Diets Fermented with Bacteria and Enzymes in China Improve Growth Performance and Health of Weaned Piglets
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Animal Management
2.2. Experimental Diet
3. Sample Collections
3.1. Feed Sample Collection
3.2. Faecal Sample Collection
3.3. Blood Sample Collection
3.4. Tissue Sample Collection
4. Chemical Analysis
4.1. Measurement of Apparent Digestibility of Nutrients
4.2. Measurement of Serum Indexes
4.3. Measurement of Antioxidant Indexes of the Jejunum
4.4. Measurement of Intestinal Permeability
4.5. Microbial Metabolites Analysis
4.6. Gene Expression in Intestinal Mucosa
4.7. DNA Extraction and Quantification of Intestinal Microflora
4.8. Calculation and Statistical Analysis
5. Results
5.1. Growth Performance
5.2. Nutrient Digestibility
5.3. Physiochemical Parameters in Serum
5.4. Antioxidant Capacity
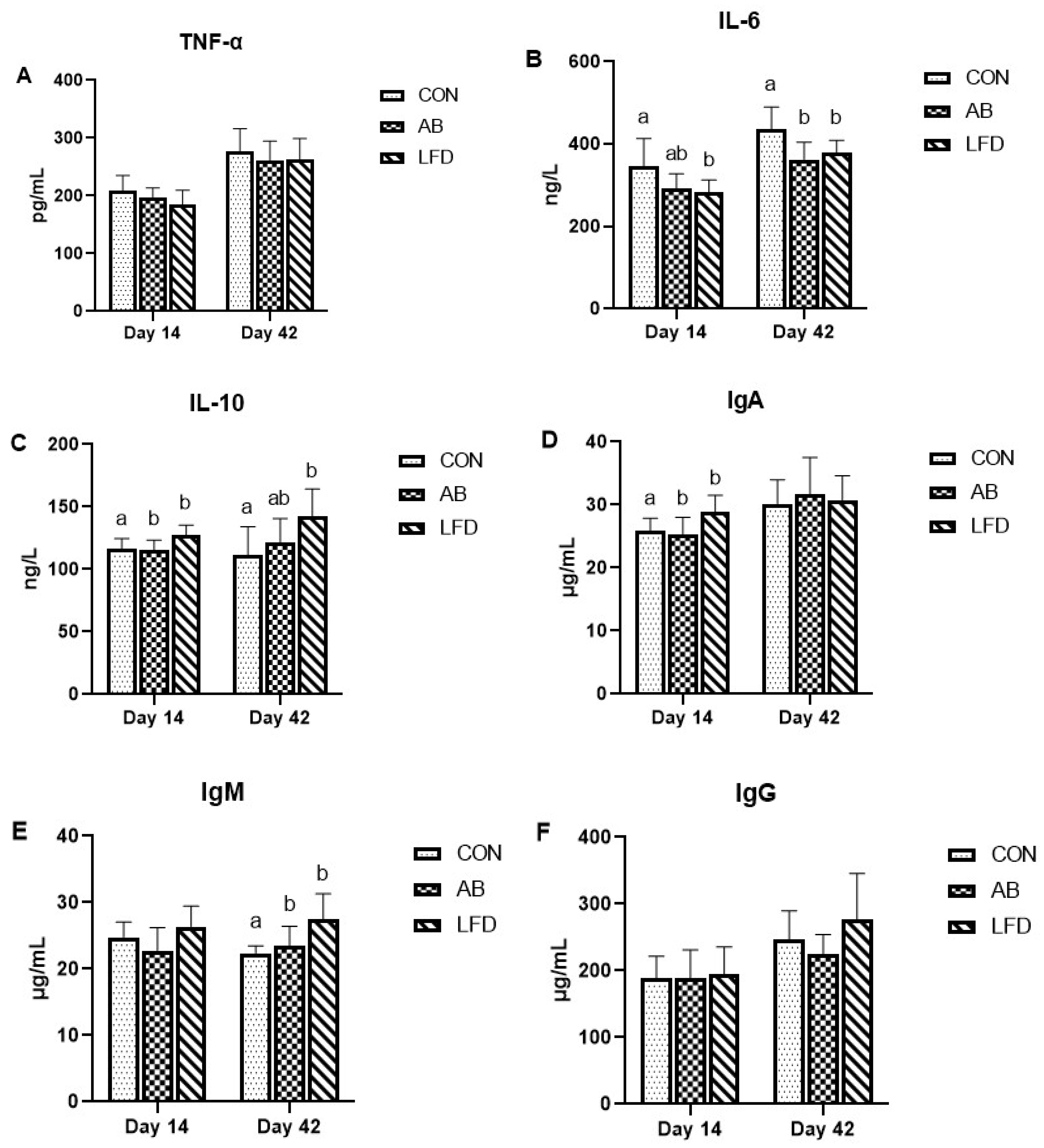
5.5. Serum Immune Index
5.6. DAO Activity and D-Lactate Concentration
5.7. Gene Expression of Tight Junction Proteins
5.8. Intestinal Microbiota
5.9. Microbial Metabolites
6. Discussion
6.1. Growth Performance
6.2. Intestinal Barrier Function, Antioxidant Capacity, and Immune Function
6.3. Intestinal Microflora and Microbial Metabolites
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, Y.H.; Hsu, T.Y.; Chen, W.J.; Horng, Y.B.; Cheng, Y.H. The Effect of Bacillus licheniformis-Fermented Products and Postpartum Dysgalactia Syndrome on Litter Performance Traits, Milk Composition, and Fecal Microbiota in Sows. Animals 2020, 10, 2044. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shi, C.; Wang, C.; Lu, Z.; Wang, F.; Feng, J.; Wang, Y. Effect of soybean meal fermented with Bacillus subtilis BS12 on growth performance and small intestinal immune status of piglets. Food Agric. Immunol. 2018, 29, 133–146. [Google Scholar] [CrossRef] [Green Version]
- Brooks, P.; Beal, J.; Niven, S.; Demeckova, V. Liquid feeding of pigs 2. Potential for improving pig health and food safety. In Proceedings of the IV Conference on Effect of Genetic and Non-Genetic Factors on Carcass and Meat Quality of Pigs, Siedlce, Poland, 24–25 April 2003. [Google Scholar]
- Russell, P.J.; Geary, T.M.; Brooks, P.H.; Campbell, A. Performance, Water Use and Effluent Output of Weaner Pigs Fedad libitumwith Either Dry Pellets or Liquid Feed and the Role of Microbial Activity in the Liquid Feed. J. Sci. Food Arg. 1996, 72, 8–16. [Google Scholar] [CrossRef]
- Deprez, P.; Deroose, P.; Van Den Hende, C.; Muylle, E.; Oyaert, W. Liquid versus dry feeding in weaned piglets: The influence on small intestinal morphology. Zent. Vet. B 1987, 34, 254–259. [Google Scholar] [CrossRef]
- Canibe, N.; Højberg, O.; Badsberg, J.H.; Jensen, B.B. Effect of feeding fermented liquid feed and fermented grain on gastrointestinal ecology and growth performance in piglets. J. Anim. Sci. 2007, 85, 2959–2971. [Google Scholar] [CrossRef] [Green Version]
- Van Winsen, R.L.; Urlings, B.A.; Lipman, L.J.; Snijders, J.M.; Keuzenkamp, D.; Verheijden, J.H.; Van Knapen, F. Effect of fermented feed on the microbial population of the gastrointestinal tracts of pigs. Appl. Environ. Microbiol. 2001, 67, 3071–3076. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Zhang, L.; Li, H.; Smidt, H.; Wright, A.-D.G.; Zhang, K.; Ding, X.; Zeng, Q.; Bai, S.; Wang, J.; et al. Different Types of Dietary Fibers Trigger Specific Alterations in Composition and Predicted Functions of Colonic Bacterial Communities in BALB/c Mice. Front. Microbiol. 2017, 8, 966. [Google Scholar] [CrossRef] [Green Version]
- Qi, H.; Xiang, Z.; Han, G.; Yu, B.; Huang, Z.; Chen, D. Effects of different dietary protein sources on cecal microflora in rats. Afr. J. Biotechnol. 2011, 10, 3704–3708. [Google Scholar]
- Xu, X.; Li, L.M.; Li, B.; Guo, W.J.; Ding, X.L.; Xu, F.Z. Effect of fermented biogas residue on growth performance, serum biochemical parameters, and meat quality in pigs. Asian-Austral. J. Anim. Sci. 2017, 30, 1464–1470. [Google Scholar] [CrossRef]
- Feng, J.; Liu, X.; Xu, Z.R.; Lu, Y.P.; Liu, Y.Y. The effect of Aspergillus oryzae fermented soybean meal on growth performance, digestibility of dietary components and activities of intestinal enzymes in weaned piglets. Anim. Feed Sci. Technol. 2007, 134, 295–303. [Google Scholar] [CrossRef]
- Ao, X.; Meng, Q.W.; Kim, I.H. Effects of Fermented Red Ginseng Supplementation on Growth Performance, Apparent Nutrient Digestibility, Blood Hematology and Meat Quality in Finishing Pigs. Asian-Austral. J. Anim. Sci. 2011, 24, 525–531. [Google Scholar] [CrossRef]
- Hung, A.T.Y.; Su, T.M.; Liao, C.W.; Lu, J.J. Effect of Probiotic Combination Fermented Soybean Meal on Growth Performance, Lipid Metabolism and Immunological Response of Growing-Finishing Pigs. J. Adv. Vet. Anim. Res. 2008, 3, 431–436. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.F.; Chen, Q.; Le, G.W.; Shi, Y.H.; Sun, J. Effect of lactic acid fermented soyabean meal on the growth performance, intestinal microflora and morphology of weaned piglets. J. Anim. Feed Sci. 2007, 16, 75–85. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, W.Q.; Li, D.F.; Liu, X.T.; Wang, H.L.; Niu, S.; Piao, X.S. Energy and ileal digestible amino Acid concentrations for growing pigs and performance of weanling pigs fed fermented or conventional soybean meal. Asian-Austral. J. Anim. Sci. 2014, 27, 706–716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, P.F.; Xue, L.F.; Zhang, R.F.; Piao, X.S.; Zeng, Z.K.; Zhan, J.S. Effects of Fermented Potato Pulp on Performance, Nutrient Digestibility, Carcass Traits and Plasma Parameters of Growing-finishing Pigs. Asian-Austral. J. Anim. Sci. 2011, 24, 1456–1463. [Google Scholar] [CrossRef]
- Liu, P.; Zhao, J.; Guo, P.; Lu, W.; Geng, Z.; Levesque, C.L.; Johnston, L.J.; Wang, C.; Liu, L.; Zhang, J.; et al. Dietary Corn Bran Fermented by Bacillus subtilis MA139 Decreased Gut Cellulolytic Bacteria and Microbiota Diversity in Finishing Pigs. Front. Cell Infect. Microbiol. 2017, 7, 526. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Kim, I.H. Effect of dietary fermented corn in different energy dense diets on growth performance, nutrient digestibility, ileal microorganisms, and fecal noxious gas emission of growing pigs. Can J. Anim. Sci. 2018, 98, 325–332. [Google Scholar] [CrossRef]
- Waititu, S.M.; Heo, J.M.; Patterson, R.; Nyachoti, C.M. Dose-response effects of in-feed antibiotics on growth performance and nutrient utilization in weaned pigs fed diets supplemented with yeast-based nucleotides. Anim. Nutr. 2015, 1, 166–169. [Google Scholar] [CrossRef]
- Jiang, J.; Chen, D.; Yu, B.; He, J.; Yu, J.; Mao, X.; Huang, Z.; Luo, Y.; Luo, J.; Zheng, P. Improvement of growth performance and parameters of intestinal function in liquid fed early weanling pigs1. J. Anim. Sci. 2019, 97, 2725–2738. [Google Scholar] [CrossRef]
- Canibe, N.; Jensen, B.B. Fermented liquid feed-Microbial and nutritional aspects and impact on enteric diseases in pigs. Anim. Feed Sci. Technol. 2012, 173, 17–40. [Google Scholar] [CrossRef]
- Kim, Y.G.; Lohakare, J.D.; Yun, J.H.; Heo, S.; Chae, B.J. Effect of feeding levels of microbial fermented soy protein on the growth performance, nutrient digestibility and intestinal morphology in weaned piglets. Asian-Austral. J. Anim. Sci. 2007, 20, 399–404. [Google Scholar] [CrossRef]
- Kim, S.W.; Van Heugten, E.; Ji, F.; Lee, C.H.; Mateo, R.D. Fermented soybean meal as a vegetable protein source for nursery pigs: I. Effects on growth performance of nursery pigs. J. Anim. Sci. 2010, 88, 214–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, M.H.; Galle, S.; Yang, Y.; Landero, J.L.; Beltranena, E.; Gänzle, M.G.; Zijlstra, R.T. Effects of feeding fermented wheat with Lactobacillus reuteri on gut morphology, intestinal fermentation, nutrient digestibility, and growth performance in weaned pigs. J. Anim. Sci. 2016, 94, 4677–4687. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.H.; Zhang, Z.F.; Kim, I.H. Effects of fermented grains as raw cereal substitutes on growth performance, nutrient digestibility, blood profiles, and fecal noxious gas emission in growing pigs. Livest. Sci. 2013, 154, 131–136. [Google Scholar] [CrossRef]
- Smith, F.; Clark, J.E.; Overman, B.L.; Tozel, C.C.; Huang, J.H.; Rivier, J.E.; Blikslager, A.T.; Moeser, A.J. Early weaning stress impairs development of mucosal barrier function in the porcine intestine. Am. J. Physiol. Gastrointest Liver Physiol. 2010, 298, G352–G363. [Google Scholar] [CrossRef] [Green Version]
- Wittish, L.M.; Mcelroy, A.P.; Harper, A.F.; Estienne, M.J. Performance and physiology of pigs administered spray-dried plasma protein during the late suckling period and transported after weaning. J. Anim. Sci. 2014, 92, 4390–4399. [Google Scholar] [CrossRef]
- Wijtten, P.J.; Van Der Meulen, J.; Verstegen, M.W. Intestinal barrier function and absorption in pigs after weaning: A review. Br. J. Nutr. 2011, 105, 967–981. [Google Scholar] [CrossRef]
- Kim, J.C.; Hansen, C.F.; Mullan, B.P.; Pluske, J.R. Nutrition and pathology of weaner pigs: Nutritional strategies to support barrier function in the gastrointestinal tract. Anim. Feed Sci. Technol. 2012, 173, 3–16. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zheng, P.; Yu, B.; He, J.; Yu, J.; Mao, X.B.; Wang, J.X.; Luo, J.Q.; Huang, Z.Q.; Cheng, G.X.; et al. Dietary spray-dried chicken plasma improves intestinal barrier function and modulates immune status in weaning piglets. J. Anim. Sci. 2016, 94, 173–184. [Google Scholar] [CrossRef] [Green Version]
- Shen, L.; Weber, C.R.; Raleigh, D.R.; Yu, D.; Turner, J.R. Tight junction pore and leak pathways: A dynamic duo. Annu. Rev. Physiol. 2011, 73, 283–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, W.Y.; Wu, K.F.; Li, X.; Luo, M.; Liu, H.C.; Zhang, S.C.; Hu, Y. Age-related changes in small intestinal mucosa epithelium architecture and epithelial tight junction in rat models. Aging Clin. Exp. Res. 2014, 26, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Ulluwishewa, D.; Anderson, R.C.; Mcnabb, W.C.; Moughan, P.J.; Wells, J.M.; Roy, N.C. Regulation of tight junction permeability by intestinal bacteria and dietary components. J. Nutr. 2011, 141, 769–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, W.B.; Wang, Y.Y.; Meng, F.S.; Zhang, Q.H.; Zeng, J.Y.; Xiao, L.P.; Yu, X.P.; Peng, D.D.; Su, L.; Xiao, B.; et al. Curcumin protects intestinal mucosal barrier function of rat enteritis via activation of MKP-1 and attenuation of p38 and NF-κB activation. PLoS ONE 2010, 5, e12969. [Google Scholar] [CrossRef]
- Zhao, Y.; Qin, G.; Sun, Z.; Che, D.; Bao, N.; Zhang, X. Effects of soybean agglutinin on intestinal barrier permeability and tight junction protein expression in weaned piglets. Int. J. Mol. Sci. 2011, 12, 8502–8512. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, F.; Odle, J.; Lin, X.; Jacobi, S.K.; Zhu, H.; Wu, Z.; Hou, Y. Fish oil enhances intestinal integrity and inhibits TLR4 and NOD2 signaling pathways in weaned pigs after LPS challenge. J. Nutr. 2012, 142, 2017–2024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Feng, J.; Xu, Z.R.; Wang, Y.Z.; Liu, J.X. Oral allergy syndrome and anaphylactic reactions in BALB/c mice caused by soybean glycinin and beta-conglycinin. Clin. Exp. Allergy. 2008, 38, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Spreeuwenberg, M.A.; Verdonk, J.M.; Gaskins, H.R.; Verstegen, M.W. Small intestine epithelial barrier function is compromised in pigs with low feed intake at weaning. J. Nutr. 2001, 131, 1520–1527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moretó, M.; PéRez-Bosque, A. Dietary plasma proteins, the intestinal immune system, and the barrier functions of the intestinal mucosa1. J. Anim. Sci. 2009, 87, E92–E100. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, D.W.; Yu, B.; He, J.; Yu, J.; Mao, X.B.; Wang, J.X.; Luo, J.Q.; Huang, Z.Q.; Cheng, G.X.; et al. Spray-dried chicken plasma improves intestinal digestive function and regulates intestinal selected microflora in weaning piglets. J. Anim. Sci. 2015, 93, 2967–2976. [Google Scholar] [CrossRef]
- Schokker, D.; Zhang, J.; Zhang, L.-L.; Vastenhouw, S.A.; Heilig, H.G.H.J.; Smidt, H.; Rebel, J.M.J.; Smits, M.A. Early-Life Environmental Variation Affects Intestinal Microbiota and Immune Development in New-Born Piglets. PLoS ONE 2014, 9, e100040. [Google Scholar] [CrossRef]
- Yan, L.; Meng, Q.W.; Kim, I.H. Effects of fermented garlic powder supplementation on growth performance, nutrient digestibility, blood characteristics and meat quality in growing-finishing pigs. Anim. Sci. J. 2012, 83, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Li, H.; Lei, Y.; Li, T.; Kim, S.; Kim, I. Effect of fermented medicinal plants on growth performance, nutrient digestibility, fecal noxious gas emissions, and diarrhea score in weanling pigs. J. Sci. Food Agric. 2016, 96, 1269–1274. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wang, C.; Ye, J.; Chen, H.; Tao, R. Effects of dietary supplementation of fermented Ginkgo biloba L. residues on growth performance, nutrient digestibility, serum biochemical parameters and immune function in weaned piglets. Anim. Sci. J. 2015, 86, 790–799. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.T.; Mun, H.S.; Islam, M.M.; Ko, S.Y.; Yang, C.J. Effects of dietary natural and fermented herb combination on growth performance, carcass traits and meat quality in grower-finisher pigs. Meat Sci. 2016, 122, 7–15. [Google Scholar] [CrossRef]
- Plumed-Ferrer, C.; Von Wright, A. Fermented pig liquid feed: Nutritional, safety and regulatory aspects. J. Appl. Microbiol. 2009, 106, 351–368. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Swine, 11th ed.; National Academy Press: Washington, DC, USA, 2012. [Google Scholar]
- Zheng, P.; Yu, B.; He, J.; Yu, J.; Mao, X.; Luo, Y.; Luo, J.; Huang, Z.; Tian, G.; Zeng, Q.; et al. Arginine metabolism and its protective effects on intestinal health and functions in weaned piglets under oxidative stress induced by diquat. Br. J. Nutr. 2017, 117, 1495–1502. [Google Scholar] [CrossRef]




| Items | Days 1–14 | Days 15–42 |
|---|---|---|
| Raw materials composition | ||
| Extruded corn | 18.48 | 30.28 |
| Corn | 29.16 | 30.01 |
| Soy protein concentrate | 10.00 | 4.00 |
| Low-protein whey powder | 10.00 | 4.00 |
| Whey protein concentrate | 4.00 | 0.00 |
| Extruded soybean | 4.00 | 10.00 |
| Soybean meal | 5.00 | 10.00 |
| Soybean oil | 0.00 | 1.80 |
| Coconut oil | 4.00 | 0.00 |
| Fish meal | 5.00 | 4.00 |
| Sucrose | 3.00 | 2.00 |
| Glucose | 5.00 | 1.00 |
| NaCl | 0.40 | 0.40 |
| Chloride choline | 0.12 | 0.12 |
| Limestone | 0.70 | 0.86 |
| Dicalcium phosphate | 0.42 | 0.52 |
| Vitamin premix 1 | 0.10 | 0.05 |
| Mineral premix 2 | 0.20 | 0.20 |
| L-Lys·HCl | 0.20 | 0.50 |
| L-Thr | 0.10 | 0.13 |
| DL-Met | 0.10 | 0.10 |
| D-Trp | 0.02 | 0.03 |
| Total | 100.00 | 100.00 |
| Nutrition level | ||
| DE, MCal/Kg | 3.62 | 3.54 |
| CP, % | 20.73 | 19.58 |
| Ca, % | 0.80 | 0.81 |
| TP, % | 0.58 | 0.59 |
| AP, % | 0.40 | 0.41 |
| D-Lys, % | 1.37 | 1.37 |
| D-Met, % | 0.44 | 0.48 |
| D-Cys, % | 0.31 | 0.26 |
| D-Met+Cys, % | 0.75 | 0.75 |
| D-Thr, % | 0.90 | 0.80 |
| D-Trp, % | 0.29 | 0.23 |
| Items 1 | Primer and Probe Sequences (5′-3′) 2 | Annealing Temperature (°C) | Product Length |
|---|---|---|---|
| β-actin | F: TCCATCGTCCACCGCAAATG R: TTCAGGAGGCTGGCATGAGG | 57.0 | 114 |
| ZO-1 | F: CAGCCCCCGTACATGGAGA R: GCGCAGACGGTGTTCATAGTT | 60.0 | 114 |
| ZO-2 | F: ATTCGGACCCATAGCAGACATAG R:GCGTCTCTTGGTTCTGTTTTAGC | 60.0 | 110 |
| OCLN | F: CTACTCGTCCAACGGGAAAG R: ACGCCTCCAAGTTACCACTG | 62.0 | 158 |
| CLDN-1 | F: TCTTAGTTGCCACAGCATGG R: CCAGTGAAGAGAGCCTGACC | 60.0 | 114 |
| Items | Primer and Probe Sequences (5′-3′) 1 | Annealing Temperature (°C) | Product Length |
|---|---|---|---|
| Total bacteria | F: ACTCCTACGGGAGGCAGCAG R: ATTACCGCGGCTGCTGG | 60 | 200 |
| Lactobacillus spp. | F: ACTCCTACGGGAGGCAGCAG R: CAACAGTTACTCTGACACCCGTTCTTC P:AAGAAGGGTTTCGGCTCGTAAAACTCTGTT | 57.5 | 126 |
| Escherichia coli | F: CATGCCGCGTGTATGAAGAA R: CGGGTAACGTCAATGAGCAAA P: AGGTATTAACTTTACTCCCTTCCTC | 59.0 | 96 |
| Items 5 | CON 2 | AB 3 | LFD 4 | SEM | p-Value |
|---|---|---|---|---|---|
| Initial body weight, kg | 8.70 | 8.69 | 8.71 | 0.45 | 1.000 |
| 14d body weight, kg | 10.34 | 10.83 | 11.04 | 0.55 | 0.657 |
| 28d body weight, kg | 14.71 | 15.78 | 17.27 | 0.86 | 0.139 |
| 42d body weight, kg | 23.02 b | 24.21 b | 27.78 a | 1.14 | 0.026 |
| Days 1–14 | |||||
| ADG, g | 117.35 b | 152.88 a | 166.53 a | 9.65 | 0.007 |
| ADFI, g | 252.48 b | 276.50 ab | 302.91 a | 9.86 | 0.009 |
| F/G | 2.22 a | 1.83 b | 1.84 b | 0.12 | 0.061 |
| Diarrhoea rate, % | 11.04 | 8.81 | 9.63 | 0.88 | 0.230 |
| Days 15–28 | |||||
| ADG, g | 312.21 b | 353.44 b | 445.47 a | 26.84 | 0.010 |
| ADFI, g | 527.68 b | 587.90 ab | 652.08 a | 35.64 | 0.078 |
| F/G | 1.70 a | 1.68 a | 1.47 b | 0.05 | 0.013 |
| Diarrhoea rate, % | 9.72 | 5.89 | 6.39 | 1.62 | 0.225 |
| Days 29–42 | |||||
| ADG, g | 593.78 b | 602.49 b | 750.34 a | 27.31 | 0.002 |
| ADFI, g | 839.44 b | 902.95 b | 1101.86 a | 39.45 | 0.001 |
| F/G | 1.42 | 1.50 | 1.47 | 0.04 | 0.491 |
| Diarrhoea rate, % | 8.34 | 5.69 | 4.55 | 1.31 | 0.143 |
| Days 1–28 | |||||
| ADG, g | 214.78 b | 253.16 b | 306.00 a | 16.72 | 0.006 |
| ADFI, g | 390.08 b | 432.21 ab | 477.49 a | 22.04 | 0.042 |
| F/G | 1.82 a | 1.72 a | 1.57 b | 0.04 | 0.001 |
| Diarrhoea rate, % | 10.38 | 7.35 | 8.01 | 1.09 | 0.155 |
| Days 1–42 | |||||
| ADG, g | 341.11 b | 369.60 b | 454.11 a | 18.76 | 0.002 |
| ADFI, g | 539.87 b | 589.12 b | 685.61 a | 27.15 | 0.006 |
| F/G | 1.59 | 1.59 | 1.51 | 0.03 | 0.192 |
| Diarrhoea rate, % | 9.70 | 6.80 | 6.85 | 1.07 | 0.123 |
| Items, % 5 | CON 2 | AB 3 | LFD 4 | SEM | p-Value |
|---|---|---|---|---|---|
| Days 1–14 | |||||
| DM | 83.60 | 82.93 | 83.05 | 0.40 | 00.466 |
| CP | 74.99 b | 74.33 b | 80.72 a | 0.75 | <0.0001 |
| EE | 60.00 b | 59.90 b | 68.81 a | 1.50 | 0.001 |
| Ash | 50.05 b | 48.84 b | 59.11 a | 1.72 | 0.001 |
| CF | 32.92 a | 26.30 b | 38.92 a | 1.99 | 0.002 |
| GE | 83.25 b | 82.78 b | 86.92 a | 0.36 | <0.0001 |
| Ca | 47.36 b | 45.12 b | 64.94 a | 2.36 | <0.0001 |
| P | 35.96 b | 34.82 b | 65.32 a | 2.69 | <0.0001 |
| Days 15–42 | |||||
| DM | 80.24 b | 81.79 a | 82.36 a | 0.41 | 0.007 |
| CP | 71.92c | 75.44 b | 84.40 a | 0.66 | <0.0001 |
| EE | 68.66 b | 70.45 b | 74.61 a | 0.74 | 0.000 |
| Ash | 41.35 b | 43.53 b | 61.78 a | 1.36 | <0.0001 |
| CF | 34.66 b | 40.71 ab | 45.24 a | 2.36 | 0.021 |
| GE | 80.68c | 82.24 b | 87.81 a | 0.42 | <0.0001 |
| Ca | 45.63 b | 43.20 b | 64.96 a | 1.36 | <0.0001 |
| P | 31.08c | 38.32 b | 75.54 a | 2.07 | <0.0001 |
| Items 5 | CON 2 | AB 3 | LFD 4 | SEM | p-Value |
|---|---|---|---|---|---|
| Day 14 | |||||
| TP, g/L | 45.55 b | 48.38 a | 48.56 a | 0.86 | 0.046 |
| ALB, g/L | 30.50 | 31.50 | 31.98 | 0.63 | 0.269 |
| GLU, mmol/L | 4.44 b | 5.72 a | 6.02 a | 0.34 | 0.011 |
| UN, mmol/L | 3.51 a | 3.03 ab | 2.03 b | 0.37 | 0.034 |
| ALT, U/mL | 28.93 ab | 25.17 b | 33.34 a | 2.17 | 0.055 |
| AST, U/mL | 23.77 ab | 20.55 b | 26.55 a | 1.47 | 0.036 |
| ALP, U/mL | 42.08 | 36.14 | 42.79 | 2.32 | 0.117 |
| IGF-1, µg/L | 8.56 | 9.08 | 9.08 | 0.34 | 0.475 |
| Cortisol, µg/L | 148.64 | 144.14 | 132.68 | 6.38 | 0.223 |
| Day 42 | |||||
| TP, g/L | 52.50 | 54.67 | 55.07 | 1.27 | 0.334 |
| ALB, g/L | 32.40 | 33.85 | 35.85 | 1.52 | 0.301 |
| GLU, mmol/L | 4.61 | 5.40 | 5.14 | 0.35 | 0.294 |
| UN, mmol/L | 2.94 a | 2.87 a | 2.24 b | 0.19 | 0.037 |
| ALT, U/mL | 36.28 | 35.61 | 44.23 | 3.26 | 0.149 |
| AST, U/mL | 29.01 | 27.98 | 32.34 | 2.02 | 0.309 |
| ALP, U/mL | 39.77 | 34.11 | 40.38 | 2.18 | 0.114 |
| IGF-1, µg/L | 12.03 | 12.46 | 13.49 | 0.74 | 0.380 |
| Cortisol, µg/L | 175.31 | 169.24 | 168.96 | 9.11 | 0.858 |
| Items 5 | CON 2 | AB 3 | LFD 4 | SEM | p-Value |
|---|---|---|---|---|---|
| Day 14 | |||||
| T-AOC, U/mL | 2.08 b | 2.56 ab | 2.93 a | 0.21 | 0.034 |
| CAT, U/mL | 8.60 | 12.25 | 12.46 | 1.19 | 0.064 |
| GPx, U/mL | 420.67 b | 512.06 ab | 529.01 a | 33.97 | 0.084 |
| SOD, U/mL | 153.59 b | 200.79 a | 173.95 ab | 10.25 | 0.018 |
| MDA, nmol /mL | 2.93 a | 2.65 ab | 2.47 b | 0.14 | 0.087 |
| Day 42 | |||||
| T-AOC, U/mL | 1.80 b | 2.00 b | 2.50 a | 0.14 | 0.009 |
| CAT, U/mL | 5.71 | 5.76 | 7.08 | 0.55 | 0.175 |
| GPx, U/mL | 467.48 b | 574.20 a | 602.00 a | 25.21 | 0.005 |
| SOD, U/mL | 242.47 | 239.08 | 236.48 | 10.32 | 0.919 |
| MDA, nmol /mL | 2.94 | 2.95 | 2.83 | 0.21 | 0.899 |
| Items 5 | CON 2 | AB 3 | LFD 4 | SEM | p-Value |
|---|---|---|---|---|---|
| T-AOC, U/mg prot | 0.64 | 0.59 | 0.74 | 0.09 | 0.495 |
| CAT, U/mg prot | 10.23 b | 9.63 b | 14.08 a | 0.90 | 0.006 |
| GPx, U/mg prot | 46.65 | 51.00 | 42.30 | 3.14 | 0.182 |
| SOD, U/mg prot | 13.31 ab | 14.35 a | 11.91 b | 0.74 | 0.097 |
| MDA, nmol/mg prot | 0.50 | 0.58 | 0.43 | 0.08 | 0.445 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Z.; Xia, Z.; Cozannet, P.; de Nanclares, M.P.; Xin, H.; Wang, M.; Chen, D.; Yu, B.; He, J.; Yu, J.; et al. Diets Fermented with Bacteria and Enzymes in China Improve Growth Performance and Health of Weaned Piglets. Agriculture 2022, 12, 1984. https://doi.org/10.3390/agriculture12121984
Fan Z, Xia Z, Cozannet P, de Nanclares MP, Xin H, Wang M, Chen D, Yu B, He J, Yu J, et al. Diets Fermented with Bacteria and Enzymes in China Improve Growth Performance and Health of Weaned Piglets. Agriculture. 2022; 12(12):1984. https://doi.org/10.3390/agriculture12121984
Chicago/Turabian StyleFan, Zequn, Zou Xia, Pierre Cozannet, Marta Perez de Nanclares, Huailu Xin, Mingyu Wang, Daiwen Chen, Bing Yu, Jun He, Jie Yu, and et al. 2022. "Diets Fermented with Bacteria and Enzymes in China Improve Growth Performance and Health of Weaned Piglets" Agriculture 12, no. 12: 1984. https://doi.org/10.3390/agriculture12121984
APA StyleFan, Z., Xia, Z., Cozannet, P., de Nanclares, M. P., Xin, H., Wang, M., Chen, D., Yu, B., He, J., Yu, J., Mao, X., Huang, Z., Luo, Y., Luo, J., Yan, H., & Zheng, P. (2022). Diets Fermented with Bacteria and Enzymes in China Improve Growth Performance and Health of Weaned Piglets. Agriculture, 12(12), 1984. https://doi.org/10.3390/agriculture12121984









