Screening Fungicides for Controlling Wheat Crown Rot Caused by Fusarium pseudograminearum across Hebei Province in China
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Strain, Wheat Cultivar, Fungicides
2.2. Inoculum Preparation for F. pseudograminearum
2.3. In Vitro Evaluation of Inhibition Effect on F. pseudograminearum
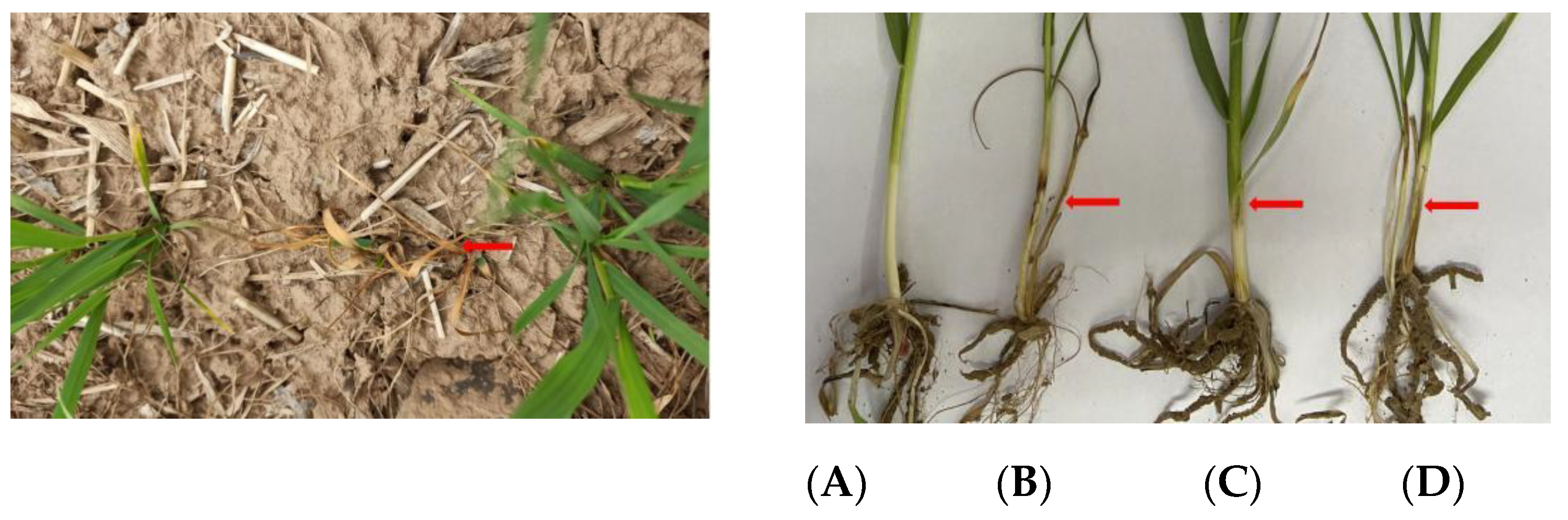
2.4. Pot Assay under Greenhouse Conditions
2.5. Plot Assay for the FCR
2.6. Field Experiments
2.7. Data Analysis
3. Results
3.1. Fungicide Sensitivity Assay of F. pseudograminearum
3.2. Pot Efficacy of Seed Dressing at the Seedling Stage
3.3. Field Plot Assay Using Artificial Inoculation
3.4. Control Efficacy of Cruiser Plus and Celest in the Field
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FCR | Fusarium crown rot |
| FHB | Fusarium head blight |
| EC50 | effective concentration for 50% growth inhibition |
| WP | wettable powder |
| SC | suspension concentrate |
| PDA | potato dextrose agar |
| DI | disease index |
| RCE | relative control efficacy |
| TGW | thousand grain weight |
| IPM | integrated pest management |
References
- Magee, C.J. News from New South Wales. Commonw. Phytopathol. News 1957, 3, 26. [Google Scholar]
- Cromey, M.G.; Parkes, R.A.; Fraser, P.M. Factors associated with stem base and root diseases of New Zealand wheat and barley crops. Australas. Plant Pathol. 2006, 35, 391–400. [Google Scholar] [CrossRef]
- Poole, G.J.; Smiley, R.W.; Walker, C.; Huggins, D.; Rupp, R.; Abatzoglou, J.; Garland-Campbell, K.; Paulitz, T.C. Effect of climate on the distribution of Fusarium spp. causing crown rot of wheat in the Pacific Northwest of the United States. Phytopathology 2013, 103, 1130–1140. [Google Scholar] [CrossRef]
- Smiley, R.W.; Gourlie, J.A.; Easley, S.A.; Patterson, L.M.; Whittaker, R. Crop damage estimates for crown rot of wheat and barley in the Pacific Northwest. Plant Dis. 2005, 89, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Jevtić, R.; Stošić, N.; Župunski, V.; Lalošević, M.; Orbović, B. Variability of stem-base infestation and coexistence of Fusarium spp. causing crown rot of winter wheat in Serbia. Plant Pathol. J. 2019, 35, 553–563. [Google Scholar] [CrossRef]
- Agustí-Brisach, C.; Raya-Ortega, M.C.; Trapero, C.; Roca, L.F.; Luque, F.; López-Moral, A.; Fuentes, M.; Trapero, A. First report of Fusarium pseudograminearum causing crown rot of wheat in Europe. Plant Dis. 2018, 102, 1670. [Google Scholar] [CrossRef]
- Gargouri, S.; Mtat, I.; Gargouri Kammoun, L.; Zid, M.; Hajlaoui, M.R. Molecular genetic diversity in populations of Fusarium pseudograminearum from Tunisia. J. Phytopathol. 2011, 159, 306–313. [Google Scholar] [CrossRef]
- Hameed, M.A.; Rana, R.M.; Ali, A. Identification and characterization of a novel Iraqi isolate of Fusarium pseudograminearum causing crown rot in wheat. Genet. Mol. Res. 2012, 11, 1341–1348. [Google Scholar] [CrossRef]
- Shikur Gebremariam, E.; Sharma-Poudyal, D.; Paulitz, T.C.; Erginbas-Orakci, G.; Karakay, A.; Dababat, A.A. Identity and pathogenicity of Fusarium species associated with crown rot on wheat (Triticum spp.) in Turkey. Eur. J. Plant Pathol. 2018, 150, 378–399. [Google Scholar] [CrossRef]
- Li, H.L.; Yuan, H.X.; Fu, B.; Xing, X.P.; Sun, B.J. First report of Fusarium pseudogruminearum causing crown rot of wheat in Henan, China. Plant Dis. 2012, 96, 1065. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Yang, G.Q.; Wang, J.M.; Song, Y.L.; Liu, L.L.; Zhao, K.; Li, Y.H.; Han, Z.H. Spatial distribution of root and crown rot fungi associated with winter wheat in the North China Plain and its relationship with climate variables. Front. Microbiol. 2018, 9, 1054. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.X.; Sun, H.Y.; Shen, C.M.; Li, W.; Yu, H.S.; Chen, H.G. Survey of Fusarium spp. causing wheat crown rot in major winter wheat growing regions of China. Plant Dis. 2015, 99, 1610–1615. [Google Scholar] [CrossRef] [PubMed]
- Hollaway, G.J.; Evans, M.L.; Wallwork, H.; Dyson, C.B.; McKay, A.C. Yield loss in cereals, caused by Fusarium culmorum and F. pseudograminearum, is related to fungal DNA in soil prior to planting, rainfall, and cereal type. Plant Dis. 2013, 97, 977–982. [Google Scholar] [CrossRef]
- Yang, X.F.; Li, J.M.; Liu, Q.R.; Wang, L. Harmfulness trend and comprehensive control measures of wheat crown rot. J. Seed Ind. Guide 2020, 3, 29–31. (In Chinese) [Google Scholar] [CrossRef]
- Xu, F.; Song, Y.L.; Yang, G.Q.; Liu, L.L.; Li, H.L. First report of Fusarium pseudograminearum from wheat heads with Fusarium head blight in North China Plain. Plant Dis. 2015, 99, 156. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.J.; Kong, L.X.; Li, Q.S.; Wang, L.S.; Chen, D.; Ma, P. First report of Fusarium pseudograminearum causing Fusarium Head Blight of wheat in Hebei Province, China. Plant Dis. 2016, 100, 220. [Google Scholar] [CrossRef]
- Aoki, T.; O’Donnell, K. Morphological and molecular characterization of Fusurium pseudograminearum sp. nov, formerly recognized as the Group 1 population of F. graminearum. Mycologia 1999, 91, 597–609. [Google Scholar] [CrossRef]
- Matny, O.N. Fusarium head blight and crown rot on wheat and barley: Losses and health risks. Adv. Plants Agric. Res. 2015, 2, 2–7. [Google Scholar] [CrossRef]
- Malosetti, M.; Zwep, L.B.; Forrest, K.; van Eeuwijk, F.A.; Dieters, M. Lessons from a GWAS study of a wheat pre-breeding program: Pyramiding resistance alleles to Fusarium crown rot. Theor. Appl. Genet. 2021, 134, 897–908. [Google Scholar] [CrossRef]
- Khudhair, M.; Melloy, P.; Lorenz, D.J.; Obanor, F.; Aitken, E.; Datta, S.; Luck, J.; Fitzgerald, G.; Chakraborty, S. Fusarium crown rot under continuous cropping of susceptible and partially resistant wheat in microcosms at elevated CO2. Plant Pathol. 2014, 63, 1033–1043. [Google Scholar] [CrossRef]
- Minati, M.H.; Mohammed-Ameen, M.K. Interaction between Fusarium head blight and crown rot disease incidence and cultural practices on wheat in the south of Iraq, Basra Province. Bull. Natl. Res. Cent. 2019, 43, 200. [Google Scholar] [CrossRef]
- Akgül, D.S.; Erkilic, A. Effect of wheat cultivars, fertilizers, and fungicides on Fusarium foot rot disease of wheat. Turk. J. Agric. For. 2016, 40, 101–108. [Google Scholar] [CrossRef]
- Moya-Elzondo, E.A.; Jacobsen, B.J. Integrated management of Fusarium crown rot of wheat using fungicide seed treatment, cultivar resistance, and induction of systemic acquired resistance (SAR). Biol. Control. 2016, 92, 153–163. [Google Scholar] [CrossRef]
- Kazan, K.; Gardiner, D.M. Fusarium crown rot caused by Fusarium pseudograminearum in cereal crops: Recent progress and future prospects. Mol. Plant Pathol. 2018, 19, 1547–1562. [Google Scholar] [CrossRef] [PubMed]
- Bhatta, M.; Morgounov, A.; Belamkar, V.; Wegulo, S.N.; Dababat, A.A.; Erginbas-Orakci, G.; El Bouhssini, M.; Gautam, P.; Poland, J.; Akci, N.; et al. Genome-wide association study for multiple biotic stress resistance in synthetic hexaploid wheat. Int. J. Mol. Sci. 2019, 20, 3667. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; He, X.L.; Hu, Y.F.; Hou, Y.; Niu, Y.J.; Dai, J.L.; Yuan, H.X.; Li, H.L. Resistance of wheat cultivars in Huang-Huai Region of China to crown rot caused by Fusarium pseudograminearum. J. Triticeae Crops 2015, 35, 339–345. (in Chinese). [Google Scholar]
- Winter, M.; Samuels, P.L.; Otto-Hanson, L.K.; Dill-Macky, R.; Kinkel, L. Biological control of Fusarium crown and root rot of wheat by Streptomyces isolates-it’s complicated. Phytobiomes J. 2019, 3, 52–60. [Google Scholar] [CrossRef]
- Moussa, T.A.A.; Almaghrabi, O.A.; Abdel-Moneim, T.S. Biological control of the wheat root rot caused by Fusarium graminearum using some PGPR strains in Saudi Arabia. Ann. Appl. Biol. 2013, 163, 72–81. [Google Scholar] [CrossRef]
- Liang, R.; Li, X.Y.; Yuan, W.L.; Jin, S.Y.; Hou, S.T.; Wang, M. Antifungal activity of Nanochitin Whisker against crown rot diseases of wheat. J. Agric. Food Chem. 2018, 66, 9907–9913. [Google Scholar] [CrossRef]
- Dehghanpour-Farashah, S.; Taheri, P.; Falahati-Rastegar, M. Effect of polyamines and nitric oxide in Piriformospora indica- induced resistance and basal immunity of wheat against Fusarium pseudograminearum. Biol. Control. 2019, 136, 104006. [Google Scholar] [CrossRef]
- Bouanaka, H.; Bellil, I.; Harrat, W.; Boussaha, S.; Benbelkacem, A.; Khelifi, D. On the biocontrol by Trichoderma afroharzianum against Fusarium culmorum responsible of Fusarium head blight and crown rot of wheat in Algeria. Egypt. J. Biol. Pest Control. 2021, 31, 68. [Google Scholar] [CrossRef]
- Wei, X.J.; Xu, Z.H.; Zhang, N.; Yang, W.X.; Liu, D.Q.; Ma, L.S. Synergistic action of commercially available fungicides for protecting wheat from common root rot caused by Bipolaris sorokiniana in China. Plant Dis. 2020, 105, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Breunig, M.; Chilvers, M.I. Baseline sensitivity of Fusariun graminearm from wheat, corn, dry bean and soybean to pydiflumetofen in Michigan, USA. Crop Prot. 2021, 140, 105419. [Google Scholar] [CrossRef]
- Spagnoletti, F.N.; Carmona, M.; Balestrasse, K.; Chiocchio, V.; Giacometti, R.; Lavado, R.S. The arbuscular mycorrhizal fungus Rhizophagus intraradices reduces the root rot caused by Fusarium pseudograminearum in wheat. Rhizosphere 2021, 19, 100369. [Google Scholar] [CrossRef]
- Hysing, S.C.; Wiik, L. Fusarium seedling blight of wheat and oats: Effects of infection level and fungicide seed treatments on agronomic characters. Acta Agric. Scand. Sect. B 2014, 64, 537–546. [Google Scholar] [CrossRef]
- Shah, L.; Ali, A.; Yahya, M.; Zhu, Y.; Wang, S.; Si, H.; Rahman, H.; Ma, C. Integrated control of Fusarium head blight and deoxynivalenol mycotoxin in wheat. Plant Pathol. 2018, 67, 532–548. [Google Scholar] [CrossRef]
- Feksa, H.R.; Do Couto, H.T.Z.; Garozi, R.; De Almeida, J.L.; Gardiano, C.G.; Tessmann, D.J. Pre- and postinfection application of strobilurin-triazole premixes and single fungicides for control of Fusarium head blight and deoxynivalenol mycotoxin in wheat. Crop Prot. 2019, 117, 128–134. [Google Scholar] [CrossRef]
- Hellin, P.; Scauflaire, J.; Van Hese, V.; Munaut, F.; Legrève, A. Sensitivity of Fusarium culmorum to Triazoles: Impact of trichothecene chemotypes, oxidative stress response and genetic diversity. Pest Manag. Sci. 2017, 73, 1244–1252. [Google Scholar] [CrossRef]
- Lamichhane, J.R.; You, M.P.; Laudinot, V.; Barbetti, M.J.; Aubertot, J.N. Revisiting sustainability of fungicide seed treatments for field crops. Plant Dis. 2020, 104, 610–623. [Google Scholar] [CrossRef]
- White, K.E.; Hoppin, J.A. Seed treatment and its implication for fungicide exposure assessment. J. Expo. Environ. Epidemiol. 2004, 14, 195–203. [Google Scholar] [CrossRef][Green Version]
- Garthwaite, D.; Parrish, G.; Couch, V. Amenity Pesticide Usage in the UK. Fera Science Ltd. 2016. Available online: https://secure.fera.defra.gov.uk/pusstats/surveys/index.cfm (accessed on 26 April 2018).
- Zhan, Z.L. Study on the technology of wheat seed coating to control diseases and insect pests. Sci. Technol. Seeds 2020, 38, 99–100. (In Chinese) [Google Scholar]
- Hitaj, C.; Smith, D.J.; Code, A.; Wechsler, S.; Esker, P.D.; Douglas, M.R. Sowing uncertainty: What we do and don’t know about the planting of pesticide-treated seed. Bio Sci. 2020, 70, 390–403. [Google Scholar] [CrossRef]
- Lamichhane, J.R.; Laudinot, V. Unveiling the unknown: Knowledge and risk perception about the planting of pesticide-treated seed among French arable farmers. J. Plant Dis. Prot. 2020, 128, 501–509. [Google Scholar] [CrossRef]
- Lamichhane, J.R. Parsimonious use of pesticide-treated seeds: An integrated pest management framework. Trends Plant Sci. 2020, 25, 1070–1073. [Google Scholar] [CrossRef] [PubMed]

| Fungicide | Toxicity Regression Equation (y = a + bx) | The Correlation Coefficient (r) | EC50 (mg/L) | Credible Interval (95%) | Slope | p-Value |
|---|---|---|---|---|---|---|
| Azoxystrobin | y = 3.8865 + 2.6816x | 0.9990 | 2.6018 ± 0.0021 a† | 2.4625~2.7484 | 2.6816 | 0.0001 |
| Difenoconazole | y = 5.3641 + 0.8768x | 0.9972 | 0.3845 ± 0.0005 c | 0.3128~0.4724 | 0.8768 | 0.0002 |
| Fludioxonil | y = 6.9780 + 1.4623x | 0.9317 | 0.0445 ± 0.0127 d | 0.0201~0.0980 | 1.4623 | 0.0212 |
| Tebuconazole | y = 5.4074 + 1.3212x | 0.9822 | 0.4919 ± 0.0118 b | 0.3395~0.7120 | 1.3212 | 0.0028 |
| Fungicides | RCE (%) |
|---|---|
| Raxil | 49.25 ± 3.29 b† |
| Dividend | 52.07 ± 1.65 ab |
| Cruiser Plus | 58.40 ± 1.95 a |
| Celest | 57.21 ± 2.20 a |
| Control | - |
| Treatments | Seedling Stage | Adult Stage | ||
|---|---|---|---|---|
| DI | RCE (%) | DI | RCE (%) | |
| Cruiser Plus | 6.39 ± 1.38 c | 72.34 | 15.71 ± 2.00 b† | 56.76 |
| Celest | 8.65 ± 0.84 b | 62.55 | 18.97 ± 0.77 b | 47.78 |
| Control | 23.11 ± 0.34 a | - | 36.33 ± 2.55 a | - |
| F | 272.071 | 99.816 | ||
| df | 2 | 2 | ||
| Treatments | Wheat Growth at Seedling Stage | Parameters at Adult Stage | ||||||
|---|---|---|---|---|---|---|---|---|
| Density Plant/m2 | Tiller/Plant | Secondary Roots | Fertile Tillers/m2 | Grain/Spike | TGW (g) | Yield/kg/m2 | Increased Yield (%) | |
| Cruiser Plus | 1565 ± 48.80 a† | 3.7 ± 0.13 a | 13.5 ± 0.13 a | 616 ± 22.17 a | 33.6 ± 0.38 ab | 44.5 ± 0.35 ab | 0.783 ± 0.01 ab | 8.7 |
| Celest | 1418 ± 26.86 b | 3.4 ± 0.16 b | 12.7 ± 0.23 ab | 619 ± 32.70 a | 33.2 ± 0.35 b | 43.4 ± 0.52 c | 0.758 ± 0.02 b | 5.3 |
| Cruiser Plus + Horizon | - | - | - | 622 ± 38.69 a | 33.7 ± 0.33 a | 44.6 ± 0.56 a | 0.795 ± 0.03 a | 10.4 |
| Celest + Horizon | - | - | - | 627 ± 40.07 a | 33.2 ± 0.27 b | 43.7 ± 0.89 bc | 0.773 ± 0.02 ab | 7.4 |
| Control | 1452 ± 71.39 b | 3.4 ± 0.14 b | 11.2 ± 0.29 b | 624 ± 38.93 a | 32.1 ± 0.19 c | 42.3 ± 0.58 d | 0.720 ± 0.03 c | |
| F | 10.870 | 8.258 | 134.390 | 0.071 | 21.355 | 12.239 | 8.394 | |
| df | 2 | 2 | 2 | 4 | 4 | 4 | 4 | |
| p-value | 0.002 | 0.006 | 0 | 0.990 | 0 | 0 | 0 | |
| Treatments | Seedling Stage | Adult Stage | Increased Control Efficacies (%) | |||
|---|---|---|---|---|---|---|
| DI | RCE (%) | DI | RCE (%) | Dressing + Spraying vs. Dressing | Cruiser Plus vs. Celest | |
| Cruiser Plus | 6.98 ± 0.67 b | 45.17 | 17.69 ± 0.96 b† | 35.76 | 15.21 | |
| Celest | 7.82 ± 0.44 b | 38.57 | 18.99 ± 1.66 b | 31.04 | ||
| Cruiser Plus + Horizon | - | - | 17.03 ± 1.39 b | 38.16 | 6.71 | 9.59 |
| Celest + Horizon | - | - | 17.95 ± 0.56 b | 34.82 | 12.18 | |
| Control | 12.73 a | - | 27.54 ± 3.27 a | - | ||
| F | 47.900 | 17.133 | ||||
| df | 2 | 4 | ||||
| p-value | 0 | 0 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, N.; Yuan, S.; Zhang, Q.; Liu, W.; Zhou, Y.; Yang, W. Screening Fungicides for Controlling Wheat Crown Rot Caused by Fusarium pseudograminearum across Hebei Province in China. Agriculture 2022, 12, 1643. https://doi.org/10.3390/agriculture12101643
Zhang N, Yuan S, Zhang Q, Liu W, Zhou Y, Yang W. Screening Fungicides for Controlling Wheat Crown Rot Caused by Fusarium pseudograminearum across Hebei Province in China. Agriculture. 2022; 12(10):1643. https://doi.org/10.3390/agriculture12101643
Chicago/Turabian StyleZhang, Na, Shengliang Yuan, Qi Zhang, Wenze Liu, Ying Zhou, and Wenxiang Yang. 2022. "Screening Fungicides for Controlling Wheat Crown Rot Caused by Fusarium pseudograminearum across Hebei Province in China" Agriculture 12, no. 10: 1643. https://doi.org/10.3390/agriculture12101643
APA StyleZhang, N., Yuan, S., Zhang, Q., Liu, W., Zhou, Y., & Yang, W. (2022). Screening Fungicides for Controlling Wheat Crown Rot Caused by Fusarium pseudograminearum across Hebei Province in China. Agriculture, 12(10), 1643. https://doi.org/10.3390/agriculture12101643







