, 126–131. [Google Scholar] [CrossRef] [Green Version]
- Kemp, A.; Geurink, J.; Haalstra, R.; Malestein, A. Nitrate poisoning in cattle. 2. Changes in nitrite in rumen fluid and methemoglobin formation in blood after high nitrate intake. Neth. J. Agric. Sci. 1977, 25, 51–62. [Google Scholar] [CrossRef]
| Item | n | CTR Mean (Standard Deviation) | Means Difference (95% CI) | Heterogeneity | Bias Assessment (p-Values) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Effect Size | p-Value | τ2 | I2 | p-Value | Fail-Safe N * | Rank Correlation | Egger’s Regression * | |||
| Reducing sugars | 34 | 0.88 (0.71) | 0.14 (0.06, 0.21) | <0.001 | 0.040 | 93.28 | <0.001 | <0.001 | 0.906 | 0.859 |
| Ammonia-N | 38 | 0.072 (0.020) | −0.005 (−0.007, −0.003) | <0.001 | 0.005 | 76.86 | <0.001 | <0.001 | 0.549 | 0.192 |
| pH | 56 | 3.77 (0.12) | −0.03 (−0.04, −0.01) | <0.001 | 0.003 | 95.57 | <0.001 | <0.001 | 0.527 | 0.346 |
| Lactic acid | 50 | 4.95 (1.16) | −0.21 (−0.33, −0.08) | <0.001 | 0.090 | 56.04 | <0.001 | <0.001 | 0.598 | 0.969 |
| Acetic acid | 50 | 1.30 (0.40) | −0.02 (−0.08, 0.04) | 0.52 | 0.024 | 74.47 | <0.001 | 0.070 | 0.947 | 0.619 |
| Ethanol | 50 | 1.46 (0.99) | −0.66 (−0.89, −0.44) | <0.001 | 0.636 | 98.77 | <0.001 | <0.001 | 0.271 | 0.914 |
| LAB | 52 | 7.18 (1.17) | 0.001 (−0.07, 0.07) | 0.98 | 0.034 | 89.83 | <0.001 | 0.033 | 0.004 | 0.147 |
| Yeasts | 56 | 4.13 (1.09) | −0.73 (−0.95, −0.52) | <0.001 | 0.546 | 99.84 | <0.001 | <0.001 | 0.616 | 0.002 |
| DM recovery | 56 | 96.09 (3.27) | 1.24 (0.56, 1.91) | <0.001 | 4.663 | 83.03 | <0.001 | <0.001 | 0.773 | 0.883 |
| Aerobic stability | 46 | 70 (47) | 117 (99, 136) | <0.001 | 3643 | 99.82 | <0.001 | <0.001 | 0.596 | 0.224 |
| Dependent Variables | n | Covariates | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Forage DM Content * | Length of Ensiling ** | ||||||||
| Intercept (p-Value) | Moderator (p-Value) | τ2 | Heterogeneity p-Value | Intercept (p-Value) | Moderator (p-Value) | τ2 | Heterogeneity p-Value | ||
| Reducing sugars | 34 | 0.004 (0.97) | 0.059 (0.25) | 0.040 | <0.001 | 0.40 (0.002) | −0.12 (0.03) | 0.035 | <0.001 |
| Ammonia-N | 38 | −0.005 (0.19) | 0.0001 (0.95) | 0.000 | <0.001 | −0.006 (0.12) | 0.0006 (0.75) | 0.000 | <0.001 |
| pH | 56 | −0.07 (0.01) | 0.02 (0.11) | 0.003 | <0.001 | −0.05 (0.03) | 0.01 (0.28) | 0.003 | <0.001 |
| Lactic acid | 50 | 0.09 (0.69) | −0.12 (0.18) | 0.088 | <0.001 | 0.01 (0.95) | −0.11 (0.14) | 0.084 | <0.001 |
| Acetic acid | 50 | −0.03 (0.78) | 0.01 (0.91) | 0.025 | <0.001 | 0.01 (0.90) | −0.02 (0.69) | 0.025 | <0.001 |
| Ethanol | 50 | −1.41 (<0.001) | 0.31 (0.03) | 0.577 | <0.001 | −0.60 (0.07) | −0.03 (0.83) | 0.642 | <0.001 |
| LAB | 52 | −0.02 (0.86) | 0.01 (0.85) | 0.035 | <0.001 | 0.01 (0.93) | −0.004 (0.94) | 0.035 | <0.001 |
| Yeasts | 56 | −0.17 (0.64) | −0.24 (0.10) | 0.519 | <0.001 | −0.80 (0.01) | 0.03 (0.82) | 0.557 | <0.001 |
| DM recovery | 56 | 2.00 (0.08) | −0.32 (0.46) | 4.703 | <0.001 | 2.16 (0.02) | −0.46 (0.30) | 4.602 | <0.001 |
| Aerobic stability | 46 | 118 (<0.001) | −0.08 (1.00) | 3728 | <0.001 | 72 (0.004) | 22 (0.052) | 3395 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benjamim da Silva, É.; Kung, L., Jr. A Meta-Analysis of the Effects of a Chemical Additive on the Fermentation and Aerobic Stability of Whole-Plant Maize Silage. Agriculture 2022, 12, 132. https://doi.org/10.3390/agriculture12020132
Benjamim da Silva É, Kung L Jr. A Meta-Analysis of the Effects of a Chemical Additive on the Fermentation and Aerobic Stability of Whole-Plant Maize Silage. Agriculture. 2022; 12(2):132. https://doi.org/10.3390/agriculture12020132
Chicago/Turabian StyleBenjamim da Silva, Érica, and Limin Kung, Jr. 2022. "A Meta-Analysis of the Effects of a Chemical Additive on the Fermentation and Aerobic Stability of Whole-Plant Maize Silage" Agriculture 12, no. 2: 132. https://doi.org/10.3390/agriculture12020132
APA StyleBenjamim da Silva, É., & Kung, L., Jr. (2022). A Meta-Analysis of the Effects of a Chemical Additive on the Fermentation and Aerobic Stability of Whole-Plant Maize Silage. Agriculture, 12(2), 132. https://doi.org/10.3390/agriculture12020132






