Abstract
Guanidinoacetic acid can improve pork quality. Previous studies have demonstrated that pork quality is closely linked to the muscle fiber type mediated by PPARGC1A. Therefore, this study aimed to evaluate the influence of dietary GAA supplementation on the skeletal muscle fiber type transformation. A total of 180 healthy Duroc × Landrace × Meishan cross castrated male pigs with a similar average weight (90 ± 1.5 kg) were randomly divided into three treatments with five replicates per treatment and 12 pigs per replicate, including a GAA-free basal diet and basal diet with 0.05% or 0.10% GAA for 15 days. Our results showed that 0.10% GAA supplementation increased the contents of Ca2+ in sarcoplasm (p < 0.05). Compared with the control group, both GAA supplementation groups upregulated the expression of Troponin I-ss (p < 0.05), and 0.10% GAA supplementation downregulated the expression of Troponin T3 (p < 0.05). GAA supplementation increased the expression of peroxisome proliferator activated receptor-γ coactivator-1alpha (PPARGC1A) (p < 0.05), and further upregulated the mitochondrial transcription factor A (TFAM), increased the level of membrane potential, and the activities of mitochondrial respiratory chain complex I, III (p < 0.05). The 0.10% GAA supplementation upregulated the protein expression of calcineurin catalytic subunit α (CnAα) and nuclear factor of activated T cells (NFATc1) (p < 0.05). Overall, dietary GAA supplementation promotes skeletal muscle fiber types transformation from fast-to-slow-twitch via increasing the PPARGC1A based mitochondrial function and the activation of CaN/NFAT pathway in finishing pigs.
1. Introduction
In mammals, different fiber types, which are the basis of muscle plasticity in response to a variety of functional demands, can be distinguished in skeletal muscle. Moreover, the muscle fiber characteristics are the major factors for the evaluation of meat quality, especially the fiber type composition [1]. According to the diverse structural properties and myosin heavy chain subtypes, muscle fiber can be classified into four fiber types (MyHC-I, MyHC-IIa, MyHC-IIx, MyHC-IIb), and these muscle fiber types can be transformed into each other (I ↔ IIa ↔ IIx ↔ IIb) [2]. MyHC-I slow-twitch muscle fiber (oxidative fiber), has an oxidative phosphorylation profile. Fast-twitch type fibers include IIa, IIx, and IIb muscle fibers [3]. It is easy to bring about the production of more lactic acid in animals after slaughter. Meanwhile, the excessive accumulation of postmortem lactic acid could further lead to a decline in pH and meat quality. As a consequence, it is particularly important to promote the transformation of skeletal muscle fibers from fast-twitch to slow-twitch. Moreover, skeletal muscle fiber transformation can be caused by some factors, such as heredity, exercise, and nutrition [4]. Meanwhile, the Troponin I-ss and Troponin T3 are specifically expressed in the slow and fast muscle fibers, and their content can indicate the proportion of the slow and fast muscle fibers, respectively [5]. Previous studies showed that skeletal muscle can be changed via decreasing the proportion of fast fibers and increasing slow fibers through some nutritional modulation strategies [6,7].
Mitochondria contain a variety of aerobic metabolism enzymes and all components of the electron-transport system. Therefore, the changes of mitochondrial content and function will affect the aerobic metabolism of the body [8]. It is worth noting that type I fibers have more mitochondria and a high capacity for the oxidation of energy substrates. In addition, type IIb fibers are poor in mitochondria, whereas rich in glycolytic enzymes, resulting in a higher potential for glycolysis [9]. A previous study has demonstrated that the mitochondria function played an important role in the muscle fiber-type transformation [10]. On the other hand, peroxisome proliferative activated receptor gamma coactivator 1 alpha (PPARGC1A) is involved in the regulation of energy metabolism and the transformation of skeletal muscle fiber-type [11]. PPARGC1A can promote the biosynthesis of mitochondria and the improvement of mitochondrial function during the metabolic process of skeletal muscle cells [12]. Once the increase of intracytoplasmic Ca2+ content increases, the expression of PPARGC1A would be upregulated [13]. The high expression of PPARGC1A can upregulate the DNA content of mitochondria and the activity of mitochondrial transcription factors A (TFAM) and DNA junctions, and then promote the biosynthesis of mitochondria [14]. Calcineurin (CaN)/nuclear factor of activated T cells (NFAT) signal pathway is a crucial signal transduction pathway to regulate the differentiation of skeletal muscle and the transformation of muscle fiber [13]. Moreover, PPARGC1A, as the substrate of CaN, may also mediate the conversion of muscle fibers from fast-twitch to slow-twitch through the modulation of CaN/NFAT signaling pathway [15].
Guanidinoacetic acid (GAA), which is synthesized from the glycine and arginine in the kidney, is the main endogenous substance of creatine synthesis in animals [16]. Creatine participates in the creatine phosphate system and generates ATP to provide energy for the body [17]. It was reported that dietary GAA supplementation significantly improved the meat quality of livestock and poultry [18]. It is well known that the skeletal muscle fiber types are highly related to the meat quality. A previous study found that creatine subjects demonstrated significant increase in muscle fiber type I [19]. Meanwhile, Zhu et al. have also found that GAA improved the meat quality by regulating the change of muscle fiber type [20]. However, the mechanism was not clear. Therefore, this study was conducted to evaluate the effects of dietary GAA supplementation on the skeletal muscle fiber type transformation, mitochondrial function, and CaN/NFAT pathway in finishing pigs.
2. Materials and Methods
2.1. Experimental Design, Animals, and Treatment
All of the experimental procedures involving the use of finishing pigs were in alignment with the Animal Care and Use Committee of Nanjing Agricultural University under the protocol number of SYXK 2017-0027 and its scope of application involves rabbit, dog, and pig. A total of 180 Duroc × Landrace × Meishan cross castrated male pigs with a similar average weight (90 ± 1.5 kg) were randomly allocated to three treatments with five replicates (pens) per treatment and 12 pigs per pen. All of the pigs were fed in the form of Jiangsu MingTian Agriculture and Animal Husbandry Technology Co., LTD (Nanjing, China). In addition, all of the pigs were housed in solid concrete floor pens in an environmentally controlled room (temperature: 22 ± 2.5 °C, humidity: 65 ± 2.4%). These experimental treatments included the following: The control group was fed with a GAA-free basal diet, and two GAA groups were fed with basal diets with 0.05% and 0.10% GAA, respectively. The experiment lasted for 15 days. The GAA additive was obtained from Beijing Gendone Biotechnology Co., Ltd. (Beijing, China). All of the trail finishing pigs were allowed free access to feed and water. The formulation of basal diet (Table 1) was referred to the nutritional requirements of NRC (1998).

Table 1.
Composition and nutrient level of basal diet.
2.2. Sample Collection
After 15 days of the feeding trial, 30 pigs (10 pigs per treatment, 2 per pen with medium bodyweight) were chosen and transported to abattoir (Su Meat Products Co., Ltd., Huaian, Jiangsu, China) for slaughter, according to the slaughtering procedures after a 12 h fast except for water. All of the pigs were slaughtered by exsanguination after electrical stunning (in front of the ears, at the temporal fossae: 50 Hz, 1.40 A, and 350 V). The longissimus thoracis muscle and semitendinosus muscle samples of the right carcass were collected in frozen tubes, and immediately placed in liquid nitrogen and stored at −80 °C for the determination of all indices included in this study.
2.3. Antibodies
The primary antibodies were as follows: Anti-peroxisome proliferator activated receptor-γ coactivator-1 alpha (PPARGC1A) (ABclonal Technology, Cat. No. A12348, Wuhan, China); anti-nuclear factor of activated T cells (NFATc1) (ABclonal Technology, Cat. No. A1539, Wuhan, China); anti-troponin I slow type (Troponin I-ss) (CLOUD-CLONE CORP, Cat. No. PAD227Hu01, Wuhan, China); anti-troponin IIb fast skeletal type (Troponin T3) (Abbkine, Cat. No. ABP60722, Wuhan, China); anti-calcineurin catalytic subunit α (CnAα) (Proteintech Group, Cat. No. 13422-1-AP, Chicago, IL, USA); anti-mitochondrial transcription factor A (TFAM) (Affinity biosciences, Cat. No. AF0531, Changzhou, China); anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Servicebio Technology, Cat. No. GB11002, Wuhan, China).
2.4. Measurement of Sarcoplasmic Ca2+ Contents
The concentration of Ca2+ in sarcoplasm was detected using the modified method of Wang et al. [21]. About 2 g of muscle sample was homogenized twice in 5 mL of ice-cold 0.15 M KCl at a speed of 12,000 rpm for 1 min each time, and then centrifuged at 27,000× g for 10 min at 4 °C to obtain the supernatant. Supernatants (4 mL) were obtained and added to 10% trichloroacetic acid to a final concentration of 0.8%. After 10 min, strontium chloride (5.4 mg) was added. After centrifugation at 10,000× g for 10 min at room temperature, the supernatants were collected to determine the concentration of sarcoplasmic Ca2+ with the method of Inductively Coupled Plasma (iCAP, 7000, SERIES, Thermo Fisher Scientific, Waltham, MA, USA).
2.5. Measurement of Mitochondrial Membrane Potential, Mitochondrial Respiratory Chain Complex I, and Mitochondrial Respiratory Chain Complex III
The mitochondrial membrane potential (MMP) was detected using a commercial kit (JC-1, Cat. No. C2006, Beyotime, Nanjing, China). First, the mitochondria were separated from the muscle using a commercial kit (Cat. No. C3606, Beyotime, Nanjing, China): About 80 mg of muscle sample was placed in a centrifuge tube with 800 µL pre-cooled PBS, and ice-chilled for 3 min. The muscle samples were precipitated by centrifugation at 600× g for 15 s, and the supernatant was discarded. Then, 640 µL of precooled mitochondrial separation reagent B was added. An ice bath homogenate was performed 25 times, and then centrifugation was performed at 600× g for 5 min at 4 °C. The obtained supernatant was transferred to another centrifugal tube and centrifuged at 11,000× g for 10 min at 4 °C. The precipitates were mitochondria. The 0.9 mL of a JC-1 staining solution was added to the 0.1 mL mitochondria. In addition, the fluorescence spectrophotometer was used for MMP detection (excitation wavelength: 485 nm, emission wavelength: 590 nm).
Mitochondrial respiratory chain complex I and mitochondrial respiratory chain complex III were measured using kits, according to the manufacturer’s instructions (Cat. No. D799471-0500 and D799480-0100, respectively, Sangon Biotech Co., Ltd., Shanghai, China). About 0.1 g of muscle sample was homogenized using the extract at 4 °C. The supernatant was centrifuged at 600× g for 10 min at 4 °C, and then transferred to another centrifuge tube, centrifuged at 11,000× g, 4 °C for 15 min. The obtained precipitation was added into a 400 µL extract, which was used for activity detection after ultrasonic crushing.
2.6. Gene Expression Analysis
Total mRNA from longissimus thoracis and semitendinosus muscle samples (about 70 mg) was isolated with RNAiso plus reagent (Takara Biotechnology Co., Ltd., Dalian, China). The mRNA was reverse transcribed into cDNA using a commercial kit (Takara Biotechnology Co., Ltd., Dalian, China). The reaction system volume was 40 µL and the conditions of the program were operated at 37 °C for 15 min, and 85 °C for 5 s.
The RT-qPCR was performed on an ABI PRISM 7500 (Applied Biosystems, Foster City, CA, USA). The PCR cycle conditions used were at 95 °C for 30 s, 40 cycles at 95 °C for 5 s, and 60 °C for 30 s, then 95 °C for 15 s, 60 °C for 1 min, and 95 °C for 15 s. The primer sequences designed by the NCBI Primer blast are listed in Table 2. The targeted genes expressions were quantified using the method of 2−ΔΔCt with GAPDH as an endogenous control.

Table 2.
Primer sequence of target genes and housekeeping gene.
2.7. Western Blot Analysis
Total protein of muscle samples (about 30 mg) was extracted using the RIPA buffer. The content of total protein was measured using a bicinchoninic acid (BCA) kit (Cat. No. C503061-1250, Sangon Biotech Co., Ltd., Shanghai, China), and then adjusted to 4 μg/μL. Each protein sample was mixed with 5 × loading buffer at the ratio of 1:4, incubated at boiling water for 5 min.
The protein sample was separated with 12.5% sodium lauryl sufate-polyacrylamide gel (Cat. No. PG113, EpiZyme Biotechnology Co., Ltd., Shanghai, China) electrophoresis (100 V, 1.5 h), and then transferred to a polyvinylidene fluoride membrane (Millipore; Billerica, MA, USA). After blocking with 5% skimmed milk solution for 2 h at room temperature, the membranes were incubated with a primary antibody overnight at 4 °C before incubation with the secondary antibody for 1 h at room temperature. The secondary antibody was purchased from Cell Signaling Technology Inc. (Cat. No. 7074S, Danvers, MA, USA) and diluted by 1 × TBST at the ratio of 1:3000. The membranes were visualized using the ChemiDocTM Imaging System (BIO-RAD, Singapore). The densities of the target bands were quantified using ImageJ software (1.6.0-20, 1.53e, National Institutes of Health, Bethesda, MD, USA) and were normalized with GAPDH as an endogenous control. Using the computer’s own photo function, the western blot images were cropped. The antibodies of PPARGC1A, NFATc1, Troponin I-ss, Troponin T3, CnAα, and TFAM were diluted by 5% skimmed milk at the ratio of 1:1000, 1:1000, 1:530, 1:800, 1:4000, 1:1000, respectively. The ECL substrate used was purchased from Vazyme Biotech Co., Ltd., Nanjing, China (Cat. No. E412-02).
2.8. Statistical Analysis
All of the data analyses were performed by the one-way ANOVA procedure using the IBM SPSS Statistics 20.0 software (SPSS Inc, Chicago, IL, USA). The experimental data were analyzed with pen as the experimental unit [n = 5 (the means of two pigs per pen were used to represent the pens)]. Data were expressed as the mean ± SEM. p < 0.05 was considered statistically significant.
3. Results
3.1. Skeletal Muscle Fiber Type Transformation
According to Figure 1A,B, the GAA treatment increased the troponin I slow type (Troponin I-ss) protein level in longissimus thoracis and semitendinosus muscle (p < 0.05), as compared with the control. Compared with the control group, the GAA addition downregulated the expression of troponin IIb fast skeletal type (Troponin T3) in semitendinosus muscle (p < 0.05). Likewise, as shown in Figure 1A, the expression of Troponin T3 was the lowest in the 0.10% GAA group of longissimus thoracis muscle among all of the treatments (p < 0.05). The full blots of Troponin T3 were shown in Supplementary Figures S1 and S2.
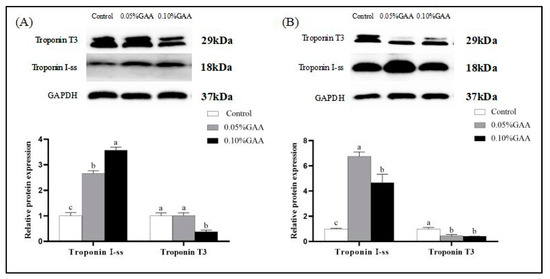
Figure 1.
Effects of guanidinoacetic acid supplementation on the protein expressions of Troponin I-ss, Troponin T3 of (A) longissimus thoracis muscle, and (B) semitendinosus muscle in finishing pigs. Means with different letters differ significantly (p < 0.05). Values are presented as the mean ± SEM (n = 5). Control: Finishing pigs were fed a basal diet; 0.05% GAA or 0.10% GAA: Finishing pigs were fed a basal diet supplemented with GAA at 500 or GAA at 1000 mg kg−1; Troponin I-ss: Troponin I slow type; Troponin T3: Troponin IIb fast skeletal type; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase.
3.2. Sarcoplasmic Ca2+ Contents
Compared with the control group, the 0.10% GAA treatment increased the sarcoplasmic Ca2+ content in longissimus thoracis muscle (p < 0.05; Figure 2A). However, no significant difference was observed in the sarcoplasmic Ca2+ content of semitendinosus muscle among the three groups (p > 0.05; Figure 2B).
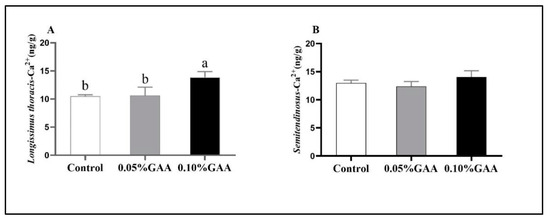
Figure 2.
Effects of guanidinoacetic acid supplementation on the sarcoplasmic Ca2+ contents in (A) longissimus thoracis muscle and (B) semitendinosus muscle of finishing pigs. Means with different letters differ significantly (p < 0.05). Values are presented as the mean ± SEM (n = 5). Control: Finishing pigs were fed a basal diet; 0.05% GAA or 0.10% GAA: Finishing pigs were fed a basal diet supplemented with GAA at 500 or GAA at 1000 mg kg−1.
3.3. Gene and Protein Expressions of PPARGC1A
Dietary 0.05% GAA supplementation upregulated the mRNA expression of PPARGC1A in longissimus thoracis muscle compared with the control (p < 0.05; Figure 3A). Meanwhile, the GAA supplementation also increased the protein level of PPARGC1A in longissimus thoracis muscle (p < 0.05; Figure 3B). Moreover, the 0.10% GAA addition upregulated the protein expression of PPARGC1A in semitendinosus muscle (p < 0.05; Figure 3C).
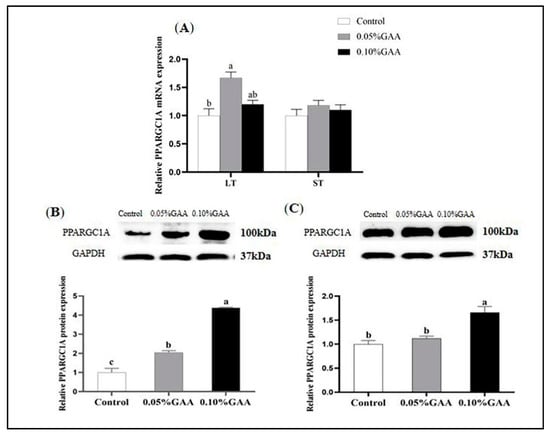
Figure 3.
Effects of guanidinoacetic acid supplementation on the mRNA (A) and protein expressions of PPARGC1A of longissimus thoracis muscle (B) and semitendinosus muscle (C) in finishing pigs. Means with different letters differ significantly (p < 0.05). Values are presented as the mean ± SEM (n = 5). Control: Finishing pigs were fed a basal diet; 0.05% GAA or 0.10% GAA: Finishing pigs were fed a basal diet supplemented with GAA at 500 or GAA at 1000 mg kg−1. LT: Longissimus thoracis muscle; ST: Semitendinosus muscle; PPARGC1A: Peroxisome proliferator activated receptor-γ coactivator-1alpha.
3.4. Indexes Related to Mitochondrial Function
As shown in Figure 4B, compared with the control group, GAA supplementation increased the level of mitochondrial membrane potential in semitendinosus muscle (p < 0.05).
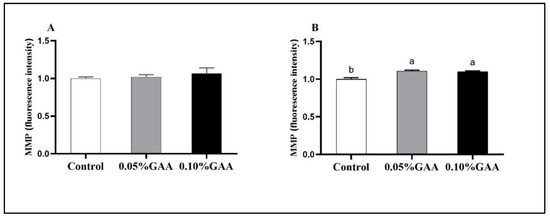
Figure 4.
Effects of guanidinoacetic acid supplementation on the mitochondrial membrane potential (MMP) of (A) longissimus thoracis muscle and (B) semitendinosus muscle in finishing pigs. Means with different letters differ significantly (p < 0.05). Values are presented as the mean ± SEM (n = 5). Control: Finishing pigs were fed a basal diet; 0.05% GAA or 0.10% GAA: Finishing pigs were fed a basal diet supplemented with GAA at 500 or GAA at 1000 mg kg−1.
Compared with the control group, the addition of GAA increased the activity of mitochondrial respiratory chain complex III in longissimus thoracis muscle (p < 0.05; Table 3). Meanwhile, dietary 0.10% GAA increased the activity of mitochondrial respiratory chain complex I in semitendinosus muscle (p < 0.05).

Table 3.
Effects of guanidinoacetic acid supplementation on the activities of mitochondrial respiratory chain complex I and III in the longissimus thoracis and semitendinosus muscle of finishing pigs.
3.5. Related Genes and Protein Expressions of Mitochondrial Synthesis
According to Figure 5A, compared with the control group, 0.05% GAA supplementation upregulated the mRNA expression of TFAM in longissimus thoracis muscle (p < 0.05). Moreover, as shown in Figure 6B, the 0.10% GAA supplementation upregulated the protein expression of TFAM in semitendinosus muscle compared with the control group (p < 0.05).
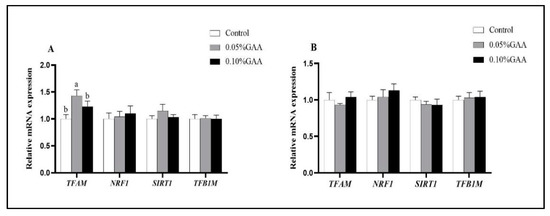
Figure 5.
Effects of guanidinoacetic acid supplementation on the related genes of mitochondrial synthesis of (A) longissimus thoracis muscle and (B) semitendinosus muscle in finishing pigs. Means with different letters differ significantly (p < 0.05). Values are presented as the mean ± SEM (n = 5). Control: Finishing pigs were fed a basal diet; 0.05% GAA or 0.10% GAA: Finishing pigs were fed a basal diet supplemented with GAA at 500 or GAA at 1000 mg kg−1; TFAM: Mitochondrial transcription factor A; NRF1: Nuclear respiratory factor 1; SIRT1: Silent information regulator 1; TFB1M: Mitochondrial transcription factor B1.
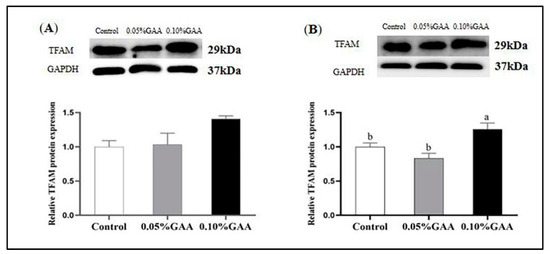
Figure 6.
Effects of guanidinoacetic acid supplementation on the protein expression of TFAM of longissimus thoracis muscle (A) and semitendinosus muscle (B) in finishing pigs. Means with different letters differ significantly (p < 0.05). Values are presented as the mean ± SEM (n = 5). Control: Finishing pigs were fed a basal diet; 0.05% GAA or 0.10% GAA: Finishing pigs were fed a basal diet supplemented with GAA at 500 or GAA at 1000 mg kg−1; TFAM: Mitochondrial transcription factor A.
3.6. Protein Expressions of CnAα and NFATc1
As exhibited in Figure 7A, dietary 0.10% GAA upregulated the protein expression of CnAα in longissimus thoracis muscle compared with the control group (p < 0.05). Meanwhile, 0.10% GAA treatment increased the protein expression of NFATc1 in semitendinosus muscle, as shown in Figure 7B (p < 0.05).
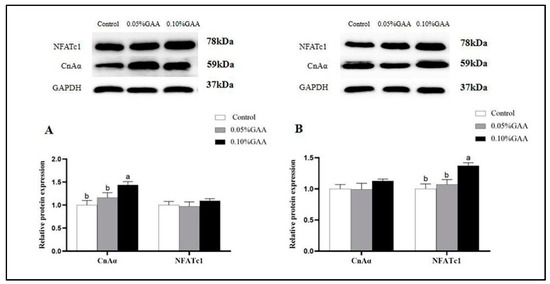
Figure 7.
Effects of guanidinoacetic acid supplementation on the protein expressions of CnAα, NFATc1 of (A) longissimus thoracis muscle, and (B) semitendinosus muscle in finishing pigs. Means with different letters differ significantly (p < 0.05). Values are presented as the mean ± SEM (n = 5). Control: Finishing pigs were fed a basal diet; 0.05% GAA or 0.10% GAA: Finishing pigs were fed a basal diet supplemented with GAA at 500 or GAA at 1000 mg kg−1; CnAα: Calcineurin catalytic subunit α; NFATc1: Nuclear factor of activated T cells.
4. Discussion
Muscle fiber is the basic constituent unit of skeletal muscle. Based on the expression of the predominant MyHC isoforms, the mammalian muscle fibers can be generally classified into four types, including MyHC-I, MyHC-IIa, MyHC-IIx, MyHC-IIb. [22]. Meanwhile, the proportion of different muscle fiber types directly affects the meat quality [23]. Therefore, an investigation of the effects and potential mechanisms of feed additives on skeletal muscle development and fiber type transformation in the production of livestock and poultry has extremely vital roles in improving meat quality. Without affecting the growth performance, we found that the dietary supplementation with GAA can increase the troponin I slow type (Troponin I-ss) protein level and downregulate the expression of troponin IIb fast skeletal type (Troponin T3) in finishing pigs. Moreover, we found this function of GAA involved in promoting the PPARGC1A-based mitochondria function and CaN/NFAT pathway.
The composition of muscle fiber types is an important factor to determine the meat quality. Generally, meat quality is positively correlated with the ratio of slow muscle fibers [24]. Troponin I-ss and Troponin T3 are two subtypes existing in slow and fast muscle fibers, respectively, and their expression levels can reflect the content of slow and fast muscle fibers in muscle [25,26]. Our unpublished data demonstrated that GAA supplementation increased pH45min and decreased the drip loss in semitendinosus muscle significantly, which was consistent with the muscle fiber types transformation in our present study. In addition, our unpublished data have also found that dietary GAA significantly increased the mRNA expression of MyHC-I and decreased the expression of MyHC-IIb in longissimus thoracis and semitendinosus muscle. Meanwhile, our present data revealed that GAA addition increased the expression of Troponin I-ss and downregulated the expression of Troponin T3 in longissimus thoracis and semitendinosus muscle, suggesting that GAA could promote the muscle fiber type transformation from fast-to-slow twitch. Similarly, Zhu et al. [20] found that dietary GAA improved the meat quality and upregulated the MyHC-I mRNA expression in finishing gilts, which was in accordance with our results.
The transformation of muscle fiber types is mediated by several independent signaling pathways, and PPARGC1A is one of the most important regulatory pathways [27]. The expression of PPARGC1A is higher in slow muscle type than in fast muscle type [28]. Russell et al. [29] found that PPARGC1A transgenic mice increased mitochondrial concentrations and the level of oxidative phosphorylation in skeletal muscle and contained more MyHC-I and MyHC-IIa fibers. The increase of PPARGC1A expression can be achieved by AMPK activation [30]. On the other hand, a previous study has also found that the signaling of Ca2+ can activate the expression of PPARGC1A in skeletal muscle [31]. Theoretically, the change of Ca2+ levels depends on the activity of Ca2+-ATPase, which is closely related to cellular energy metabolism [32]. With the increase of intracellular ATP concentration, the activity of Ca2+-ATPase increases. This can in turn activate the calcium pump on the sarcoplasmic reticulum to release more Ca2+ into the cytoplasm [32]. The increased concentration of Ca2+ has the potential to activate PPARGC1A. A previous study has demonstrated that dietary GAA can significantly increase the content of ATP in animal cells [33]. Meanwhile, the present study indicated that GAA treatment increased the concentration of Ca2+ in longissimus thoracis muscle. Finally, our data showed that the mRNA and protein levels of PPARGC1A were upregulated in GAA supplementation treatments, suggesting that GAA could promote the expression of PPARGC1A through the Ca2+ signal.
There are four fiber types found in the skeletal muscle (MyHC-I MyHC-IIa, MyHC-IIx, MyHC-IIb). MyHC-I is called slow-twitch fiber, which has a predominantly oxidative metabolism. MyHC-IIb is based on anaerobic metabolism due to the lower mitochondria and higher glycogen, which leads to a decrease in meat quality [2]. In other words, the change in muscle fiber type distribution from fast-to-slow twitch is the result of changes in key oxidative phosphorylation and glycolytic enzymes. Mitochondria contain cytochrome and enzymes for oxidative phosphorylation [34]. Therefore, the changes in mitochondrial content and function would affect the body’s aerobic metabolic functions [34]. A previous study showed that the improvement of mitochondrial biosynthesis and function could lead to an increased oxidative phosphorylation capacity of fibers, and finally upregulated the proportion of slow muscle fibers [35]. In addition, PPARGC1A is involved in energy metabolism regulation. It has been demonstrated that PPARGC1A plays a vital role in the improvement of mitochondrial biosynthesis and function [36]. Furthermore, the improvement of mitochondrial biosynthesis and function promotes the conversion of skeletal muscle fiber types [37].
To prove the improvement of mitochondrial function in muscle due to GAA addition, the present study measured the mitochondrial membrane potential (MMP) and the activities of mitochondrial respiratory chain complex I and III. In the process of respiratory oxidation, mitochondria store the generated energy as electrochemical potential energy in the inner membrane of mitochondria. This state results in asymmetric distribution of ion concentrations on both sides of the inner membrane, and then forms the MMP [38]. The normal MMP is a necessary condition to maintain the mitochondrial function, oxidative phosphorylation, and ATP production [39,40]. On the other hand, the main effect of mitochondrial respiratory chain, representing the basic function of mitochondria, is to form energy through hydrogen and electron transmitters and a series of redox reactions. Moreover, mitochondrial respiratory chain complex I is the main element to drive electrons into the respiratory chain and complex III is the essential protein of mitochondrial oxidative phosphorylation and the gatekeeper of respiratory chain [41]. In our study, dietary GAA significantly increased the level of MMP in semitendinosus muscle and upregulated the activity of mitochondrial respiratory chain complex I, III in semitendinosus and longissimus thoracis muscle, respectively. Additionally, we detected the levels of mitochondrial synthesis-related genes and proteins to evaluate the state of mitochondrial synthesis. Mitochondrial transcription factor A (TFAM) is a key factor in the replication of mitochondrial DNA and mitochondrial formation [42]. In mitochondria, TFAM exerts its transcriptional activation ability through the recruitment of mitochondrial RNA polymerase and mitochondrial transcription factor B1 (TFB1M) [43]. Previous evidences indicated that the increased expression of PPARGC1A upregulated the DNA content of mitochondria and promoted the activity of TFAM with DNA junctions, and then increased the biosynthesis of mitochondria [44]. Our present results indicated that GAA significantly increased the mRNA expression of TFAM in longissimus thoracis muscle. Meanwhile, 0.10% GAA group upregulated the protein expression of TFAM in semitendinosus muscle. To summarize, these results indicated that the increased expression of PPARGC1A promoted the improvement of mitochondrial biosynthesis and function, which may further increase the proportion of slow muscle fibers.
CaN/NFAT is one of the most important pathways that regulates the transformation of skeletal muscle fiber types after animal birth [45]. In skeletal muscle, once the concentrations of intracytoplasmic Ca2+ elevates, the catalytic subtypes CnAα of CaN will integrate Ca2+, resulting in the excitation of CaN [46]. A previous study demonstrated that PPARGC1A, as the substrate of CaN, also mediated the conversion of muscle fibers from fast-twitch to slow-twitch through CaN/NFAT signaling pathway [47]. PPARGC1A directly interacts with myocyte enhancer factor 2 (MEF2), which physically binds to the NFATc1 site to upregulate the expression of slow-twitch fibers genes. [48]. Myogenic differentiation (MyoD) and myogenic factor 5 (Myf5), as the downstream genes of CaN/NFAT signal pathway, play a crucial role in regulating the proliferation and differentiation of muscle cells [49]. Our unpublished data found that GAA treatment significantly upregulated the mRNA expression of MyoD in longissimus dorsi muscle. Lu et al. [50] also found that dietary GAA enhanced the expression of MyoD and Myf5 in longissimus thoracis muscle of finishing pigs. In our previous results (unpublished data), GAA addition upregulated the mRNA expressions of CnA and NFATc1 in semitendinosus muscle. Moreover, in the current study, the protein expressions of CnAα and NFATc1 were upregulated in the muscle of finishing pigs from the GAA supplementation groups, which were also in accordance with the increased expression of PPARGC1A.
5. Conclusions
Dietary GAA supplementation promotes the skeletal muscle fiber type transformation from fast-to-slow-twitch in finishing pigs by increasing the expression of PPARGC1A to improve the mitochondrial function and the mitochondrial biosynthesis, and by upregulating the key factors of CaN/NFAT pathway. These findings may expand our knowledge regarding the function of dietary GAA in finishing pigs.
Supplementary Materials
TThe following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agriculture12010087/s1. Figure S1: Effects of guanidinoacetic acid supplementation on the expressions of Troponin T3 of longissimus thoracis muscle in finishing pigs (n = 5), Figure S2: Effects of guanidinoacetic acid supplementation on the expressions of Troponin T3 in semitendinosus muscle in finishing pigs (n = 5).
Author Contributions
Data curation and writing—original draft, J.L. (Jingzheng Li); methodology, project administration, supervision and writing—review, J.L. (Jiaolong Li); advice, writing—review, L.Z.; advice and writing—review, T.X.; supervision and writing—review, Y.J.; funding acquisition, project administration, supervision and writing—review, F.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China (grant number: 31902194), National Key Research and Development Program of China (grant number: 2018YFD0500405), and Jiangsu Planned Projects for Postdoctoral Research Funds (grant number: 1601030A).
Institutional Review Board Statement
All of the experimental procedures involving the use of finishing pigs were in alignment with the Animal Care and Use Committee of Nanjing Agricultural University, under protocol number SYXK 2017-0027.
Informed Consent Statement
No applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
Acknowledgments
The authors thank Bingbing Ma, Zuodong Chen, Dongdong Yang, and Binbin Duan for their assistance during the whole animal trials and the follow-up experiment.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lefaucheur, L. A second look into fibre typing–relation to meat quality. Meat Sci. 2010, 84, 257–270. [Google Scholar] [CrossRef]
- Talmadge, R.J.; Roy, R.R.; Edgerton, R.V. Muscle fiber types and function. Curr. Opin. Rheumatol. 1993, 5, 695–705. [Google Scholar] [CrossRef]
- Schiaffino, S. Muscle fiber type diversity revealed by anti-myosin heavy chain antibodies. FEBS J. 2018, 285, 3688–3694. [Google Scholar] [CrossRef]
- Men, X.M.; Deng, B.; Xu, Z.W.; Tao, X.; Qi, K.K. Age-related changes and nutritional regulation of myosin heavy-chain composition in longissimus thoracis of commercial pigs. Animal 2013, 7, 1486–1492. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Jin, J.P. TNNT1, TNNT2, and TNNT3: Isoform genes, regulation, and structure-function relationships. Gene 2016, 582, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.; Chen, X.; Huang, Z.; Chen, D.; Yu, B.; Chen, H.; Luo, Y.; Zheng, P.; Yu, J.; He, J. Grape seed proanthocyanidin extract promotes skeletal muscle fiber type transformation via AMPK signaling pathway. J. Nutr. Biochem. 2020, 84, 108462. [Google Scholar] [CrossRef]
- Luo, P.; Luo, L.; Zhao, W.; Wang, L.; Sun, L.; Wu, H.; Li, Y.; Zhang, R.; Shu, G.; Wang, S.; et al. Dietary thymol supplementation promotes skeletal muscle fibre type switch in longissimus thoracis of finishing pigs. J. Anim. Physiol. Anim. Nutr. 2020, 104, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; He, Y.; Du, H.; Yang, J.; Wan, H. Output Regulation and Function Optimization of Mitochondria in Eukaryotes. Front. Cell Dev. Biol. 2020, 8, 598112. [Google Scholar] [CrossRef]
- Shen, L.Y.; Luo, J.; Lei, H.G.; Jiang, Y.Z.; Bai, L.; Li, M.Z.; Tang, G.Q.; Li, X.W.; Zhang, S.H.; Zhu, L. Effects of muscle fiber type on glycolytic potential and meat quality traits in different Tibetan pig muscles and their association with glycolysis-related gene expression. Genet. Mol. Res. 2015, 14, 14366–14378. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Okutsu, M.; Akhtar, Y.N.; Lira, V.A. Regulation of exercise-induced fiber type transformation, mitochondrial biosynthesis, and angiogenesis in skeletal muscle. J. Appl. Physiol. 2011, 110, 264–274. [Google Scholar] [CrossRef] [Green Version]
- Popov, D.V. Adaptation of Skeletal Muscles to Contractile Activity of Varying Duration and Intensity: The Role of PGC-1α. Biochemistry 2018, 83, 613–628. [Google Scholar] [CrossRef]
- Ying, F.; Zhang, L.; Bu, G.; Xiong, Y.; Zuo, B. Muscle fiber-type conversion in the transgenic pigs with overexpression of PGC-1α gene in muscle. Biochem. Biophys. Res. Commun. 2016, 480, 669–674. [Google Scholar] [CrossRef]
- Ducreux, S.; Gregory, P.; Schwaller, B. Inverse regulation of the cytosolic Ca2+ buffer parvalbumin and mitochondrial volume in muscle cells via Sirt1/PGC-1α axis. PLoS ONE 2012, 7, e44837. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Tao, S.; Li, X.; Yao, Q. Resistin destroys mitochondrial biosynthesis by inhibiting the PGC-1α/NRF1/TFAM signaling pathway. Biochem. Biophys. Res. Commun. 2018, 504, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Wang, L.; Luo, L.; Wang, L.; Yang, K.; Shu, G.; Wang, S.; Zhu, X.; Gao, P.; Jiang, Q. Ca2+-Calcineurin-NFAT pathway mediates the effect of thymol on oxidative metabolism and fiber-type switch in skeletal muscle. Food Funct. 2019, 10, 5166–5173. [Google Scholar] [CrossRef] [PubMed]
- Ostojic, S.M. Benefits and drawbacks of guanidinoacetic acid as a possible treatment to replenish cerebral creatine in AGAT deficiency. Nutr. Neurosci. 2019, 22, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Edison, E.E.; Brosnan, M.E.; Meyer, C.; Brosnan, J.T. Creatine synthesis: Production of guanidinoacetate by the rat and human kidney in vivo. Am. J. Physiol. Ren. Physiol. 2007, 293, 1799–1804. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.; Li, J.L.; Xing, T.; Zhang, L.; Gao, F. Effects of guanidinoacetic acid and complex antioxidant supplementation on growth performance, meat quality, and antioxidant function of broiler chickens. J. Sci. Food Agric. 2020, 101, 3961–3968. [Google Scholar] [CrossRef]
- Volek, J.S.; Duncan, N.D.; Mazzetti, S.A.; Staron, R.S.; Putukian, M.; Gómez, A.L.; Pearson, D.R.; Fink, W.J.; Kraemer, W.J. Performance and muscle fiber adaptations to creatine supplementation and heavy resistance training. Med. Sci. Sports Exerc. 1999, 31, 1147–1156. [Google Scholar] [CrossRef]
- Zhu, Z.; Gu, C.; Hu, S.; Li, B.; Zeng, X.; Yin, J. Dietary guanidinoacetic acid supplementation improved carcass characteristics, meat quality and muscle fibre traits in growing-finishing gilts. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1454–1461. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, R.; Tian, X.; Fan, X.; Shi, Y.; Zhang, W.; Hou, Q.; Zhou, G. Comparison of Activity, Expression, and S-Nitrosylation of Calcium Transfer Proteins between Pale, Soft, and Exudative and Red, Firm, and Non-exudative Pork during Post-Mortem Aging. J. Agric. Food Chem. 2019, 67, 3242–3248. [Google Scholar] [CrossRef]
- Serrano, A.L.; Petrie, J.L.; Rivero, J.L.; Hermanson, J.W. Myosin isoforms and muscle fiber characteristics in equine gluteus medius muscle. Anat. Rec. 1996, 244, 444–451. [Google Scholar] [CrossRef]
- Bao, G.; Liu, X.; Wang, J.; Hu, J.; Shi, B.; Li, S.; Luo, Y. Effects of Slaughter Age on Myosin Heavy Chain Isoforms, Muscle Fibers, Fatty Acids, and Meat Quality in Longissimus Thoracis Muscle of Tibetan Sheep. Front. Vet. Sci. 2021, 8, 689589. [Google Scholar] [CrossRef] [PubMed]
- Reiser, P.J. Current understanding of conventional and novel co-expression patterns of mammalian sarcomeric myosin heavy chains and light chains. Arch. Biochem. Biophys. 2019, 662, 129–133. [Google Scholar] [CrossRef]
- Orzuna-Orzuna, J.F.; Dorantes-Iturbide, G.; Lara-Bueno, A.; Mendoza-Martínez, G.D.; Miranda-Romero, L.A.; López-Ordaz, R.; Hernández-García, P.A. Productive Performance, Carcass Traits, and Meat Quality in Finishing Lambs Supplemented with a Polyherbal Mixture. Agriculture 2021, 11, 942. [Google Scholar] [CrossRef]
- Muroya, S.; Nakajima, I.; Chikuni, K. Amino acid sequences of multiple fast and slow troponin T isoforms expressed in adult bovine skeletal muscles. J. Anim. Sci. 2003, 81, 1185–1192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selsby, J.T.; Morine, K.J.; Pendrak, K.; Barton, E.R.; Sweeney, H.L. Rescue of dystrophic skeletal muscle by PGC-1α involves a fast to slow fiber type shift in the mdx mouse. PLoS ONE 2012, 7, e30063. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Duan, Y.; Yao, K.; Li, F.; Hou, Y.; Wu, G.; Yin, Y. β-Hydroxy-β-methylbutyrate, mitochondrial biogenesis, and skeletal muscle health. Amino Acids 2016, 48, 653–664. [Google Scholar] [CrossRef]
- Russell, A.P.; Feilchenfeldt, J.; Schreiber, S.; Praz, M.; Crettenand, A.; Gobelet, C.; Meier, C.A.; Bell, D.R.; Kralli, A.; Giacobino, J.P.; et al. Endurance training in humans leads to fiber type-specific increases in levels of peroxisome proliferator-activated receptor-gamma coactivator-1 and peroxisome proliferator-activated receptor-alpha in skeletal muscle. Diabetes 2003, 52, 2874–2881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jäger, S.; Handschin, C.; St-Pierre, J.; Spiegelman, B.M. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc. Natl. Acad. Sci. USA 2007, 104, 12017–12022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, Y.K.; Park, J.H.; Baek, Y.Y.; Won, M.H.; Jeoung, D.; Lee, H.; Ha, K.S.; Kwon, Y.G.; Kim, Y.M. Carbon monoxide stimulates astrocytic mitochondrial biogenesis via L-type Ca2+ channel-mediated PGC-1α/ERRα activation. Biochem. Biophys. Res. Commun. 2016, 479, 297–304. [Google Scholar] [CrossRef]
- Brookes, P.S.; Yoon, Y.; Robotham, J.L.; Anders, M.W.; Sheu, S.S. Calcium, ATP, and ROS: A mitochondrial love-hate triangle. Am. J. Physiol. Cell Physiol. 2004, 287, C817–C833. [Google Scholar] [CrossRef] [PubMed]
- He, D.T.; Gai, X.R.; Yang, L.B.; Li, J.T.; Lai, W.Q.; Sun, X.L.; Zhang, L.Y. Effects of guanidinoacetic acid on growth performance, creatine and energy metabolism, and carcass characteristics in growing-finishing pigs. J. Anim. Sci. 2018, 96, 3264–3273. [Google Scholar] [CrossRef]
- Papa, S.; Martino, P.L.; Capitanio, G.; Gaballo, A.; De Rasmo, D.; Signorile, A.; Petruzzella, V. The oxidative phosphorylation system in mammalian mitochondria. Adv. Exp. Med. Biol. 2012, 942, 3–37. [Google Scholar] [CrossRef] [PubMed]
- Venhoff, N.; Lebrecht, D.; Pfeifer, D.; Venhoff, A.C.; Bissé, E.; Kirschner, J.; Walker, U.A. Muscle-fiber transdifferentiation in an experimental model of respiratory chain myopathy. Arthritis Res. Ther. 2012, 14, R233. [Google Scholar] [CrossRef] [Green Version]
- Rasbach, K.A.; Gupta, R.K.; Ruas, J.L.; Wu, J.; Naseri, E.; Estall, J.L.; Spiegelman, B.M. PGC-1-alpha regulates a HIF-2-alpha-dependent switch in skeletal muscle fiber types. Proc. Natl. Acad. Sci. USA 2010, 107, 21866–21871. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.; Wu, H.; Tarr, P.T.; Zhang, C.Y.; Wu, Z.; Boss, O.; Michael, L.F.; Puigserver, P.; Isotani, E.; Olson, E.N.; et al. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature 2002, 418, 797–801. [Google Scholar] [CrossRef] [PubMed]
- Casas, F.; Pessemesse, L.; Grandemange, S.; Seyer, P.; Gueguen, N.; Baris, O.; Lepourry, L.; Cabello, G.; Wrutniak-Cabello, C. Overexpression of the mitochondrial T3 receptor p43 induces a shift in skeletal muscle fiber types. PLoS ONE 2008, 3, e2501. [Google Scholar] [CrossRef] [Green Version]
- Teodoro, J.S.; Palmeira, C.M.; Rolo, A.P. Mitochondrial Membrane Potential (ΔΨ) Fluctuations Associated with the Metabolic States of Mitochondria. Methods Mol. Biol. 2018, 1782, 109–119. [Google Scholar] [CrossRef]
- Bagkos, G.; Koufopoulos, K.; Piperi, C. A new model for mitochondrial membrane potential production and storage. Med. Hypotheses 2014, 83, 175–181. [Google Scholar] [CrossRef]
- Guo, R.; Gu, J.; Zong, S.; Wu, M.; Yang, M. Structure and mechanism of mitochondrial electron transport chain. Biomed. J. 2018, 41, 9–20. [Google Scholar] [CrossRef]
- Kang, I.; Chu, C.T.; Kaufman, B.A. The mitochondrial transcription factor TFAM in neurodegeneration: Emerging evidence and mechanisms. FEBS Lett. 2018, 592, 793–811. [Google Scholar] [CrossRef] [Green Version]
- Mulder, H. Transcribing β-cell mitochondria in health and disease. Mol. Metab. 2017, 6, 1040–1051. [Google Scholar] [CrossRef]
- Wenz, T. Regulation of mitochondrial biosynthesis and PGC-1α under cellular stress. Mitochondrion 2013, 13, 134–142. [Google Scholar] [CrossRef]
- Liu, W.; Chen, G.; Li, F.; Tang, C.; Yin, D. Calcineurin-NFAT Signaling and Neurotrophins Control Transformation of Myosin Heavy Chain Isoforms in Rat Soleus Muscle in Response to Aerobic Treadmill Training. J. Sports Sci. Med. 2014, 13, 934–944. [Google Scholar]
- Parsons, S.A.; Wilkins, B.J.; Bueno, O.F.; Molkentin, J.D. Altered skeletal muscle phenotypes in calcineurin Aalpha and Abeta gene-targeted mice. Mol. Cell. Biol. 2003, 23, 4331–4343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts-Wilson, T.K.; Reddy, R.N.; Bailey, J.L.; Zheng, B.; Ordas, R.; Gooch, J.L.; Price, S.R. Calcineurin signaling and PGC-1alpha expression are suppressed during muscle atrophy due to diabetes. Biochim. Biophys. Acta 2010, 1803, 960–967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, H.; Rothermel, B.; Kanatous, S.; Rosenberg, P.; Naya, F.J.; Shelton, J.M.; Hutcheson, K.A.; DiMaio, J.M.; Olson, E.N.; Bassel-Duby, R.; et al. Activation of MEF2 by muscle activity is mediated through a calcineurin-dependent pathway. EMBO J. 2001, 20, 6414–6423. [Google Scholar] [CrossRef] [PubMed]
- Zammit, P.S. Function of the myogenic regulatory factors Myf5, MyoD, Myogenin and MRF4 in skeletal muscle, satellite cells and regenerative myogenesis. Semin. Cell Dev. Biol. 2017, 72, 19–32. [Google Scholar] [CrossRef]
- Lu, Y.; Zou, T.; Wang, Z.; Yang, J.; Li, L.; Guo, X.; He, Q.; Chen, L.; You, J. Dietary guanidinoacetic acid improves the growth performance and skeletal muscle development of finishing pigs through changing myogenic gene expression and myofibre characteristics. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1875–1883. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).