Ripeness Evaluation of Achacha Fruit Using Hyperspectral Image Data
Abstract
1. Introduction
2. Materials and Methods
2.1. Hyperspectral Image Data Acquisition and Preparation
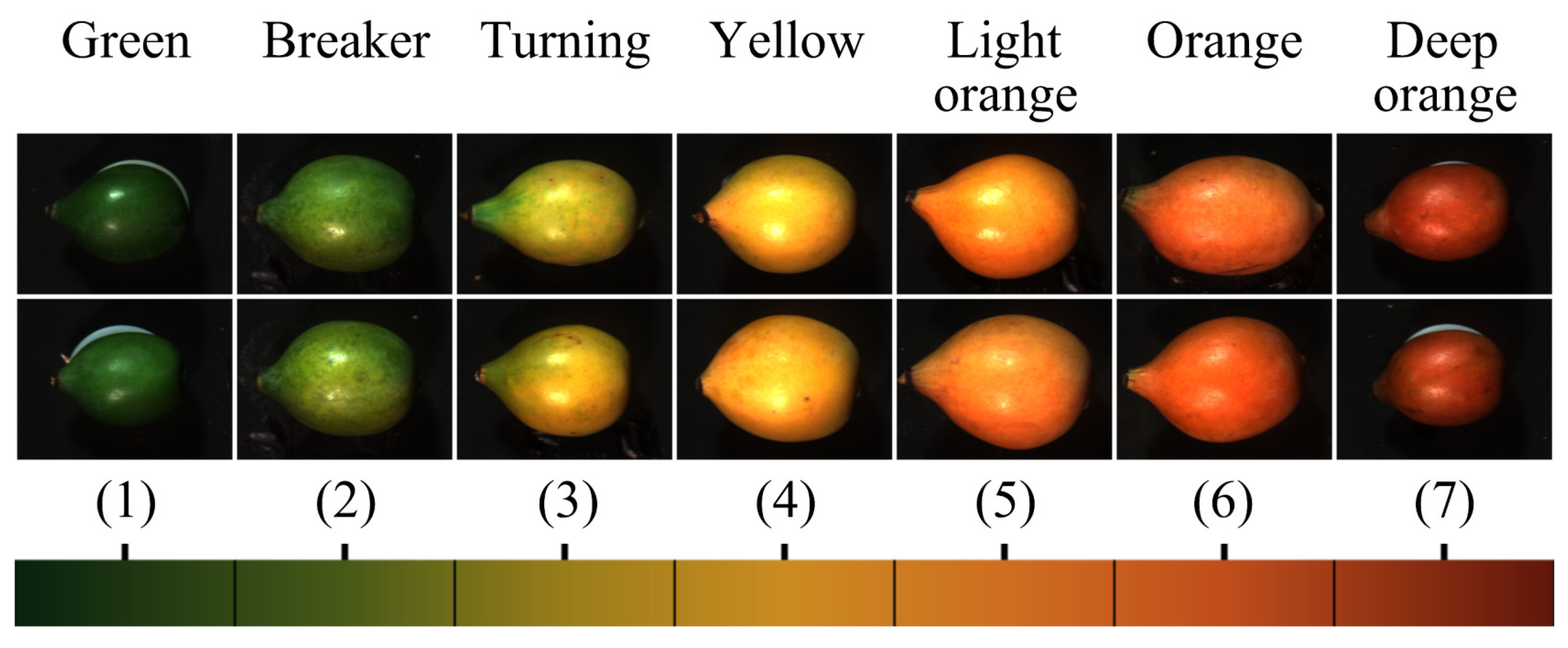
2.2. Achacha Samples
2.3. Training and Validation Data Preparation
2.4. Ripeness Evaluation Models
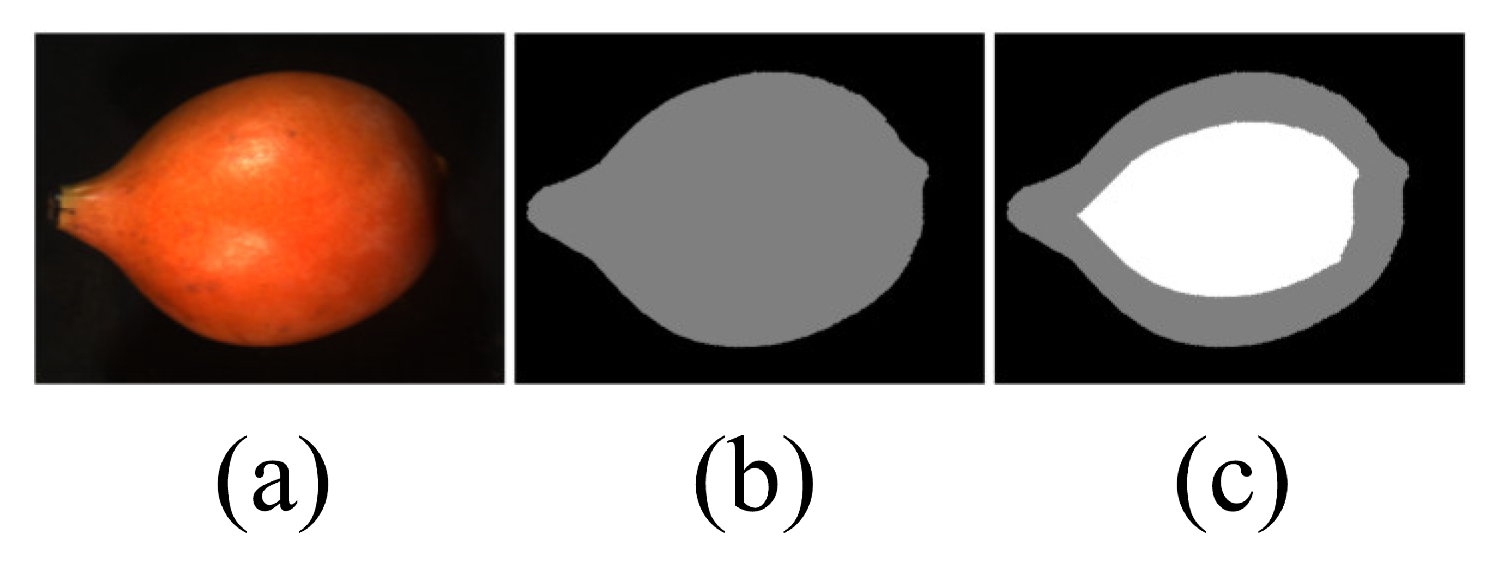
2.5. Pixel-Based Classification and One-Level Error Prediction
3. Results and Discussion
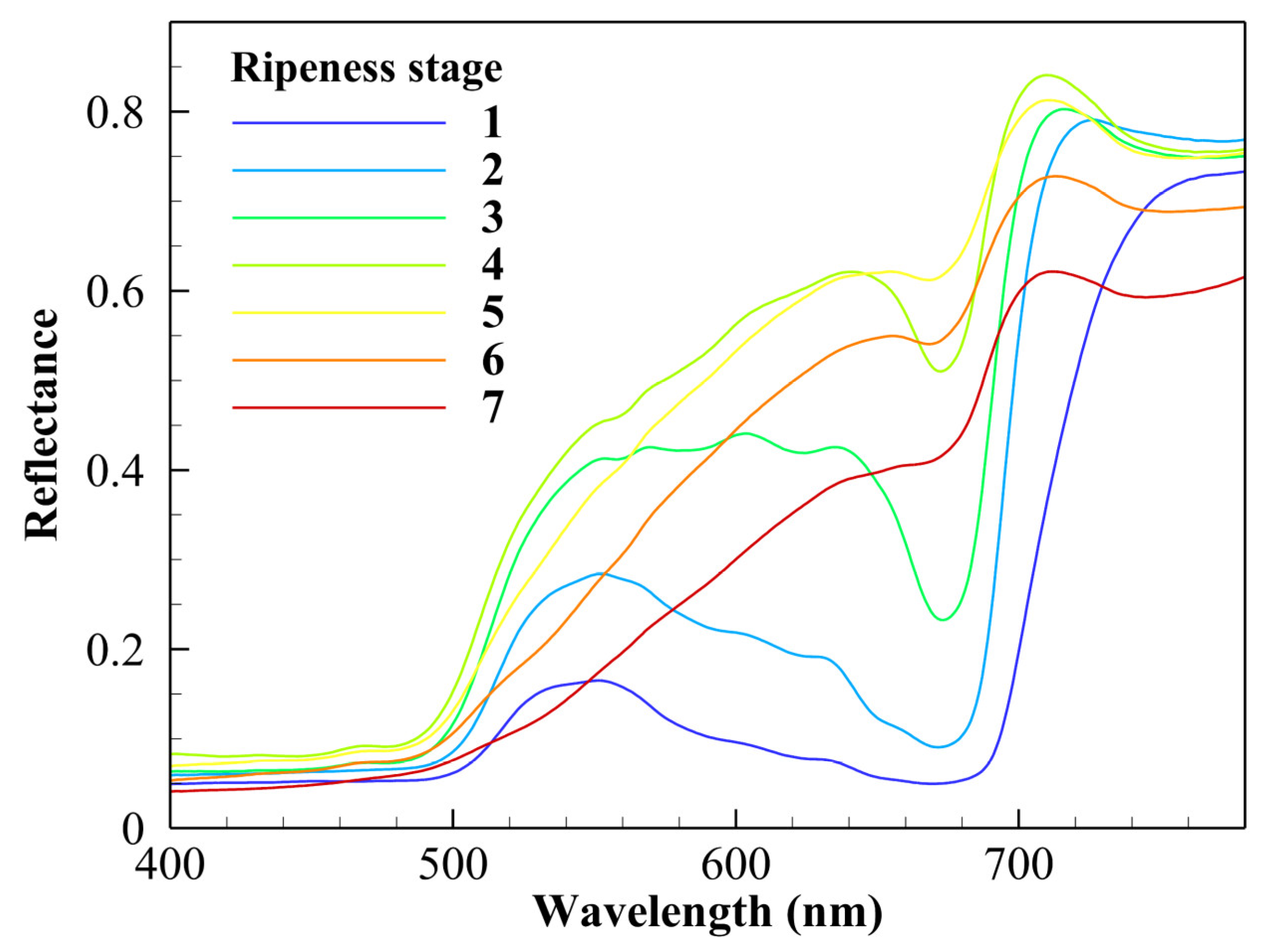
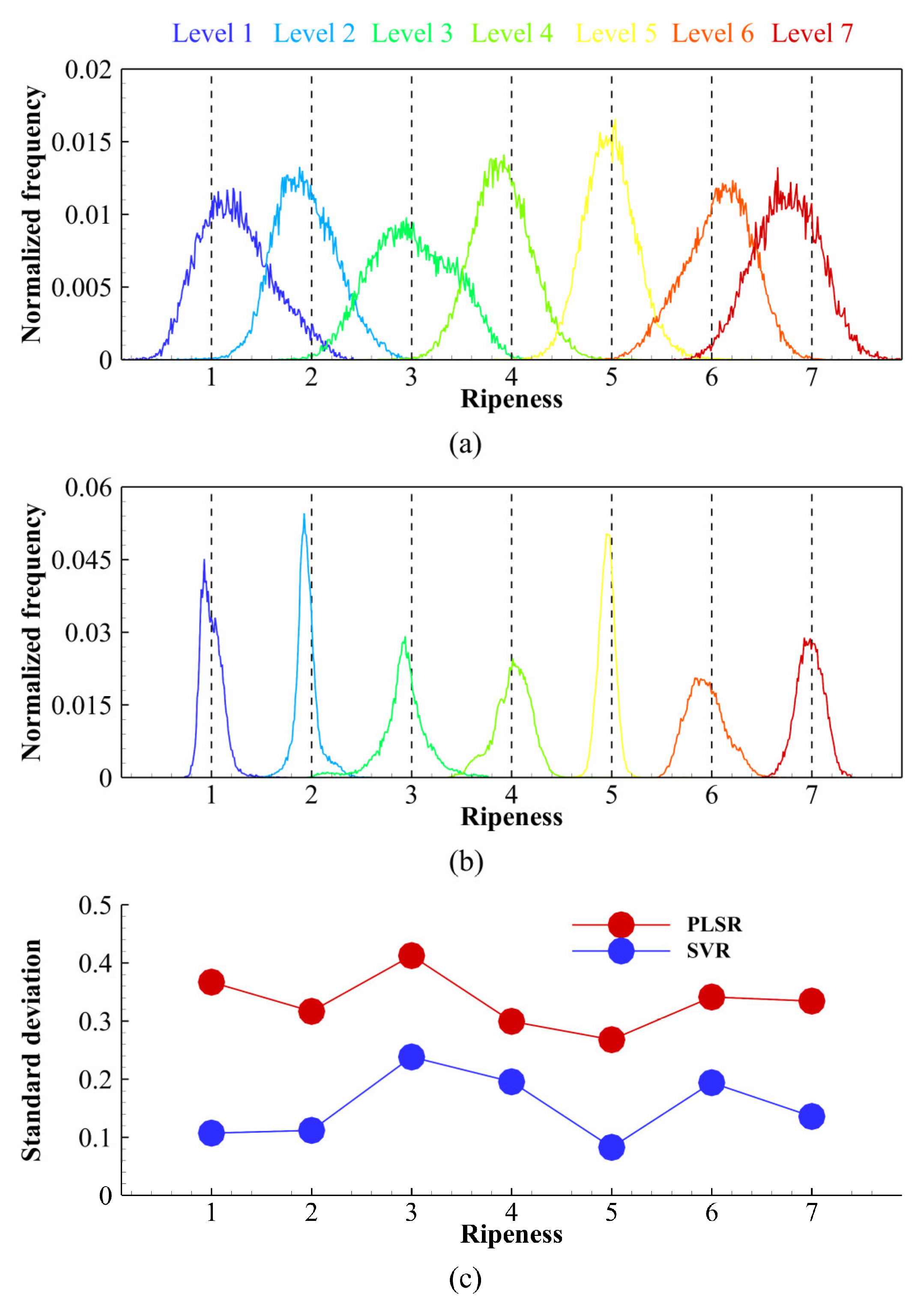
3.1. Spectral Characteristics of Different Ripeness Stages
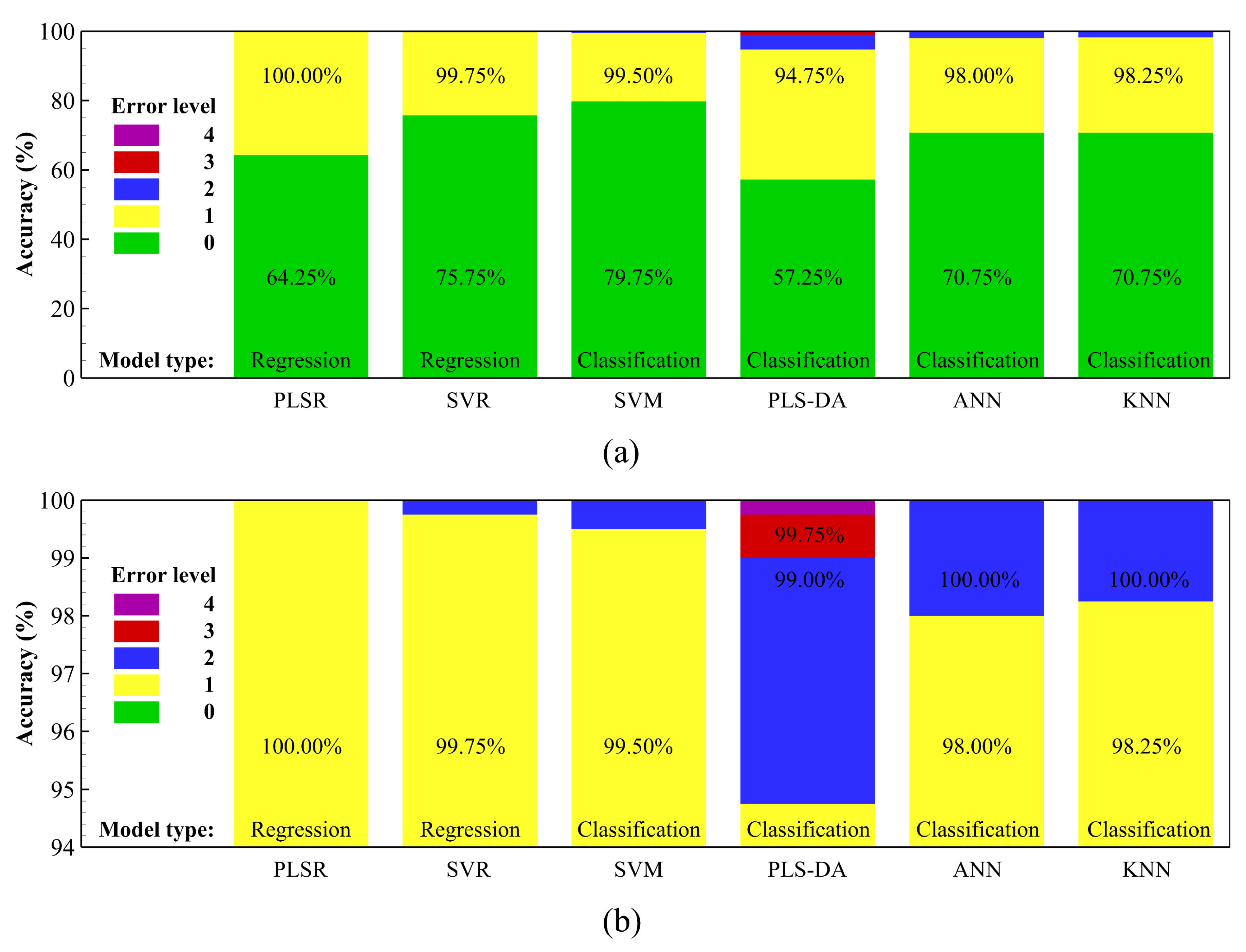
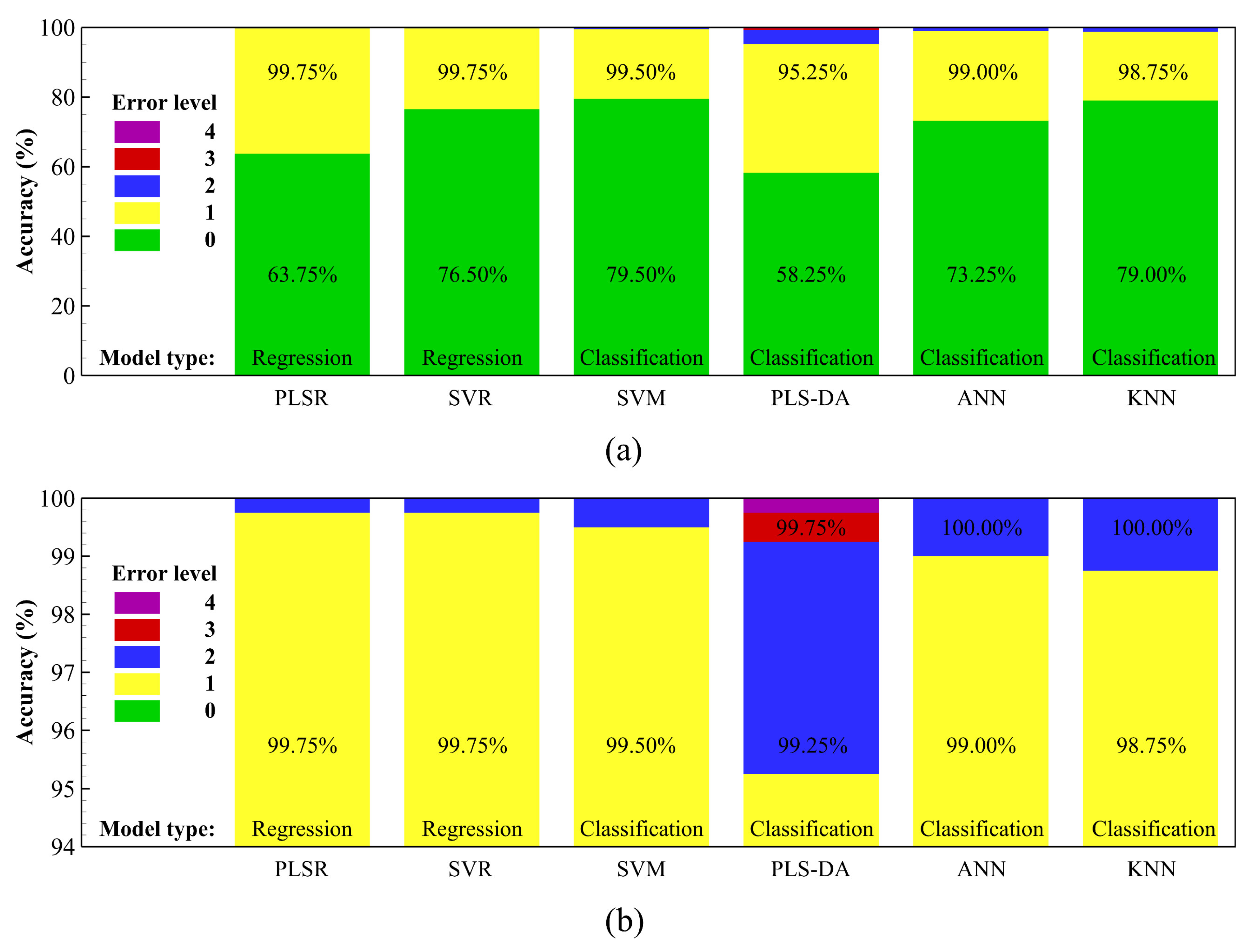
3.2. Average-Spectrum Approach Using Regression and Classification Algorithms
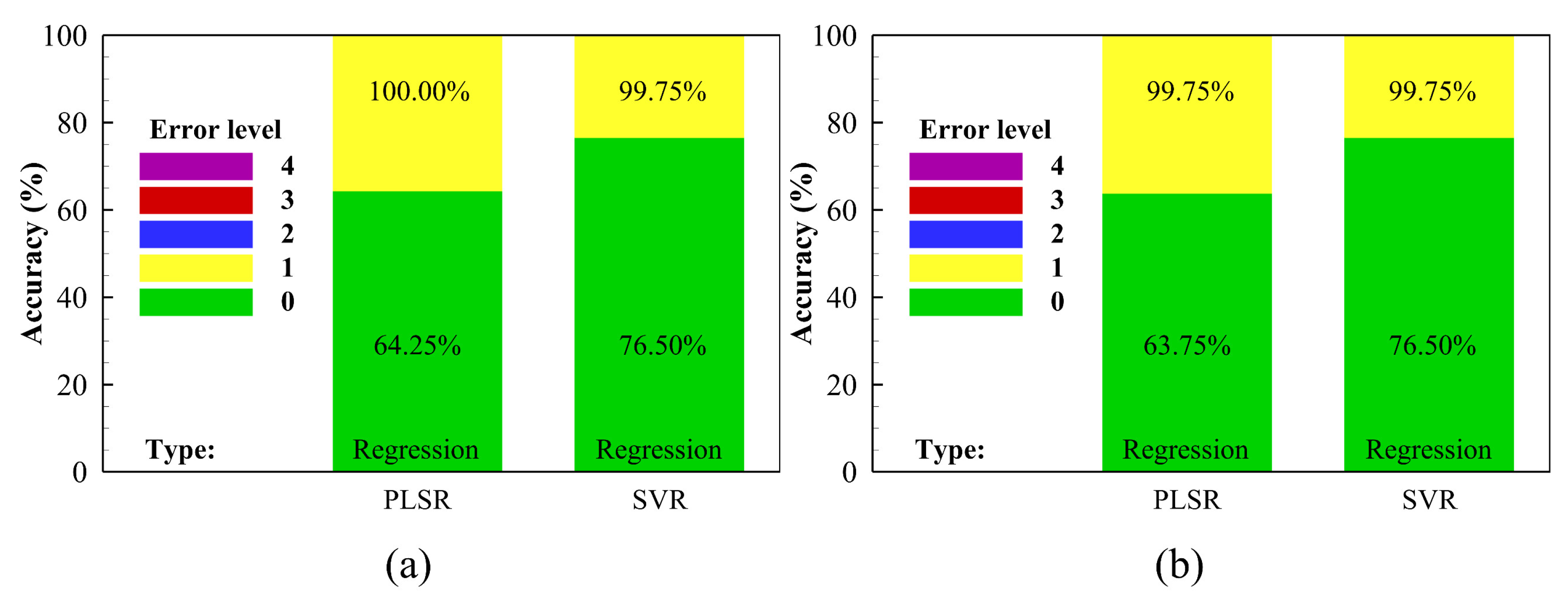
3.3. The Effect of Curvature on Ripeness Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Toivonen, P.M. Fruit maturation and ripening and their relationship to quality. Stewart Postharvest Rev. 2007, 3, 1–5. [Google Scholar] [CrossRef]
- Nunes, R.; Broering, M.F.; De Faveri, R.; Goldoni, F.C.; Mariano, L.N.B.; Mafessoli, P.C.M.; Delle Monache, F.; Cechinel, V.; Niero, R.; Santin, J.R.; et al. Effect of the metanolic extract from the leaves of Garcinia humilis Vahl (Clusiaceae) on acute inflammation. Inflammopharmacology 2021, 29, 423–438. [Google Scholar] [CrossRef] [PubMed]
- Almeida, R.L.J.; Santos, N.C.; Alves, I.L.; Andre, A.M.M.C.N. Evaluation of thermodynamic properties and antioxidant activities of Achachairu (Garcinia humilis) peels under drying process. Flavour. Frag. J. 2021, 36, 213–222. [Google Scholar] [CrossRef]
- Valero, D.; Serrano, M. Growth and ripening stage at harvest modulates postharvest quality and bioactive compounds with antioxidant activity. Stewart Postharvest Rev. 2013, 3, 7. [Google Scholar]
- Li, B.; Lecourt, J.; Bishop, G. Advances in Non-Destructive Early Assessment of Fruit Ripeness towards Defining Optimal Time of Harvest and Yield Prediction-A Review. Plants 2018, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Birth, G.S.; Norris, K.H. An instrument using light transmittance for nondestructive measurement of fruit maturity. Food Technol. 1958, 12, 592–595. [Google Scholar]
- Gonzalez-Araiza, J.R.; Ortiz-Sanchez, M.C.; Vargas-Luna, F.M.; Cabrera-Sixto, J.M. Application of electrical bio-impedance for the evaluation of strawberry ripeness. Int. J. Food Prop. 2017, 20, 1044–1050. [Google Scholar] [CrossRef]
- Llobet, E.; Hines, E.L.; Gardner, J.W.; Franco, S. Non-destructive banana ripeness determination using a neural network-based electronic nose. Meas. Sci. Technol. 1999, 10, 538. [Google Scholar] [CrossRef]
- Choi, K.; Lee, G.; Han, Y.J.; Bunn, J.M. Tomato Maturity Evaluation Using Color Image-Analysis. Trans. Asae 1995, 38, 171–176. [Google Scholar] [CrossRef]
- Yang, H.Q. Nondestructive Prediction of Optimal Harvest Time of Cherry Tomatoes Using VIS-NIR Spectroscopy and PLSR Calibration. Adv. Eng. Forum 2011, 1, 92–96. [Google Scholar] [CrossRef]
- Sivakumar, S.S.; Qiao, J.; Wang, N.; Gariépy, Y.; Raghavan, G.S.V.; McGill, J. Detecting Maturity Parameters of Mango Using Hyperspectral Imaging Technique; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2006. [Google Scholar]
- Lleo, L.; Barreiro, P.; Ruiz-Altisent, M.; Herrero, A. Multispectral images of peach related to firmness and maturity at harvest. J. Food Eng. 2009, 93, 229–235. [Google Scholar] [CrossRef]
- Flitsanov, U.; Mizrach, A.; Liberzon, A.; Akerman, M.; Zauberman, G. Measurement of avocado softening at various temperatures using ultrasound. Postharvest Biol. Technol. 2000, 20, 279–286. [Google Scholar] [CrossRef][Green Version]
- El-Bendary, N.; El Hariri, E.; Hassanien, A.E.; Badr, A. Using machine learning techniques for evaluating tomato ripeness. Expert Syst. Appl. 2015, 42, 1892–1905. [Google Scholar] [CrossRef]
- Wan, P.; Toudeshki, A.; Tan, H.Q.; Ehsani, R. A methodology for fresh tomato maturity detection using computer vision. Comput. Electron. Agric. 2018, 146, 43–50. [Google Scholar] [CrossRef]
- Cardenas-Perez, S.; Chanona-Perez, J.; Mendez-Mendez, J.V.; Calderon-Dominguez, G.; Lopez-Santiago, R.; Perea-Flores, M.J.; Arzate-Vazquez, I. Evaluation of the ripening stages of apple (Golden Delicious) by means of computer vision system. Biosyst. Eng. 2017, 159, 46–58. [Google Scholar] [CrossRef]
- Mohammadi, V.; Kheiralipour, K.; Ghasemi-Varnamkhasti, M. Detecting maturity of persimmon fruit based on image processing technique. Sci. Hortic. 2015, 184, 123–128. [Google Scholar] [CrossRef]
- Kheiralipour, K.; Nadimi, M.; Paliwal, J. Development of an Intelligent Imaging System for Ripeness Determination of Wild Pistachios. Sensors 2022, 22, 7134. [Google Scholar] [CrossRef] [PubMed]
- Azadnia, R.; Kheiralipour, K. Evaluation of hawthorns maturity level by developing an automated machine learning-based algorithm. Ecol. Inform. 2022, 71, 101804. [Google Scholar] [CrossRef]
- Jiang, Y.P.; Chen, S.F.; Bian, B.; Li, Y.H.; Sun, Y.; Wang, X.C. Discrimination of Tomato Maturity Using Hyperspectral Imaging Combined with Graph-Based Semi-supervised Method Considering Class Probability Information. Food Anal. Methods 2021, 14, 968–983. [Google Scholar] [CrossRef]
- Arias, R.; Lee, T.C.; Logendra, L.; Janes, H. Correlation of lycopene measured by HPLC with the L*, a* b* color readings of a hydroponic tomato and the relationship of maturity with color and lycopene content. J. Agric. Food Chem. 2000, 48, 1697–1702. [Google Scholar] [CrossRef]
- Wang, H.T.; Hu, R.; Zhang, M.Y.; Zhai, Z.Q.; Zhang, R.Y. Identification of tomatoes with early decay using visible and near infrared hyperspectral imaging and image-spectrum merging technique. J. Food Process Eng. 2021, 44, e13654. [Google Scholar] [CrossRef]
- Prasanna, V.; Prabha, T.N.; Tharanathan, R.N. Fruit ripening phenomena–An overview. Crit. Rev. Food Sci. Nutr. 2007, 47, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Riley, D.L.; Bryan, S.; Jacob, S.; Neil, S.W.; Amy, P.; John, W.S.; Joseph, A.S. Hyperspectral imaging and machine learning for monitoring produce ripeness. In Proceedings of the Sensing for Agriculture and Food Quality and Safety XII, Online, 22 April 2020; p. 114210O. [Google Scholar]
- Zhang, C.; Guo, C.T.; Liu, F.; Kong, W.W.; He, Y.; Lou, B.G. Hyperspectral imaging analysis for ripeness evaluation of strawberry with support vector machine. J. Food Eng. 2016, 179, 11–18. [Google Scholar] [CrossRef]
- Wei, X.; Liu, F.; Qiu, Z.J.; Shao, Y.N.; He, Y. Ripeness Classification of Astringent Persimmon Using Hyperspectral Imaging Technique. Food Bioprocess Technol. 2014, 7, 1371–1380. [Google Scholar] [CrossRef]
- Yang, C.; Lee, W.S.; Gader, P. Hyperspectral band selection for detecting different blueberry fruit maturity stages. Comput. Electron. Agric. 2014, 109, 23–31. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, Z.; Chen, Z.; Liu, J.; Wang, W.C.; Zheng, W.Y. Hyperspectral Surface Analysis for Ripeness Estimation and Quick UV-C Surface Treatments for Preservation of Bananas. J. Appl. Spectrosc. 2016, 83, 254–260. [Google Scholar] [CrossRef]
- Pu, Y.Y.; Sun, D.W.; Buccheri, M.; Grassi, M.; Cattaneo, T.M.P.; Gowen, A. Ripeness Classification of Bananito Fruit (Musa acuminata, AA): A Comparison Study of Visible Spectroscopy and Hyperspectral Imaging. Food Anal. Methods 2019, 12, 1693–1704. [Google Scholar] [CrossRef]
- Khodabakhshian, R.; Emadi, B. Application of Vis/SNIR hyperspectral imaging in ripeness classification of pear. Int. J. Food Prop. 2018, 20, S3149–S3163. [Google Scholar] [CrossRef]
- Polder, G.; van der Heijden, G.; Young, I. Hyperspectral image analysis for measuring ripeness of tomatoes. Trans. ASAE 2000, 45, 1155–1161. [Google Scholar]
- Zhang, M.S.; Zhang, B.; Li, H.; Shen, M.S.; Tian, S.J.; Zhang, H.H.; Ren, X.L.; Xing, L.B.; Zhao, J. Determination of bagged ‘Fuji’ apple maturity by visible and near-infrared spectroscopy combined with a machine learning algorithm. Infrared Phys. Technol. 2020, 111, 103529. [Google Scholar] [CrossRef]
- Van Der Heijden, G.W.; Polder, G.; Gevers, T. Comparison of multispectral images across the Internet. In Proceedings of the Internet Imaging, San Jose, CA, USA, 26–28 January 2000; pp. 196–206. [Google Scholar]
- Wei, X.; He, J.C.; Ye, D.P.; Jie, D.F. Navel Orange Maturity Classification by Multispectral Indexes Based on Hyperspectral Diffuse Transmittance Imaging. J. Food Qual. 2017, 2017, 1023498. [Google Scholar] [CrossRef]
- Xuan, G.T.; Gao, C.; Shao, Y.Y.; Wang, X.Y.; Wang, Y.X.; Wang, K.L. Maturity determination at harvest and spatial assessment of moisture content in okra using Vis-NIR hyperspectral imaging. Postharvest Biol. Technol. 2021, 180, 111597. [Google Scholar] [CrossRef]
- Shao, Y.Y.; Wang, Y.X.; Xuan, G.T.; Gao, Z.M.; Hu, Z.C.; Gao, C.; Wang, K.L. Assessment of Strawberry Ripeness Using Hyperspectral Imaging. Anal. Lett. 2020, 54, 1547–1560. [Google Scholar] [CrossRef]
- Munera, S.; Amigo, J.M.; Blasco, J.; Cubero, S.; Talens, P.; Aleixos, N. Ripeness monitoring of two cultivars of nectarine using VIS-NIR hyperspectral reflectance imaging. J. Food Eng. 2017, 214, 29–39. [Google Scholar] [CrossRef]
- Drucker, H.; Burges, C.J.; Kaufman, L.; Smola, A.; Vapnik, V. Support vector regression machines. In Advances in Neural Information Processing Systems; MIT Press: Cambridge, MA, USA, 1996; Volume 9. [Google Scholar]
- Awad, M.; Khanna, R. Efficient Learning Machines: Theories, Concepts, and Applications for Engineers and System Designers; Springer Nature: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Silva, R.; Gomes, V.; Mendes-Faia, A.; Melo-Pinto, P. Using Support Vector Regression and Hyperspectral Imaging for the Prediction of Oenological Parameters on Different Vintages and Varieties of Wine Grape Berries. Remote Sens. 2018, 10, 312. [Google Scholar] [CrossRef]
- Azarmdel, H.; Jahanbakhshi, A.; Mohtasebi, S.S.; Munoz, A.R. Evaluation of image processing technique as an expert system in mulberry fruit grading based on ripeness level using artificial neural networks (ANNs) and support vector machine (SVM). Postharvest Biol. Technol. 2020, 166, 111201. [Google Scholar] [CrossRef]
- Jaramillo-Acevedo, C.A.; Choque-Valderrama, W.E.; Guerrero-Alvarez, G.E.; Meneses-Escobar, C.A. Hass avocado ripeness classification by mobile devices using digital image processing and ANN methods. Int. J. Food Eng. 2020, 16, 20190161. [Google Scholar] [CrossRef]
- Amirulah, R.; Mokji, M.; Ibrahim, Z. Starfruit color maturity classification using Cr as feature. In Proceedings of the 2010 Sixth International Conference on Signal-Image Technology and Internet Based Systems, Kuala Lumpur, Malaysia, 15–18 December 2010; pp. 93–97. [Google Scholar]
- Garcia, M.B.; Ambat, S.; Adao, R.T. Tomayto, tomahto: A machine learning approach for tomato ripening stage identification using pixel-based color image classification. In Proceedings of the 2019 IEEE 11th International Conference on Humanoid, Nanotechnology, Information Technology, Communication and Control, Environment, and Management (HNICEM), Laoag, Philippines, 29 November–1 December 2019; pp. 1–6. [Google Scholar]
- Polder, G.; van der Heijden, G.W.A.M.; Young, I.T. Spectral image analysis for measuring ripeness of tomatoes. Trans. Asae 2002, 45, 1155–1161. [Google Scholar] [CrossRef]
- Seager, S.; Turner, E.L.; Schafer, J.; Ford, E.B. Vegetation’s red edge: A possible spectroscopic biosignature of extraterrestrial plants. Astrobiology 2005, 5, 372–390. [Google Scholar] [CrossRef]
- Gates, D.M.; Keegan, H.J.; Schleter, J.C.; Weidner, V.R. Spectral properties of plants. Appl. Opt. 1965, 4, 11–20. [Google Scholar] [CrossRef]
- Delwiche, S.R.; Baek, I.; Kim, M.S. Effect of curvature on hyperspectral reflectance images of cereal seed-sized objects. Biosyst. Eng. 2021, 202, 55–65. [Google Scholar] [CrossRef]
- Rogelj, L.; Simoncic, U.; Tomanic, T.; Jezersek, M.; Pavlovcic, U.; Stergar, J.; Milanic, M. Effect of curvature correction on parameters extracted from hyperspectral images. J. Biomed. Opt. 2021, 26, 096003. [Google Scholar] [CrossRef] [PubMed]
- Valero, D.; Serrano, M. Postharvest Biology and Technology for Preserving Fruit Quality; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Omar, A.F.; MatJafri, M.Z. Near infrared spectral linearisation in quantifying soluble solids content of intact carambola. Sensors 2013, 13, 4876–4883. [Google Scholar] [CrossRef] [PubMed]
- Li, X.L.; Wei, Y.Z.; Xu, J.; Feng, X.P.; Wu, F.Y.; Zhou, R.Q.; Jin, J.J.; Xu, K.W.; Yu, X.J.; He, Y. SSC and pH for sweet assessment and maturity classification of harvested cherry fruit based on NIR hyperspectral imaging technology. Postharvest Biol. Technol. 2018, 143, 112–118. [Google Scholar] [CrossRef]
- Blanke, M.M. Non-invasive Assessment of Firmness and NIR Sugar (TSS) Measurement in Apple, Pear and Kiwi Fruit. Erwerbs-Obstbau 2013, 55, 19–24. [Google Scholar] [CrossRef]
- Teerachaichayut, S.; Ho, H.T. Non-destructive prediction of total soluble solids, titratable acidity and maturity index of limes by near infrared hyperspectral imaging. Postharvest Biol. Technol. 2017, 133, 20–25. [Google Scholar] [CrossRef]
- Chauchard, F.; Cogdill, R.; Roussel, S.; Roger, J.M.; Bellon-Maurel, V. Application of LS-SVM to non-linear phenomena in NIR spectroscopy: Development of a robust and portable sensor for acidity prediction in grapes. Chemom. Intell. Lab. Syst. 2004, 71, 141–150. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, N.M.T.; Liou, N.-S. Ripeness Evaluation of Achacha Fruit Using Hyperspectral Image Data. Agriculture 2022, 12, 2145. https://doi.org/10.3390/agriculture12122145
Nguyen NMT, Liou N-S. Ripeness Evaluation of Achacha Fruit Using Hyperspectral Image Data. Agriculture. 2022; 12(12):2145. https://doi.org/10.3390/agriculture12122145
Chicago/Turabian StyleNguyen, Ngo Minh Tri, and Nai-Shang Liou. 2022. "Ripeness Evaluation of Achacha Fruit Using Hyperspectral Image Data" Agriculture 12, no. 12: 2145. https://doi.org/10.3390/agriculture12122145
APA StyleNguyen, N. M. T., & Liou, N.-S. (2022). Ripeness Evaluation of Achacha Fruit Using Hyperspectral Image Data. Agriculture, 12(12), 2145. https://doi.org/10.3390/agriculture12122145



