The Use of Compost Increases Bioactive Compounds and Fruit Yield in Calafate Grown in the Central South of Chile
Abstract
1. Introduction
2. Materials and Methods
2.1. General Characteristics of the Site of the Study and Orchard Establishment
2.2. Characterization of Physiological and Environmental Conditions of the Plant
2.2.1. PAR Radiation and Leaf Area Index
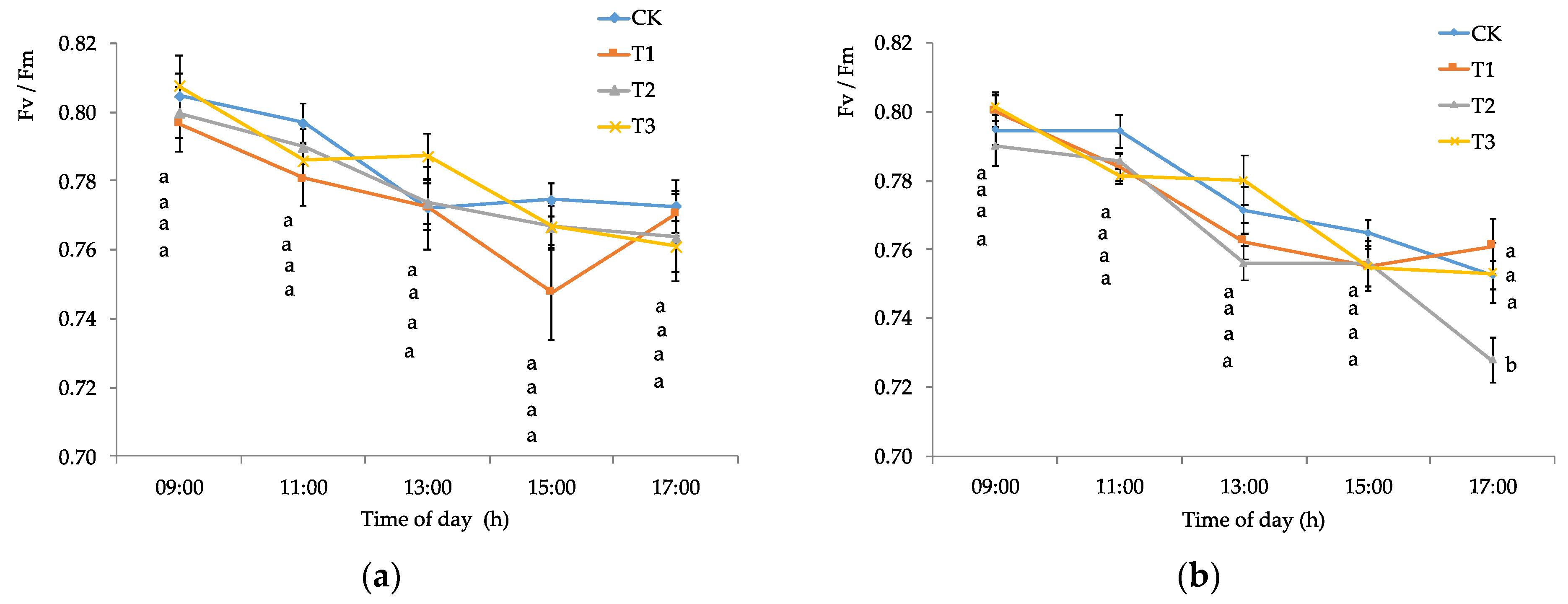
2.2.2. Chlorophyll Fluorescence and Stomatal Conductance
2.3. Yield and Chemical Parameters of the Fruit
2.3.1. Calafate Fruit Productivity
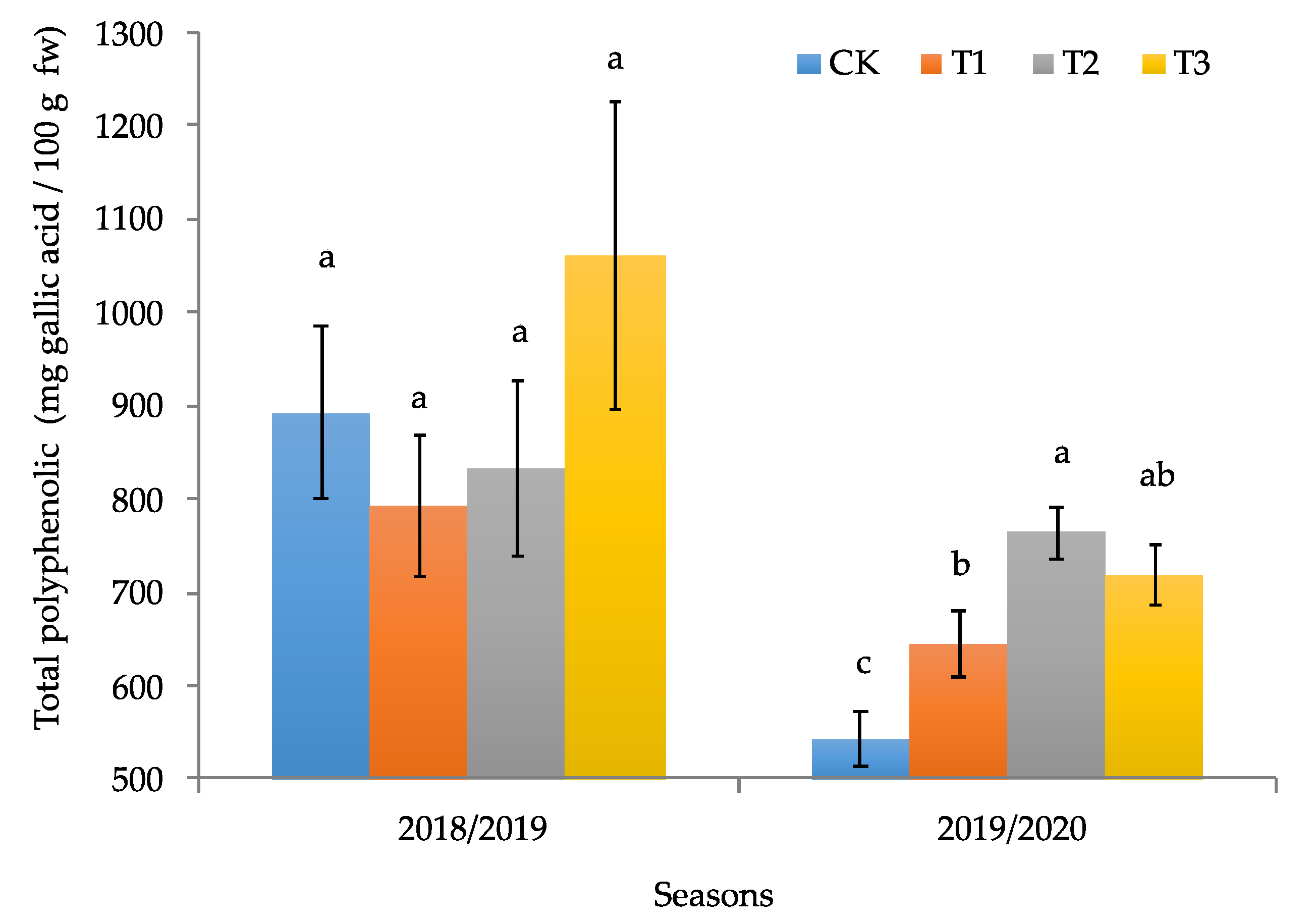
2.3.2. Determination of Total Polyphenol Concentration
2.3.3. Determination of Anthocyanin Content
2.3.4. Determination of Antioxidant Capacity
2.4. Statistical Analysis
3. Results
3.1. Edaphoclimatic and Physiological Parameters of Calafate
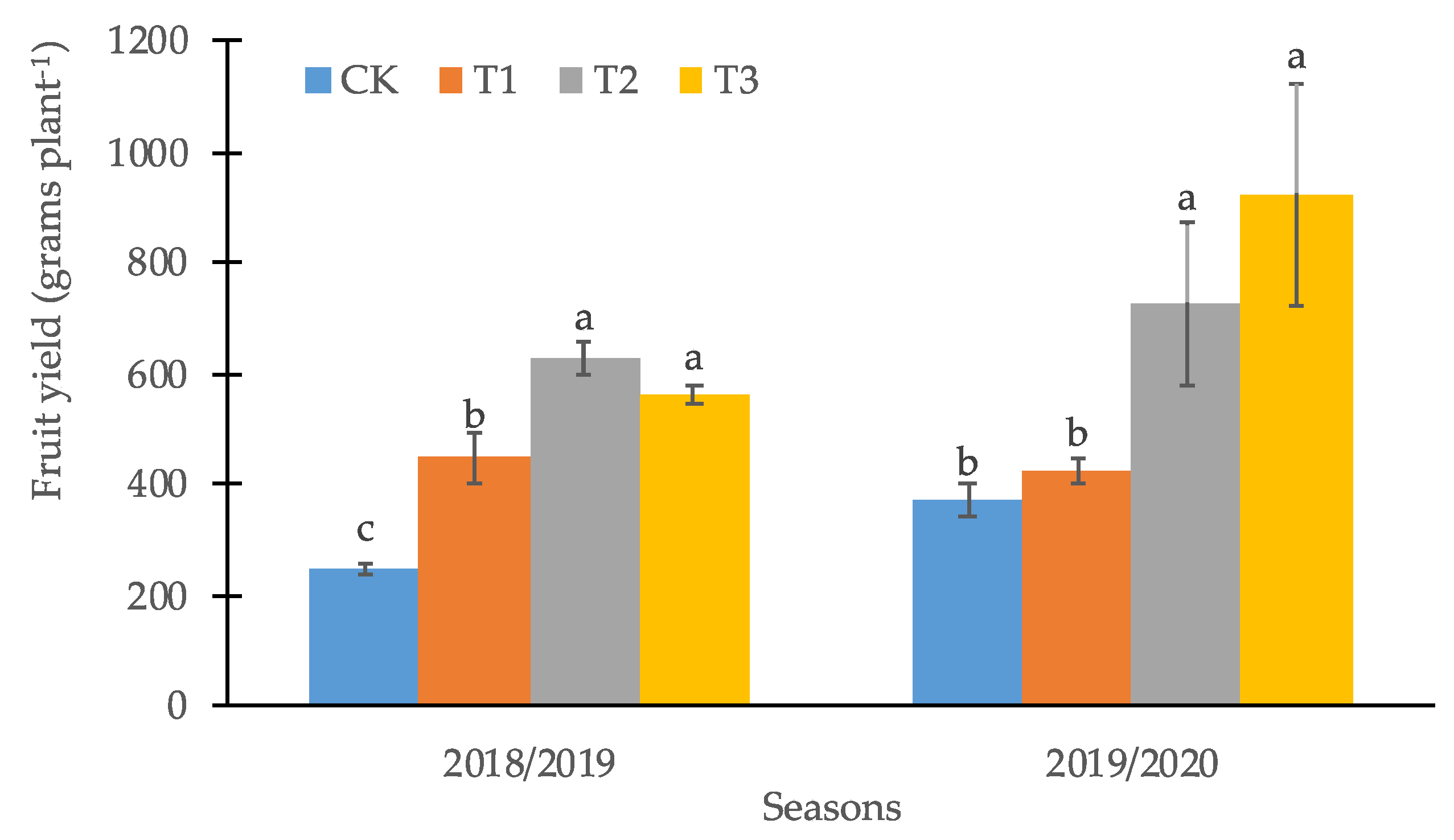
3.2. Productive and Quality Parameters of the Calafate Fruit
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mc Leod, C.; Pino, M.T.; Aguila, K. Calafate (Berberis microphylla): Otro Superberries Chileno. Available online: https://biblioteca.inia.cl/bitstream/handle/123456789/4637/NR40444.pdf?sequence=1 (accessed on 10 November 2021).
- Pino, M.T.; Mc Leod, C.; Ojeda, A.; Zamora, O.; Saavedra, J. Characterization and Clonal Selection of Berberis Microphylla G. Forst in the Chilean Patagonia Region for Natural Colorant Purposes. In XIV EUCARPIA Symposium on Fruit Breeding and Genetics; ISHS: Bologna, Italy, 2017; pp. 249–254. [Google Scholar] [CrossRef]
- Arena, M.E.; Zuleta, A.; Dyner, L.; Constenla, D.; Ceci, L.; Curvetto, N. Berberis Buxifolia Fruit Growth and Ripening: Evolution in Carbohydrate and Organic Acid Contents. Sci. Hortic. 2013, 158, 52–58. [Google Scholar] [CrossRef]
- Rodoni, L.M.; Feuring, V.; Zaro, M.J.; Sozzi, G.O.; Vicente, A.R.; Arena, M.E. Ethylene Responses and Quality of Antioxidant-Rich Stored Barberry Fruit (Berberis microphylla). Sci. Hortic. 2014, 179, 233–238. [Google Scholar] [CrossRef]
- Ruiz, A.; Hermosín-Gutiérrez, I.; Mardones, C.; Vergara, C.; Herlitz, E.; Vega, M.; Dorau, C.; Winterhalter, P.; von Baer, D. Polyphenols and Antioxidant Activity of Calafate (Berberis microphylla) Fruits and Other Native Berries from Southern Chile. J. Agric. Food Chem. 2010, 58, 6081–6089. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, A.; Zapata, M.; Sabando, C.; Bustamante, L.; von Baer, D.; Vergara, C.; Mardones, C. Flavonols, Alkaloids, and Antioxidant Capacity of Edible Wild Berberis Species from Patagonia. J. Agric. Food Chem. 2014, 62, 12407–12417. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Mazur, A.; Scalbert, A. Polyphenols and prevention of cardiovascular diseases. Curr. Opin. Lipidol. 2005, 16, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, C.; Pellarin, G.; Rojas, M.J.; Albesa, I.; Eraso, A.J. Beneficial Effect of Berberis Buxifolia Lam., Zizyphus Mistol Griseb and Prosopis Alba Extracts on Oxidative Stress Induced by Chloramphenicol. Medicina 2010, 70, 65–70. [Google Scholar]
- Arena, M.E.; Vater, G.; Peri, P. Fruit Production of Berberis Buxifolia Lam. in Tierra Del Fuego. HortScience 2003, 38, 200–202. [Google Scholar] [CrossRef]
- Radice, S.; Arena, M.E. Environmental Effect on the Leaf Morphology and Anatomy of Berberis Microphylla G. Forst. Int. J. Plant Biol. 2015, 6. [Google Scholar] [CrossRef]
- Sandri, M.A.; Andriolo, J.L.; Witter, M.; Dal Ross, T. Effect of Shading on Tomato Plants Grow under Greenhouse. Hortic. Bras. 2003, 21, 642–645. [Google Scholar] [CrossRef]
- Challa, H.; Schapendonk, A.H.C.M. Quantification of Effects of Light Reduction in Greenhouses on Yield. Acta Hortic. 1984, 501–510. [Google Scholar] [CrossRef]
- Medina, C.L.; Souza, R.P.; Machado, E.C.; Ribeiro, R.V.; Silva, J.A.B. Photosynthetic Response of Citrus Grown under Reflective Aluminized Polypropylene Shading Nets. Sci. Hortic. 2002, 96, 115–125. [Google Scholar] [CrossRef]
- Retamal-Salgado, J.; Vásquez, R.; Fischer, S.; Hirzel, J.; Zapata, N. Decrease in Artificial Radiation with Netting Reduces Stress and Improves Rabbit-Eye Blueberry (Vaccinium Virgatum Aiton) Cv. Ochlockonee Productivity. Chil. J. Agric. Res. 2017, 77, 226–233. [Google Scholar] [CrossRef]
- Arena, M.E.; Pastur, G.M.; Lencinas, M.V.; Soler, R.; Bustamante, G. Changes in the Leaf Nutrient and Pigment Contents of Berberis Microphylla G. Forst. in Relation to Irradiance and Fertilization. Heliyon 2020, 6, e03264. [Google Scholar] [CrossRef] [PubMed]
- Romero, M.E.; Noriega Vásquez, F.; Farías Villagra, M.; López Belchi, M.D.; Jara Zapata, P.; Vera Flores, B. Nuevas fuentes de antioxidantes naturales: Caracterización de compuestos bioactivos en cinco frutos nativos de Chile. Perfiles 2019, 2, 34–41. [Google Scholar] [CrossRef]
- Valenzuela, C.; Pérez, P. Actualización En El Uso de Antioxidantes Naturales Derivados de Frutas y Verduras Para Prolongar La Vida Útil de La Carne y Productos Cárneos. Rev. Chil. Nutr. 2016, 43, 188–195. [Google Scholar] [CrossRef]
- Radice, S.; Alonso, M.; Arena, M. Berberis Microphylla: A Species with Phenotypic Plasticity in Different Climatic Conditions. Int. J. Agric. Biol. 2018, 20, 2221–2229. [Google Scholar] [CrossRef]
- Hirzel, J. Diagnostico Nutricional y Principios de Fertilizacion en Frutales y Vides. Segunda Edicion Aumentada y Corregida; Colección Libros INIA—Instituto de Investigaciones Agropecuarias Trama Impresores S.A.: Hualpén, Chile, 2014. [Google Scholar]
- Vásquez, H.V.; Maraví, C. Efecto de fertilización orgánica (biol y compost) en el establecimiento de morera (Morus alba L.). Rev. De Investig. En Cienc. Y Biotecnol. Anim. 2017, 1, 33–39. [Google Scholar] [CrossRef][Green Version]
- Wang, S.Y.; Lin, H.-S. Compost as a Soil Supplement Increases the Level of Antioxidant Compounds and Oxygen Radical Absorbance Capacity in Strawberries. J. Agric. Food Chem. 2003, 51, 6844–6850. [Google Scholar] [CrossRef]
- Bittenbender, H.C.; Hue, N.V.; Fleming, K.; Brown, H. Sustainability of organic fertilization of macadamia with macadamia husk-manure compost. Commun. Soil Sci. Plant Anal. 1998, 29, 409–419. [Google Scholar] [CrossRef]
- Mugnai, S.; Masi, E.; Azzarello, E.; Mancuso, S. Influence of Long-Term Application of Green Waste Compost on Soil Characteristics and Growth, Yield and Quality of Grape (Vitis Vinifera L.). Compost Sci. Util. 2012, 20, 29–33. [Google Scholar] [CrossRef]
- Zhong, W.; Gu, T.; Wang, W.; Zhang, B.; Lin, X.; Huang, Q.; Shen, W. The Effects of Mineral Fertilizer and Organic Manure on Soil Microbial Community and Diversity. Plant Soil 2010, 326, 511–522. [Google Scholar] [CrossRef]
- INIA Red Agrometeorológica de INIA. Available online: http://agromet.inia.cl (accessed on 27 April 2020).
- Romero, P.; Fernández-Fernández, J.I.; Martinez-Cutillas, A. Physiological Thresholds for Efficient Regulated Deficit-Irrigation Management in Winegrapes Grown under Semiarid Conditions. Am. J. Enol. Vitic. 2010, 61, 300–312. [Google Scholar]
- Evanylo, G.; Sherony, C.; Spargo, J.; Starner, D.; Brosius, M.; Haering, K. Soil and Water Environmental Effects of Fertilizer-, Manure-, and Compost-Based Fertility Practices in an Organic Vegetable Cropping System. Agric. Ecosyst. Environ. 2008, 127, 50–58. [Google Scholar] [CrossRef]
- Bohórquez, W. El proceso de Compostaje; Universidad de la Salle: Bogota, DC, Colombia, 2019; ISBN 978-958-54-8668-3. [Google Scholar]
- Sayara, T.; Basheer-Salimia, R.; Hawamde, F.; Sánchez, A. Recycling of Organic Wastes through Composting: Process Performance and Compost Application in Agriculture. Agronomy 2020, 10, 1838. [Google Scholar] [CrossRef]
- Sonnentag, O.; Talbot, J.; Chen, J.M.; Roulet, N.T. Using Direct and Indirect Measurements of Leaf Area Index to Characterize the Shrub Canopy in an Ombrotrophic Peatland. Agric. For. Meteorol. 2007, 144, 200–212. [Google Scholar] [CrossRef]
- Retamal-Salgado, J.; Bastías, R.M.; Wilckens, R.; Paulino, L. Influence of Microclimatic Conditions under High Tunnels on the Physiological and Productive Responses in Blueberry Cv. O’Neal. Chil. J. Agric. Res. 2015, 75, 291–297. [Google Scholar] [CrossRef]
- Reyes-Diaz, M.; Alberdi, M.; de la Luz Mora, M. Short-Term Aluminum Stress Differentially Affects the Photochemical Efficiency of Photosystem II in Highbush Blueberry Genotypes. J. Am. Soc. Hortic. Sci. 2009, 134, 14–21. [Google Scholar] [CrossRef]
- van Kooten, O.; Snel, J.H. The Use of Chlorophyll Fluorescence Nomenclature in Plant Stress Physiology. Photosynth. Res. 1990, 25, 147–150. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll Fluorescence—A Practical Guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Yıldırım, A.; Mavi, A.; Kara, A.A. Determination of Antioxidant and Antimicrobial Activities of Rumex Crispus L. Extracts. J. Agric. Food Chem. 2001, 49, 4083–4089. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Giordani, E.; Gori, M.; Arena, M.; Radice, S. Caracterización molecular de especies nativas de Berberis del NOA. Investig. Cienc. Univ. 2019, 3, 118. [Google Scholar]
- Giordani, E.; Müller, M.; Gambineri, F.; Paffetti, D.; Arena, M.; Radice, S. Genetic and Morphological Analysis of Berberis Microphylla G. Forst. Accessions in Southern Tierra Del Fuego. Plant Biosyst.—Int. J. Deal. Asp. Plant Biol. 2017, 151, 715–728. [Google Scholar] [CrossRef]
- Arena, M.E.; Lencinas, M.V.; Radice, S. Variability in Floral Traits and Reproductive Success among and within Populations of Berberis Microphylla G. Forst., an Underutilized Fruit Species. Sci. Hortic. 2018, 241, 65–73. [Google Scholar] [CrossRef]
- Arena, M.E.; Postemsky, P.D.; Curvetto, N.R. Changes in the Phenolic Compounds and Antioxidant Capacity of Berberis Microphylla G. Forst. Berries in Relation to Light Intensity and Fertilization. Sci. Hortic. 2017, 218, 63–71. [Google Scholar] [CrossRef]
- Arena, M.E.; Radice, S. Shoot Growth and Development of Berberis Buxifolia Lam. in Tierra Del Fuego (Patagonia). Sci. Hortic. 2014, 165, 5–12. [Google Scholar] [CrossRef]
- Romero-Román, M.E.; Schoebitz, M.; Bastías, R.M.; Fernández, P.S.; García-Viguera, C.; López-Belchi, M.D. Native Species Facing Climate Changes: Response of Calafate Berries to Low Temperature and UV Radiation. Foods 2021, 10, 196. [Google Scholar] [CrossRef] [PubMed]
- Peri, P.L.; Arena, M.; Martínez Pastur, G.; Lencinas, M.V. Photosynthetic Response to Different Light Intensities, Water Status and Leaf Age of Two Berberis Species (Berberidaceae) of Patagonian Steppe, Argentina. J. Arid Environ. 2011, 75, 1218–1222. [Google Scholar] [CrossRef]
- Guo, X.; Li, S.; Wang, D.; Huang, Z.; Sarwar, N.; Mubeen, K.; Shakeel, M.; Hussain, M. Effects of water and fertilizer coupling on the physiological characteristics and growth of rabbiteye blueberry. PLoS ONE 2021, 16, e0254013. [Google Scholar] [CrossRef] [PubMed]
- Hasnain, M.; Chen, J.; Ahmed, N.; Memon, S.; Wang, L.; Wang, Y.; Wang, P. The Effects of Fertilizer Type and Application Time on Soil Properties, Plant Traits, Yield and Quality of Tomato. Sustainability 2020, 12, 9065. [Google Scholar] [CrossRef]
- Medina, J.; Calabi-Floody, M.; Aponte, H.; Santander, C.; Paneque, M.; Meier, S.; Panettieri, M.; Cornejo, P.; Borie, F.; Knicker, H. Utilization of Inorganic Nanoparticles and Biochar as Additives of Agricultural Waste Composting: Effects of End-Products on Plant Growth, C and Nutrient Stock in Soils from a Mediterranean Region. Agronomy 2021, 11, 767. [Google Scholar] [CrossRef]
- Maselesele, D.; Ogola, J.B.O.; Murovhi, R.N. Macadamia Husk Compost Improved Physical and Chemical Properties of a Sandy Loam Soil. Sustainability 2021, 13, 6997. [Google Scholar] [CrossRef]
- Mohamed, M.H.M.; Petropoulos, S.A.; Ali, M.M.E. The Application of Nitrogen Fertilization and Foliar Spraying with Calcium and Boron Affects Growth Aspects, Chemical Composition, Productivity and Fruit Quality of Strawberry Plants. Horticulturae 2021, 7, 257. [Google Scholar] [CrossRef]
- Wen, M.; Zhang, J.; Zheng, Y.; Yi, S. Effects of Combined Potassium and Organic Fertilizer Application on Newhall Navel Orange Nutrient Uptake, Yield, and Quality. Agronomy 2021, 11, 1990. [Google Scholar] [CrossRef]
- Arena, M.E.; Curvetto, N. Berberis Buxifolia Fruiting: Kinetic Growth Behavior and Evolution of Chemical Properties during the Fruiting Period and Different Growing Seasons. Sci. Hortic. 2008, 118, 120–127. [Google Scholar] [CrossRef]
- Cojocaru, A.; Vlase, L.; Munteanu, N.; Stan, T.; Teliban, G.C.; Burducea, M.; Stoleru, V. Dynamic of Phenolic Compounds, Antioxidant Activity, and Yield of Rhubarb under Chemical, Organic and Biological Fertilization. Plants 2020, 9, 355. [Google Scholar] [CrossRef]
- Fredes, C.; Parada, A.; Salinas, J.; Robert, P. Phytochemicals and Traditional Use of Two Southernmost Chilean Berry Fruits: Murta (Ugni Molinae Turcz) and Calafate (Berberis Buxifolia Lam.). Foods 2020, 9, 54. [Google Scholar] [CrossRef] [PubMed]
- Giordani, E.; Radice, S.; Lencinas, M.V.; Arena, M. Variabilidad fenotípica de Berberis microphylla G. Forst. en poblaciones naturales de Tierra del Fuego, una especie frutal forestal no maderable con posibilidad de diversificar la producción agroforestal patagónica. Investig. Cienc. Univ. 2019, 3, 91. [Google Scholar]
- Arena, M.E. Estudio de Algunos Fenómenos Morfofisiológicos y Cambios Bioquímicos en Berberis microphylla G. Forst. (sinónimo B. Buxifolia Lam.) Asociados a la Formación y Maduración de Frutos en Tierra de Fuego y su Relación con la Producción de Metabolitos Útiles; Sebelas Maret University: Surakarta City, Indonesia, 2016. [Google Scholar]

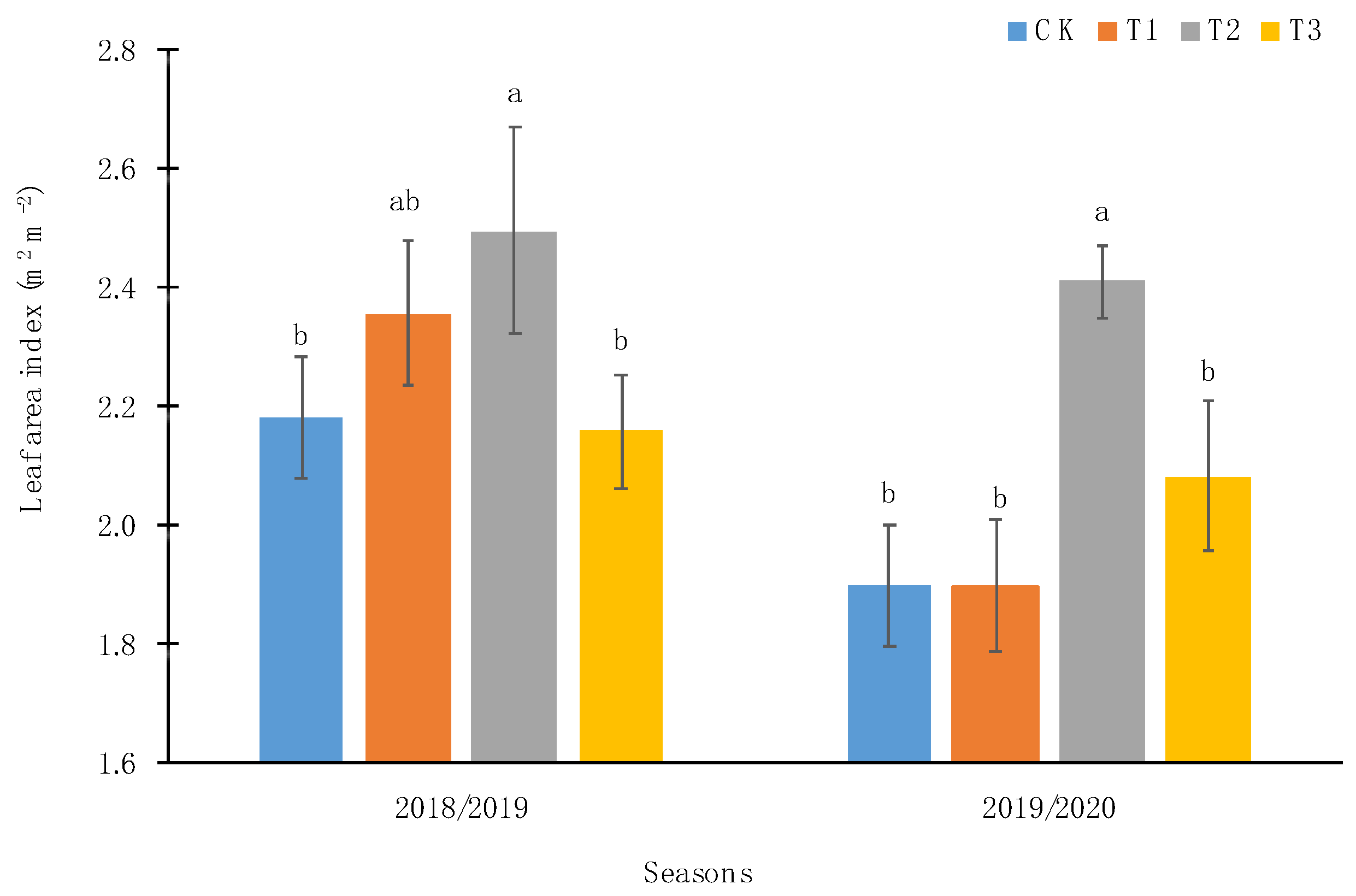
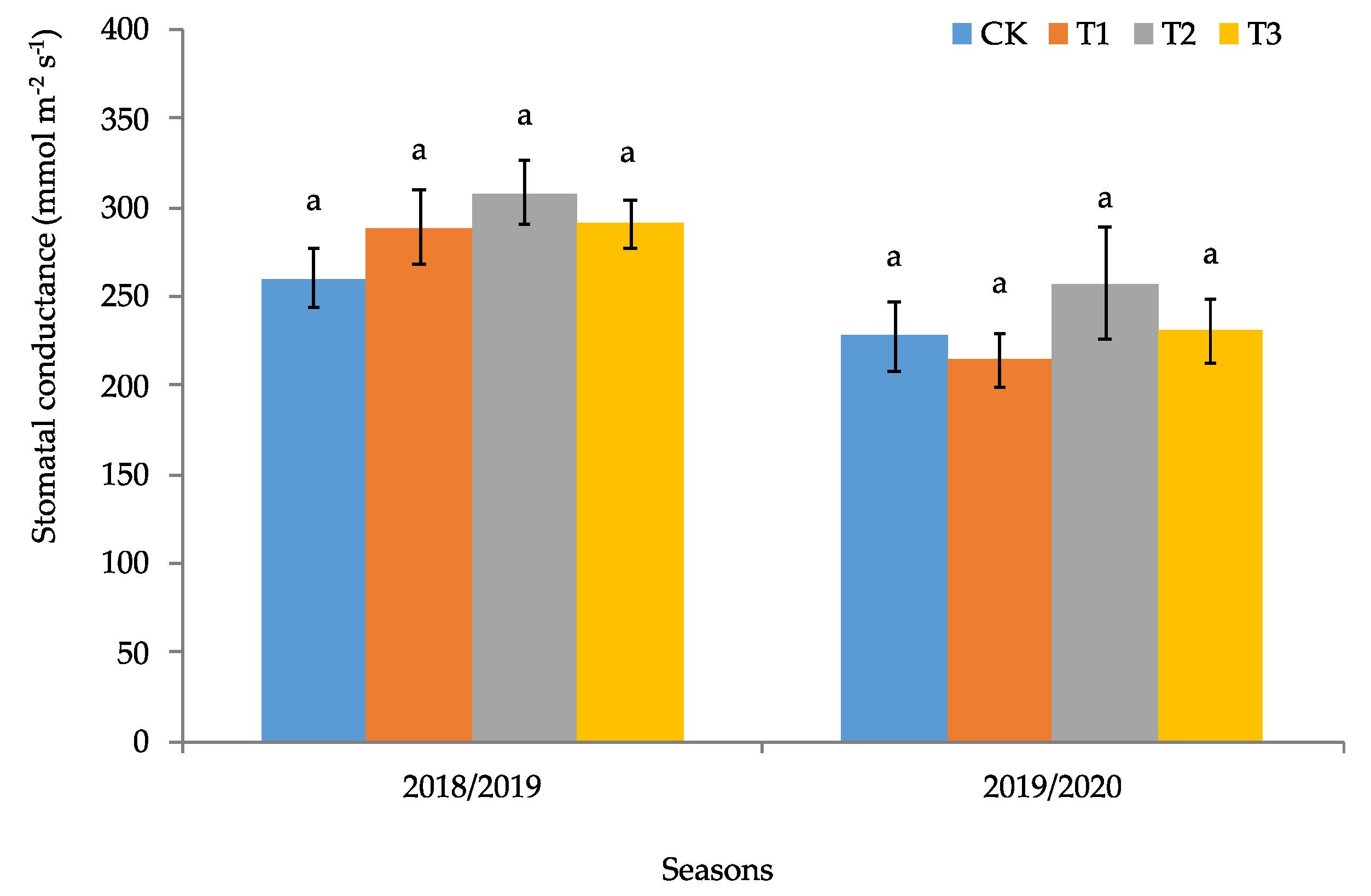


| Analysis 1 | Unit | Result |
|---|---|---|
| Organic matter | % | 9.70 |
| Water pH | 6.40 | |
| Nitrogen availability | mg kg−1 | 19.00 |
| Phosohorus availability | mg kg−1 | 15.30 |
| Potassium availability | mg kg−1 | 496.00 |
| Sulfur availability | mg kg−1 | 24.00 |
| Exchangeable calcium | cmol + kg−1 | 8.70 |
| Exchangeable magnesium | cmol + kg−1 | 1.60 |
| Exchangeable potassium | cmol + kg−1 | 1.30 |
| Exchangeable sodium | cmol + kg−1 | 0.01 |
| Sum of bases | cmol + kg−1 | 11.60 |
| Interchangeable aluminum | cmol + kg−1 | 0.02 |
| CEC | cmol + kg−1 | 11.59 |
| Aluminum saturation | % | 0.14 |
| Boron | mg kg−1 | 0.40 |
| Copper | mg kg−1 | 1.63 |
| Zinc | mg kg−1 | 0.90 |
| Iron | mg kg−1 | 44.00 |
| Manganese | mg kg−1 | 3.02 |
| Analysis | Units | Result |
|---|---|---|
| Humidity (dry basis) | % | 22.20 |
| Apparent density (dry basis ˂16 mm) | Kg m−3 | NS * |
| Porosity (sample ˂16 mm) | mg kg−1 | NS * |
| pH in water 1:5 | 7.41 | |
| Electric conductivity 1:5 | dS/m | 0.19 |
| Organic matter | % | 21.60 |
| Organic carbon | % | 12.00 |
| Total nitrogen | % | 0.87 |
| Nitrogen–ammonia (N–+) | mg kg−1 | 0.50 |
| Nitrogen–Nitric (N–−) | mg kg−1 | 59.64 |
| Carbon/Nitrogen Ratio | -- | 13.78 |
| Ammonium/Nitrate Ratio | -- | 0.008 |
| Analysis | Units | Treatments 1 | |||
|---|---|---|---|---|---|
| CK | T1 | T2 | T3 | ||
| Organic matter | % | 9.80 | 10.40 | 11.00 | 11.90 |
| Water pH | --- | 6.59 | 6.56 | 6.50 | 6.60 |
| Nitrogen available | mg kg−1 | 16.00 | 18.00 | 16.00 | 13.00 |
| Available phosphorus | mg kg−1 | 10.00 | 14.00 | 9.00 | 13.00 |
| Available potassium | mg kg−1 | 342.00 | 332.00 | 372.00 | 359.00 |
| Available sulfur | mg kg−1 | 9.00 | 12.00 | 29.00 | 27.00 |
| Exchangeable calcium | cmol+ kg−1 | 9.25 | 10.90 | 10.04 | 10.86 |
| Exchangeable magnesium | cmol+ kg−1 | 2.17 | 2.33 | 1.82 | 2.19 |
| Exchangeable potassium | cmol+ kg−1 | 0.87 | 0.85 | 0.95 | 0.92 |
| Exchangeable sodium | cmol+ kg−1 | 0.30 | 0.27 | 0.29 | 0.29 |
| Sum of bases | cmol+ kg−1 | 12.59 | 14.36 | 13.10 | 14.26 |
| Interch. aluminum | cmol+ kg−1 | 0.010 | 0.001 | 0.010 | 0.010 |
| CEC | cmol+ kg−1 | 12.60 | 14.37 | 13.11 | 14.27 |
| Aluminum saturation | % | 0.08 | 0.07 | 0.08 | 0.07 |
| Boron | mg kg−1 | 0.45 | 0.48 | 0.50 | 0.45 |
| Copper | mg kg−1 | 2.06 | 2.11 | 2.14 | 1.85 |
| Zinc | mg kg−1 | 0.90 | 1.62 | 1.17 | 1.74 |
| Iron | mg kg−1 | 35.00 | 33.10 | 31.30 | 30.80 |
| Manganese | mg kg−1 | 3.02 | 3.50 | 3,20 | 6.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinto-Morales, F.; Retamal-Salgado, J.; Lopéz, M.D.; Zapata, N.; Vergara-Retamales, R.; Pinto-Poblete, A. The Use of Compost Increases Bioactive Compounds and Fruit Yield in Calafate Grown in the Central South of Chile. Agriculture 2022, 12, 98. https://doi.org/10.3390/agriculture12010098
Pinto-Morales F, Retamal-Salgado J, Lopéz MD, Zapata N, Vergara-Retamales R, Pinto-Poblete A. The Use of Compost Increases Bioactive Compounds and Fruit Yield in Calafate Grown in the Central South of Chile. Agriculture. 2022; 12(1):98. https://doi.org/10.3390/agriculture12010098
Chicago/Turabian StylePinto-Morales, Fernando, Jorge Retamal-Salgado, María Dolores Lopéz, Nelson Zapata, Rosa Vergara-Retamales, and Andrés Pinto-Poblete. 2022. "The Use of Compost Increases Bioactive Compounds and Fruit Yield in Calafate Grown in the Central South of Chile" Agriculture 12, no. 1: 98. https://doi.org/10.3390/agriculture12010098
APA StylePinto-Morales, F., Retamal-Salgado, J., Lopéz, M. D., Zapata, N., Vergara-Retamales, R., & Pinto-Poblete, A. (2022). The Use of Compost Increases Bioactive Compounds and Fruit Yield in Calafate Grown in the Central South of Chile. Agriculture, 12(1), 98. https://doi.org/10.3390/agriculture12010098








