Abstract
Flash flooding (FF) along with bacterial blight (BB) outbreak are very destructive for rice production in the rainfed shallow-lowland (RSL) ecosystem. The presence of dynamic Xoo races with varying levels of genetic diversity and virulence renders their management extremely challenging under RSL. In this context, the marker-assisted improvement of plant resistance/tolerance has been proven as one of the most promising strategies towards the development of sustainable cultivars. The present study demonstrates the marker-assisted introgression of the submergence tolerant (Sub1) and three bacterial blight resistant genes (Xa21 + xa13 + xa5) into the genetic background of Hasanta, a long duration popular rice variety in the eastern coastal region of India. The rice genotypes, Swarna Sub1 (carrying Sub1) and IRBB66 (carrying Xa21 + xa13 + Xa7 + xa5 + Xa4) had maximum genetic similarity (0.84 and 0.73, respectively) with Hasanta; recurrent parent (RP) was used as donor. The forward analysis of target genes in F1s, IC1F1s and backcross (BC) generations was performed by linked/genic markers (Sub1bc2; pTA248, xa13prom and RM122), whereas background recovery of RP in each BC and segregating generations was performed utilizing 108 hypervariable SSR markers. Intervened speed breeding (SB) strategy and intensive phenotyping could lead the development of near isogenic lines (NILs) as to the RP in all basic traits. The performance of the near isogenic lines (NILs, BC2F3 and BC2F4), HS 232-411-391-756-37, HS 232-411-391-809-8, HS 232-411-391-756-18, HS 110-224-197-10-36, HS 232-411-391-809-81, HS 110-224-197-10-41 and HS 232-411-391-809-63 establishes the utility of marker-assisted backcross-breeding (MAB) and SB in accelerated trait introgression. The introgressed lines carrying Sub1 + Xa21 + xa13 + xa5 showed 76% to 91% survival under 14 days of submergence and durable BB resistance (percent disease index-PDI of 2.68 ± 0.26 to 6.22 ± 1.08 and lesion length, LL of 1.29 ± 0.12 to 4.2 ± 0.64 cm). Physiological analysis revealed that improved NILs, carrying Sub1 gene conquered adaptive physiological modulations, had reduced the consumption of soluble sugar and the degradation of total chlorophyll contents (TCC), and an enhanced level of Alcohol Dehydrogenase activity (ADH) and proline accumulation in all submergence regimes. The pyramided lines attained complete product profile of RP, that will contribute to sustainable rice production under RSL, particularly in the coastal region that has substantial acreage under the variety Hasanta.
1. Introduction
Global climate change has affected the agro-ecosystem of the planet significantly in various way [1]. The frequent occurrence of intensive flooding, drought and heat has obstructed cropping patterns and the production of many crops [2]. Submergence due to FF is considered the third most important abiotic constraint of rice production in rainfed shallow lowland (RSL) ecologies, causing substantial yield loss annually [3]. In India, ~16 mha of rice acreage are submergence prone, of that ~90% areas are confined to the eastern region where the rice crop is a major source of livelihood [4]. Rice is a semi-aquatic crop, able to modulate its adaptive patterns and pathways to sustain under a wide range of habitats/niches [5], but, intense and prolonged flooding is fatal for its survivability [6,7,8]. Under submergence condition, the rice canopy faces several challenges for proper growth and survival. Owing to a lower rate of gaseous and light diffusion under water, submerged plants face energy shortage and nutrient deficiency and subsequently perish [9]. Additionally, flooding offers a very conducive environment for the growth of BB and other biotic stresses, which along with water hypoxia/anoxia causes havoc to rice sustainability. Further, climatic patterns predicting a very distressing situation due to severe flooding in future, particularly in coastal regions [10], necessitates step up yield potential in rice by 70% to feed our ever-growing population [11]. The frequent outbreak of BB, sheath blight and the resurgence of minor diseases and pests are other challenges for rice production in RSL [12], demands breeding climate resilient and multiple disease resistance rice cultivars [13,14,15]. Typically, rice plants sustain up to one week of submergence [16,17], but beyond that, usually only tolerant genotypes survive [18]. Tolerant rice genotypes make several adaptive modulations, which enables it to survive under hypoxia/anoxia without major damage [2,4,8,19,20]. Plant growth regulators like ethylene, gibberellic acid (GA) and abscisic acid (ABA), and their successive manipulation, are regulating shoot elongation and leaf senescence in submerged canopies and thus enhances plant survival under flood [21,22,23]. Tolerant genotypes under flooding condition switch-on their alcohol dehydrogenase activity, which regulates judicious energy consumption in plants and enhances plant survivability [6].
Genomic regions responding to adaptive intonations in rice under submergence have been mapped, validated and successfully deployed in several intolerant genotypes [24,25,26,27,28,29,30,31,32]. The Sub1 gene exerts two weeks of submergence tolerance in mapped on chromosome-9, along with other minor QTLs in the Indian rice landrace FR13A [33,34,35,36,37]. FR13A is utilized as a donor of Sub1 in several rice improvement programs [4,9,28]. Popular rice varieties, Ranjit et al. [24], BRRI Dhan52 [25], Aiswarya et al. [27], Swarna et al. [28], CO3 [30], IR64 [32], Chehrang [32] and CR 1009 [32], etc., have been introgressed with Sub1 through the markers-assisted selection (MAS)/MAB approach. A submergence tolerant version of mega rice variety, Swarna Sub1 [28], has similar agro-morphology as Swarna, yielding a 2-fold or higher yield advantage over parent under 10 or more days’ vegetative stage flooding [8,28]. Recently, two climate smart rice varieties, CR Dhan 801 and CR Dhan 802, carrying dual resilient traits (drought and submergence tolerance), have been developed through MAS, which are working based on the shut-down and shut-on mechanism [29].
Furthermore, BB disease (caused by Xanthomonas oryzae pv. Oryzae; Xoo) is devastating under RSL, causing 20–100% yield loss in rice [38]. Warm temperatures (25–30 °C), high humidity, heavy rainfall and water stagnation coupled with excessive nitrogen application, promote BB outbreak. BB disease also impairs photosynthesis; once synchronizes with FF it causes huge yield and quality loss in rice [4,14,15]. The Xoo inoculum of BB disease enters in the host plant through injured epidermis and perpetuates in the xylem vessels. The pathogenic Xoo races are highly dynamic and diverse in nature, produce a variety of toxic secretions including extracellular polysaccharides, enzymes, iron chelating siderophores and the type III-secretion dependent effectors [39] that compromise the rice plant’s immune response [7]. Rice cultivars with inbuilt resistance are reported substantial in BB disease management [13,40,41,42]. More than 40 genes conferring resistance (R) to BB in rice have been mapped, some of those are successfully deployed into the popular genetic backgrounds [4,13,41,42,43,44,45,46]. Even though, the dynamic nature of Xoo pathotypes and the varied extent of their virulency necessitates stacking multiple R genes for durable resistance [4,13,14,15,41,42,43,47,48].
The above findings highlighted the opportunity to develop additional high-yielding varieties that are adapted to other regions or adverse environments. Improving plant sustainability through the incorporation of resistance/tolerance genes/QTLs is an effective strategy for managing submergence stress and BB disease in rice [2,4,49,50]. Recent advances in biotechnological tools and techniques have facilitated mapping and more importantly the stacking of the desirable genes/QTLs into elite agronomic backgrounds [13,24,49]. Molecular markers enabling the selection of desired traits/types, irrespective of the growth stage and habitats of plants, is simple [44,51]. MAB has been widely accepted as an established tool for accelerative and precise trait improvement in rice [2,4,13,14,24,41,42,44,52,53]. Besides, the utility of speed breeding, which harnesses the modified environment (compact spacing 2.5–5.0 cm and nutritional and physiological stress) to shorten growth duration in plants, was also displayed successfully; we could advance three generations in a year. Hasanta, a medium-long duration (145–150 days) rice variety is very popular among farmers. It has an average productivity of 6.5–7.0 ton/ha and has considerable acreage (>2000 ha) in the coastal region of state Odisha, India (DAC indent, 2021). However, its vulnerability to flooding and bacterial blight disease received severe yield inconsistency under the flash flood situation. This research work was framed to enhance the sustainability of Hasanta under submergence and bacterial blight stress, through the MAB approach.
2. Materials and Methods
2.1. Experimental Materials and Breeding Strategy
We have selected Swarna Sub1 (Sub1 gene) and IRBB66 (Xa21 + xa13 + Xa7 + xa5 + Xa4) as donors (have genetic similarity i.e., 0.84 and 0.73, respectively, with RP) for the marker-aided improvement of submergence tolerant and BB resistance in the popular rice variety, Hasanta (Table 1 and Table S1; Figure 1 and Figure S1). The hybridity of the individual F1 and IC1F1 plants of the cross Hasanta/Swarna Sub1 and Hasanta/IRBB66 was confirmed with the tightly linked DNA markers, Sub1bc2 [2]; pTA248 [54] and xa13prom [13,44,55] and RM122 [2] (Table 2). The true hybrids were intercrossed and positive IC1F1s were backcrossed with the RP to generate BC1F1 seeds. The generation advancement of target gene(s) with maximum RP genome recovery was assessed in every BC generation with linked foreground and 108 background SSR markers (Table S2), followed by robust phenotyping. The molecular data were also subjected for genetic divergence analysis among parents and NIL derivatives by using the DARwin-6.0 software [56]. The best BC1F1 plants with maximum RP genome and desirable phenome (days to 50% flowering, DFF; plant height, PH; grain length-breadth ration, L/B ratio; head rice recovery, HRR; and amylose content, AC) were advanced to the next generation. The seven BC2F1 plants carried heteroalleles of the targeted genes were selfed to produce BC2F2. Further, BC2F2 population was subjected to FS, BS and phenotyping, to identify the plants with maximum RP genome and phenome. In each BC and BC2F2 generation, desirable plants were advanced adopting single seed descent (SSD) strategy under field rapid generation advance (RGA) to accelerate the development of the NILs. Selection differential (Δd) analysis for the product profile traits, DFF (days), PH (cm), grain L/B ratio, HRR and AC content was performed in BC and segregating generation. The submergence screening of NILs (BC2F3 and BC2F4) along with parents under the standard evaluation system (SES) [57] were performed at research farm of the ICAR-National Rice Research Institute, India. Biochemical parameters such as ADH [58,59], total soluble sugar content [60,61], total chlorophyll content [62] and proline content (PC) [63] were also analyzed to assess the biochemical changes and their relation to the Sub1 in NILs under flooding. In addition, disease bioassay with eight virulent Xoo type was conducted and percent disease index (PDI) and area under disease progress curve (AUDPC) were calculated to analyze the extent of improvement in the derivatives lines [64].

Table 1.
Details of parental lines, their pedigrees, maturity duration and zone of adoption.
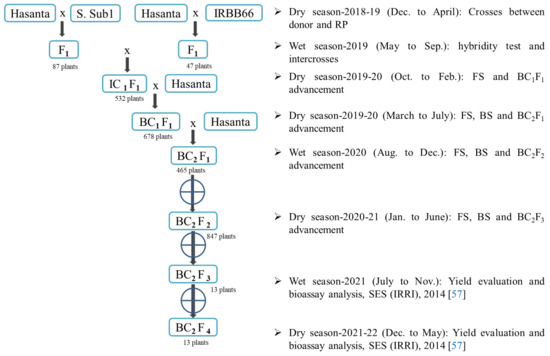
Figure 1.
MAB strategy followed for the development of improved Hasanta.

Table 2.
Genic and linked markers for FS and recombinant selection (RS).
2.2. PCR and Marker Analysis
The genomic DNA from the leaf sample of each plant (20–22 days old seedling) was extracted and purified using the CTAB method [66]. The PCR reaction was carried out using 15 ng of template DNA, 1x Taq assay buffer, 0.3 mM of MgCl2, 133.0 µM of dNTPs, 1 U/µL of Taq DNA polymerase (Thermo-scientific, Life Science Products, Mumbai, Maharashtra, India) and 1.25 µM of each primer (Eurofins, Genomics, Hyderabad, India). The PCR was carried out in an Eppendorf Thermo Cycler (Enfield, CT06082, USA) with the following program: (1) initial denaturation at 94 °C for 3 min; (2) 39 cycles for denaturation for 30 s at 94 °C, annealing for 30 s at 56 °C, extension for 1 min at 72 °C; and (3) final extension at 72 °C for 5 min. The amplified products were resolved with 2.5% MetaphorTM Agarose gel (Typhoon FLA 700, Alpha Innotech, Marlborough, MA 01752, USA) and visualized under a UV light source in photographed gel documentation (Gel-Doc) system (Gel DocTM XR, Bio-Rad Laboratories Inc., Portsmouth, NH 03801, USA).
Target traits in F1, IC1F1 and BC generations were traced using trait linked-DNA markers, Sub1bc2 with 0.0 cm distant to Sub1 [2], pTA248 lying 0.2 cm distant to Xa21 [23,67], xa13prom with 0 cm distant to xa13 [13,44,55] and RM122 with 0.4 cm distance to xa5 [65] (Table 2). Moreover, genome recovery of the RP was monitored with 108 polymorphic SSR markers (Table 3 and Table S2) that uniformly span entire rice genome [13,34,68,69]. These 108 SSRs were selected from 421 SSRs based on the polymorphism survey between Hasanta and Swarna Sub1. Recombinant analysis was carried out using markers, Sub1-A203, and Sub1-C173 (Sub1); RM26969 (Xa21), RM23356 and RM22914 (xa13) and RM17941 (xa5) flanking the Sub1 and BB resistant genes on chromosome 9, 11, 8 and 5. The molecular data in the BC2F2 generation were used to visualize genotype of individual NILs using the Graphical Genotyper (GGT2.0) software [70].

Table 3.
Brief summary of BC generations RP genome recuperation during the development of Hasanta’s NILs (P1-Hasanta/Swarna Sub1 and P2-Hasanta/IRBB66).
2.3. Screening for Submergence Tolerance
Twenty days old seedlings (BC2F3 and BC2F4) carrying the homo-quadruplet of target genes (Sub1 + Xa21+ xa13 + xa5) were grown along with parents in a screening tank. Each NIL was transplanted in three rows with 22 plants per row at 20 cm × 15 cm spacing under complete block design with two replications. Subsequently, submergence was imposed for 14 days with 1.5-m water depth. After 14 days of submergence, the water was removed from the tanks and then after 1 week of desubmergence, regeneration was recorded [57].
2.4. Disease Bioassay Analysis
The NILs and each BC generations carrying effective hetero/homo-alleliec combinations of R genes were grown in field condition along with parents and bio-assayed with eight virulent Xoo races (Xa17, Xa7, xa2, Xb7, Xc4, xd1, xa1 and xa5) prevalent in the eastern region of the country. The pathotypes were maintained in peptone sucrose agar (PSA) medium [71] and single spore culture with 108 cfu/mL bacterial Xoo suspension was used for inoculation. The top five leaves of each plant were clipped off and inoculated [53]. Post inoculation, the plants were observed after every 24 h time interval to note the appearance of disease symptoms. The lesion lengths were measured at 14, 21 and 28 days after inoculation (DAI) [72] using a disease score index of 0–9 [73]. The lesion length of <5 cm was considered resistant (R), 5–10 cm was considered moderately resistant (MR), 10–15 cm was moderately susceptible and >15 cm was taken as highly susceptible. The epidemiological parameters, such as PDI and AUDPC, which indicates overtime disease accumulation, were calculated to assess the disease severity [64].
2.5. Speed Breeding and Single Seed Descent (SSD) Strategies for Generation Advancement
Speed breeding (SB) is a suite of techniques which involves the manipulation of environments under which plants are grown, aiming to accelerate flowering and seed set. We have adopted a field rapid generation advance (RGA) along with SSD strategy (compact spacing 2.5–5.0 cm; nutritional and moisture stress, physiological stress-maintained solitary culm), which makes 30–35 days’ flowering duration reduction in rice, and thus accommodates three generations in a year (Figure 2).

Figure 2.
Photo views of field rapid generation advance, RGA. (a) Two seeds sown in each cup of 104-cavity seedling tray, after germination one seedling with solitary culm maintained up to flowering. (b) 12–14 old seedling planted in field with 5 cm spacing and solitary culm maintained till flowering. (c) Two time pruned seedlings in flowering stage.
2.6. Agro-Morpho Evaluation of NILs
The NILs (BC2F3 and BC2F4) along with RP and donor parents were evaluated for yield and other agro-morphological traits in a randomized complete block design (RCBD) with three replications. Standard agronomic management practices were followed for raising the rice crop at two locations (ICAR-National Rice Research Institute, Cuttack and Odisha Agricultural University, Bhubaneshwar) in India. The data were recorded on five plants from each of the entries for the characters namely: days to 50% flowering (DFF), days to maturity (DM), plant height (PH), flag leaf length (FLL), flag leaf width (FLW), number of effective tillers per plant (NETPP), panicle length (PL), panicle weight (PW), number of grains per panicle (NGPP), number of chaffs per panicle (NCPP), Spikelets fertility % (SF%), test weight (TW), grain yield per plant (GYPP) and % survival (%S). Further, the lines were also analyzed for grain and cooking quality parameters, such as head rice recovery (HRR) [34], kernel length before cooking (KLBC), kernel breadth before cooking (KBBC), length/breadth ratio (L/B), amylose content (AC) and panel test for palatability trait, as described by [74]. The statistical analysis was performed using the DARwin-6.0, XLSTAT, R Package Version 1.0.7. [75]. The use of plant material comply with relevant institutional, national, and international guidelines and legislation.
3. Results
3.1. Molecular Characterization of Parents and Selection of Donor
Of the total 421 SSRs screened among the 11 rice genotypes (Hasanta, Mrunalini, Swarna Sub1, IRBB66, Swarna Shreya, Upahar, Chehrang Sub1, improved CRMS 31B, improved CRMS 32B, CR Dhan 801 and CRL22), 108 markers (26.03%) showed polymorphism among the recurrent parent (RP) Hasanta and donors (Table S2). The diversity analysis revealed varying degree of genetic relatedness (similarity metrics of 0.66 to 0.84) (Table S1 and Figure S1) among the parents. The genotypes, Swarna Sub1 (carrying Sub1) and IRBB66 (carrying Xa21 + xa13 + Xa7 + xa5 + Xa4) had closest genetic similarity with RP (0.84 and 0.73, respectively). The bioassay analysis of IRBB66 also showed incompatible or HR disease reaction against eight Xoo races (LL of 1.67 ± 0.26 cm to 2.68 ± 0.23 cm) (Table 4). Therefore, to avoid undesirable linkage drag and allow a rapid fixation of segregating loci in derivatives, the IRBB66 [76] and Swarna Sub1 [77] were used as donor for Sub1 and R genes of BB disease in the our trait improvement scheme.

Table 4.
Disease reaction (after 21 days of inoculation) in improved NILs (BC2F3 and BC2F4) carrying resistance genes against eight different races of Xoo.
3.2. MAB Based Introgression of Sub1 and Three BB Resistance Genes in Hasanta
The F1s (87 and 47 plants) and intercrossed F1s (IC1F1s, 532 plants) of Hasanta/Swarna Sub1 and Hasanta/IRBB66 combinations were screened for hybridity using gene linked markers Sub1bc2 (~0.0 cm) [2], pTA-248 (~0.2 cm from Xa21) [23], xa13prom (0.0 cm from xa13) [13,44,55,78] and xa5 (0.4 cm from xa5) [65] (Table 2). The ten IC1F1s with confirmed hybridity were crossed with RP to generate BC1F1s that were genotyped with foreground markers and also compared with RP for agro-morphological traits. Eight out of the total 678 BC1F1 plants were found hetero-alelliec for target genes (Sub1 + Xa21 + xa13 + xa5) with 68.43 to 80.08% of RP genome and desirable phenome (agro-morphology and MR disease reaction, percent disease index (PDI) of 11.82 ± 0.460 to 35.14 ± 0.344, area under disease curve (AUDPC) of 278.34 to 622.35) (Table 3 and Table S3; Figure 3). Six BC1F1s carrying >80.0% RP genome and desirable phenome were advanced to BC2F1 with 465 seeds (Table 3). Further, seven positive BC2F1 plants carrying 80.65 to 89.40% RP genome and had desirable phenome (PDI = 3.82 ± 0.10 to 16.6 ± 0.55, AUDPC = 102.11 to 208.86, MR) were selfed to develop near isogenic lines (NILs) (Table 3 and Table S3; Figure 4). The BS in the BC1F1 and BC2F1 revealed 22.25% and 7.84% heteroallelism for background SSR markers, respectively, which were further analyzed in BC2F2. FS in 847 BC2F2 plants could identify 13 plants with fixed target genes Sub1+ Xa21 + xa13 + xa5 (Figure 5 and Table S4). The BS analysis in BC2F2 with 108 informative SSR markers revealed recovery of >90.0% RP genome in the plants, HS 110-224-197-10, HS 110-224-197-13, HS 110-224-197-135, HS 110-224-197-263, HS 110-224-197-379, HS 110-224-197-424, HS 232-411-391-603, HS 232-411-391-619, HS 232-411-391-702, HS 232-411-391-735, HS 232-411-391-756, HS 232-411-391-809 and HS 232-411-391-831 (Table 3 and Table S4 and Figure 5, Figures S2 and S3). Thirteen BC2F2 plants fixed with target genes and had maximum RP genome were further advanced to BC2F4 generation to allow selection of the most desirables submergence tolerant and BB resistant NILs. The submergence screening and disease bioassay analysis in 13 BC2F3 NILs confirmed their tolerance to flooding (Table 5) and durable resistance to BB disease (LL of 1.29 ± 0.12 to 4.2 ± 0.64; PDI of 2.68 ± 0.26 to 6.22 ± 1.08) (Table 4 and Table S4; Figure 6).
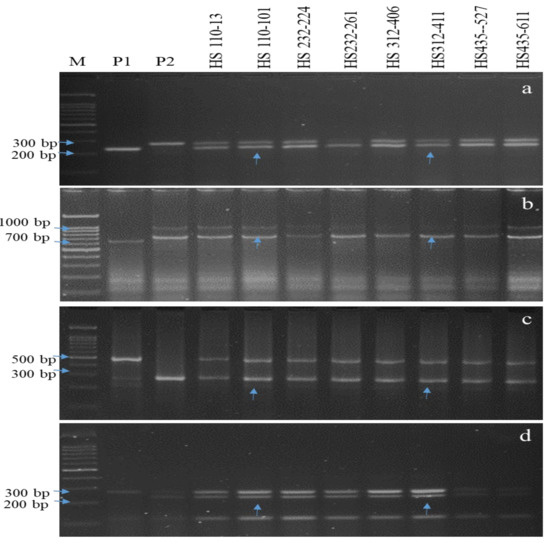
Figure 3.
PCR amplification of tolerance/resistance gene(s) in BC1F1. (a) Amplicons of the Sub1 using Sub1bc2 primer. (b) Amplicons of the Xa21 using pTA248 primer. (c) Amplicons of the xal3 using xal3prom primer. (d) Amplicons of the xa5 using RM122 primer; M, marker; P1, donor parent (Swarna Sub1 and IRBB66); P2, recurrent parent (Hasanta) Lanes 4-11 represent BC1F1 plants; vertical arrows indicate positive plants homozygous/heterozygotes for all targeted genes.
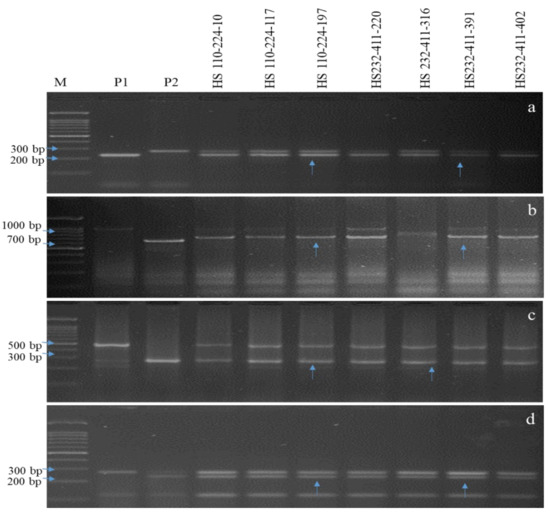
Figure 4.
PCR amplification of submergence tolerance and bacterial blight resistant gene(s) in BC2F1. (a) Amplicons of the Sub1 using Sub1bc2 primer. (b) Amplicons of the Xa21 using pTA248 primer. (c) Amplicons of the xal3 using xal3prom primer. (d) Amplicons of the xa5 using RM122 primer; M, marker; P1, donor parent (Swarna Sub1, IRBB66); P2, recurrent parent (Hasanta); Lanes 4-10 represent BC2F1 (HS 110-224-10, HS 110-224-117, HS 110-224-197, HS 232-411-220, HS 232-411-316, HS 232-411-391, HS 232-411-402); vertical arrows indicate positive plant homozygous/heterozygous for all targeted genes.
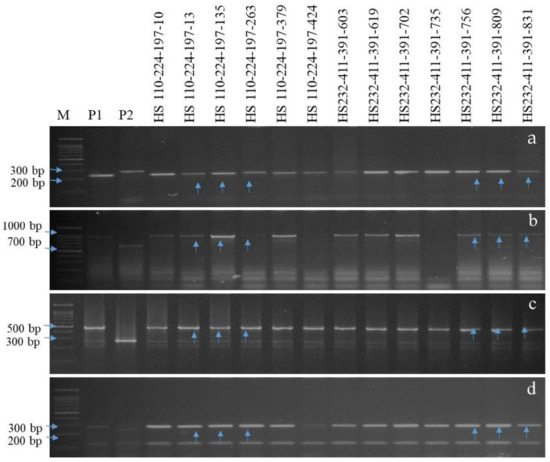
Figure 5.
PCR amplification of submergence tolerance and bacterial blight resistant gene(s) in BC2F2. (a) Amplicons of the Sub1 using Sub1bc2 primer. (b) Amplicons of the Xa21 using pTA248 primer. (c) Amplicons of the xal3 using xal3prom primer. (d) Amplicons of the xa5 using RM122 primer; M, marker; P1, donor parents (Swarna Sub1, IRBB66); P2, recurrent parent, RP (Hasanta); Lanes 4-16 represent NILs (HS 110-224-197-10, HS 110-224-197-13, HS 110-224-197-135, HS 110-224-197-263, HS 110-224-197-379, HS 110-224-197-424, HS 232-411-391-603, HS232-411-391-619, HS232-411-391-702, HS232-411-391-735, HS 232-411-391-756, HS 232-411-391-809, HS 232-411-391-831); vertical arrows indicate positive plant homozygous/heterozygous for all targeted genes.

Table 5.
Comparison of agronomic and grain quality traits of the NILs vis-à-vis parents in the BC2F3 and BC2F4 generation (pooled) under stressed (S) and non-stressed (NS) condition.
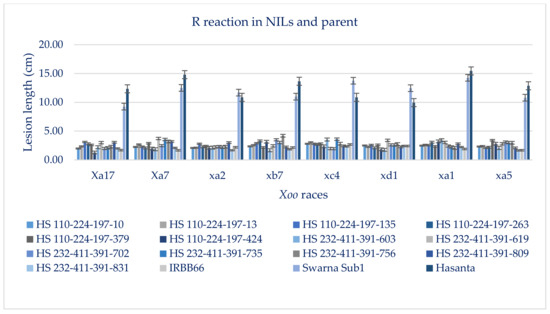
Figure 6.
Extent of BB resistance in parents and derivative NILs (BC2F3 and BC2F4) inoculated with 8 virulent Xoo isolates after 21 days of inoculation.
The phenotypic data of selected positive plants in BC generations were recorded progressing but least selection differential (Δd) for the product profile traits like, days to 50% flowering (DFF) (Δd: 1.35–2.42), plant height (PH) (Δd: 2.08–2.65), grain L/B ratio (Δd: 0.01–0.02), head rice recovery (HRR) (Δd: 0.23–1.54) and amylose content (AC%, Δd: 0.01–0.04) (Table S3). The improved NILs, HS 110-224-197-10-2, HS 110-224-197-10-5, HS 110-224-197-10-36, HS 110-224-197-10-41, HS 110-224-197-10-68, HS 232-411-391-756-10, HS 232-411-391-756-18, HS 232-411-391-756-20, HS 232-411-391-756-37, HS 232-411-391-809-1, HS 232-411-391-809-8, HS 232-411-391-809-63 and HS 232-411-391-809-81 carrying Sub1 and R genes, Xa21 + xa13 + xa5 showed 76% to 91% survival under 14 days submergence (Table 5 and Table S7) and broad spectrum of BB disease resistance (LL of 1.29 ± 0.12 to 4.2 ± 0.64 cm; PDI of 2.55 ± 0.82 to 19.08 ± 0.31) (Table 4 and Table S3; Figure 6). These improved lines were also phenotyped for grain quality, and the results revealed similar quality parameters as of RP with selection differentials (Δd) of 0.02 (Grain L/B ration), Δd = 1.54 (HRR) and same palatability as of RP (Table 5 and Table S5). Furthermore, genetic similarity amongst RP and improved NILs were assessed based on similarity metrics data of 108 SSR markers, which ranged from 0.87 to 0.93 (Figure S2). All 15 genotypes (parents + NILs) could be distinguished into two major clusters, where cluster I-A consisted of only one genotype i.e., Swarna Sub1 with similarity coefficient value of 0.77. Whereas, cluster-II consisted of RP and 13 NILs with maximum similarity coefficient value of 0.93 between Hasanta (RP) and HS 232-411-391-809-8 followed by HS 232-411-391-809-63 (0.92), HS 110-224-197-10-2 and HS 110-224-197-10-41 (0.91). The majority of the NILs were similar to the recipient parent, Hasanta, for the yield, quality and sustainability traits (Table 5, Figure 7). The improved NILs carrying targeted genes (Sub1 + Xa 21+ xa13+ xa5) and >90% RP genome achieved complete product profile as of Hasanta with at par yielding ability (Table 5 and Table S7). The NILs, HS 232-411-391-756-37, HS 232-411-391-809-8, HS 232-411-391-756-18, HS 110-224-197-10-36, HS 232-411-391-809-81, HS 110-224-197-10-41 and HS 232-411-391-809-63 were found to have similar yield, 1000-grain weight (test weight), head rice recovery (HRR), amylose content (AC) and survivability as of RP under 14 days submergence (Table S7). The quality assessment of improved NILs suggested that the introgressed lines had similar quality parameters including grain type, HRR (53.1% to 64.64%) and AC content (21.66% to 25.00%) (Table 5 and Table S7). Besides, sensory evaluation results revealed that there was no significant difference between RP and NILs in respect to palatability (Table S5).
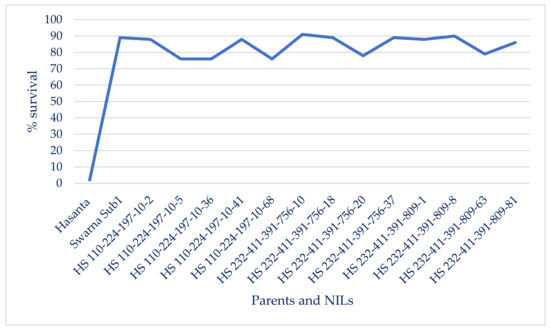
Figure 7.
Survivability of introgressed lines (BC2F3 and BC2F4) under 14 days submergence [56].
3.3. Genome Introgression on the Carrier and Non-Carrier Chromosomes
The success of the trait improvement strategy relies upon the extent of the RP genome recovery in the resulting products, while retaining originality of the RP. Our results suggested an average of 92.86% RP genome recovery in the pyramided lines, representing >90% genomic segments from the chromosomes 1, 2, 4, 5, 6, 7, 10 and 12 (Figure 8 and Figure S3, Table S4). RP genome of chromosomes 1, 2 and 10 were fully recovered in all NILs. The Sub1 gene located on chromosome 9 was stacked with negligible linkage drag (LD), which is delimited up to 1.0 mb upstream and 1.0 mb downstream with markers A203 and C173, respectively (Figure S4). R genes (xa5, xa13 and Xa21), which are located on chromosomes 5, 8 and 11, were also pyramided with negligible LD from donor IRBB66. The recombinant analysis of xa5 revealed that LD is delimited up to 3.4 Mb downstream with RM17941. Whereas, it was delimited up to 2.6 Mb upstream and 4.1 Mb downstream of xa13 with markers RM23358 and RM22914; and 1.1 Mb upstream of Xa21 with RM26969 marker (Figure 8 and Figure S4). The result of genic marker, Sub1Bc2 (InDels) revealed that Sub1 gene is introgressed successfully in all improved NILs with >90% RP genome (Figure 7). Besides, the intervention of the marker RM122 supported successful introgression of xa5 in all 13 NILs along with >90% RP genome (Figure 8). Likewise, the marker pattern of xa13prom suggested the successful introgression of xa13 in all NILs (1-13) with >80%% RP genome, where HS 232-411-391-809-63 and HS 232-411-391-809-81 were recovered with the whole region of chromosome-8. Moreover, all NILs having Xa21 had >80.0% RP genome with desirable phenotypic traits (Table 5 and Table S7, Figure 9). In BC2F2 generation, on an average, 93.52 (86.59%) background markers confirmed homozygosity for RP alleles, 5.31 markers (4.91%) had heterozygosity and 4.69 markers (4.34%) showed homozygosity for donor alleles (Table S4). The pyramids, HS 232-411-391-809-63-8 (95.19%), HS 232-411-391-809-63 (93.75%), HS 110-224-197-10-68 (93.75%), HS 110-224-197-10-2 (93.75%), HS 110-224-197-10-5 (93.27%) and HS232-411-391-809-63-8-20 (93.27%) with all target genes were recovered with substantial RP genome (Table S4, Figure S3) and morphological traits (Table 5 and Table S7, Figures S5 and S6).
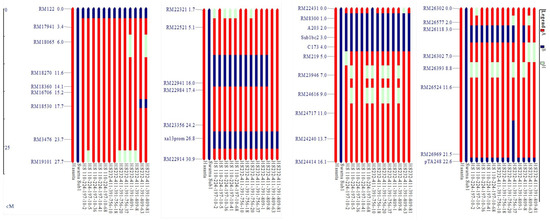
Figure 8.
Graphical representation of genomic contributions from parents in NILs derived from the cross between Hasanta (RP) and Swarna Sub1 and IRBB66 (donor), NILs: 1-13, Legend A represent RP, B as donor and H as heterozygosity; Chromosome 5, 8, 9 and 11 harbors R genes, xa5, xa13, Sub1 and Xa21, respectively.
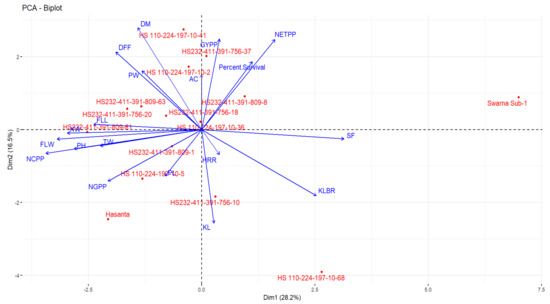
Figure 9.
Biplot diagram of 19 traits including yield, quality and other agro-morphological traits in the parents and NILs.
3.4. Screening of NILs for Submergence Tolerance and BB Resistance
The results of bioassay analysis in BC2F3 and BC2F4 NILs suggested BB resistance reactions (LL of 1.29 ± 0.12 cm to 4.2 ± 0.64 cm, PDI of 2.68 ± 0.26 to 6.22 ± 1.08), which remained at par with the extent of resistance in donor parent (Table 4, Figure 6 and Figure S5). All 13 NILs that attained homozygosity for the four targeted genes had 76% to 91% survival (14 days of submergence) and a broad spectrum of BB resistance against eight virulent Xoo pathotypes prevalent in the eastern region of the country. The NILs harbored homo-alleles of Sub1 and three R genes, Xa21+ xa13+ xa5 and 91.35% to 95.19% RP genome had LL in the range of 1.29 ± 0.12 to 4.2 ± 0.64 cm, which corresponded to the resistant category (disease score of 1). HS 232-411-391-756-10 has recorded maximum (91.0%) survivability and BB resistant reaction (Mean LL of 1.29 to 3.22 cm) attained perfect product profile as of RP for the traits, DFF (117 days), TW (20.2 g), HRR (554.60%), AC (22.08%) and grain size (2.51, L/B ratio), followed by HS 232-411-391-809-8 (90.0% survivability, mean LL of 2.08 cm to 4.22 cm; DFF of 116 days; TW of 19.69 g; HRR of 64.2%; AC of 25.0% and L/B ration of 2.51), HS 232-411-391-756-37 and HS 232-411-391-756-18 (89.0% survivability, Mean LL of 1.68 cm to 3.54 cm; DFF of 115 days; TW of 22.2 g; HRR of 64.64%; AC of 25.12% and grain size of 2.51, L/B ratio), HS 232-411-391-809-81 (86.0% survivability under 14 days submergence, mean LL of 1.64 cm to 2.44 cm; DFF of 118 days; TW of 21.62 g; HRR of 59.85%; AC of 22.05% and grain L/B ratio of 2.48) and HS 110-224-197-10-2 (88.0% survivability; mean LLof 1.98 cm to 2.80 cm; DFF of 118 days; TW of 20.1 g; HRR of 53.12%; AC of 23.4% and grain L/B ratio of 2.43) (Table 5 and Table S7 and Figure 6 and Figure 7). The lesion lengths observed on the lines containing Xa21 + xa5 genes varied from 4.38 ± 1.08 cm to 9.22 ± 0.87 cm, while it is ranged from 3.85 ± 0.64 to 6.90 ± 1.24 cm in the NILs carrying xa13 + xa5 gene combination (data not presented).
3.5. Physiological Responses of Sub1 Gene in NILs
The pyramided lines (BC2F4) along with parents were evaluated for physiological traits, such as total chlorophyll content, total soluble sugar, ADH activities and proline accumulation under 0, 7, 14 and 21 days of flooding regimes (Figure 10, Figure 11, Figure 12 and Figure 13, Table S6). The analysis of variance (ANOVA) for the RBD is given in Table 6. The variation among the NILs was higher for all physiological response studied in all submergence regimes. The total chlorophyll (Chl a and Chl b) content was measured in the leaf and culm of 60 days old plants after 7, 14 and 21 days of submergence (Figure 10). The result revealed that the maximum chlorophyll content was reported under control condition (ranged from 1.80 to 3.20 mg g−1) and it was highest in Sub1 carrying genotypes. It recorded a decreasing trend in proportion to the duration of submergence in all lines (parent and NILs) and regimes. The lowest value was recorded in RP (1.40 mg g−1) followed by in HS 110-224-197-10-5 (1.80 mg g−1) and HS 232-411-391-756-10 (1.90 mg g−1) after 14 days’ submergence. The result indicates fast depletion of total chlorophyll in intolerant cultivars. Notably, Swarna Sub1 along with HS 110-224-197-10-36, HS 232-411-391-756-37, HS 232-411-391-809-1 recorded comparatively increased level of chlorophyll content after desubmergence (i.e., 21 days).
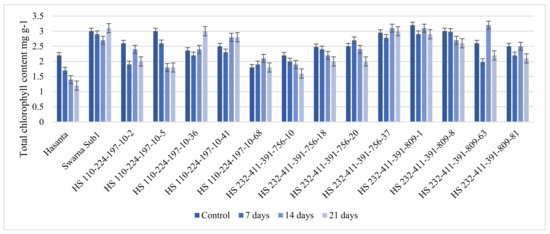
Figure 10.
Total chlorophyll content in leaf and culm under different submergence regimes (0, 7, 14, and 21 days).
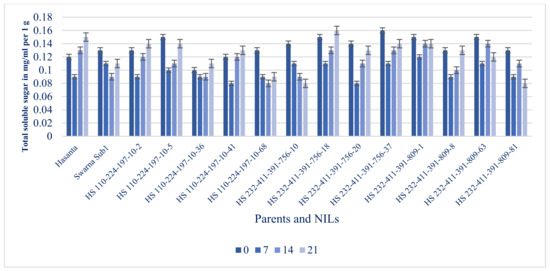
Figure 11.
Total soluble sugar content in leaf under different submergence regimes (0, 7, 14, and 21 days).
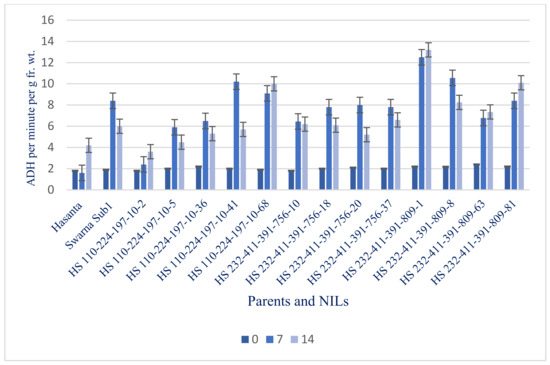
Figure 12.
ADH activities in parents and NILs under different submergence regimes (0, 7, and 14 days).
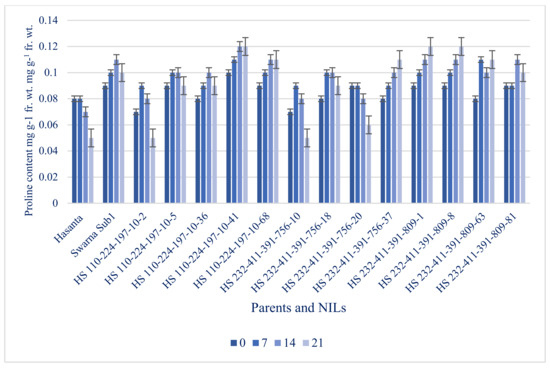
Figure 13.
Proline content in parents and NILs under different submergence regimes (0, 7, 14, and 21 days).

Table 6.
ANOVA of physiological traits in parents and NILs (Hasanta/Swarna Sub1//Hasanta/IRBB66) under submergence (0, 7, 14, 21 days) condition.
Furthermore, total soluble sugar (mg g−1 fr. wt.) was recorded maximum in non-submerged leaves in all genotypes, which slowly decreased with increase in submergence period (Figure 11). Under non-submergence (control condition), all genotypes had a high level of soluble sugar content, which showed considerable reduction up to 7 days of submergence then after recorded increasing trend. Tolerant genotypes (NILs and donor) had comparatively slowed increase in soluble sugar with prolonged submergence period (beyond 7 days flooding). The result indicates that the slowed consumption of insoluble sugar during flooding led to submergence tolerance in rice.
The ADH, an enzyme catalyzes anaerobic respiration (ethanolic) was reported increasing trend, recorded maximum (3.60 to 13.20 per min per g fr. wt.) in the lines carrying Sub1 gene even after 14 days of submergence. ADH activities was found highest in the HS 232-411-391-809-1 (13.20 per min per g fr. wt.) followed by HS 232-411-391-809-81 (10.10 per min per g fr. wt.) and HS 110-224-197-10-68 (10.0 per min per g fr. wt.) (Figure 12).
Proline maintains normal osmoregulation in plant tissue under submergence. Our results revealed an increasing trend of proline accumulation under submergence in all improved NILs and donor (Figure 13). The genotypes carrying Sub1 gene showed maximum proline accumulation even beyond 14 days of submergence. The lines, HS 232-411-391-809-1, HS 232-411-391-809-8, HS 110-224-197-10-41 has recorded maximum (0.12 mg g−1 fr. wt.) proline accumulation followed by HS 232-411-391-809-63, HS 232-411-391-756-37 and HS 110-224-197-10-68 (0.11 mg g−1 fr. wt.), which was substantially greater than RP (0.07 mg g−1 fr. wt.).
4. Discussion
A rainfed ecosystem is acquainted with frequent submergence and drought, amongst submergence stress, and is detrimental to rice production in Asian countries, accounting ~600 million USD annual monetary loss [6]. Turbid water along with dense algal growth hinders light and gaseous transmission under submergence and affects the photosynthetic activity, which led plants to die due to starvation [6,74,79]. Submergence stress, along with bacterial blight disease, causes substantial yield and quality reduction in rice [6]. The frequent occurrence of FF and the prevalence of Xoo races with varying levels of genetic diversity and virulence renders their management extremely challenging [6,39,49]. Despite varietal abundancy, the productivity of rice under RSL and a deep water ecosystem remains inconsistent and low [24]. Prolonged submergence creates very conducive environments to grow several biotic (bacterial blight, sheath blight, sheath rot etc.) and other abiotic (salinity) stresses, which threatens rice sustainability in a rainfed ecology [59,79]. Enhancing the resistance level in the host plant presents a standard practice to mitigate the risks of biotic and abiotic stresses, and is ecofriendly [2,4,13,24,41,42,76,80,81,82,83]. Evidence suggests that stacking tolerant/multiple R genes resulting in quantitative complementation or synergistic response, improves the resistance/tolerant level of plants [2,39,81,84]. In recent years, advances in genomic tools [34,80,84,85] have made significant contributions to the trait improvement in plant breeding. In this context, the marker-assisted improvement of plant resistance/tolerance has been proven as one of the most promising strategies for sustainable enhancement in crop plants [2,4,13,24,42,76,81,82,83].
The present study demonstrates the utility of MAB based introgression of the submergence tolerant (Sub1) and bacterial blight resistant genes (Xa21 + xa13 + xa5) into the background of Hasanta, a popular long duration rice in the eastern coastal region of India. MAB coupled with robust phenomics and speed breeding strategies led to fast and precise introgression of four genes, with perfect product profile of RP in two BC generation [2,39,81]. The variety Swarna Sub1 (carrying Sub1 gene) having >02 week flooding tolerance [2,28] and IRBB66 (NIL of IR 24, carrying five R genes, Xa21 + xa13 + Xa7 + xa5 + Xa4) has a high level of resistance against hyper-virulent Xoo pathotypes (Table 4) [86]. In addition, both genotypes, Swarna Sub1 and IRBB66, had maximum genetic proximity with RP (0.84 and 0.73 similarity coefficient, respectively) (Table S1 and Figure S1) and were used donor to avoid linkage drag and accelerative recovery of RP genome in the derivatives [39,81]. The F1s with a hetero-alleliec combination of target genes (Sub1 + Xa21+ Xa7 + Xa4) showed submergence tolerant and BB resistant reaction owing to the presence of Sub1 and R genes in functional complementarity. Whereas, BC progenies (Bc1F1s, Bc2F1s) with effective combination of Sub1 and Xa21 gene had complete submergence tolerant and moderately R reaction (Table S3) is fully corroborated with previous findings [2,39,81]. The improved lines did not show any linkage drag from the donors (Table 5, Tables S7 and S8), which might be due to the close genetic similarity among donor and RP genotypes. Besides, recombinant analysis delimited the linkage drags below threshold level, which on the other hand helped in the precise introgression of target genes [2,81]. Notably, selection differentials for most of the desirable traits changed progressively throughout the BC and segregating generations. The sensory panel test revealed no substantial differences for palatability in NILs and RP, which validated the successful recovery of all major gene(s) responsible for quality traits in Hasanta. The targeted product profile in NILs was attained since most of the traits considered in the study were attributed to a number of small effect genes/QTLs. The adoption of limited backcrosses in this study was found to have substantial edge over the burden of more backcrosses as our rapid backcross scheme (coupled with speed breeding strategy) led the accelerated accumulation of desirable genes (minor) in the derivatives. Thus, the inclusion of genetically close parents in MAS not only reduces the number of breeding generations, but it can also contribute to enhanced response to selection for important traits in later stage of the population [42,87,88]. Stepwise BS analysis coupled with robust phenotyping led to the fast recovery of RP genomes in early generation [2,39,42,49,76,82]. The majority of the NILs carrying Sub1 and three R genes (Sub1 + Xa21 + xa13 + xa5) had similar agro-morphology (duration, grain, yield and quality parameters) as to the RP with least selection differential and >0.90 similarity index (Table 5 and Table S3, Figures S2 and S6) and at par tolerance/resistance with the donor, Swarna Sub1 and IRBB66 (Table 5) indicates the utility of MAB in trait introgression [2,4,13,24,41,42,49,76,80,81,82,83,89]. Phenotyping in combination with MAS in BC generations was proven equally important as this strategy helped fast-tracking the transfer of genomic regions of functional relevance. Whereas, SSR markers employed in the BS usually target the non-coding and heterochromatic regions and therefore may not be suitable to quantify the recovery of functional part of the genome [42,81].
Being a semi aquatic plant, rice is able to make several modulations to sustain under flood adversity [6]. The results of the physiological analysis for total soluble sugar content, total chlorophyll content, ADH activities and proline accumulation under submergence revealed the considerable impact of Sub1 introgression in the NILs (Table S6, Figure 10, Figure 11, Figure 12 and Figure 13). The delayed degradation of the total chlorophyll content under complete flooding helps the plant to sustain longer [6,90]. SUB1A delays dark-induced senescence through the conservation of carbohydrate reserves and chlorophyll in the photosynthetic tissue [91,92], which helps in post submergence fast recovery [36]. Our result revealed comparatively slowed degradation in total chlorophyll content in NILs and donor genotypes carrying Sub1 is fully corroborated with previous findings [93,94,95]. Introgression or over expression of SUB1A-1 conferred significant submergence tolerance in the susceptible cultivars viz., Swarna and IR64 lines that lack SUB1A-1 or have the SUB1A-2 allele, but, due to a point mutation within the coding region SUB1A-2 allele is non-functional [32,36,96,97].
Under submergence, tolerant plants are capable of restricting their growth, and thus conserve ~25 to 50% more nonstructural carbohydrate, which helps in faster post submergence recovery [6,89,98,99,100]. The slowed release of non-structural sugar or total soluble sugar in submerged canopy indicates its judicious consumption of stored sugar, which helps prolonged survivability and post submergence fast recovery [8,89,101]. We have reported in our study that lines carrying the Sub1 gene had showed a slowed release of soluble sugar even beyond two weeks of submergence and followed ‘quiescence strategies’ of submergence tolerant [6,39,101]; whereas intolerant lines showed rapid increment in the non-structural sugar after 1 week of submergence. Contrariwise, growth regulators like ethylene, abscisic acid (ABA) and gibberellins (GA) promotes internode elongation and help plants to get exposed and survive [79]. The increased activity of hydrolase enzymes like ADH, α-amylase etc. facilitates energy to submerged canopy through anaerobic respiration [6,91]. The results of ADH activities revealed that lines carrying Sub1 had progressive ADH activity during submergence [44,102], which helped in the reduction of aldehyde production, and thus increases tolerance under hypoxia/anoxia in rice [30]. The increased accumulation of proline content in stressed plant is also an adaptive mechanism, which help to maintain osmoregulation in the submerged condition [103,104]. NILs that harbored Sub1 gene showed an increasing trend in proline accumulation under submergence, and further validated the impact of Sub1 gene.
Furthermore, BB bioassay of NILs carrying three R genes confirmed the successful introgression of the R genes, and the lines manifested incompatible disease response against all virulent Xoo pathotypes considered in the study [2,4,15,39,42,49]. The improved lines with two or more effective R gene combinations showed resistance levels comparable to that of the donor, which validated the efficacy of the introgression strategy that followed [2,4,15,39,42,49].
The success of this study has again validated the utility of marker assisted gene introgression with more precision and succession [13,14,41,42,53,65,73,86,105,106,107,108]. We could have achieved exactly the same product profile of RP in derivatives with 2 weeks of flooding tolerance and the broad spectrum of BB resistance. This research is fully corroborated with the results of improved Tapaswini, improved Lalat, Swarna Sub1, Ranjit Sub1 and Improved Rajalaxmi, improved Pusa RH10 [41,109,110]. The application of markers to recuperate full genome of RP in end product is again validated in this research, ~100 markers are found to be enough to retain originality of RP in MAB strategy, which is also fully corroborated with previous study [13,42,49]. The results of this study opened the path for the introgression of multiple genes in suitable genetic background with more than 95% originality very quickly and precisely.
5. Conclusions
Genomics-aided trait improvement to combat submergence stress due to FF and BB disease is an environmentally-sound and economically-viable strategy to achieve sustainable rice production under RSL worldwide. The prevalence of genetically diverse virulent Xoo strains over agro-climatic zone renders this disease extremely challenging to manage. The results of this study revealed that Sub1 along with R genes (Xa21 + xa13 + xa5) in combination could impart submergence tolerance and broad spectrum BB resistance along with ~10-fold yield enhancement. We demonstrated the utility of speed breeding strategies along with MAS in quick and precise stacking of Sub1 and R genes into the background of the popular rice variety, Hasanta.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agriculture12111815/s1, Figure S1: genetic relationship amongst the parents; Figure S2: genetic relationship amongst the recurrent parent Hasanta and NILs; Figure S3: extant of RP genome recovery in derivative NILs; Figure S4: physical position of SSR markers used for gene introgression and genome recovery of the recurrent parent; Figure S5: bacterial blight disease severity in parents and NILs after 21 days of inoculation of eight virulent Xoo races; Figure S6: field view parents and NILs of Hasanta/Swarna Sub1//Hasanta/IRBB66; (a) comparative field view of parents and derivatives, (b) screening of NILs and parents under 14 days flooding, and (c) panicle size of NILs and parents; Table S1: similarity matrix of parental lines taken for genetic diversity analysis; Table S2: a list of polymorphic SSR markers between parents Hasanta, Swarna Sub1 and IRBB66; Table S3: brief summary of selection differential and bacterial blight disease index in BC and advanced segregating generations; Table S4: RP genome recovery in BC2F2 generation; Table S5: X2 analysis for palatability in RP and improved lines based on panel test; Table S6: effect of Sub1 in improved lines for physiological traits under different submergence regimes. (a) total chlorophyll content mg g−1, (b) total soluble sugar in mg/mL per 1 g, (c) alcohol dehydrogenase per minute per gm fr. Wt, and (d) amount of free proline content (mg g−1 fr. wt.); Table S7: performance of NILs (BC2F3 and BC2F4) for yield and other agro-morphological traits under control condition-pooled data; Table S8: performance of NILs (BC2F3 and BC2F4) for yield and other agro-morphological traits under 14 days submergence-pooled data.
Author Contributions
Conceptualization, B.P. and R.V.; data curation, C.M.; formal analysis, G.D., D.J. and P.B.A.; investigation, G.D.; methodology, S.S., D.R. and R.V.; project administration, B.P. and D.B.; resources, R.V.; software, D.R., P.B.A. and A.K.M.; supervision, B.P. and R.V.; validation, D.J. and A.K.M.; visualization, D.J.; writing—original draft, G.D., D.R. and V.S.; writing—review and editing, C.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Authors are thankful to VC, OUAT, Bhubaneshwar, for providing all the necessary facilities. The study is a part of the Ph.D. research work of the first author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ismail, A.M.; Singh, U.S.; Singh, S.; Dar, M.H.; Mackill, D.J. The contribution of submergence tolerant (Sub1) rice varieties to food security in flood prone rainfed lowland areas in Asia. Field Crops Res. 2013, 152, 83–93. [Google Scholar] [CrossRef]
- Pradhan, S.K.; Pandit, E.; Pawar, S.; Baksh, S.Y.; Mukherjee, A.K.; Mohanty, S.P. Development of flash-flood tolerant and durable bacterial blight resistant versions of mega rice variety ‘Swarna’ through marker-assisted backcross breeding. Sci. Rep. 2019, 9, 12810. [Google Scholar] [CrossRef] [PubMed]
- Iftekharuddaula, K.M.; Ahmed, H.U.; Ghosal, S.; Moni, Z.R.; Amin, A.; Ali, M.S. Development of a new submergence tolerant rice variety for Bangladesh using marker-assisted backcrossing. Rice Sci. 2015, 22, 15–26. [Google Scholar] [CrossRef]
- Pradhan, S.K.; Nayak, D.K.; Mohanty, S.; Behera, L.; Barik, S.R.; Pandit, E.; Lenka, S.; Anandan, A. Pyramiding of three bacterial blight resistance genes for broad-spectrum resistance in deepwater rice variety, Jalmagna. Rice 2015, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Khush, G.S. Origin, dispersal, cultivation and variation of rice. Plant Mol. Biol. 1997, 35, 25–34. [Google Scholar] [CrossRef]
- Kumar, A.; Nayak, A.K.; Hanjagi, P.S.; Kumari, K.; Vijaykumar, S.; Mohanthy, S.; Tripathi, R.; Pannerselvam, P. Submergence stress in rice: Adaptive mechanism, coping strategies and future research needs. Environ. Exp. Bot. 2021, 186, 104448. [Google Scholar] [CrossRef]
- Mackill, D.J.; Ismail, A.M.; Singh, U.S.; Labios, R.V.; Paris, T.R. Development and rapid adoption of submergence-tolerant (Sub1) rice varieties. Ad. Agron. 2012, 115, 252–299. [Google Scholar]
- Sarkar, R.K.; Das, K.K.; Panda, D.; Reddy, J.N.; Patnaik, S.S.C.; Patra, B.C.; Singh, D.P. Submergence Tolerance in Rice: Biophysical Constraints, Physiological Basis and Identification of Donors; Central Rice Research Institute: Cuttack, India, 2014; Volume 36. [Google Scholar]
- Pradhan, K.C.; Barik, S.R.; Mohapatra, S.; Nayak, D.K.; Pandit, E.; Jena, B.K.; Sangeeta, S.; Pradhan, A.; Samal, A.; Meher, J.; et al. Incorporation of two bacterial blight resistance genes into the popular rice variety, ranidhan through marker-assisted breeding. Agriculture 2022, 12, 1287. [Google Scholar] [CrossRef]
- Sanchez, A.C.; Brar, D.S.; Huang, N.; Li, Z.; Khush, G.S. Sequence tagged site marker-assisted selection for three bacterial blight resistance genes in rice. Crop Sci. 2000, 40, 792–797. [Google Scholar] [CrossRef]
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Toulmin, C. Food security: The challenge of feeding 9 billion people. Science 2010, 327, 812–818. [Google Scholar] [CrossRef]
- Hasan, M.M.; Rafii, M.Y.; Ismail, M.R.; Mahmood, M.; Rahim, H.A.; Alam, M.A. Marker assisted backcrossing: A useful method for rice improvement. Biotech. Biotechnol. Equip. 2015, 29, 237–254. [Google Scholar] [CrossRef]
- Dash, A.K.; Rao, R.N.; Rao, G.J.N.; Verma, R.L.; Katara, J.L.; Mukherjee, A.K.; Singh, O.N.; Bagchi, T.B. Phenotypic and Marker-Assisted Genetic Enhancement of Parental Lines of Rajalaxmi, an Elite Rice Hybrid. Front. Plant Sci. 2016, 7, 1005. [Google Scholar] [CrossRef]
- Kumar, M.; Singh, R.P.; Singh, O.N.; Singh, P.; Arsode, P.; Jena, D.; Samantaray, S.; Verma, R.L. Generation mean analysis for bacterial blight resistance and yield traits in rice. J. Pharm. Phytochem. 2019, 8, 2120–2124. [Google Scholar]
- Ronald, P.C.; Albano, B.; Tabien, R.; Abenes, M.L.P.; Wu, K.S.; McCouch, S.R. Genetic and physical analysis of the rice bacterial blight disease resistance locus Xa21. Mol. Gen. Genet. 1992, 236, 113–120. [Google Scholar] [CrossRef]
- Adkins, S.W.; Shiraishi, T.; McComb, J.A. Submergence tolerance of rice; a new glasshouse method for the experimental submergence of plants. Plant Physiol. 1990, 80, 642–646. [Google Scholar] [CrossRef]
- Park, C.; Ronald, P.C. Cleavage and nuclear localization of the rice Xa21 immune receptor. Nat. Commun. 2012, 3, 920. [Google Scholar] [CrossRef]
- Catling, D. Rice in Deep Water; International Rice Research Institute: Manila, Philippines, 1992. [Google Scholar]
- Cen, M.M.; Li, W.; Wang, R.; Luo, S. The molecular regulatory pathways and metabolic adaptation in the seed germination and early seedling growth of rice in response to low O2 stress. Plants 2020, 9, 1363. [Google Scholar]
- Samanta, P.; Ganie, S.A.; Chakraborty, A.; Dey, N. Study on regulation of carbohydrate usage in a heterogeneous rice population under submergence. J. Plant Biochem. Biotechnol. 2021, 30, 138–146. [Google Scholar] [CrossRef]
- Das, K.K.; Sarkar, R.K.; Ismail, A.M. Elongation ability and non-structural carbohydrate levels in relation to submergence tolerance in rice. Plant Sci. 2005, 168, 131–136. [Google Scholar] [CrossRef]
- Kende, H.; Knaap, E.; Cho, H. Deep water rice: A model plant to study stem elongation. Plant Physiol. 1998, 118, 1105–1110. [Google Scholar] [CrossRef]
- Rajpurohit, D.; Kumar, R.; Kumar, M.; Paul, P.; Awasthi, A.; Osman, B.P.; Puri, A.; Jhang, T.; Singh, K.; Dhaliwal, H.S. Pyramiding of two bacterial blight resistance and a semi dwarfing gene in type 3 Basmati using marker-assisted selection. Euphytica 2011, 178, 111–126. [Google Scholar] [CrossRef]
- Chetia, S.K.; Kalita, M.; Verma, R.K.; Barua, B.; Ahmed, T.M.; Modi, K.; Singh, N.K. Flood proofing of Ranjit, a popular variety of North-Eastern India through transfer of Sub1 rice QTL by modified marker-assisted backcross breeding. Indian J. Genet. 2018, 78, 166–173. [Google Scholar] [CrossRef]
- Kabir, M.E.; Iftekharuddaula, K.M.; Khan, M.A.I.; Mian, M.A.K.; Ivy, N.A. Marker assisted introgression of bacterial blight resistant gene into submergence tolerance rice variety BRRI Dhan52. Bangladesh J. Agril. Res. 2017, 42, 403–411. [Google Scholar] [CrossRef][Green Version]
- Manivong, P.; Korinsak, K.; Korinsak, S.; Siangliw, J.L.; Vanavichit, A.; Toojinda, T. Marker-assisted selection to improve submergence tolerance, blast resistance and strong fragrance in glutinous rice. Thai J. Genet. 2014, 7, 110–122. [Google Scholar]
- Nair, M.M.; Shylaraj, K.S. Introgression of dual abiotic stress tolerance QTLs (Saltol QTL and Sub1 gene) into rice (Oryza sativa L.) variety Aiswarya through marker assisted backcross breeding. Physiol. Mol. Biol. Plants 2021, 27, 497–514. [Google Scholar] [CrossRef] [PubMed]
- Neeraja, C.N.; Rodriguez, M.R.; Pamplona, A.; Heuer, S.; Collard, B.C.; Septiningsih, E.M.; Vergara, G.; Sanchez, D.; Xu, K.; Ismail, A.M.; et al. A marker-assisted backcross approach for developing submergence-tolerant rice cultivars. Theor. Appl. Genet. 2007, 115, 767–776. [Google Scholar] [CrossRef] [PubMed]
- ICAR-National Rice Research Institute (NRRI). Annual-Report; NRRI: Cuttack, India, 2018; pp. 5–8. [Google Scholar]
- Rahman, H.; Dakshinamurthi, V.; Ramasamy, S.; Manickam, S.; Kaliyaperumal, A.K.; Raha, S.; Paneerselvam, N.; Ramanathan, V.; Nallathambi, J.; Sabariyappan, R.; et al. Introgression of submergence tolerance into CO43, a popular rice variety of india, through marker-assisted backcross breeding. Czech J. Genet. Plant Breed. 2018, 54, 101–108. [Google Scholar]
- Singh, P.; Sinha, A.K. A positive feedback loop governed by SUB1A1 interaction with MITOGEN-ACTIVATED PROTEIN KINASE3 imparts submergence tolerance in rice. Plant Cell 2016, 28, 1127–1143. [Google Scholar] [CrossRef]
- Singh, R.; Singh, Y.; Xalaxo, S.; Verulkar, S.; Yadav, N.; Singh, S.; Singh, N.; Prasad, K.S.N.; Kondayya, K.; Rao, P.V.R.; et al. From QTL to variety- harnessing the benefits of QTLs for drought, flood and salt tolerance in mega rice varieties of India through a multi-institutional network. Plant Sci. 2016, 242, 278–287. [Google Scholar] [CrossRef]
- Chen, M.; Presting, G.; Barbazuk, W.B.; Goicoechea, J.L.; Blackmon, B.; Fang, G.; Kim, H.; Frisch, D.; Yu, Y.; Sun, S.; et al. An integrated physical and genetic map of the rice genome. Plant Cell 2002, 14, 537–545. [Google Scholar] [CrossRef]
- Toojinda, T.; Siangliw, M.; Tragoonrung, S.; Vanavichit, A. Molecular genetics of submergence tolerance in rice: QTL analysis of key traits. Ann. Bot. 2003, 91, 243–253. [Google Scholar] [CrossRef]
- Verma, R.L.; Singh, S.; Singh, P.; Kumar, V.; Singh, S.P.; Singh, S.; Samantaray, S.; Singh, O.N. Genetic purity assessment of indica rice hybrids through DNS fingerprinting and grow-out test. J. Environ. Biol. 2017, 38, 1321–1331. [Google Scholar] [CrossRef]
- Xu, K.; Mackill, D.J. A major locus for submergence tolerance mapped on rice chromosome 9. Mol. Breed. 1996, 2, 219–224. [Google Scholar] [CrossRef]
- Xu, K.; Xu, X.; Fukao, T.; Canlas, P.; Maghirang-Rodriguez, R.; Heuer, S.; Ismail, A.M.; Bailey- Serres, J.; Ronald, P.C.; Mackill, D.J. Sub1A is an ethylene-response-factor-like gene that confers submergence tolerance to rice. Nature 2006, 442, 705–708. [Google Scholar] [CrossRef]
- Ou, S.H. Rice Diseases, 2nd ed.; Commonwealth Mycological Institute: Kew, UK, 1985; p. 380. [Google Scholar]
- Rout, D.; Jena, D.; Singh, V.; Kumar, M.; Arsode, P.; Singh, P.; Katara, J.L.; Samantaray, S.; Verma, R.L. Hybrid rice research: Current status and prospects. In Recent Advances in Rice Research, 1st ed.; Ansari, M.R., Ed.; IntechOpen: London, UK, 2020; p. 93668. [Google Scholar]
- Khush, G.S.; Mackill, D.J.; Sidhu, G.S. Breeding Rice for Resistance to Bacterial Leaf Blight; IRRI, Ed.; IRRI: Manila, Philippines, 1989; pp. 207–217. [Google Scholar]
- Das, G.; Rao, G.J.N.; Varier, M.; Prakash, A.; Prasad, D. Improved Tapaswini having four BB resistance genes pyramided with six genes/QTLs, resistance/ tolerance to biotic and abiotic stresses in rice. Sci. Rep. 2018, 8, 2413. [Google Scholar] [CrossRef]
- Ellur, R.K.; Khanna, A.; Yadav, A.; Pathania, S.; Rajashekar, H.; Singh, V.K.; Gopala Krishnan, S.; Bhowmicka, P.K.; Nagaraj, M.; Vinod, K.K.; et al. Improvement of basmati rice varieties for resistance to blast and bacterial blight diseases using marker assisted back-cross breeding. Plant Sci. 2016, 242, 330–341. [Google Scholar] [CrossRef]
- Dokku, P.; Das, K.M.; Rao, G.J.N. Pyramiding of four resistance genes of bacterial blight in Tapaswini, an elite rice cultivar, through marker-assisted selection. Euphytica 2013, 192, 87–96. [Google Scholar] [CrossRef]
- Suh, J.P.; Jeung, J.U.; Noh, T.H.; Cho, Y.C.; Park, S.H.; Park, H.S.; Shin, M.S.; Kim, C.K.; Jena, K.K. Development of breeding lines with three pyramided resistance genes that confer broad-spectrum bacterial blight resistance and their molecular analysis in rice. Rice 2013, 6, 5. [Google Scholar] [CrossRef]
- Yamada, S.; Katsuhara, M.W.; Michalowski, C.B.; Bohnert, H.J. A family or transcripts encoding MIP: Homologues tissue specificity of expression under salt-stress in Masembryanthemum crystallinum. Plant Cell 1995, 7, 1129–1142. [Google Scholar]
- Yoshimura, S.; Yoshimura, A.; Iwata, N.; McCouch, S.R.; Abenes, M.L.; Baraoidan, M.R.; Mew, T.W.; Nelson, R.J. Tagging and combining of bacterial blight resistance genes in rice using RAPD and RFLP markers. Mol. Breed. 1995, 1, 375–387. [Google Scholar] [CrossRef]
- Stafford, H.A.; Vennesland, B. Alcohol dehydrogenase of wheat germ. Arch. Biochem. Biop. 1953, 44, 404–414. [Google Scholar] [CrossRef]
- Toojinda, T.; Tragoonrung, S.; Vanavichit, A.; Siangliw, J.L.; Pa-In, N.; Jantaboon, J.; Siangliw, M.; Fukai, S. Molecular breeding for rainfed lowland rice in the Mekong region. Plant Prod. Sci. 2005, 8, 330–333. [Google Scholar] [CrossRef]
- Pradhan, S.K.; Chakraborti, M.; Chakraborty, K.; Behera, L.; Meher, J.; Subudhi, H.N.; Mishra, S.K.; Pandit, E.; Reddy, J.N. Genetic improvement of rainfed shallowlowland rice for higher yield and climate resilience. In Rice Research for Productivity, Profitability and Climate Resilience, 1st ed.; NRRI: Cuttack, India, 2018; pp. 107–121. [Google Scholar]
- Sundaram, R.M.; Vishnupriya, M.R.; Biradar, S.K.; Laha, G.S.; Reddy, G.A.; Rani, S.N.; Sharma, N.P.; Sonti, R.V. Marker assisted introgression of bacterial blight resistance in Samba Mahsuri, an elite indica rice variety. Euphytica 2008, 160, 411–422. [Google Scholar] [CrossRef]
- Nandakumar, N.; Singh, A.K.; Sharma, R.K.; Mohapatra, T.; Prabhu, K.V.; Zaman, F.U. Molecular fingerprinting of hybrids and assessment of genetic purity of hybrid seeds in rice using microsatellite markers. Euphytica 2004, 136, 257–264. [Google Scholar] [CrossRef]
- Dutta, S.S.; Divya, D.; Durga, R.C.V.; Reddy, D.T.; Visalakshmi, V.; Cheralu, C. Characterization of gall midge resistant rice genotypes using resistance gene specific markers. J. Exp. Biol. Agric. Sci. 2014, 2, 339–446. [Google Scholar]
- Kauffman, H.E.; Reddy, A.; Hsieh, S.P.Y.; Merca, S.D. An improved technique for evaluating resistance of varieties to Xanthomonas oryzae pv. oryzae. Plant Dis. Rep. 1973, 57, 537–541. [Google Scholar]
- Raskin, I.; Kende, H. Role of gibberellin in the growth response of submerged deep water rice. Plant Physiol. 1985, 76, 947–950. [Google Scholar] [CrossRef]
- Septiningsih, E.M.; Pamplona, A.M.; Sanchez, D.L.; Neeraja, C.N.; Vergara, G.V.; Heuer, S.; Ismail, A.M.; Mackill, D.J. Development of submergence tolerant rice cultivars: The Sub1 locus and beyond. Ann. Bot. 2009, 103, 151–160. [Google Scholar] [CrossRef]
- Perrier, X.; Flori, A.; Bonnot, F. Data analysis methods. In Genetic Diversity of Cultivated Tropical Plants; Hamon, P., Seguin, M., Perrier, X., Glaszmann, J.C., Eds.; Enfield, Science Publishers: Montpellier, France, 2003; pp. 43–76. [Google Scholar]
- IRRI. Injuries caused by diseases. In Standard Evaluation System for Rice (SES), 5th ed.; International Rice Research Institute: Los Baños, Philippines, 2013; pp. 18–27. [Google Scholar]
- Lowry, O.H.; Rosenbrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- SSR Markers. Available online: https://www.gramene.org/SSR (accessed on 27 July 2019).
- Krishnaveni, S.; Balasubramanian, T.; Sadasivam, S. Sugar distribution in sweet stalk sorghum. Food Chem. 1984, 15, 229–232. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.; Hamilton, J.; Rebers, P.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Witham, F.H.; Blaydes, D.F.; Devlin, R.M. Experiments in Plant Physiology; Van Nostrand Reinhold: New York, NY, USA, 1971. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid Determination of free proline for water stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Gnanamanickam, S.S.; Brindha, P.V.; Narayanan, N.N.; Vasudevan, P.; Kavitha, S. An overview of bacterial blight disease of rice and strategies for its management. Curr. Sci. 1999, 77, 1435–1443. [Google Scholar]
- Chen, X.; Temnykh, S.; Xu, Y.; Cho, Y.G.; McCouch, S.R. Development of a microsatellite framework map providing genome wide coverage in rice (Oryza sativa L.). Theor. Appl. Genet. 1997, 95, 553–567. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Khush, G.S. What it will take to feed 5.0 billion rice consumers in 2030. Plant Mol. Biol. 2005, 59, 1–6. [Google Scholar] [CrossRef]
- Singh, V.K.; Singh, A.; Singh, S.P.; Ellur, R.K.; Singh, D.; Krishnan, S.G.; Bhowmick, P.K.; Nagarajan, M.; Vinod, K.K.; Singh, U.D.; et al. Marker-assisted simultaneous but stepwise backcross breeding for pyramiding blast resistance genes Pi2 and Pi54 into an elite basmati rice restorer line PRR78. Plant Breed. 2013, 132, 486–495. [Google Scholar]
- Yazid, S.N.; Ahmad, K.; Razak, M.S.F.A.; Rahman, Z.A.; Ramachandran, K.; Mohamad, S.N.A.; Ghaffar, M.B.A. Introgression of bacterial leaf blight (BLB) resistant gene, Xa7 into MARDI elite variety, MR219 by marker assisted backcrossing (MABC) approach. Braz. J. Biol. 2021, 84, e248359. [Google Scholar] [CrossRef]
- Berloo, R.V. GGT 2.0: Versatile software for visualization and analysis of genetic data. J. Hered. 2008, 99, 232–236. [Google Scholar] [CrossRef]
- Fahy, P.C.; Persley, G.J. Plant Bacterial Diseases: A Diagnostic Guide; Academic Press: New York, NY, USA, 1983; p. 393. [Google Scholar]
- IRRI. International Rice Research Institute, Rice Fact Sheet, Bacterial Blight. March 2010. Available online: https://download.ceris.purdue.edu/file/1503 (accessed on 22 July 2022).
- Mondal, K.K.; Meena, B.R.; Junaid, A.; Verma, G.; Mani, C.; Majumder, D.; Khicher, M.; Kumar, S.; Banik, S. Pathotyping and genetic screening of type III effectors in Indian strains of Xanthomonas oryzae pv. oryzae causing bacterial leaf blight of rice. Physiol. Mol. Plant Pathol. 2014, 86, 98–106. [Google Scholar] [CrossRef]
- Bhaduri, D.; Chakraborty, K.; Nayak, A.K.; Shahid, M.; Tripathi, R.; Behera, R.; Singh, S.; Srivastava, A.K. Alteration in plant spacing improves submergence tolerance in Sub1 and non-Sub1 rice (cv. IR64) by better light interception and effective carbohydrate utilisation under stress. Funct. Plant Biol. 2020, 47, 891–903. [Google Scholar] [CrossRef] [PubMed]
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses. 2020. R Package Version 1.0.7. Available online: https://CRAN.R-project.org/package=factoextra (accessed on 10 December 2021).
- Hsu, Y.C.; Chiu, C.H.; Yap, R.; Tseng, U.C.; Wu, Y.P. Pyramiding bacterial blight resistance genes in Tainung82 for broad-spectrum resistance using marker-assisted selection. Inter. J. Mol. Sci. 2020, 21, 1281. [Google Scholar] [CrossRef] [PubMed]
- Bailey-Serres, J.; Fukao, F.; Ronald, P.; Ismail, A.; Heuer, S.; Mackill, D. Submergence tolerant rice: Sub1’s journey from landrace to modern cultivar. Rice 2010, 3, 138–147. [Google Scholar] [CrossRef]
- Singh, A.K.; Gopalakrishnan, S.; Singh, V.P.; Prabhu, K.V.; Mohapatra, T.; Singh, N.K.; Sharma, T.R.; Nagarajan, M.; Vinod, K.K.; Singh, D.; et al. Marker assisted selection: A paradigm shift in Basmati breeding. Indian J. Genet. 2011, 71, 120–128. [Google Scholar]
- Bailey-Serres, J.; Voesenek, L.A.C.J. Flooding stress: Acclimations and genetic diversity. Ann. Rev. Plant Biol. 2008, 59, 313–339. [Google Scholar] [CrossRef] [PubMed]
- Chukwu, S.C.; Rafii, M.Y.; Ramlee, S.I.; Ismail, S.I.; Hasan, M.M.; Oladosu, Y.A.; Magaji, U.G.; Akos, I.; Olalekan, K.K. Bacterial leaf blight resistance in rice: A review of conventional breeding to molecular approach. Mol. Biol. Rep. 2019, 46, 1519–1532. [Google Scholar] [CrossRef]
- Khanna, A.; Sharma, V.; Ellur, R.K.; Shikari, A.B.; Gopalakrishnan, S.; Singh, U.D.; Prakash, G.; Sharma, T.R.; Rathour, R.; Variar, M.; et al. Development and evaluation of near-isogenic lines for major blast resistance gene(s) in Basmati rice. Theor. Appl. Genet. 2015, 128, 1243–1259. [Google Scholar] [CrossRef]
- Balachiranjeevi, C.; Bhaskar, N.S.; Abhilash, V.; Akanksha, S.; Viraktamath, B.C.; Madhav, M.S.; Hariprasad, A.S.; Laha, G.S.; Prasad, M.S.; Balachandran, S.; et al. Marker-assisted introgression of bacterial blight and blast resistance into DRR17B, an elite, fine-grain type maintainer line of rice. Mol. Breed. 2015, 35, 151. [Google Scholar] [CrossRef]
- Singh, G.P.; Singh, S.R.; Singh, R.V.; Singh, R.M. Variation and qualitative losses caused by bacterial blight in different rice varieties. Indian Phytopathol. 1997, 30, 180–185. [Google Scholar]
- Singh, S.; Mackill, D.J.; Ismail, A.M. Responses of Sub1 rice introgression lines to submergence in the field: Yield and grain quality. Field Crops Res. 2009, 113, 12–23. [Google Scholar] [CrossRef]
- Cobb, J.N.; Biswas, P.S.; Platten, J.D. Back to the future: Revisiting MAS as a tool for modern plant breeding. Theor. Appl. Genet. 2019, 132, 647–667. [Google Scholar] [CrossRef]
- Cheema, K.; Grewal, N.; Vikal, Y.; Sharma, R.; Lore, J.S.; Das, A.; Bhatia, D.; Mahajan, R.; Gupta, V.; Bharaj, T.S.; et al. A novel bacterial blight resistant gene from Oryza nivara mapped to 38 kb region on chromosome 4L and transferred to Oryza sativa L. Genet. Res. Camb. 2008, 90, 397–407. [Google Scholar] [CrossRef]
- Ikmal, A.M.; Noraziyah, A.A.S.; Wickneswari, R.; Amira, I.; Ellina, Z.P.D. Interaction of submergence tolerance and drought yield QTLs (Sub1 and qDTYs) enhances morpho-physiological traits and survival of rice (Oryza sativa L.) under submergence. Ann. Appl Biol. 2021, 178, 355–366. [Google Scholar] [CrossRef]
- Singh, V.K.; Singh, A.; Singh, P.; Ellur, R.K.; Choudhary, V.; Sarkel, S.; Singh, D.; Gopalakrishnan, S.; Nagarajan, M.; Vinod, K.K.; et al. Incorporation of blast resistance into “PRR78”, an elite basmati rice restorer line, through marker assisted backcross breeding. Field Crops Res. 2012, 128, 8–16. [Google Scholar] [CrossRef]
- Sarkar, R.K.; Reddy, J.N.; Sharma, S.G.; Ismail, A.M. Physiological basis of submergence tolerance in rice and implications for crop improvement. Cur. Sci. 2006, 91, 899–906. [Google Scholar]
- Adak, M.K.; Gupta, D.K.D. Prolonged waterlogging on photosynthesis and related characters in rice. Indian J. Plant Physiol. 2000, 5, 380–382. [Google Scholar]
- Fukao, T.; Yeung, E.; Bailey-Serres, J. The submergence tolerance gene SUB1A delays leaf senescence under prolonged darkness through hormonal regulation in rice. Plant Physiol. 2012, 4, 1795–1807. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Nanda, B.B.; Swami, S.G.; Nayak, B.B. Influence of nitrogen on the physico-chemical characteristics of rice grain. Oryza 1971, 891, 87–97. [Google Scholar]
- Ashraf, M. Relationships between leaf gas exchange characteristics and growth of differently adapted populations of blue panicgrass panicum antidotale Retz. under salinity or water logging. Plant Sci. 2003, 165, 69–75. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Roles glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Deka, M.; Baruah, K.K. Comparable studies of rainfed upland winter rice (Oryza sativa) cultivars for drought tolerance. Indian J. Agril. Sci. 2000, 70, 135–139. [Google Scholar]
- Fukao, T.; Xu, K.; Ronald, P.C.; Bailey-Serres, J. A variable cluster of ethylene response factor-like genes regulates metabolic and developmental acclimation responses to submergence in rice. Plant Cell 2006, 18, 2021–2034. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Verma, R.L.; Singh, R.S.; Singh, R.P.; Singh, H.B.; Arsode, P.; Kumar, M.; Singh, P.K. Biotic stress management in rice (Oryza sativa L.) through conventional and molecular approaches. In New Frontiers in Stress Management for Durable Agriculture; Springer: Singapore, 2020; pp. 609–644. [Google Scholar]
- Luo, F.L.; Chen, Y.; Huang, L.; Wang, A.; Zhang, M.X.; Yu, F.H. Shifting effects of physiological integration on performance of a clonal plant during submergence and de-submergence. Ann. Bot. 2014, 113, 1265–1274. [Google Scholar] [CrossRef] [PubMed]
- Loreti, E.; Striker, G.G. Plant responses to hypoxia: Signaling and adaptation. Plants 2020, 9, 1704. [Google Scholar] [CrossRef] [PubMed]
- Joshi, E.; Kumar, D.; Lal, B.V.; Gautam, P.; Vyas, A.K. Management of direct seeded rice for enhanced resource—Use efficiency. Plant Knowl. J. 2013, 2, 119–134. [Google Scholar]
- Xu, K.; Xu, X.; Ronald, P.C.; Mackill, D.J. A high-resolution linkage map in the vicinity of the rice submergence tolerance locus Sub1. Mol. Gen. Genet. 2000, 263, 681–689. [Google Scholar] [CrossRef]
- Ismail, A.M.; Ella, E.S.; Vergara, G.V.; Mackill, D.J. Mechanisms associated with tolerance to flooding during germination and early seedling growth in rice (Oryza sativa). Ann. Bot. 2009, 103, 197–209. [Google Scholar] [CrossRef]
- Chen, C.T.; Kao, C.H. Osmotic stress and water stress have opposite effects on putrescine and proline production in excised rice leaves. Plant Growth Regul. 1993, 13, 197–202. [Google Scholar] [CrossRef]
- Caballero, J.L.; Verderzco, C.V.; Galam, J.; Jimenez, E.S.D. Proline accumulation as a symptom of water stress in maize: A tissue differentiation requirement. J. Exp. Bot. 2005, 39, 889–897. [Google Scholar] [CrossRef]
- Bradbury, L.M.T.; Fitzgerald, T.L.; Henry, R.J.; Jin, Q.S.; Waters, D.L.E. The gene for fragrance in rice. Plant Biotechnol. J. 2005, 3, 363–370. [Google Scholar] [CrossRef]
- Juliano, B.O. A simplified assay for milled rice amylose. Cereal Sci. Today 1971, 16, 340–360. [Google Scholar]
- Kumar, M.; Singh, R.P.; Singh, O.N.; Singh, P.; Arsode, P.; Namrata; Chaudhary, M.; Jena, D.; Singh, V.; Rout, D.; et al. Genetic analysis for bacterial blight resistance in indica rice (Oryza sativa L.) cultivars. Oryza 2019, 56, 247–255. [Google Scholar] [CrossRef]
- Rahman, M.; Grover, A.; Peacock, W.J.; Dennis, E.S.; Ellis, M.H. Effects of manipulation of pyruvate decarboxylase and alcohol dehydrogenase levels on the submergence tolerance of rice. Aust. J. Plant Physiol. 2001, 28, 1231–1241. [Google Scholar] [CrossRef]
- Peng, H.; Chen, Z.; Fang, Z.; Zhou, J.; Xia, Z.; Gao, L.; Chen, L.; Li, L.; Li, T.; Zhai, W.; et al. Rice Xa21 primed genes and pathways that are critical for combating bacterial blight infection. Sci. Rep. 2015, 5, 12165. [Google Scholar] [CrossRef]
- Katara, J.L.; Verma, R.L.; Parida, M.; Ngangkham, U.; Molla, K.A.; Barbadikar, K.M.; Mukherjee, M.; Samantaray, S.; Ravi, N.R.; Singh, O.N.; et al. Differential expression of genes at panicle initiation and grain filling stages implied in heterosis of rice hybrids. Int. J. Mol. Sci. 2020, 21, 1080. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).