Impacts of the Inoculation of Piriformospora indica on Photosynthesis, Osmoregulatory Substances, and Antioxidant Enzymes of Alfalfa Seedlings under Cadmium Stress
Abstract
1. Introduction
2. Materials and Method
2.1. Preparation of Endophytic Fungi
2.2. Plant Material and Experimental Design
2.3. Estimation of Growth Parameters
2.4. Gaseous Exchange Measurement
2.5. Chlorophyll Content (Chl)
2.6. Soluble Protein (SP) and Proline Concentrations (Pro)
2.7. Antioxidant Enzyme Activity
2.8. Statistical Analysis
3. Results
3.1. Number of Leaves, Plant Height, and Biomass
3.2. Chlorophyll Content
3.3. Leaf Gas Exchange
3.4. Proline and Soluble Protein Concentrations
3.5. Antioxidant Parameters of the Leaves
3.6. Effects of P. indica on Alfalfa Growth by Affecting Photosynthesis, The Content of Osmotic Regulatory Substances, and Activity of Antioxidant Enzymes
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grant, C.A. Influence of Phosphate Fertilizer on Cadmium in Agricultural Soils and Crops (Symposium 3.5.1 Heavy Metal Contaminated Soils, International Symposium: Soil Degradation Control, Remediation, and Reclamation, Tokyo Metropolitan University Symposium Series No.2, 2010). Pedologist 2011, 54, 143–155. [Google Scholar] [CrossRef]
- Jiang, X.; Dai, J.; Zhang, X.; Wu, H.; Tong, J.; Shi, J.; Fang, W. Enhanced Cd Efflux Capacity and Physiological Stress Resistance: The Beneficial Modulations of Metarhizium Robertsii on Plants under Cadmium Stress. J. Hazard. Mater. 2022, 437, 129429. [Google Scholar] [CrossRef] [PubMed]
- Rao, G.; Huang, S.; Ashraf, U.; Mo, Z.; Duan, M.; Pan, S.; Tang, X. Ultrasonic Seed Treatment Improved Cadmium (Cd) Tolerance in Brassica Napus L. Ecotoxicol. Environ. Saf. 2019, 185, 109659. [Google Scholar] [CrossRef] [PubMed]
- Greger, M.; Kabir, A.H.; Landberg, T.; Maity, P.J.; Lindberg, S. Silicate Reduces Cadmium Uptake into Cells of Wheat. Environ. Pollut. 2016, 211, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, X.; Gao, B.; Li, Z.; Xia, H.; Li, H.; Li, J. Effect of Cadmium on Growth, Photosynthesis, Mineral Nutrition and Metal Accumulation of an Energy Crop, King Grass (Pennisetum Americanum × P. Purpureum). Biomass Bioenergy 2014, 67, 179–187. [Google Scholar] [CrossRef]
- Dalla Vecchia, F.; Rocca, N.L.; Moro, I.; De Faveri, S.; Andreoli, C.; Rascio, N. Morphogenetic, Ultrastructural and Physiological Damages Suffered by Submerged Leaves of Elodea Canadensis Exposed to Cadmium. Plant Sci. 2005, 168, 329–338. [Google Scholar] [CrossRef]
- Zhao, Y. Cadmium Accumulation and Antioxidative Defenses in Leaves of Triticum Aestivum L. and Zea Mays L. AJB 2011, 10, 2936–2943. [Google Scholar] [CrossRef]
- Gu, Q.; Chen, Z.; Yu, X.; Cui, W.; Pan, J.; Zhao, G.; Xu, S.; Wang, R.; Shen, W. Melatonin Confers Plant Tolerance against Cadmium Stress via the Decrease of Cadmium Accumulation and Reestablishment of MicroRNA-Mediated Redox Homeostasis. Plant Sci. 2017, 261, 28–37. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, Y.; Xu, W.; Chi, S.; Li, T.; Li, Y.; He, Z.; Yang, M.; Feng, D. Resistance of Alfalfa and Indian Mustard to Cd and the Correlation of Plant Cd Uptake and Soil Cd Form. Env. Sci. Pollut. Res. 2019, 26, 13804–13811. [Google Scholar] [CrossRef]
- Dias, M.C.; Monteiro, C.; MoutinhoPereira, J.; Correia, C.; Gonçalves, B.; Santos, C. Cadmium Toxicity Affects Photosynthesis and Plant Growth at Different Levels. Acta Physiol. Plant. 2013, 35, 1281–1289. [Google Scholar] [CrossRef]
- Oelmüller, R.; Sherameti, I.; Tripathi, S.; Varma, A. Piriformospora Indica, a Cultivable Root Endophyte with Multiple Biotechnological Applications. Symbiosis 2009, 49, 1–17. [Google Scholar] [CrossRef]
- Evelin, H.; Kapoor, R.; Giri, B. Arbuscular Mycorrhizal Fungi in Alleviation of Salt Stress: A Review. Ann. Bot. 2009, 104, 1263–1280. [Google Scholar] [CrossRef] [PubMed]
- Tsimilli Michael, M.; Strasser, R. Biophysical Phenomics: Evaluation of the Impact of Mycorrhization with Piriformospora Indica. Soil Biol. 2013, 33, 173–190. [Google Scholar] [CrossRef]
- Sherameti, I.; Venus, Y.; Drzewiecki, C.; Tripathi, S.; Dan, V.M.; Nitz, I.; Varma, A.; Grundler, F.M.; Oelmüller, R. PYK10, a β-Glucosidase Located in the Endoplasmatic Reticulum, Is Crucial for the Beneficial Interaction between Arabidopsis Thaliana and the Endophytic Fungus Piriformospora Indica. Plant J. 2008, 54, 428–439. [Google Scholar] [CrossRef] [PubMed]
- Bagde, U.S.; Prasad, R.; Varma, A. Impact of Culture Filtrate of Piriformospora Indica on Biomass and Biosynthesis of Active Ingredient Aristolochic Acid in Aristolochia Elegans Mart. IJB 2013, 6, 29. [Google Scholar] [CrossRef]
- Li, L.; Zhu, P.; Wang, X.; Zhang, Z. Phytoremediation Effect of Medicago Sativa Colonized by Piriformospora Indica in the Phenanthrene and Cadmium Co-Contaminated Soil. BMC Biotechnol. 2020, 20, 20. [Google Scholar] [CrossRef]
- Ansari, M.W.; Trivedi, D.K.; Sahoo, R.K.; Gill, S.S.; Tuteja, N. A Critical Review on Fungi Mediated Plant Responses with Special Emphasis to Piriformospora Indica on Improved Production and Protection of Crops. Plant Physiol. Biochem. 2013, 70, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Sagonda, T.; Adil, M.F.; Sehar, S.; Rasheed, A.; Joan, H.I.; Ouyang, Y.; Shamsi, I.H. Physio-Ultrastructural Footprints and ITRAQ-Based Proteomic Approach Unravel the Role of Piriformospora Indica-Colonization in Counteracting Cadmium Toxicity in Rice. Ecotoxicol. Environ. Saf. 2021, 220, 112390. [Google Scholar] [CrossRef]
- Goussi, R.; Manaa, A.; Derbali, W.; Ghnaya, T.; Abdelly, C.; Barbato, R. Combined Effects of NaCl and Cd2+ Stress on the Photosynthetic Apparatus of Thellungiella Salsuginea. Biochim. Biophys. Acta (BBA) Bioenerg. 2018, 1859, 1274–1287. [Google Scholar] [CrossRef]
- Jiang, W.; Pan, R.; Wu, C.; Xu, L.; Abdelaziz, M.E.; Oelmüller, R.; Zhang, W. Piriformospora Indica Enhances Freezing Tolerance and Post-Thaw Recovery in Arabidopsis by Stimulating the Expression of CBF Genes. Plant Signal. Behav. 2020, 15, 1745472. [Google Scholar] [CrossRef]
- Su, Z.; Zeng, Y.; Li, X.; Perumal, A.B.; Zhu, J.; Lu, X.; Dai, M.; Liu, X.; Lin, F. The Endophytic Fungus Piriformospora Indica-Assisted Alleviation of Cadmium in Tobacco. J. Fungi 2021, 7, 675. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Bodjrenou, D.M.; Zhang, S.; Wang, B.; Pan, H.; Yeh, K.W.; Lai, Z.; Cheng, C. The Endophytic Fungus Piriformospora Indica Reprograms Banana to Cold Resistance. Int. J. Mol. Sci. 2021, 22, 4973. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, D.K.; Ansari, M.W.; Bhavesh, N.S.; Johri, A.K.; Tuteja, N. Response of PiCypA Tobacco T2 Transgenic Matured Plant to Potential Tolerance to Salinity Stress. Plant Signal. Behav. 2014, 9, e27538. [Google Scholar] [CrossRef]
- Sepehri, M.; Khatabi, B. Combination of Siderophore-Producing Bacteria and Piriformospora Indica Provides an Efficient Approach to Improve Cadmium Tolerance in Alfalfa. Microb. Ecol. 2021, 81, 717–730. [Google Scholar] [CrossRef] [PubMed]
- Cardona, C.A.; Muñoz Cuervo, I.; Rincón, A. Simulación de Una Columna de Destilación Reactiva Para La Síntesis de Butilacetato. Rev. Univ. Eafit 2006, 42, 79–87. [Google Scholar]
- Baltruschat, H.; Fodor, J.; Harrach, B.; Niemczyk, E.; Barna, B.; Gullner, G.; Janeczko, A.; Kogel, K.-H.; Schäfer, P.; Schwarczinger, I.; et al. Salt Tolerance of Barley Induced by the Root Endophyte Piriformospora Indica Is Associated with a Strong Increase in Antioxidants. New Phytol. 2008, 180, 501–510. [Google Scholar] [CrossRef]
- Asilian, E.; Ghasemi-Fasaei, R.; Ronaghi, A.; Sepehri, M.; Niazi, A. Chemical and Microbial Enhanced Phytoremediation of Cadmium-Contaminated Calcareous Soil by Maize. Toxicol. Ind. Health 2019, 35, 378–386. [Google Scholar] [CrossRef]
- Amani, S.; Mohebodini, M.; Khademvatan, S.; Jafari, M.; Kumar, V. Piriformospora Indica Based Elicitation for Overproduction of Phenolic Compounds by Hairy Root Cultures of Ficus Carica. J. Biotechnol. 2021, 327, 43–53. [Google Scholar] [CrossRef]
- Ouledali, S.; Ennajeh, M.; Zrig, A.; Gianinazzi, S.; Khemira, H. Estimating the Contribution of Arbuscular Mycorrhizal Fungi to Drought Tolerance of Potted Olive Trees (Olea Europaea). Acta Physiol. Plant. 2018, 40, 81. [Google Scholar] [CrossRef]
- Shahabivand, S.; Parvaneh, A.; Aliloo, A.A. Root Endophytic Fungus Piriformospora Indica Affected Growth, Cadmium Partitioning and Chlorophyll Fluorescence of Sunflower under Cadmium Toxicity. Ecotoxicol. Environ. Saf. 2017, 145, 496–502. [Google Scholar] [CrossRef]
- Singh, M.; Tiwari, N. Microbial Amelioration of Salinity Stress in HD 2967 Wheat Cultivar by Up-Regulating Antioxidant Defense. Commun. Integr. Biol. 2021, 14, 136–150. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Lu, C. Effects of Abscisic Acid on Photoinhibition in Maize Plants. Plant Sci. 2003, 165, 1403–1410. [Google Scholar] [CrossRef]
- Gill, S.S.; Gill, R.; Trivedi, D.K.; Anjum, N.A.; Sharma, K.K.; Ansari, M.W.; Ansari, A.A.; Johri, A.K.; Prasad, R.; Pereira, E.; et al. Piriformospora Indica: Potential and Significance in Plant Stress Tolerance. Front Microbiol. 2016, 7, 332. [Google Scholar] [CrossRef]
- Parmar, P.; Kumari, N.; Sharma, V. Structural and Functional Alterations in Photosynthetic Apparatus of Plants under Cadmium Stress. Bot. Stud. 2013, 54, 45. [Google Scholar] [CrossRef] [PubMed]
- Kilic, S.; Karaboyacı, M.; Sencan, A.; Kiliç, M. Ecotoxicological Responses of Morphological and Physiological Parameters of Cadmium-Stressed Maize Seeds. Bangladesh J. Bot. 2017, 46, 211–216. [Google Scholar]
- Khalid, M.; Hassani, D.; Liao, J.; Xiong, X.; Bilal, M.; Huang, D. An Endosymbiont Piriformospora Indica Reduces Adverse Effects of Salinity by Regulating Cation Transporter Genes, Phytohormones, and Antioxidants in Brassica Campestris Ssp. Chinensis. Environ. Exp. Bot. 2018, 153, 89–99. [Google Scholar] [CrossRef]
- Naqqash, T.; Aziz, A.; Babar, M.; Hussain, S.B.; Haider, G.; Shahid, M.; Qaisrani, M.M.; Arshad, M.; Hanif, M.K.; Mancinelli, R.; et al. Lead-Resistant Morganella Morganii Rhizobacteria Reduced Lead Toxicity in Arabidopsis Thaliana by Improving Growth, Physiology, and Antioxidant Activities. Agriculture 2022, 12, 1155. [Google Scholar] [CrossRef]
- Arora, M.; Saxena, P.; Abdin, M.; Varma, A. Interaction between Piriformospora Indica and Azotobacter Chroococcum Diminish the Effect of Salt Stress in Artemisia Annua L. by Enhancing Enzymatic and Non-Enzymatic Antioxidants. Symbiosis 2020, 80, 61–73. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Matowe, W. Drought Tolerance in Two Mosses: Correlated with Enzymatic Defence Against Lipid Peroxidation. J. Exp. Bot. 1981, 32, 79–91. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper Enzymes in Isolated Chloroplasts. Polyphenoloxidase in Beta Vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid Determination of Free Proline for Water-Stress Studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Maehly, A.C.; Chance, B. The Assay of Catalases and Peroxidases. Methods Biochem. Anal. 1954, 1, 357–424. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in Vitro. Methods Enzym. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Demmig Adams, B.; Gilmore, A.M.; Adams, W.W. Carotenoids 3: In Vivo Function of Carotenoids in Higher Plants. FASEB J. 1996, 10, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Małkowski, E.; Sitko, K.; Szopiński, M.; Gieroń, Ż.; Pogrzeba, M.; Kalaji, H.M.; Zieleźnik-Rusinowska, P. Hormesis in Plants: The Role of Oxidative Stress, Auxins and Photosynthesis in Corn Treated with Cd or Pb. Int. J. Mol. Sci. 2020, 21, 2099. [Google Scholar] [CrossRef]
- Zhang, T.; Hu, Y.; Zhang, K.; Tian, C.; Guo, J. Arbuscular Mycorrhizal Fungi Improve Plant Growth of Ricinus Communis by Altering Photosynthetic Properties and Increasing Pigments under Drought and Salt Stress. Ind. Crops Prod. 2018, 117, 13–19. [Google Scholar] [CrossRef]
- Ramanjulu, S.; Sreenivasalu, N.; Kumar, S.; Sudhakar, C. Photosynthetic Characteristics in Mulberry during Water Stress and Rewatering. Photosynthetica 1998, 35, 259–263. [Google Scholar] [CrossRef]
- Liu, E.K.; Mei, X.R.; Yan, C.R.; Gong, D.Z.; Zhang, Y.Q. Effects of Water Stress on Photosynthetic Characteristics, Dry Matter Translocation and WUE in Two Winter Wheat Genotypes. Agric. Water Manag. 2016, 167, 75–85. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Z.W.; Tu, S.H.; Feng, W.Q.; Xu, F.; Zhu, F.; Zhang, D.W.; Du, J.B.; Yuan, S.; Lin, H.H. Comparative Study of Four Rice Cultivars with Different Levels of Cadmium Tolerance. Biologia 2013, 68, 74–81. [Google Scholar] [CrossRef]
- Xue, Z.C.; Gao, H.Y.; Zhang, L.T. Effects of Cadmium on Growth, Photosynthetic Rate and Chlorophyll Content in Leaves of Soybean Seedlings. Biol. Plant. 2013, 57, 587–590. [Google Scholar] [CrossRef]
- Jalal, A.; de Oliveira Junior, J.C.; Ribeiro, J.S.; Fernandes, G.C.; Mariano, G.G.; Trindade, V.D.R.; Reis, A.R. dos Hormesis in Plants: Physiological and Biochemical Responses. Ecotoxicol. Environ. Saf. 2021, 207, 111225. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Yang, X.; He, Z.; Baligar, V.C. Morphological and Physiological Responses of Plants to Cadmium Toxicity: A Review. Pedosphere 2017, 27, 421–438. [Google Scholar] [CrossRef]
- Ying, R.R.; Qiu, R.L.; Tang, Y.T.; Hu, P.J.; Qiu, H.; Chen, H.R.; Shi, T.H.; Morel, J.L. Cadmium Tolerance of Carbon Assimilation Enzymes and Chloroplast in Zn/Cd Hyperaccumulator Picris Divaricata. J. Plant Physiol. 2010, 167, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Shukla, U.C.; Murthy, R.C.; Kakkar, P. Combined Effect of Ultraviolet-B Radiation and Cadmium Contamination on Nutrient Uptake and Photosynthetic Pigments in Brassica Campestris L. Seedlings. Environ. Toxicol 2008, 23, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Wang, Y.; Chen, H.; Deng, F.; Liu, Y.; Cao, G. Changes in Metal Distribution, Vegetative Growth, Reactive Oxygen and Nutrient Absorption of Tagetes Patula under Soil Cadmium Stress. Horticulturae 2022, 8, 69. [Google Scholar] [CrossRef]
- Hernández, J.A.; Ferrer, M.A.; Jiménez, A.; Barceló, A.R.; Sevilla, F. Antioxidant Systems and O2−/H2O2 Production in the Apoplast of Pea Leaves. Its Relation with Salt-Induced Necrotic Lesions in Minor Veins. Plant Physiol. 2001, 127, 817–831. [Google Scholar] [CrossRef]
- Anamul Hoque, M.; Okuma, E.; Nasrin Akhter Banu, M.; Nakamura, Y.; Shimoishi, Y.; Murata, Y. Exogenous Proline Mitigates the Detrimental Effects of Salt Stress More than Exogenous Betaine by Increasing Antioxidant Enzyme Activities. J. Plant Physiol. 2007, 164, 553–561. [Google Scholar] [CrossRef]
- Pang, J.; Chan, G.S.Y.; Zhang, J.; Liang, J.; Wong, M.H. Physiological Aspects of Vetiver Grass for Rehabilitation in Abandoned Metalliferous Mine Wastes. Chemosphere 2003, 52, 1559–1570. [Google Scholar] [CrossRef]
- Patakas, A.; Nikolaou, N.; Zioziou, E.; Radoglou, K.; Noitsakis, B. The Role of Organic Solute and Ion Accumulation in Osmotic Adjustment in Drought-Stressed Grapevines. Plant Sci. 2002, 163, 361–367. [Google Scholar] [CrossRef]
- Jackson, M.B.; Campbell, D.J. Movement of Ethylene from Roots to Shoots, a Factor in the Responses of Tomato Plants to Waterlogged Soil Conditions. New Phytol. 1975, 74, 397–406. [Google Scholar] [CrossRef]
- Plesa, I.M.; GonzálezOrenga, S.; Al Hassan, M.; Sestras, A.F.; Vicente, O.; Prohens, J.; Sestras, R.E.; Boscaiu, M. Effects of Drought and Salinity on European Larch (Larix Decidua Mill.) Seedlings. Forests 2018, 9, 320. [Google Scholar] [CrossRef]
- Islam, M.M.; Hoque, M.A.; Okuma, E.; Banu, M.N.A.; Shimoishi, Y.; Nakamura, Y.; Murata, Y. Exogenous Proline and Glycinebetaine Increase Antioxidant Enzyme Activities and Confer Tolerance to Cadmium Stress in Cultured Tobacco Cells. J. Plant Physiol. 2009, 166, 1587–1597. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, K.; Yamaguchi-Shinozaki, K. Molecular Responses to Drought and Cold Stress. Curr. Opin. Biotechnol. 1996, 7, 161–167. [Google Scholar] [CrossRef]
- Valentovičová, K.; Halušková, L.; Huttová, J.; Mistrík, I.; Tamás, L. Effect of Cadmium on Diaphorase Activity and Nitric Oxide Production in Barley Root Tips. J. Plant Physiol. 2010, 167, 10–14. [Google Scholar] [CrossRef]
- Somashekaraiah, B.V.; Padmaja, K.; Prasad, A.R.K. Phytotoxicity of Cadmium Ions on Germinating Seedlings of Mung Bean (Phaseolus Vulgaris): Involvement of Lipid Peroxides in Chlorphyll Degradation. Physiol. Plant 1992, 85, 85–89. [Google Scholar] [CrossRef]
- Decros, G.; Baldet, P.; Beauvoit, B.; Stevens, R.; Flandin, A.; Colombié, S.; Gibon, Y.; Pétriacq, P. Get the Balance Right: ROS Homeostasis and Redox Signalling in Fruit. Front. Plant Sci. 2019, 10, 1091. [Google Scholar] [CrossRef]
- Sytar, O.; Kumar, A.; Latowski, D.; Kuczynska, P.; Strzałka, K.; Prasad, M.N.V. Heavy Metal-Induced Oxidative Damage, Defense Reactions, and Detoxification Mechanisms in Plants. Acta Physiol. Plant. 2013, 35, 985–999. [Google Scholar] [CrossRef]
- Nath, M.; Bhatt, D.; Prasad, R.; Gill, S.S.; Anjum, N.A.; Tuteja, N. Reactive Oxygen Species Generation-Scavenging and Signaling during Plant-Arbuscular Mycorrhizal and Piriformospora Indica Interaction under Stress Condition. Front. Plant Sci. 2016, 7, 1574. [Google Scholar] [CrossRef]
- Moradi, R.; Pourghasemian, N.; Naghizadeh, M. Effect of Beeswax Waste Biochar on Growth, Physiology and Cadmium Uptake in Saffron. J. Clean. Prod. 2019, 229, 1251–1261. [Google Scholar] [CrossRef]
- Bashir, W.; Anwar, S.; Zhao, Q.; Hussain, I.; Xie, F. Interactive Effect of Drought and Cadmium Stress on Soybean Root Morphology and Gene Expression. Ecotoxicol. Environ. Saf. 2019, 175, 90–101. [Google Scholar] [CrossRef]
- Sun, T.; Tan, W.; Yang, Y.; Mu, H. AM Fungi and Piriformospora Indica Improve Plant Growth of Pinus ElliottiiSeedlings. Phyton 2021, 90, 171–178. [Google Scholar] [CrossRef]
- Abdelaziz, M.E.; Abdelsattar, M.; Abdeldaym, E.A.; Atia, M.A.M.; Mahmoud, A.W.M.; Saad, M.M.; Hirt, H. Piriformospora Indica Alters Na+/K+ Homeostasis, Antioxidant Enzymes and LeNHX1 Expression of Greenhouse Tomato Grown under Salt Stress. Sci. Hortic. 2019, 256, 108532. [Google Scholar] [CrossRef]
- Tan, S.Y.; Jiang, Q.Y.; Zhuo, F.; Liu, H.; Wang, Y.T.; Li, S.S.; Ye, Z.H.; Jing, Y.X. Effect of Inoculation with Glomus Versiforme on Cadmium Accumulation, Antioxidant Activities and Phytochelatins of Solanum Photeinocarpum. PLoS ONE 2015, 10, e0132347. [Google Scholar] [CrossRef] [PubMed]
- Forouzi, A.; Ghasemnezhad, A.; Nasrabad, R.G. Phytochemical Response of Stevia Plant to Growth Promoting Microorganisms under Salinity Stress. S. Afr. J. Bot. 2020, 134, 109–118. [Google Scholar] [CrossRef]
- Prasad, R.; Kamal, S.; Sharma, P.K.; Oelmüller, R.; Varma, A. Root Endophyte Piriformospora Indica DSM 11827 Alters Plant Morphology, Enhances Biomass and Antioxidant Activity of Medicinal Plant Bacopa Monniera. J. Basic Microbiol. 2013, 53, 1016–1024. [Google Scholar] [CrossRef]
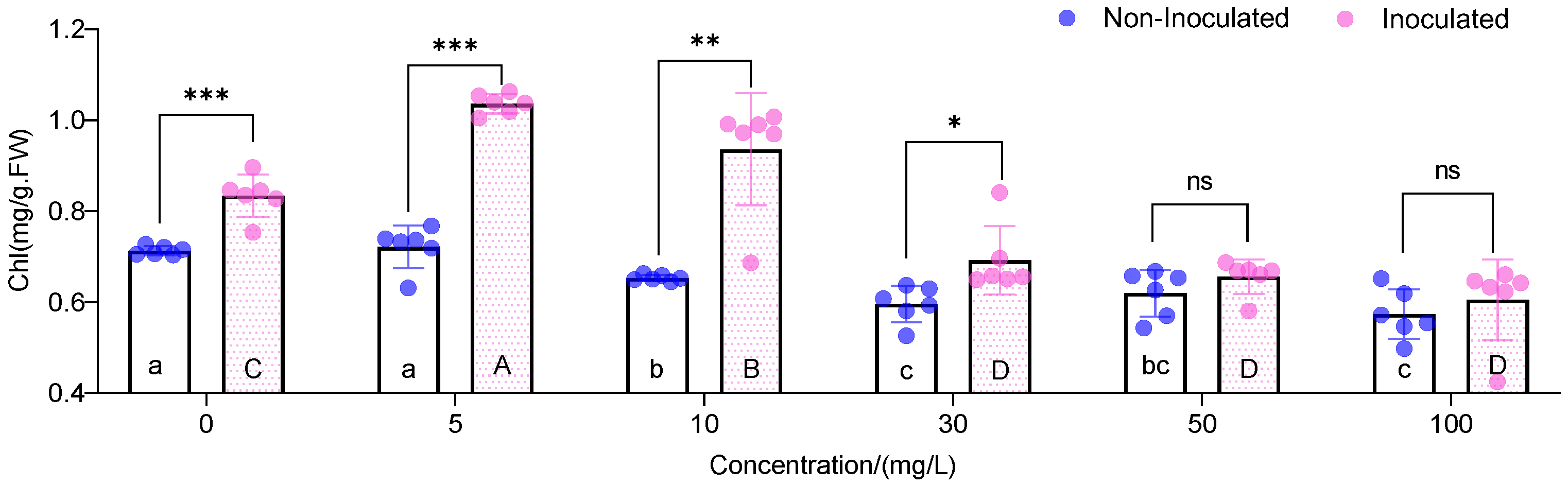

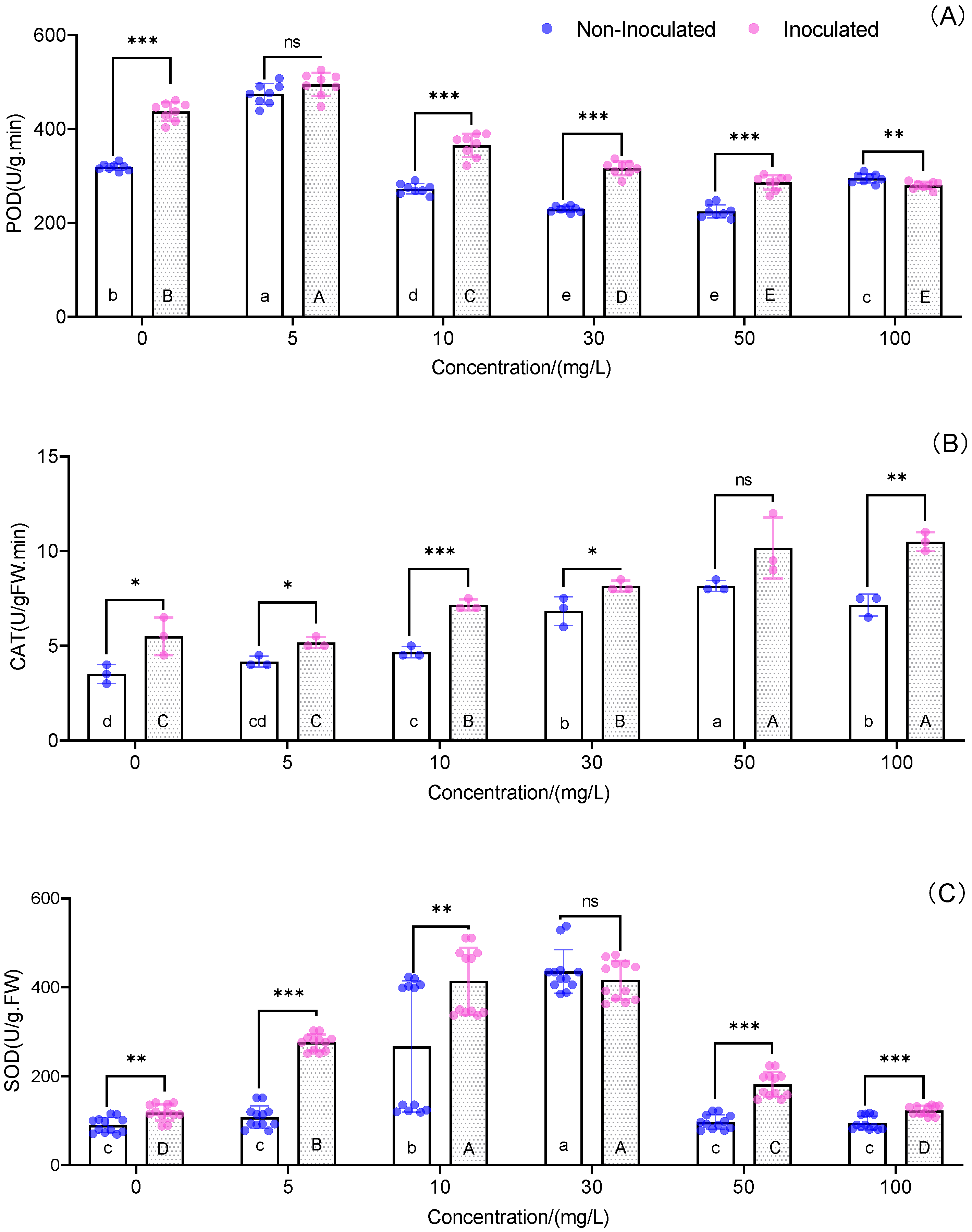
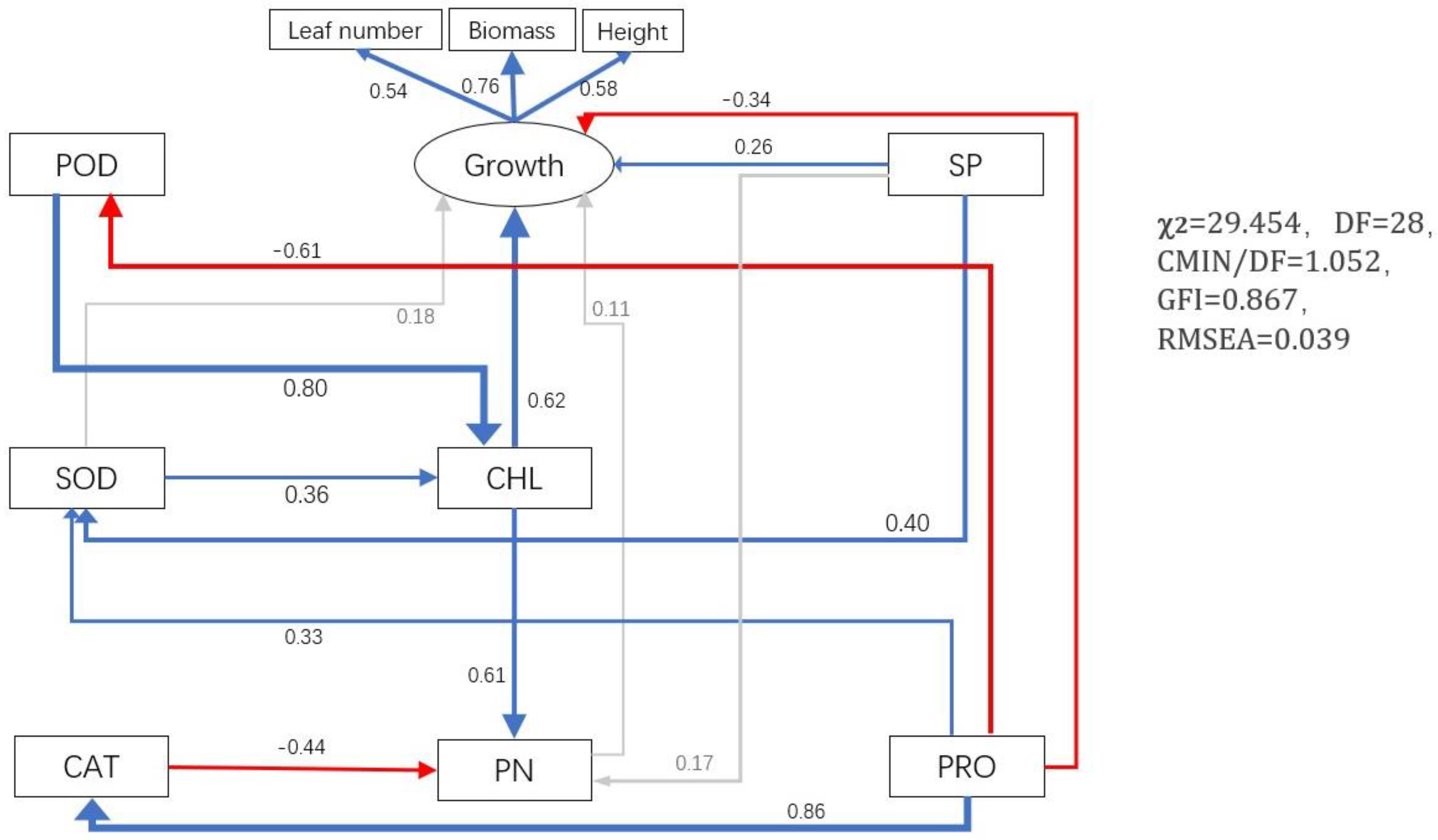
| Cd (mg L−1) | Treatments | Number of Leaves (Pieces) | Plant Height (cm) | Biomass (g/Plant) |
|---|---|---|---|---|
| 0 | non-inoculated | 12.00 ± 3.00 ab | 22.83 ± 2.02 ab | 0.39 ± 0.00 a |
| inoculated | 12.67 ± 2.08 A | 25.20 ± 2.61 A | 0.39 ± 0.01 B | |
| 5 | non-inoculated | 14.00 ± 1.00 a | 24.30 ± 2.79 a | 0.40 ± 0.01 a |
| inoculated | 15.67 ± 4.04 A | 26.00 ± 6.00 A | 0.40 ± 0.01 B | |
| 10 | non-inoculated | 10.00 ± 1.73 b | 23.13 ± 1.11 ab | 0.37 ± 0.01 b |
| inoculated | 11.67 ± 2.89 A | 24.33 ± 3.06 A | 0.44 ± 0.01A *** | |
| 30 | non-inoculated | 9.67 ± 1.53 b | 22.50 ± 3.91 ab | 0.36 ± 0.00 bc |
| inoculated | 11.67 ± 3.21 A | 23.00 ± 1.00 A | 0.38 ± 0.00 C *** | |
| 50 | non-inoculated | 9.00 ± 1.00 b | 22.00 ± 1.73 ab | 0.35 ± 0.01 bc |
| inoculated | 11.00 ± 3.61 A | 22.50 ± 1.48 A | 0.37 ± 0.01 CD | |
| 100 | non-inoculated | 8.67 ± 1.15 b | 18.27 ± 2.00 b | 0.34 ± 0.01 c |
| inoculated | 10.00 ± 1.73 A | 20.80 ± 4.85 A | 0.35 ± 0.01 D |
| Cd (mg L−1) | Treatments | Pn (µmol m−2 s−1) | Tr (mmol m−2 s−1) | Ci (µmol mol−1) | Gs (mmol m−2 s−1) |
|---|---|---|---|---|---|
| 0 | non-inoculated | 8.10 ± 1.41 a | 2.39 ± 0.41 a | 275.44 ± 38.32 b | 0.12 ± 0.02 b |
| inoculated | 9.89 ± 0.64 A | 4.69 ± 1.22 A * | 315.30 ± 13.35 A | 0.23 ± 0.06 A * | |
| 5 | non-inoculated | 5.42 ± 0.28 b | 3.00 ± 0.08 b | 296.69 ± 33.24 a | 0.15 ± 0.00 a |
| inoculated | 9.97 ± 1.26 A ** | 4.14 ± 2.27 A | 328.73 ± 4.68 AB | 0.21 ± 0.12 A | |
| 10 | non-inoculated | 5.74 ± 0.34 b | 2.84 ± 0.06 b | 287.76 ± 7.94 a | 0.14 ± 0.00 a |
| inoculated | 8.15 ± 0.16 B *** | 2.56 ± 0.18 B | 322.64 ± 2.66 BC ** | 0.13 ± 0.01 AB | |
| 30 | non-inoculated | 2.26 ± 0.03 c | 0.59 ± 0.24 c | 226.32 ± 5.14 c | 0.03 ± 0.01 c |
| inoculated | 6.12 ± 0.77 C ** | 1.26 ± 0.14 C * | 234.55 ± 46.84 C | 0.06 ± 0.01 BC * | |
| 50 | non-inoculated | 2.51 ± 0.34 c | 0.17 ± 0.01 c | 251.49 ± 4.26 d | 0.01 ± 0.01 d |
| inoculated | 2.08 ± 0.05 D | 0.73 ± 0.02 D *** | 329.46 ± 0.17 C *** | 0.01 ± 0.01 BC | |
| 100 | non-inoculated | 1.01 ± 0.30 d | 0.12 ± 0.07 d | 268.87 ± 5.00 d | 0.01 ± 0.01 d |
| inoculated | 1.28 ± 0.12 D | 0.72 ± 0.22 D * | 341.54 ± 10.70 C *** | 0.04 ± 0.01 C * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; An, C.; Jiao, S.; Jia, F.; Liu, R.; Wu, Q.; Dong, Z. Impacts of the Inoculation of Piriformospora indica on Photosynthesis, Osmoregulatory Substances, and Antioxidant Enzymes of Alfalfa Seedlings under Cadmium Stress. Agriculture 2022, 12, 1928. https://doi.org/10.3390/agriculture12111928
Liu B, An C, Jiao S, Jia F, Liu R, Wu Q, Dong Z. Impacts of the Inoculation of Piriformospora indica on Photosynthesis, Osmoregulatory Substances, and Antioxidant Enzymes of Alfalfa Seedlings under Cadmium Stress. Agriculture. 2022; 12(11):1928. https://doi.org/10.3390/agriculture12111928
Chicago/Turabian StyleLiu, Bingqian, Chunchun An, Shuying Jiao, Fengyuan Jia, Ruilin Liu, Qicong Wu, and Zhi Dong. 2022. "Impacts of the Inoculation of Piriformospora indica on Photosynthesis, Osmoregulatory Substances, and Antioxidant Enzymes of Alfalfa Seedlings under Cadmium Stress" Agriculture 12, no. 11: 1928. https://doi.org/10.3390/agriculture12111928
APA StyleLiu, B., An, C., Jiao, S., Jia, F., Liu, R., Wu, Q., & Dong, Z. (2022). Impacts of the Inoculation of Piriformospora indica on Photosynthesis, Osmoregulatory Substances, and Antioxidant Enzymes of Alfalfa Seedlings under Cadmium Stress. Agriculture, 12(11), 1928. https://doi.org/10.3390/agriculture12111928





