Abstract
TGA transcription factor (TF) family genes play a major role in the regulation of plant growth and development as well as in the defense against pathogen attack. Little is known about the TGA family genes and their functions in sugarcane. Here, a total of 16 TGA members were identified in the sugarcane genome by bioinformatic approaches. All members exhibited similar conserved motifs and contained a bZIP domain and a DOG1 domain, except for ShTGA15/16. Phylogenetic analysis demonstrated that 16 ShTGA family genes could be divided into eight clades, and evolved differently from Arabidopsis TGAs. All ShTGA family genes suffered a purifying selection during evolution. A wide range of cis-regulatory elements were found in the promoter of ShTGA genes including hormone regulatory elements, adversity response elements, light responsive elements, and growth and development regulatory elements. Most ShTGA expressions were increased in bud growth and developmental processes except for ShTGA10/11. It is worth noting that the expression of ShTGA13 was decreased after sugarcane was infected with Sporisorium scitamineum, and it was highly expressed in the resistant variety compared to the susceptible variety. Adding IAA, GA3 and SA restored the expression of ShTGA13, suggesting an association with plant hormone regulatory pathways. Our study provides a framework for further functional studies of important ShTGA genes in development and stress response, and uncovered a previously unrecognized role of ShTGA13 in regulating resistance against S. scitamineum.
1. Introduction
TGACG-Binding (TGA) transcription factors (TFs) are the subfamily of basic region/leucine zipper (bZIP) TFs which are extensively present in all eukaryotes. These TGAs act at the interface between the DNA and the regulatory proteins by binding to cis-regulatory elements with TGACG (also called activation sequence-1, as-1) [1,2]. The first plant TGA transcription factor was tobacco TGA1a, characterized in 1989 [3]. In 1992, 10 TGA members were discovered and were divided into five clades in Arabidopsis [4]. Identification of the TGA transcription factor family in other plant species has been reported recently [5,6,7,8,9]. The function and regulatory mechanism of TGAs have been well studied in Arabidopsis TGAs mutants, revealing their importance in a wide range of biological processes. ATTGA1/2/3/4/5/6/7 were found to constitutively interact with the non-repressor of pathogenesis-related gene 1 (NPR1), which is a key positive regulator of the salicylic acid (SA)-dependent signaling pathway [10,11,12]. Thus, these ATTGAs played the essential role of inducing pathogenesis-related (PR) 1 expression and subsequently SAR activation in response to pathogen attack [13,14]. ATTGA2/5/6 were believed to be essential activators of jasmonic acid/ethylene-induced defense responses [15,16]. ATTGAs were also found to be involved in regulating the detoxification pathway [17], UV-B stress [18], Cr6+ tolerance [19], drought resistance [20] and SA-induce redox state [21]. Several studies showed that the expression of TGAs was differentially regulated subsequent to pathogen infection and abiotic stress in plants [22,23,24]. Their regulatory roles were usually connected to plant hormonal pathways and they could affect plant immunity by modulating the basal promoter activity of the PR-1 gene [25,26]. Beyond their importance for biotic and abiotic stress responses, the TGA gene family is associated with plant growth and development including shoot apical meristem (SAM) maintenance, flowering, inflorescence architecture development, root growth and circadian rhythm [27,28,29,30].
Sugarcane (Saccharum spp. hybrid) is the world’s largest tropical and subtropical crop constituting the chief source of sugar. It has a long growth season, and is attacked by many fungal pathogens. Sugarcane smut, caused by Sporisorium scitamineum, is the most challenging fungous disease. It was first reported in Natal province, South Africa, in 1877, and is now widely prevalent in major sugarcane planting areas around the world. To cope with the smut, sugarcane developed various mechanisms to impair pathogen colonization, proliferation and spread [31]. It was documented that the progression of S. scitamineum infection was accompanied by distinct gene transcriptional changes in plant hormone biosynthesis and signal transduction [32,33,34]. Auxin, gibberellin (GA), abscisic acid (ABA), ethylene (ETH), jasmonic acid (JA) and SA were more apparent in response to smut fungus invasion and played complex roles in regulating plant defense responses in cooperative or antagonistic mode [33,34,35,36,37,38]. The regulatory mechanisms of these hormones are still unspecified, some of which achieved varied roles in different circumstances. Studies on the roles of plant hormones in the interaction between sugarcane and smut fungus are still lacking.
The activity of TGA was connected to different plant hormone signaling pathways including auxin, GA, ABA, JA and SA [38,39,40,41,42,43]. The research suggests a role of the plant hormone signaling pathway in the defense of sugarcane smut, revealing a potential mechanism by which sugarcane TGA transcription factors mediated appropriate adjustment of gene expression in hormone signal transduction cascades. However, little is known about sugarcane TGA family members and their roles in plant developmental processes and pathogens defense. In this study, we identified the sugarcane TGA family members at the genome level and investigated the physico-chemical properties, gene structure, molecular evolution, and promoter elements. Furthermore, we analyzed the expression of sugarcane TGA family genes in response to S. scitamineum infection combined with different plant hormone treatments. Our finding suggests a unique role of ShTGA13 in sugarcane–smut interaction, which can be further explored in explaining the smut-resistant mechanism.
2. Materials and Methods
2.1. Materials
The study was conducted on a smut-susceptible sugarcane genotype, ROC22 and a smut-resistant sugarcane genotype, YZ05-51 [44], which were produced by the China National Germplasm Repository of Sugarcane, Kaiyuan, China. Robust stems were selected and cut into single bud setts, which were then immersed under flowing water for 24 h at room temperature so as to remove dirt and microorganisms. The setts were dried at room temperature and were transferred to the light incubator (12 h light-dark cycle, 32℃, 80% humidity) for germination. The source of S. scitamineum inoculum was collected from the main cultivar ROC22 at Kaiyuan City, Yunnan Province, China in 2021, and was stored at 4℃. The germination capacity of the teliospores of S. scitamineum was checked in 1% water agar; teliospores that showed over 90% germination were used for inoculation.
2.2. S. scitamineum Inoculation and Applied Treatments
After three days of germination, sugarcane buds were injected with S. scitamineum inoculum via puncture as described previously [33]. Five different treatments and at least forty setts per treatment were used including control (inoculated with sterile water), sugarcane smut infected plants, and infected plants supplemented with plant hormones (IAA, GA3, and SA were used separately). The specific phytohormones and their concentrations were 2 × 10−3 M IAA, 1 × 10−3 M gibberellin (GA3), 5 × 10−3 M SA referring to previous studies [45,46]. The treated bud setts continued to culture in the light incubator, and the shoot apical meristem samples were collected at 0, 1, 3 and 7 d after inoculation. At least two bud setts were mixed into one sample, and three biological replicates were adopted at each condition. Samples were frozen immediately in liquid N2 for further analysis.
2.3. Sequence Retrieval
The whole-genome shotgun contigs database of smut-resistant sugarcane cultivar SP80-3280 (txid: 193079, accession number in PRJNA431722) was obtained from the National Center for Biotechnology Information (NCBI), and the genomic data of S. spontaneum AP85-441, a wild Saccharum species, were also retrieved (accession number in QVOL00000000). The TGA nucleotide sequences and amino acid sequences of Oryza sativa Indica Group (txid: 39946) and Arabidopsis thaliana (L.) Heynh (txid: 3702) were obtained by querying the reported TGA gene name [42,47] from the Arabidopsis Information Resource and the Rice Genome Annotation Project (http://rice.plantbiology.msu.edu/, accessed on 3 July 2022), respectively. The gene IDs of Arabidopsis TGA were ATTGA1 (At5g65210), ATTGA2 (At5g06950), ATTGA3 (At1g22070), ATTGA4 (At5g10030), ATTGA5 (At5g06960), ATTGA6 (At3g12250), ATTGA7 (At1g77920), ATTGA8 (At1g68640), ATTGA9 (At1g08320), and ATTGA10 (At5g06839). The gene IDs of rice TGA were OSTGA1 (Os05t0443900), OSTGAP1 (Os04t0637000), OSTGA2 (Os01t0808100), OSTGA3 (Os03t0318600), OSTGA5 (Os01t0279900), OSTGA10 (Os09t0489500), OSTGAL11 (Os12t0152900), OSbZIP47 (Os06t0265400) and OSbZIP49 (Os06t0614100).
2.4. Screening and Identification of TGA Family Genes in Sugarcane
ATTGA family genes and OSTGA family genes were used as query sequences to search for sugarcane TGA family genes in the genome database of Saccharum spp. hybrid SP80-3280 and S. spontaneum AP85-441 (downloaded from NCBI) through the Basic Local Alignment Search Tool and default parameters [48]. The resulting hits were filtered by E-value (1 × 10−8), and only the longest sequence was retained if several results were found for the same gene. The open reading frames were sought by the Open Reading Frame Finder program (https://www.ncbi.nlm.nih.gov/orffinder/, accessed on 3 July 2022). The conserved domain analysis was performed and checked by the NCBI-Conserved Domain Database [49] and PFAM protein family database (http://pfam.xfam.org/, accessed on 3 July 2022) [50]. All sugarcane TGA protein sequences were analyzed by ExPASy to obtain their basic physical and chemical properties, such as molecular weight (MW), isoelectric point (pI), amino acid composition, instability coefficient and total average hydrophilicity (https://www.expasy.org/protparam/, accessed on 3 July 2022) [51]. The protein subcellular localization was predicted by CELLO v.2.5 [52].
2.5. Analysis of Gene Structure and Conserved Motifs
The homology and structural characteristics of all ShTGA family members were conducted by BioEdit v7.0 with the default parameters [53]. Gene structures of ShTGA genes were analyzed and visualized by TBtools [54], and the motif structures in the ShTGA protein sequences were analyzed by the MEME program (Version 5.1.1, University of Nevada, Reno) with a maximum number of motifs set to 10 [55]. Letters that appeared in each position constituted a position-specific probability matrix, which could be used to judge the possible motifs in the sequence group.
2.6. Phylogenetic Analysis of ShTGA Proteins and Calculation of Ka/Ks
The alignment of multiple amino acid sequences of the selected 35 TGA family members, composed of 10 Arabidopsis TGAs, 9 rice TGAs and 16 sugarcane TGAs, was performed using MUSCLE in MEGA 11 software with default parameters [56]. The phylogenetic tree based on the alignments was constructed using the maximum likelihood method with 1000 bootstrap replicates, the Jones–Taylor–Thornton (JTT) model, gamma distribution and partial deletion [56]. The non-synonymous (Ka) and synonymous (Ks) substitution ratios were also calculated using Compute Pairwise Distances in MEGA 11, and the divergence time (T) was calculated according to the Ks value by T = Ks/2λ × 10−6 Mya (λ = 1.5 × 10−8).
2.7. Cis-Regulatory Elements Prediction of ShTGA Gene Family
The 2000-bp upstream sequence of the coding region of each ShTGA family gene was obtained to investigate cis-regulatory elements. The putative cis-regulatory elements in the promoter sequences were analyzed via PlantCARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/, accessed on 3 July 2022) and visualized by Microsoft Excel version 2010.
2.8. Experimental Validation of ShTGA Gene Expression Levels by qRT-PCR
The shoot apical meristem samples were used to extract RNA using the FastPure Plant Total RNA Isolation Kit (Vazyme Biotech Co., Ltd., Nanjing, China), and the RNA was reverse transcribed into cDNA using HiScript III RT SuperMix for qPCR (Vazyme Biotech Co., Ltd., Nanjing, China). Gene-specific primer synthesis was conducted by the Beijing Genome Institute (Shenzhen, China). The specific nucleotide sequences of primers for quantifying every ShTGA expression were listed in Supplementary Table S1. Gene expressions were normalized against an internal reference GAPDH gene. The volume of the qRT-PCR reaction was 20 μL, including 10 μL FastStart Universal SYBR Green PCR Master, 0.4 μL primer and 2 μL template cDNA. The qRT-PCR program was set at 95 °C for 2 min, with 40 cycles of 95 °C for 10 s and 60 °C for 30 s. Three independent biological samples were evaluated for every condition and all reactions of each sample were performed in triplicate for the analysis of ShTGA gene expression. The relative expression of ShTGA genes was calculated using the 2−∆∆c(t) method.
2.9. Statistical Analysis
In the qRT-PCR analysis, the differences between relative gene expressions were analyzed using the one-way ANOVA test, and the least significant differences (LSD) method was used for further comparison between two groups at p < 0.05 (marked with *) or p < 0.01 (marked with **) (SPSS 20.0, Inc., Chicago, IL, USA).
3. Results
3.1. Screening TGA Transcription Factors Family Genes for Basic Physic-Chemical Properties of ShTGA Coding Proteins
Sugarcane TGA family genes, named ShTGA1 to ShTGA16, were identified from the sugarcane genome database by sequence homology blast and domain confirmation. The number of nucleotides of sixteen TGA genes ranged from 2172 to 11238, and the sequence identity of their coding DNA sequences varied from 2.6% to 23.7%. The sixteen TGA proteins had from as few as 330 (ShTGA5) to as many as 652 (ShTGA16) amino acids (Table 1). The molecular weights varied from 36.7 to 68.7 kD. The isoelectric points ranged from 5.86 (ShTGA15) to 9.67 (ShTGA13). The bZIP domain was contained in all ShTGA proteins, while the DELAY OF GERMINATION1 (DOG1) domain was presented in fourteen ShTGA proteins except for in ShTGA15 and ShTGA16. The grand average of the hydropathicity of all ShTGA proteins was <0, suggesting that those proteins were hydrophilic. All sixteen ShTGA proteins were classified unstable as they had a high instability coefficient (instability coefficient >40) and a low aliphatic index (<90). Protein subcellular prediction showed that all ShTGA proteins were located in the nucleus.

Table 1.
Physico-chemical parameters and subcellular predictions of TGA family members in Saccharum spp. hybrid.
3.2. Gene Structures and Protein Domains Analysis of ShTGAs
Converse motif analysis of the sixteen ShTGA proteins showed that motif numbers ranged from 2 (ShTGA16) to 8 (ShTGA8/9/14) (Figure 1). Motif 2 was present in all ShTGAs and more than 81% of the ShTGA proteins had motif 5, 1, 4, 6 and 3, which were partially absent in ShTGA13 and entirely absent in ShTGA15/16. Only ShTGA8/9 contained motif 10, and only ShTGA15/16 had motif 9. Gene structure analysis suggested that the number of exons of the sixteen ShTGA genes ranged from 2 (ShTGA16) to 12 (ShTGA8/10/11/12). Correspondingly, the number of introns ranged from 1 (ShTGA16) to 11 (ShTGA8/10/11//12). The maximum number of exons was eight, which was observed in ShTGA3/4/5/6/13 genes. The exon and intron distribution patterns varied considerably, suggesting that there are function variations among different ShTGA genes.
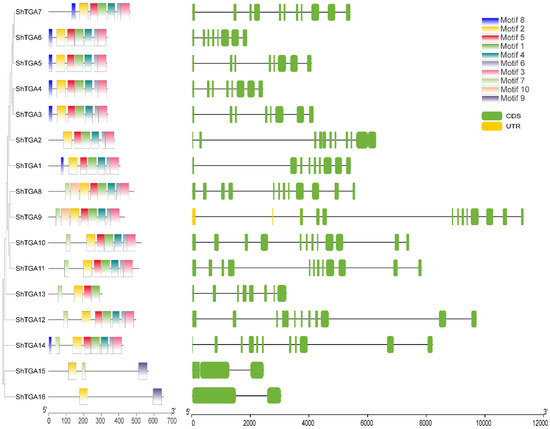
Figure 1.
Analysis of conserved motifs and gene structure domains in all ShTGA family members. Phylogenetic tree was constructed using the ShTGA protein sequences. Ten types of conserved motifs were predicted, and different motifs are shown in different color boxes. The sequence information for each motif is provided in Supplementary Table S2. The gene structures of ShTGA members were visualized; coding sequence (CDS) and untranslated regions (UTR) are shown as light green boxes and yellow boxes, respectively.
3.3. Evolution Analysis of ShTGA Family Members
Phylogenetic analysis demonstrated that 35 TGA proteins were separated into eight groups with high bootstrap support (Figure 2). The numbers of sugarcane, Arabidopsis, and rice TGA genes in each of groups were I (6, 4, 4), II (1, 4, 1), Ⅲ (2, 0, 1), Ⅳ (2, 1, 1), Ⅴ (1, 1, 1), Ⅵ (1, 0, 1), Ⅶ (1, 0, 0) and Ⅷ (2, 0, 0), respectively. Group I had the largest number of TGA family members, and TGAs in the same group may have similar functions. The ShTGA proteins were distributed in each group, while Arabidopsis and rice TGAs were present in half and three-quarters of the groups. This indicated that species differences might cause the TGA family members to cluster separately.
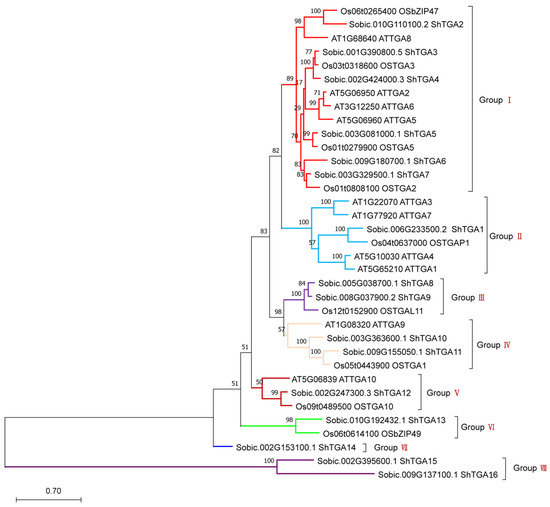
Figure 2.
Maximum likelihood phylogenetic analysis of TGA families from Arabidopsis, rice and sugarcane. Protein sequences were aligned using MUSCLE, and phylogenetic tree reconstruction was made using maximum likelihood method under the best model selection in MEGA11 software with 1000 replicates of rapid bootstrap and LRT statistics. The bootstrap value in which the associated taxa clustered together are shown next to the branches. The scale bar represents 0.07 units of amino acid substitutions per site.
In addition, we analyzed the substitution rate ratio Ka/Ks and divergence time between the TGA gene family members (Table S3). The results showed that all Ka/Ks ratios were less than 1, revealing that the evolution of TGA family genes was under a purifying selection. The differentiation times of these gene pairs occurred from 25.2 to 105.3 Mya.
3.4. Cis-Elements Analysis in ShTGAs Promoter Regions
To better understand the regulatory functions of the ShTGAs, the cis-regulatory elements were identified in the 2000 bp upstream promoter sequences of sixteen ShTGA genes. The gridding diagram showed that the cis-regulatory elements of the ShTGA family genes were divided into four categories: hormone regulatory element, adversity response element, light responsive element, and growth and development regulatory elements (Figure 3). There was the largest number of cis-regulatory elements in the category of hormone regulatory elements including abscisic acid, auxin, ethylene, GA, MeJA and SA responsiveness. The second largest category was adversity response elements, which contained anaerobic induction, stress responsiveness, drought responsiveness, wound-responsive element, etc. The major types of the light responsive element were G-box, GT1-motif, Box4, Sp1 and GATA-motif. The growth and development regulatory elements mainly had meristem specific activation, seed-specific regulation and zein metabolism regulation. The hormone regulatory elements frequently presented in the promoter regions of all ShTGA genes, indicating that these ShTGAs might be involved in various phytohormone signaling pathways. For individual genes, ShTGA6 had a considerable number of abscisic acid responsive elements including six ABRE elements, two AAGAA-motif and one ABRE3a. ShTGA9 contained five TGA-elements which were involved in auxin responsiveness. The MeJA-responsiveness elements were found in the promoter regions of all ShTGA genes. Except for ShTGA4 and ShTGA13, all ShTGA genes had the TGACG motif, also called the as-1 (activation sequence-1) element, which was usually bound to the SA-induced PR-1 gene. In addition, ShTGA genes were found to contain various adversity response elements, such as anaerobic environment, drought, low-temperature and wound. This suggested that ShTGA genes were predicted to be involved in the response to various environmental stimuli.
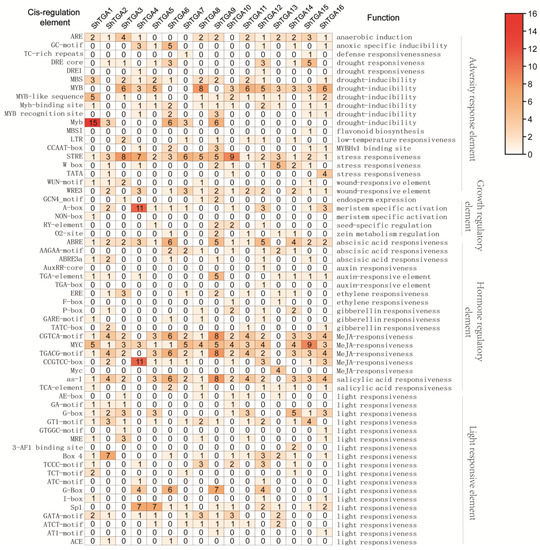
Figure 3.
Number of varied cis-regulatory elements identified in the promoter regions from TGA family genes in sugarcane. Cis-regulatory elements related to hormone response, environmental stress and development were identified by Plant Promoter Analysis Navigator from PlantPAN 3.0 database, using 2000 bp upstream from the translation start site from each gene.
3.5. Expression Profile Analysis of ShTGA Genes in the Response to S. scitamineum Infection
Since TGAs were originally found for their crucial roles in plant immunity response by coordinated plant hormone signaling pathways, we want to examine whether ShTGAs play a role in regulating the smut-resistance of sugarcane accompanied with IAA, GA3 and SA treatment. The expression levels of fifteen ShTGA family genes were observed under the infection of S. scitamineum as well as when dding hormone, while ShTGA6 was not detected (Figure 4). Under the condition of inoculation with sterile water (control group), thirteen ShTGA family genes, except for ShTGA10/11, were shown to be up-regulated during bud growth, indicating their roles in regulating these developmental processes. When sugarcane was inoculated with S. scitamineum, there were eleven ShTGA family gene expression increases except for ShTGA10/11/13/14. Compared with the control treatment, the expressions of ShTGA13 were inhibited at day3 and day7 after inoculation; the expressions of ShTGA14 were also decreased at day7 after inoculation. In the circumstance of S. scitamineum inoculum supplemented with IAA, it was found that the expressions of twelve ShTGA family genes except for ShTGA2/10/11 were obviously elevated. Apart from ShTGA2/11, the other thirteen ShTGA family genes exhibited up-regulation in the treatment of S. scitamineum inoculum incorporated GA, as well as SA addition. After pathogens inoculation, adding hormone down-regulated the expression of ShTGA2 but restored the expression of ShTGA 13/14 to control levels. To further investigate the possible role of ShTGA13 in sugarcane-S. scitamineum interaction, the expression of ShTGA13 was compared between the susceptible (ROC22) and resistant (YZ05-51) varieties (Figure 5). The results showed that the expression of ShTGA13 was also decreased after ROC22 infected with S. scitamineum. It is noteworthy that ShTGA13 was significantly highly expressed in YZ05-51 compared to ROC22 under all experimental conditions. In general, the expression of ShTGA13 was regulated subsequent to pathogen infection, and higher expression level was maintained in resistant variety indicating that ShTGA13 had relevance for the resistance against S. scitamineum.
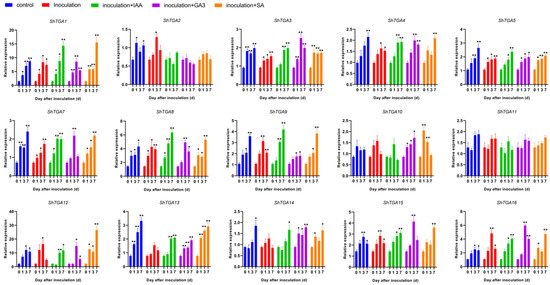
Figure 4.
Gene expression of ShTGA genes in response to S. scitamineum infection supplemented with different plant hormones. The stem bud materials were inoculated separately with sterile water (control), S. scitamineum (inoculation), S. scitamineum supplemented IAA (inoculation + IAA), S. scitamineum supplemented GA3 (inoculation + GA3) and S. scitamineum supplemented SA (inoculation + SA), and were collected at 0, 1, 3 and 7 d after inoculation. Error bars represent standard error of the mean (SEM). One way ANOVA was applied independently, and the least significant differences method was used for further comparison between two groups at p < 0.05 (marked with *) or p < 0.01 (marked with **).
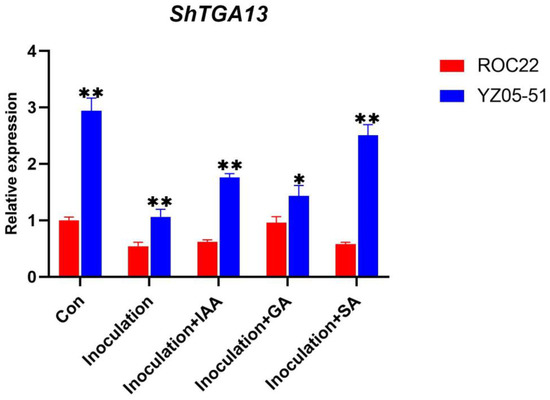
Figure 5.
Relative expression of ShTGA13 gene in the susceptible and resistant varieties subsequent to S. scitamineum infection. The stem buds of ROC22 and YZ05-51 were inoculated separately with sterile water (control), S. scitamineum (inoculation), S. scitamineum supplemented IAA (inoculation + IAA), S. scitamineum supplemented GA3 (inoculation + GA3) and S. scitamineum supplemented SA (inoculation+ SA) and were collected at 7 d after inoculation. Error bars represent standard error of the mean (SEM). One way ANOVA was applied independently, and the least significant differences method was used for further comparison between two groups at p < 0.05 (marked with *) or p < 0.01 (marked with **).
4. Discussion
In plants, TGA transcription factors play crucial roles in regulating growth and development as well as pathogen defense. Nevertheless, there is nearly no report on the systematic study of the TGA family members in sugarcane. We used a genomic survey and identified sixteen ShTGA family members. The number of ShTGA members identified was greater than that of Arabidopsis (n = 10), tomato (n = 5) [57], peach (n = 15) [5], banana (n = 9) [6], Taxus chinensis (n = 12) [7], melon (n = 9) [8], but less than that of soybean (n = 25) [9]. It is possible that the larger number of ShTGA family genes was closely related to big genome size and divergence between the species. Variations of TGA family genes may provide a genetic basis for phenotypic variability. Analysis of conserved motifs and gene structure domains showed that most TGAs exhibited a similar pattern and a highly conserved DOG1 domain and bZIP domain, indicating that they shared similar functions. It was also present in other plant TGA transcription factors [5,6,7,8]. However, ShTGA15/16 were distinct; these might be the possible pseudo-genes. Their proteins lacked the DOG1 domain, and the number of introns was far less than the other ShTGA genes. Furthermore, these two pseudo-genes were grouped into an outer group (Group Ⅷ) in the phylogenetic analysis of Arabidopsis, rice and sugarcane TGA family proteins. Subcellular localization identified that ShTGAs are located in the nucleus, suggesting they mainly functioned in the nucleus.
Phylogenetic analysis demonstrated that sugarcane TGAs evolved differently from Arabidopsis TGAs, but showed an evolutionary close relationship with rice TGAs. In Arabidopsis, ten ATTGA family members were divided into five clades based on sequence homology. TGA1/4 comprised clade I, TGA2/5/6 belonged to clade II, TGA3/7 made up clade III, TGA9/10 were grouped into clade Ⅳ, and TGA8 (also named Pan) was separately grouped in clade Ⅴ. While ShTGA transcription factor family members were evolutionarily divided into eight groups, and the sizes of ShTGA family numbers in each divided group were obviously greater than those of ATTGA family numbers except for Group II, which was comprised of ATTGA1/3/4/7 and ShTGA1. This may indicate that sugarcane has maintained more diverse functions to better survive during evolution. It was initially considered that the AtTGAs from clades I, II and III mainly participated in the response to pathogen attack and abiotic stress, whereas AtTGA8/9/10 in clades Ⅳ and Ⅴ were involved in regulating plant growth and development [4,42]. Nevertheless, an increasing number of reports show that most clades played roles in the regulation of defense response as well as developmental processes. For example, Arabidopsis TGA1 and TGA4 were found to be essential cofactors in the BLADE-ON-PETIOLE1(BOP)-dependent regulation required for SAM maintenance, flowering, and inflorescence architecture development [23]. ATTGA1/3/4/7 were found to function redundantly in the regulation of root growth and development, and TGA2/5/6/8 indeed had a common role in both promoting cell elongation and cellular redox photosynthesis [30]. The similar functions within ATTGA1/3/4/7 and ATTGA2/5/6/8 were consistent with our phylogenetical grouping. On the other hand, it was documented that clade IV AtTGAs played important roles in the defense of pathogens attack [58,59]. Similarly, it is possible that the functional division of ShTGA genes is not that evident in different groups, whereas functional redundancy is prevalent among the phylogenetically close genes in sugarcane.
It is well known that TGA are members of the bZIP transcription factors and regulate the transcription of the downstream genes to mediate a range of processes including the plant growth, development and adversity response [4]. The prediction of cis-regulatory elements in the promoter of ShTGA family genes supported these ideas that ShTGA genes were likely related to the responsiveness of hormone, adversity stress, light response, plant growth and development. In particular, a quantity of the hormone-responsive cis-regulatory elements in ShTGAs indicated that these genes may play roles in regulating resistance against pathogens in association with plant hormone signaling pathways. In this study, most test genes were involved in bud growth and developmental processes. Yet the interesting aspect here is that the levels of expression or expression patterns of ShTGA13/14 were evidently altered when sugarcane was inoculated with S. scitamineum. It is highly likely that these genes were involved in the response to fungus infection. Phylogenetic analysis showed that ShTGA13 and OSbZIP49 were clustered into one group, and they had a close evolutionary relationship with ShTGA14. It was reported that OsbZIP49 regulated shoot growth and tiller via induction of the indole-3-acetic acid-amido synthetase genes (GH3) that catalyze the conjugation of auxins to amino acids as inactive forms to mediate local auxin homeostasis [60]. OsbZIP49-overexpressing transgenic rice exhibited an abnormal phenotype with increased tiller number, reduced plant height and internode lengths. Conversely, CRISPR/Cas9-mediated knockout of OsbZIP49 displayed a compact architecture. After sugarcane suffered a pathogen attack, the expression of ShTGA13 was depressed, which might decrease the activity of GH3 to convert auxin into inactive forms. It is well known that auxin is a vital virulence factor in some host–pathogen systems [61]. The GH3 family genes were activators of plant disease resistance due to their functions in the regulation of IAA homeostasis in Arabidopsis, which was often associated with an SA-dependent pathway [43,62,63,64]. The rice GH3 family could positively regulate bacterial and fungal pathogens by suppressing the loosening of the cell wall caused by auxin signaling [65,66]. ShTGA13 was found to be highly expressed in the resistant variety compared to in the susceptible variety. These findings indicate that ShTGA13 may also have positive role in regulating resistance to smut fungus in sugarcane, which is affected by pathogen attack and by adding IAA, GA and SA. It is expected that ShTGA13 may be used for manipulating disease resistance to smut fungus in breeding sugarcane.
5. Conclusions
In this study, a total of sixteen ShTGA family genes were identified by way of a bioinformatic approach, most of which exhibited similar conserved motifs and contained a bZIP domain and a DOG1 domain. Phylogenetic analysis demonstrated that the sixteen ShTGA family members could be divided into eight clades, and evolved differently from Arabidopsis TGAs. All ShTGA family members suffered a purifying selection during evolution. A wide range of cis-regulatory elements was found in the promoter of ShTGA family genes including a hormone regulatory element, an adversity response element, a light responsive element, and growth and development regulatory elements. Most ShTGA genes were involved in bud growth and developmental processes except for ShTGA10/11. It is worth noting that the expression of ShTGA13/14 was depressed after sugarcane was infected with S. scitamineum, indicating that they may have roles in regulating resistance against S. scitamineum. Adding IAA, GA3 and SA could restore the expression of ShTGA13/14, suggesting an association with a hormone-dependent regulatory pathway.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agriculture12101644/s1, Table S1: The specific nucleotide sequences of primers for qRT-PCR; Table S2: The characteristics of conserved motifs in ShTGAs using MEME-suite; Table S3: The substitution rate ratio Ka/Ks and divergence time between the TGA gene family members.
Author Contributions
Conceptualization, C.W., Q.Z. and X.H.; methodology, Z.L., X.L. and X.H; validation, Z.W., X.L., C.W. and Q.Z.; formal analysis, Z.L., X.H. and Z.W.; resources, X.L., X.H., Z.W. and C.W; data curation, Z.L. and X.H.; writing—original draft preparation, Z.L. and X.H.; writing—review and editing, C.W., Q.Z. and Z.L.; supervision, C.W., Z.W. and Q.Z.; project administration, C.W., Q.Z. and X.H.; funding acquisition, C.W. and Q.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by China Agriculture Research System of MOF and MARA (grant number CARS-170101), Yunnan Fundamental Research Projects (grant number 2019FA016) and Science and Technology Mission of Cane Sugar Industry in Gengma County, Yunnan Province (grant number 202104BI090003).
Data Availability Statement
Not applicable.
Acknowledgments
Authors are grateful to the China National Germplasm Repository of Sugarcane for Supplementary Materials used for experiments. The authors also would like to thank Jiayong Liu, Bin Wu, Chunjia Li, Xiujuan Li, Ming Zhang, Yanhang Tang, Wei Zhang, Chenping Guan, Ying Li and Ziai Zhao for their assistance and consultations.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Izawa, T.; Foster, R.; Chua, N.H. Plant bZIP protein DNA binding specificity. J. Mol. Biol. 1993, 230, 1131–1144. [Google Scholar] [CrossRef]
- Schindler, U.; Beckmann, H.; Cashmore, A.R. TGA1 and G-box binding factors: Two distinct classes of Arabidopsis leucine zipper proteins compete for the G-box-like element TGACGTGG. Plant Cell 1992, 4, 1309–1319. [Google Scholar] [PubMed]
- Katagiri, F.; Lam, E.; Chua, N.H. Two tobacco DNA-binding proteins with homology to the nuclear factor CREB. Nature 1989, 340, 727–730. [Google Scholar] [CrossRef] [PubMed]
- Jakoby, M.; Weisshaar, B.; Droge-Laser, W.; Vicente-Carbajosa, J.; Tiedemann, J.; Kroj, T.; Parcy, F. bZIP transcription factors in Arabidopsis. Trends Plant Sci. 2002, 7, 106–111. [Google Scholar] [CrossRef]
- Li, C.H.; Wang, K.T.; Lei, C.Y.; Xu, F.; Ji, N.N.; Jiang, Y.B. Identification of TGA gene family in peach and analysis of expression mode involved in a BABA-induced disease resistance. Acta Hortic. Sin. 2022, 49, 265–280. [Google Scholar]
- Lin, P.; Wang, M.Y.; Li, Y.Q.; Liu, J.F.; Zhang, H.Y.; Lin, S.D. Identification of banana TGA transcription factor family and the expression analysis under Fusarium wilt infection. Chin. J. Trop. Crops 2021, 42, 2134–2142. [Google Scholar]
- Jin, X.F. Identification of Tga Transcription Factor and Its Functional Analysis in The Regulation of Taxol Synthesis in Taxus chinensis. Master’s Thesis, Huazhong University of Science and Technology, Wuhan, China, 2020. [Google Scholar]
- Tian, M. Genome-Wide Characterization of the TGA Gene Family in Melon and Their Roles in Disease Resistance. Master’s Thesis, Zhejiang University, Hangzhou, China, 2021. [Google Scholar]
- Ullah, I.; Magdy, M.; Wang, L.X.; Liu, M.Y.; Li, X. Genome-wide identification and evolutionary analysis of TGA transcription factors in soybean. Sci. Rep. 2019, 9, 11186. [Google Scholar] [CrossRef]
- Loake, G.; Grant, M. Salicylic acid in plant defence-the players and protagonists. Curr. Opin. Plant Biol. 2007, 10, 466–472. [Google Scholar] [CrossRef]
- Kesarwani, M.; Yoo, J.; Dong, X.N. Genetic interactions of TGA transcription factors in the regulation of pathogenesis-related genes and disease resistance in Arabidopsis. Plant Physiol. 2007, 144, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Bari, R.; Jones, J.D.G. Role of plant hormones in plant defence responses. Plant Mol. Biol. 2009, 69, 473–488. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Tessaro, M.J.; Lassner, M.; Li, X. Knockout analysis of Arabidopsis transcription factors TGA2, TGA5 and TGA6 reveals their redundant and essential roles in systemic acquired resistance. Plant Cell 2003, 15, 2647–2653. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.J.; Busta, L.; Zhang, Q.; Ding, P.T.; Jetter, R.; Zhang, Y.L. TGACG-BINDING FACTOR 1 (TGA1) and TGA4 regulate salicylic acid and pipecolic acid biosynthesis by modulating the expression of SYSTEMIC ACQUIRED RESISTANCE DEFICIENT 1 (SARD1) and CALMODULINBINDING PROTEIN 60g (CBP60g). New Phytol. 2018, 217, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Zander, M.; La Camera, S.; Lamotte, O.; Métraux, J.P.; Gatz, C. Arabidopsis thaliana class-II TGA transcription factors are essential activators of jasmonic acid/ethylene-induced defense responses. Plant J. 2010, 61, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Zander, M.; Thurow, C.; Gatz, C. TGA transcription factors activate the salicylic acid-suppressible branch of the ethylene-induced defense program by regulating ORA59 expression. Plant Physiol. 2014, 165, 1671. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.; Hilbert, B.; Dueckershoff, K.; Roitsch, T.; Krischke, M.; Mueller, M.J.; Berger, S. General detoxification and stress responses are mediated by oxidized lipids through TGA transcription factors in Arabidopsis. Plant Cell 2008, 20, 768–785. [Google Scholar] [CrossRef]
- Herrera-Vásquez, A.; Fonseca, A.; Ugalde, J.M.; Lamig, L.; Seguel, A.; Moyano, T.C.; Gutiérrez, R.A.; Salinas, P.; Vidal, E.A.; Holuigue, L. TGA class II transcription factors are essential to restrict oxidative stress in response to UV-B stress in Arabidopsis. J. Exp. Bot. 2021, 72, 1891–1905. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.H.; Liu, Z.Q.; Long, Y.P.; Liang, Y.L.; Jin, Z.P.; Zhang, L.P.; Liu, D.M.; Li, H.; Zhai, J.X.; Pei, Y.X. The Ca2+/calmodulin2-binding transcription factor TGA3 elevates LCD expression and H2S production to bolster Cr6+ tolerance in Arabidopsis. Plant J. 2017, 91, 1038–1050. [Google Scholar] [CrossRef]
- Zhong, L.; Chen, D.D.; Min, D.H.; Li, W.W.; Xu, Z.S.; Zhou, Y.B.; Li, L.C.; Chen, M.; Ma, Y.Z. AtTGA4, a bZIP transcription factor, confers drought resistance by enhancing nitrate transport and assimilation in Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 2015, 457, 433–439. [Google Scholar] [CrossRef]
- Hou, J.Y.; Sun, Q.; Li, J.J.; Ahammed, G.J.; Yu, J.Q.; Fang, H.; Xia, X.J. Glutaredoxin S25 and its interacting TGACG motif-binding factor TGA2 mediate brassinosteroid-induced chlorothalonil metabolism in tomato plants. Environ. Pollut. 2019, 255, 113256. [Google Scholar] [CrossRef]
- Chen, L.J.; Zou, W.S.; Fei, C.Y.; Wu, G.; Li, X.Y.; Lin, H.H.; Xi, D.H. α-Expansin EXPA4 positively regulates abiotic stress tolerance but negatively regulates pathogen resistance in Nicotiana tabacum. Plant Cell Physiol. 2018, 59, 2317–2330. [Google Scholar] [CrossRef]
- Wang, Y.; Salasini, B.C.; Khan, M.; Devi, B.; Bush, M.; Subramaniam, R.; Hepworth, S.R. Clade I TGA bZIP transcription factors mediate BLADE-ON-PETIOLE-dependent regulation of development. Plant Physiol. 2019, 180, 937–951. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.Y.; Wi, S.J.; Park, K.Y. Functional switching of NPR1 between chloroplast and nucleus for adaptive response to salt stress. Sci. Rep. 2020, 10, 4339. [Google Scholar] [CrossRef]
- Kumar, S.; Zavaliev, R.; Wu, Q.L.; Zhou, Y.; Cheng, J.; Dillard, L.; Powers, J.; Withers, J.; Zhao, J.S.; Guan, Z.Q.; et al. Structural basis of NPR1 in activating plant immunity. Nature 2022, 605, 561–566. [Google Scholar] [CrossRef]
- Chai, L.X.; Dong, K.; Liu, S.Y.; Zhang, Z.; Zhang, X.P.; Tong, X.; Zhu, F.F.; Zou, J.Z.; Wang, X.B. A putative nuclear copper chaperone promotes plant immunity in Arabidopsis. J. Exp. Bot. 2020, 71, 6684–6696. [Google Scholar] [CrossRef] [PubMed]
- Maier, A.T.; Stehling-Sun, S.; Offenburger, S.-L.; Lohmann, J.U. The bZIP transcription factor PERIANTHIA: A multifunctional hub for meristem control. Front. Plant Sci. 2011, 2, 79. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.R.; Xu, J.Y.; Yuan, C.; Hu, Y.K.; Liu, Q.G.; Chen, Q.Q.; Zhang, P.C.; Shi, N.N.; Qin, C. Characterization of genes associated with TGA7 during the floral transition. BMC Plant Biol. 2021, 21, 367. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, W.; Karapetyan, S.; Mwimba, M.; Marqués, J.; Buchler, N.E.; Dong, X.N. Redox rhythm reinforces the circadian clock to gate immune response. Nature 2015, 523, 472–476. [Google Scholar] [CrossRef]
- Hu, X.C.; Yang, L.Y.; Ren, M.F.; Liu, L.; Fu, J.; Cui, H.C. TGA factors promote plant root growth by modulating redox homeostasis or response. J. Integr. Plant Biol. 2022, 8, 1543–1559. [Google Scholar] [CrossRef]
- Bhuiyan, S.A.; Magarey, R.C.; McNeil, M.D.; Aitken, K.S. Sugarcane smut, caused by Sporisorium scitamineum, a major disease of sugarcane: A contemporary review. Phytopathology 2021, 111, 1905–1917. [Google Scholar] [CrossRef]
- Schaker, P.D.C.; Palhares, A.C.; Taniguti, L.M.; Peters, L.P.; Creste, S.; Aitken, K.S.; Van Sluys, M.A.; Kitajima, J.P.; Vieira, M.L.; Monteiro-Vitorello, C.B. RNA-Seq transcriptional profiling following whip development in sugarcane smut disease. PLoS ONE 2016, 11, e0162237. [Google Scholar] [CrossRef]
- Que, Y.X.; Guo, J.L.; Wu, Q.B.; Xu, L.P. A global view of transcriptome dynamics during Sporisorium scitamineum challenge in sugarcane by RNA-seq. PLoS ONE 2014, 9, e106476. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.C.; Xu, L.P.; Wang, Z.Q.; Peng, Q.; Yang, Y.T.; Chen, Y.; Que, Y.X. Comparative proteomics reveals that central metabolism changes are associated with resistance against Sporisorium scitamineum in sugarcane. BMC Genom. 2016, 17, 800. [Google Scholar] [CrossRef] [PubMed]
- Rahman, T.A.E.; Oirdi, M.E.; Gonzalez-Lamothe, R.; Bouarab, K. Necrotrophic pathogens use the salicylic acid signaling pathway to promote disease development in tomato. Mol. Plant-Microbe Interact. 2012, 25, 1584–1593. [Google Scholar] [CrossRef]
- Su, Y.C.; Xu, L.P.; Xue, B.T.; Wu, Q.B.; Guo, J.L.; Wu, L.J.; Que, Y.X. Molecular cloning and characterization of two pathogenesis-related β-1, 3-glucanase genes ScGluA1 and ScGluD1 from sugarcane infected by Sporisorium scitamineum. Plant Cell Rep. 2013, 32, 1503–1519. [Google Scholar] [CrossRef]
- Chen, J.W.; Kuang, J.F.; Peng, G.; Wan, S.B.; Liu, R.; Yang, Z.D.; Deng, H.H. Molecular cloning and expression analysis of a NPR1 gene from sugarcane. Pak. J. Bot. 2012, 44, 193–200. [Google Scholar]
- Huang, N.; Ling, H.; Su, Y.C.; Liu, F.; Xu, L.P.; Su, W.H.; Wu, Q.B.; Guo, J.L.; Gao, S.W.; Que, Y.X. Transcriptional analysis identifies major pathways as response components to Sporisorium scitamineum stress in sugarcane. Gene 2018, 678, 207–218. [Google Scholar] [CrossRef]
- Tang, N.; Ma, S.Q.; Zong, W.; Yang, N.; Lv, Y.; Yan, C.; Guo, Z.L.; Li, J.; Li, X.; Xiang, Y.; et al. MODD mediates deactivation and degradation of OsbZIP46 to negatively regulate ABA signaling and drought resistance in rice. Plant Cell 2016, 28, 2161–2177. [Google Scholar] [CrossRef]
- Yang, X.; Yang, Y.N.; Xue, L.J.; Zou, M.J.; Liu, J.Y.; Chen, F.; Xue, H.W. Rice ABI5-Like1 regulates abscisic acid and auxin responses by affecting the expression of ABRE-containing genes. Plant Physiol. 2011, 156, 1397–1409. [Google Scholar] [CrossRef]
- Tomaž, Š.; Gruden, K.; Coll, A. TGA transcription factors—Structural characteristics as basis for functional variability. Front. Plant Sci. 2022, 13, 935819. [Google Scholar] [CrossRef]
- Gatz, C. From pioneers to team players: TGA transcription factors provide a molecular link between different stress pathways. Mol. Plant Microbe Interact. 2013, 26, 151–159. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Li, Q.; Li, Z.M.; Staswick, P.E.; Wang, M.Y.; Zhu, Y.; He, Z.H. Dual regulation role of GH3.5 in salicylic acid and auxin signaling during Arabidopsis-Pseudomonas syringae interaction. Plant Physiol. 2007, 145, 450–464. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.F.; Liu, J.Y.; Yang, K.; Xia, H.M.; Wu, C.W.; Chen, X.K.; Zhao, J.; Yang, H.C.; Li, J.; Zan, F.G. Registration of ‘YZ0551’ sugarcane. J. Plant Regist. 2015, 9, 172–178. [Google Scholar] [CrossRef]
- Almeida, C.M.A.; Donato, V.M.T.; Amaral, D.O.J.; Lima, G.S.A.; Brito, G.G.; Lima, M.M.A.; Correia, M.T.S.; Silva, M.V. Differential gene expression in sugarcane induced by salicylic acid and under water deficit conditions. Agric. Sci. Res. J. 2014, 3, 38–44. [Google Scholar]
- Szőke, L.; Moloi, M.J.; Kovács, G.E.; Biró, G.; Radócz, L.; Hájos, M.T.; Kovács, B.; Rácz, D.; Danter, M.; Tóth, B. The Application of Phytohormones as Biostimulants in Corn Smut Infected Hungarian Sweet and Fodder Corn Hybrids. Plants 2021, 10, 1822. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, Y.; de la Bastide, M.; Hamilton, J.P.; Kanamori, H.; McCombie, W.R.; Ouyang, S.; Schwartz, D.C.; Tanaka, T.; Wu, J.Z.; Zhou, S.G.; et al. Improvement of the Oryza sativa Nipponbare reference genome using next generation sequence and optical map data. Rice 2013, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.; Zaretskaya, I.; Raytselis, Y.; Merezhuk, Y.; McGinnis, S.; Madden, T.L. NCBI BLAST: A better web interface. Nucleic Acids Res. 2008, 36, 5–9. [Google Scholar] [CrossRef]
- Lu, S.N.; Wang, J.Y.; Chitsaz, F.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; Gwadz, M.; Hurwitz, D.I.; Marchler, G.H.; Song, J.S.; et al. CDD/SPARCLE: The conserved domain database in 2020. Nucleic Acids Res. 2020, 48, 265–268. [Google Scholar] [CrossRef]
- El-Gebali, S.; Mistry, J.; Bateman, A.; Eddy, S.R.; Luciani, A.; Potter, S.C.; Qureshi, M.; Richardson, L.J.; Salazar, G.A.; Smart, A.; et al. The Pfam protein families database in 2019. Nucleic Acids Res. 2019, 47, 427–432. [Google Scholar] [CrossRef]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Duvaud, S.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. Protein identification and analysis tools on the ExPASy server. In The Proteomics Protocols Handbook; Humana Press: Totowa, NJ, USA, 2005; pp. 571–607. [Google Scholar]
- Yu, C.S.; Lin, C.J.; Hwang, J.K. Predicting subcellular localization of proteins for Gram-negative bacteria by support vector machines based on n-peptide compositions. Protein Sci. 1987, 13, 1402–1406. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Chen, C.J.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.H.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.Y.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, 202–208. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Schiermeyer, A.; Thurow, C.; Gatz, C. Tobacco bZIP factor TGA10 is a novel member of the TGA family of transcription factors. Plant Mol. Biol. 2003, 51, 817–829. [Google Scholar] [CrossRef] [PubMed]
- Noshi, M.; Mori, D.; Tanabe, N.; Maruta, T.; Shigeoka, S. Arabidopsis clade IV TGA transcription factors, TGA10 and TGA9, are involved in ROS mediated responses to bacterial PAMP flg22. Plant Sci. 2016, 252, 12–21. [Google Scholar] [CrossRef]
- Venturuzzi, A.L.; Rodriguez, M.C.; Conti, G.; Leone, M.; Caro, M.D.P.; Montecchia, J.F.; Zavallo, D.; Asurmendi, S. Negative modulation of SA signaling components by the capsid protein of tobacco mosaic virus is required for viral long-distance movement. Plant J. 2021, 106, 896–912. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.H.; Lin, X.H.; Zuo, Y.; Yu, Z.; Baerson, S.R.; Pan, Z.Q.; Zeng, R.S.; Song, Y.Y. Transcription factor OsbZIP49 controls tiller angle and plant architecture through the induction of indole-3-acetic acid-amido synthetases in rice. Plant J. 2021, 108, 1346–1364. [Google Scholar] [CrossRef] [PubMed]
- Glickmann, E.; Gardan, L.; Jacquet, S.; Hussain, S.; Elasri, M.; Petit, A.; Dessaux, Y. Auxin production is a common feature of most pathovars of Pseudomonas syringae. Mol. Plant-Microbe Interact. 1998, 11, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Jagadeeswaran, G.; Raina, S.; Acharya, B.R.; Maqbool, S.B.; Mosher, S.L.; Appel, H.M.; Schultz, J.C.; Klessig, D.F.; Raina, R. Arabidopsis GH3-LIKE DEFENSE GENE 1 is required for accumulation of salicylic acid, activation of defense responses and resistance to Pseudomonas syringae. Plant J. 2007, 51, 234–246. [Google Scholar] [CrossRef]
- Park, J.E.; Park, J.Y.; Kim, Y.S.; Staswick, P.E.; Jeon, J.; Yun, J.; Kim, S.Y.; Kim, J.; Lee, Y.H.; Park, C.M. GH3-mediated auxin homeostasis links growth regulation with stress adaptation response in Arabidopsis. J. Biol. Chem. 2007, 282, 10036–10046. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Pajerowska-Mukhtar, K.; Culler, A.H.; Dong, X.N. Salicylic acid inhibits pathogen growth in plants through repression of the auxin signaling pathway. Curr. Biol. 2007, 17, 1784–1790. [Google Scholar] [CrossRef]
- Ding, X.H.; Cao, Y.L.; Huang, L.L.; Zhao, J.; Xu, C.G.; Li, X.H.; Wang, S.P. Activation of the indole-3-acetic acid-amido synthetase GH3-8 suppresses expansin expression and promotes salicylate- and jasmonate-independent basal immunity in rice. Plant Cell 2008, 20, 228–240. [Google Scholar] [CrossRef]
- Domingo, C.; Andrés, F.; Tharreau, D.; Iglesias, D.J.; Talón, M. Constitutive expression of OsGH3.1 reduces auxin content and enhances defense response and resistance to a fungal pathogen in rice. Mol. Plant-Microbe Interact. 2009, 22, 201–210. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).