Survival and Feeding Behavior of Diaphorina citri (Hemiptera: Liviidae) Adults on Common Cover Crops in Citrus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plants
2.2. Insects
2.3. Feeding Behavior of D. citri Adults on Cover Crop Plants versus Citrus Using an Electrical Penetration Graph (EPG)
2.4. Behavioral Choice of D. citri to Various Citrus Cover Crop Species and Citrus
2.5. Survival of D. citri Adults on Citrus Cover Crop Species in No-Choice Assays
3. Results
3.1. Feeding Behavior of D. citri Adults on Cover Crop Plants versus Citrus Using Electrical Penetration Graph (EPG)
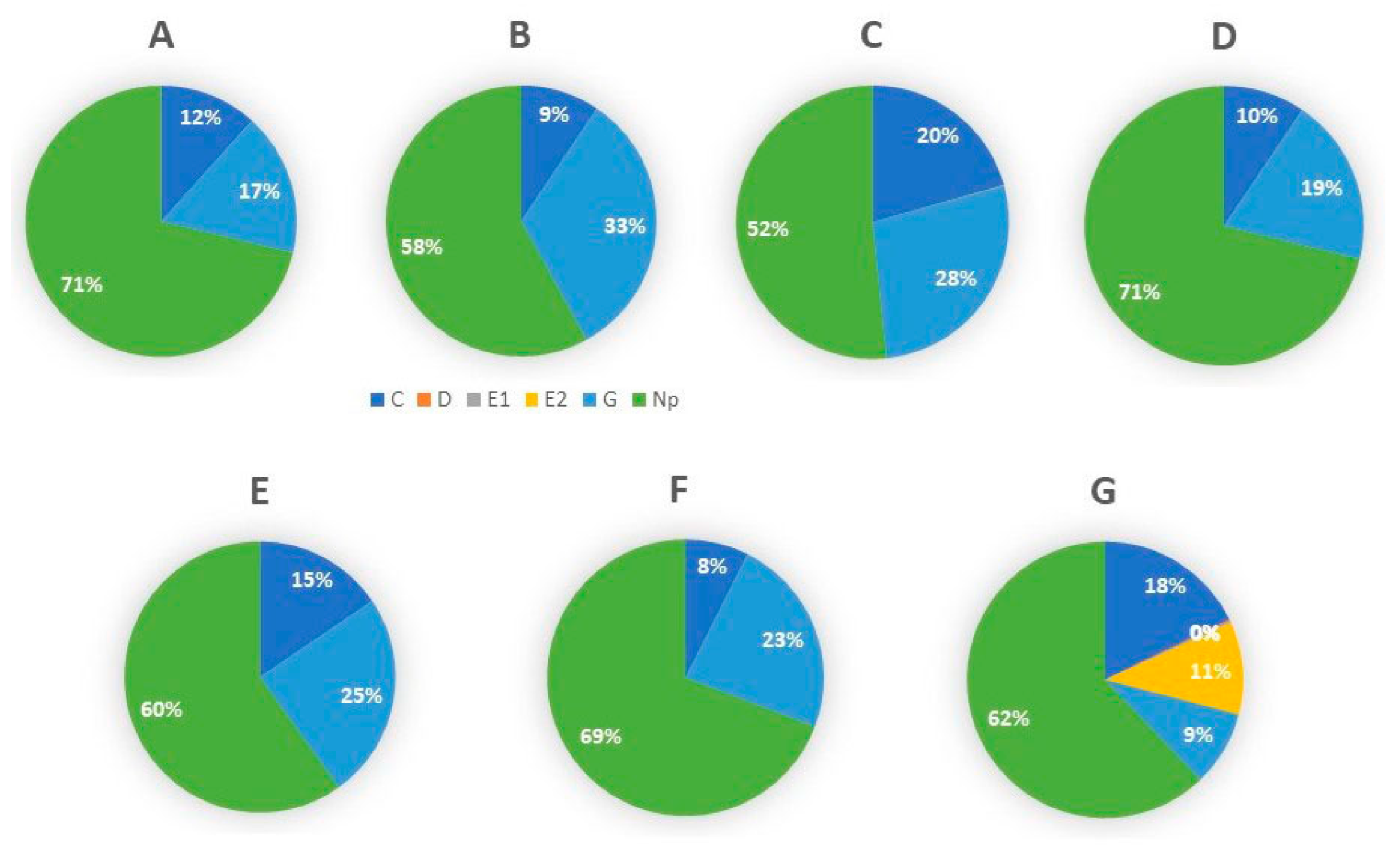
3.2. Behavioral Choice of D. citri to Various Citrus Cover Crop Species and Citrus
3.3. Survival of D. citri Adults on Citrus Cover Crop Species in No-Choice Assays
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Linares, J.; Scholberg, J.; Boote, K.; Chase, C.A.; Ferguson, J.J.; McSorley, R. Use of the cover crop weed index to evaluate weed suppression by cover crops in organic citrus orchards. HortScience 2008, 43, 27–34. [Google Scholar] [CrossRef]
- Ngouajio, M.; McGiffen, M.E. Going organic changes weed population dynamics. HortTechnology 2002, 12, 590–596. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.H.; McSorley, R.; Marshall, A.; Gallaher, R.N. Influence of organic Crotalaria juncea hay and ammonium nitrate fertilizers on soil nematode communities. Appl. Soil Ecol. 2006, 31, 186–198. [Google Scholar] [CrossRef]
- Bailey, K.L.; Lazarovits, G. Suppressing soil-borne diseases with residue management and organic amendments. Soil Tillage Res. 2003, 72, 169–180. [Google Scholar] [CrossRef]
- Letourneau, D.K.; Armbrecht, I.; Rivera, B.S.; Lerma, J.M.; Carmona, E.J.; Daza, M.C.; Trujillo, A.R. Does plant diversity benefit agroecosystems? A synthetic review. Ecol. Appl. 2011, 21, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Sáenz-Romo, M.G.; Veas-Bernal, A.; Martínez-García, H.; Ibáñez-Pascual, S.; Martínez-Villar, E.; Campos-Herrera, R.; Pérez-Moreno, I. Effects of ground cover management on insect predators and pests in a mediterranean vineyard. Insects 2019, 10, 421. [Google Scholar] [CrossRef] [Green Version]
- Aguilar-Fenollosa, E.; Ibáñez-Gual, M.V.; Pascual-Ruiz, S.; Hurtado, M.; Jacas, J.A. Effect of ground-cover management on spider mites and their phytoseiid natural enemies in clementine mandarin orchards (I): Bottom-up regulation mechanisms. Biol. Control. 2011, 59, 158–170. [Google Scholar] [CrossRef]
- Froud, K.; Stevens, P.; Steven, D. Survey of alternative host plants for Kellys citrus thrips (Pezothrips kellyanus) in citrus growing regions. NZ Plant Prot. 2001, 54, 15–20. [Google Scholar] [CrossRef]
- Karp, D.S.; Chaplin-Kramer, R.; Meehan, T.D.; Martin, E.A.; DeClerck, F.; Grab, H.; Gratton, C.; Hunt, L.; Larsen, A.; Martínez-Salinas, A.; et al. Crop pests and predators exhibit inconsistent responses to surrounding landscape composition. Proc. Natl. Acad. Sci. USA 2018, 115, 863–870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stansly, P.A.; Arevalo, H.A.; Qureshi, J.A.; Jones, M.M.; Hendricks, K.; Roberts, P.D.; Roka, F.M. Vector control and foliar nutrition to maintain economic sustainability of bearing citrus in Florida groves affected by huanglongbing. Pest. Manag. Sci. 2014, 70, 415–425. [Google Scholar] [CrossRef]
- George, J.; Kanissery, R.; Ammar, E.D.; Cabral, I.; Markle, L.T.; Patt, J.M.; Stelinski, L.L. Feeding behavior of Asian citrus psyllid (Diaphorina citri (Hemiptera: Liviidae)) nymphs and adults on common weeds occurring in cultivated citrus described using electrical penetration graph recordings. Insects 2020, 11, 48. [Google Scholar] [CrossRef] [Green Version]
- Martinez, L.; Soti, P.; Kaur, J.; Racelis, A.; Kariyat, R.R. Impact of cover crops on insect community dynamics in organic farming. Agriculture 2020, 10, 209. [Google Scholar] [CrossRef]
- Campbell, J.W.; Irvin, A.; Irvin, H.; Stanley-Stahr, C.; Ellis, J.D. Insect visitors to flowering buckwheat, Fagopyrum esculentum (Polygonales: Polygonaceae), in north-central Florida. Fla. Entomol. 2016, 99, 264–268. [Google Scholar] [CrossRef] [Green Version]
- Nicholls, C.I.; Parrella, M.; Altieri, M.A. The effects of a vegetational corridor on the abundance and dispersal of insect biodiversity within a northern California organic vineyard. Landscape Ecol. 2001, 16, 133–146. [Google Scholar] [CrossRef]
- Irvin, N.A.; Hagler, J.R.; Hoddle, M.S. Measuring natural enemy dispersal from cover crops in a California vineyard. Biol. Control 2018, 126, 15–25. [Google Scholar] [CrossRef]
- Gill, H.K.; McSorley, R. Cover crops for managing root-knot nematodes. ENY063/IN892. EDIS 2011, 7. [Google Scholar]
- Meagher, R.L., Jr.; Nagoshi, R.N.; Stuhl, C.; Mitchell, E.R. Larval development of fall armyworm (Lepidoptera: Noctuidae) on different cover crop plants. Fla. Entomol. 2004, 87, 454–460. [Google Scholar] [CrossRef]
- SARE Outreach. Cover Cropping for Pollinators and Beneficial Insects. Available online: https://www.sare.org/Learning-Center/Bulletins/Cover-Cropping-for-Pollinators-and-Beneficial-Insects/Text-Version (accessed on 30 March 2020).
- Manandhar, R.; Wright, M.G. Effects of interplanting flowering plants on the biological control of corn earworm (Lepidoptera: Noctuidae) and thrips (Thysanoptera: Thripidae) in sweet corn. J. Econ. Entomol. 2016, 109, 113–119. [Google Scholar] [CrossRef]
- Manandhar, R.; Hooks, C.R.; Wright, M.G. Influence of cover crop and intercrop systems on Bemisia argentifolii (Hemiptera: Aleyrodidae) infestation and associated squash silverleaf disorder in zucchini. Environ. Entomol. 2009, 38, 442–449. [Google Scholar] [CrossRef]
- English-Loeb, G.; Rhainds, M.; Martinson, T.; Ugine, T. Influence of flowering cover crops on Anagrus parasitoids (Hymenoptera: Mymaridae) and Erythroneura leafhoppers (Homoptera: Cicadellidae) in New York vineyards. Agric. Forest Entomol. 2003, 5, 173–181. [Google Scholar] [CrossRef] [Green Version]
- Akins, J. Cover Crop Effects on Insect Dynamics in Cropping Systems of the Southeastern US 2020. Master’s Thesis, Auburn University, Auburn, AL, USA, 12 December 2020. [Google Scholar]
- Bugg, R.L.; Ellis, R.T. Insects associated with cover crops in Massachusetts. Biol. Agric. Hortic. 1990, 7, 47–68. [Google Scholar] [CrossRef]
- Bugg, R.L.; Phatak, S.C.; Dutcher, J.D. Insects associated with cool-season cover crops in southern Georgia: Implications for pest control in truck-farm and pecan agroecosystems. Biol. Agric. Hortic. 1990, 7, 17–45. [Google Scholar] [CrossRef]
- Skelley, L.H.; Hoy, M.A. A synchronous rearing method for the Asian citrus psyllid and its parasitoids in quarantine. Biol. Control. 2004, 29, 14–23. [Google Scholar] [CrossRef]
- Li, W.B.; Hartung, J.S.; Levy, L. Quantitative real-time PCR for detection and identification of Candidatus Liberibacter species associated with citrus Huanglongbing. J. Microbiol. Methods 2006, 66, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Yen, A.L.; Powell, K.S.; Wu, F.; Wang, Y.; Xeng, L.; Yang, Y.; Cen, Y. Feeding behavior of Diaphorina citri (Hemiptera: Liviidae) and its acquisition of ‘Candidatus Liberibacter asiaticus’, on huanglongbing-infected Citrus reticulata leaves of several maturity stages. Fla. Entomol. 2015, 98, 186–192. [Google Scholar] [CrossRef]
- George, J.; Ammar, E.-D.; Hall, D.G.; Lapointe, S.L. Sclerenchymatous ring as a barrier to phloem feeding by Asian citrus psyllid: Evidence from electrical penetration graph and visualization of stylet pathways. PLoS ONE 2017, 12, e0173520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tjallingii, W. Electronic recording of penetration behaviour by aphids. Entomol. Exp. Appl. 1978, 24, 721–730. [Google Scholar] [CrossRef]
- Bonani, J.P.; Fereres, A.; Garzo, E.; Miranda, M.P.; Appezzato-Da-Gloria, B.; Lopes, J.R.S. Characterization of electrical penetration graphs of the Asian citrus psyllid, Diaphorina citri, in sweet orange seedlings. Entomol. Exp. Appl. 2010, 134, 35–49. [Google Scholar] [CrossRef] [Green Version]
- George, J.; Ammar, E.-D.; Hall, D.G.; Lapointe, S.L. Prolonged phloem feeding activities by Diaphorina citri nymphs may explain their greater acquisition of citrus greening pathogen. Sci. Rep. 2018, 8, 10352. [Google Scholar] [CrossRef] [Green Version]
- Ebert, T.A.; Backus, E.A.; Shugart, H.J.; Rogers, M.E. Behavioral plasticity in probing by Diaphorina citri (Hemiptera, Liviidae): Ingestion from phloem versus xylem is influenced by leaf age and surface. J. Insect Behav. 2018, 31, 119–137. [Google Scholar] [CrossRef] [Green Version]
- Pompon, J.; Quiring, D.; Goyer, C.; Giordanengo, P.; Pelletier, Y. A phloem-sap feeder mixes phloem and xylem sap to regulate osmotic potential. J. Insect Physiol. 2011, 57, 1317–1322. [Google Scholar] [CrossRef] [PubMed]
- Irvin, N.A.; Pierce, C.; Hoddle, M.S. Evaluating the potential of flowering plants for enhancing predatory hoverflies (Syrphidae) for biological control of Diaphorina citri (Liviidae) in California. Biol. Control 2021, 157, 104574. [Google Scholar] [CrossRef]
- Badenes-Perez, F.R.; Gershenzon, J.; Heckel, D.G. Insect attraction versus plant defense: Young leaves high in glucosinolates stimulate oviposition by a specialist herbivore despite poor larval survival due to high saponin content. PLoS ONE 2014, 9, e95766. [Google Scholar] [CrossRef]
- Carlsen, S.C.; Fomsgaard, I.S. Biologically active secondary metabolites in white clover (Trifolium repens L.)—A review focusing on contents in the plant, plant–pest interactions and transformation. Chemoecology 2008, 18, 129–170. [Google Scholar] [CrossRef]
- Bugg, R.L.; Waddington, C. Using cover crops to manage arthropod pests of orchards: A review. Agric. Ecosyst. Environ. 1994, 50, 11–28. [Google Scholar] [CrossRef]
- Castellano-Hinojosa, A.; Martens-Habbena, W.; Smyth, A.R.; Kadyampakeni, D.M.; Strauss, S.L. Short-term effects of cover crops on soil properties and the abundance of N-cycling genes in citrus agroecosystems. Appl. Soil Ecol. 2022, 172, 104341. [Google Scholar] [CrossRef]
- Grafton-Cardwell, E.E.; Ouyang, Y.; Bugg, R.L. Leguminous cover crops to enhance population development of Euseius tularensis (Acari: Phytoseiidae) in citrus. Biol. Control 1999, 16, 73–80. [Google Scholar] [CrossRef]
- Spreen, T.H.; Baldwin, J.P.; Futch, S.H. An economic assessment of the impact of Huanglongbing on citrus tree plantings in Florida. HortScience 2014, 49, 1052–1055. [Google Scholar] [CrossRef]





| Time | Plant Type | F-Ratio | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Radish | Peanut | Buckwheat | Vetch | Citrus | Cowpea | Clover | Negative Control | |||
| Day 2 | 23 ± 1 a | 22 ± 1 a | 24 ± 1 a | 23 ± 1 a | 21 ± 1 a | 22 ± 1 a | 22 ± 1 a | 16 ± 1 b | 19.78 | <0.0001 |
| Day 4 | 21 ± 1 a | 15 ± 2 b | 17 ± 2 ab | 15 ± 1 b | 20 ± 1 ab | 16 ± 1 b | 18 ± 1 ab | 10 ± 1 c | 9.1 | <0.0001 |
| Day 6 | 12 ± 1 b | 11 ± 1 bc | 12 ± 2 b | 11 ± 1 bc | 19 ± 1 a | 5 ± 2 cd | 13 ± 1 ab | 2 ± 1 d | 13.4 | <0.0001 |
| Day 8 | 10 ± 2 b | 9 ± 1 b | 8 ± 2 b | 6 ± 1 b | 18 ± 1 a | 5 ± 1 bc | 10 ± 1 b | 0 ± 0 b | 19.1 | <0.0001 |
| Day 10 | 0 ± 0 b | 1 ± 1 b | 0 ± 0 b | 0 ± 0 b | 15 ± 1 a | 0 ± 0 b | 0 ± 0 b | 0 ± 0 b | 344.3 | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
George, J.; Kanissery, R.; Bashyal, M.; Tamayo, B.; Stelinski, L.L. Survival and Feeding Behavior of Diaphorina citri (Hemiptera: Liviidae) Adults on Common Cover Crops in Citrus. Agriculture 2022, 12, 2175. https://doi.org/10.3390/agriculture12122175
George J, Kanissery R, Bashyal M, Tamayo B, Stelinski LL. Survival and Feeding Behavior of Diaphorina citri (Hemiptera: Liviidae) Adults on Common Cover Crops in Citrus. Agriculture. 2022; 12(12):2175. https://doi.org/10.3390/agriculture12122175
Chicago/Turabian StyleGeorge, Justin, Ramdas Kanissery, Mahesh Bashyal, Blessy Tamayo, and Lukasz L. Stelinski. 2022. "Survival and Feeding Behavior of Diaphorina citri (Hemiptera: Liviidae) Adults on Common Cover Crops in Citrus" Agriculture 12, no. 12: 2175. https://doi.org/10.3390/agriculture12122175
APA StyleGeorge, J., Kanissery, R., Bashyal, M., Tamayo, B., & Stelinski, L. L. (2022). Survival and Feeding Behavior of Diaphorina citri (Hemiptera: Liviidae) Adults on Common Cover Crops in Citrus. Agriculture, 12(12), 2175. https://doi.org/10.3390/agriculture12122175








