Abstract
Red raspberries (Rubus idaeus L.) are highly appreciated by consumers. However, their postharvest shelf life scarcely exceeds 5 d under the refrigeration temperatures usually applied during commercialization, due to their high susceptibility to dehydration, softening and rot incidence. Thus, the objective of this study was to investigate the ability of UV-C radiation (UV1: 2 kJ m−2 and UV2: 4 kJ m−2), passive modified atmosphere packaging (MAP) with transmission rates (TR) for O2 and CO2 of 1805 mL d−1 and 1570 mL d−1 (MAP1), and 902 mL d−1 and 785 mL d−1 (MAP2), respectively, and the combination of both technologies to prolong raspberries’ shelf life at 6 °C. Their influence on respiration, physicochemical parameters, and microbiological and nutritional quality was assessed during 12 d of storage. The combination of 4 kJ m−2 UV-C radiation and a packaging film with O2 and CO2 transmission rates of 902 mL d−1 and 785 mL d−1, respectively, produced a synergistic effect against rot development, delaying senescence of the fruit. The UV2MAP2 and MAP2 samples only showed 1.66% rot incidence after 8 d of storage. The UV2MAP2 samples also had higher bioactive content (1.76 g kg−1 of gallic acid equivalents (GAE), 1.08 g kg−1 of catechin equivalents (CE) and 0.32 g kg−1 of cyanidin 3-O-glucoside equivalents (CGE)) than the control samples at the end of their shelf life. Moreover, the mass loss was minimal (0.56%), and fruit color and firmness were maintained during shelf life. However, the rest of the batches were not suitable for commercialization after 4 d due to excessive mold development.
1. Introduction
The production and consumption of red raspberries (Rubus idaeus L.) increase every year because of their nutritional properties. However, raspberries’ shelf life lasts between 2 and 5 d after harvest, primarily caused by loss of firmness and the appearance of rot [1]. Moreover, their high respiratory metabolism (40–200 mL kg−1 h−1 at 20 °C) must be slowed down, so quick refrigeration after harvest is essential to delay senescence. However, the temperature must not fall below 0 °C in order to avoid frost injury. The temperature gradient between fruit and air may also provoke high dehydration, because the water vapor pressure deficit (VPD) is higher; however, it can be minimized to reduce the difference of water vapor partial pressure between the fruit and the external atmosphere. Postharvest technologies, such as modified atmosphere packaging (MAP), edible coatings, and ionizing (electron beam, X-rays and gamma-rays) or non-ionizing radiation (UV) have been studied to control decay and maintain the quality of fruit during storage and in sale. This may further enhance the positive effects of the aforementioned low temperature storage [2,3,4,5,6,7,8,9].
Irradiation is still considered an alternative technology and it has attracted interest in the last decade because of the effectiveness of e-beam and gamma-rays for microbial inactivation [6,8]. Moreover, these technologies allow in-package processing, preventing further cross-contamination [6,8,10]. However, the main drawbacks of e-beam and gamma-irradiation are the cost of application and restrictive legislation. On the other hand, UV-C light has been applied to inhibit the growth of bacteria and phytopathogen fungi, enhancing the shelf life of various fruits and vegetables [11,12,13,14,15]. Its benefits include affordability, ease of handling and a lack of noxious byproducts [10]. Nevertheless, the effectiveness of this treatment may be affected by the type of fruit, cultivar, chemical composition or skin roughness [14,15,16]. Additionally, radiation intensity must be considered because excessive doses provoke loss of firmness, the oxidation of compounds such as carotenes, vitamin C or polyphenols [6,8,17,18,19], and darkening of fruits and vegetables [8,17].
High CO2 MAP (12–20%) lengthens raspberries’ shelf life [7,9,20,21]. For example, MAP inhibits the development of gray rot caused by Botritys cinerea, which provokes considerable losses during storage [22]. However, CO2 concentrations exceeding 25% or O2 concentrations below 1% may induce physiological changes contributing to color modification and the appearance of off-flavors [7,23,24]. The use of MAP also raises the total concentration of phenols and anthocyanins, and the antioxidant activity commonly attributed to these compounds [7,25,26]. Anthocyanins are responsible for external color, which is one of the main attributes of visual acceptance. Stavang et al. [27] and Wang, Chen and Wang [28] found that both predominant anthocyanins and total anthocyanin concentration (TAC) increase during storage, demonstrating de novo synthesis. However, a negative correlation was reported between the majority of the anthocyanins quantified and the attractiveness of the berries during storage due to skin darkening [27]. Lower redness (a*) is also attributed to higher TAC, and to changes in anthocyanin conformation because of the decrease in carboxylic acid and the consequent increase of pH [29,30].
Although the effects of MAP and UV-C radiation alone or combined with other technologies have been studied in depth in a wide range of commodities [2,9,15,31,32,33], to the best of our knowledge there is no evidence about the benefits of merging both technologies to prolong red raspberry shelf life at 6 °C, which is the temperature commonly used in retail fruit aisles. In general terms, it is well known that low O2 and high CO2 concentrations coupled with refrigerated storage (0–4 °C) have a beneficial effect on fruit quality. These conditions are proposed as optimal to preserve some fresh fruit; however, commercial open-refrigerated display cabinets scarcely achieve air temperatures below 5 °C, depending on the position of the shelf [34]. Moreover, one study showed that the temperature for food stuffs exceeded 7 °C in 70% of the cases [35]. Despite fluctuations, display cabinets for fruit, including red raspberries, maintain an average temperature above what is recommended [36]. However, few studies consider the real temperature that the fruit is exposed to within their parameters [7,25,37,38], and most of them consider temperatures close to 0 °C [8,9,21,24,29,39]. Given this background, two lidding films with different transmission rates and two doses of UV-C radiation were evaluated individually and in all their possible combinations. The aim was to preserve red raspberry global quality during storage at 6 °C and prolong the shelf life, with the hope that this knowledge may benefit the fruit industry and consumers.
2. Materials and Methods
2.1. Plant Material
Red raspberries (Rubus idaeus L., ‘Glen Lyon’) (25 kg) produced in Huelva (Spain) were purchased from a local distributor established in Mercazaragoza (Zaragoza, Spain). The fruit were transported to the laboratory while maintaining the cold chain. There, they were visually inspected, and damaged berries were removed to ensure uniformity in color and size. Preliminary analyses were conducted to characterize the initial quality of the fruit. The rest of the berries were randomly divided into nine batches: non-treated and non-modified atmosphere packaged (control), treated with two different UV-C intensities (UV1 and UV2), packaged under two modified atmosphere conditions (MAP1 and MAP2), and irradiated with the two different UV-C doses and packaged under the two different MAPs (UV1MAP1, UV1MAP2, UV2MAP1 and UV2MAP2). After the application of the corresponding UV-C treatment, when necessary, polypropylene (PP) trays were manually filled with 0.15 kg of red raspberries, resulting in 15 trays for each batch, i.e., five trays containing 0.15 kg per batch and day of analysis (T4, T8 and T12). Controls were directly packaged in PP macroperforated clam shell containers using the same quantity of fruit and kept at 6 °C.
2.2. UV-C Treatment
The raspberries were exposed to 2 (UV1) and 4 kJ m−2 (UV2) of UV-C radiation (peak wave length: 254 nm) in a steel chamber (68 cm × 55 cm × 45 cm). These doses have pronounced effects on different quality parameters in strawberries [26], blueberries [5] and grapes [40] and have significantly reduced the presence of Penicillium expansum in organic cherries, strawberries and raspberries [15]. The berries were placed on a stainless steel mesh and radiation was homogenously applied by mirror-finished reflectors and four lamps (G15W T8, Feilo Sylvania Group, Budapest, Hungary) located in pairs at the top and bottom of the cabinet at a 26 cm distance from the sample. Irradiation intensities of 1, 2, 4, and 6 kJ m−2 were measured by a portable digital radiometer (HD 2102.2, Delta Ohm, Padova, Italy) to calculate the irradiation time for the specific UV-C dosages UV1 and UV2, which corresponded to 2 and 4 min, respectively.
2.3. Modified Atmosphere Packaging (MAP) and Atmosphere Evolution during Shelf Life
Approximately 150 g of raspberries were packaged in 750 mL PP trays (Linpac Packaging Pravia S.A.U, Pravia, Spain) using microperforated P-Plus film (Amcor Flexibles, Ledbury, UK). Two films with different O2 and CO2 transmission rates were used (1805 mL d−1 and 1570 mL d−1, respectively, (MAP1), and 902 mL d−1 and 785 mL d−1, respectively, (MAP2)). This selection of materials was based on the results obtained using a predictive model for the gas composition evolution [41] and the consideration of the preliminary results of the raspberries’ respiration rate, the amount of fruit included in the packages and the desired steady state gas composition. The gas composition within the packages was monitored with a Dansensor CheckMate3 O2 and CO2 analyzer (Ametek Mocon, Brooklyn Park, MN, USA) during storage.
2.4. Respiration Rate
The respiration rates (RRs) of fresh and UV-C treated (UV1 and UV2) raspberries were measured the day before the initial study (day 0) at 6 °C in a static respirometer equipped with electronic CO2 and O2 sensors and a closed-loop gas circulating system [42]. Approximately 300 g of fruit were placed in a 1250 mL glass jar, and once the respirometer was closed, gas was pumped to the sensors at a flow rate of 30 mL min−1. The initial composition of the atmosphere was that of air. The concentrations of O2 and CO2 (%) were measured every 50 min using an optical O2 sensor (Luminox LOX-02, SST Sensing Ltd., Coatbridge, UK) and a non-dispersive infrared CO2 sensor (CO2 Engine BLG, SenseAir AB, Delsbo, Sweden). The raw O2 and CO2 data were corrected for pressure and temperature registered with the sensors. The actual O2- and CO2-based RRs were calculated from the changes in the respective O2 and CO2 concentrations and were expressed in mL kg−1 h−1. The average O2 RR and CO2 RR were calculated from the linear regression of the O2 and CO2 concentrations from the data available between O2 concentrations of 5% and 15% , and were expressed in the same units. Respiration rates were measured in triplicate for each treatment.
2.5. Microbiological Quality and Rot Incidence
The fruit were visually evaluated in search of mold spoilage, which was reported as the percentage of rotten fruit per treatment. The total counts of aerobic mesophilic bacteria, yeasts and molds were also evaluated at day 0 and after 4, 8 and 12 d of storage. The fruit samples were cut into halves and two of them, which were equivalent to 20 cm2, were blended with 80 mL of 0.1% peptone water (Merck, Darmstadt, Germany) in a Vibromatic (JP Selecta, Barcelona, Spain) at 8.7 Hz for 3 min. After serial dilutions, 0.1 mL was inoculated in plate count agar (PCA) (Oxoid, Hampshire, UK) and dichloran Rose Bengal chloranphenicol (DRBC; Oxoid) for the analysis of aerobic mesophilic bacteria, and yeast and mold, respectively. The PCA plates were incubated (Incubig, JP Selecta, Barcelona, Spain) at 30 °C for 24–48 h and the DRBC plates were incubated for 5 d at 25 °C. The results were expressed as the average ± standard deviation of log CFU cm−2 of five replicates per batch and control day.
2.6. Physicochemical Fruit Quality Determination
2.6.1. Mass Loss
The percentage of mass loss was calculated by the difference between the initial mass before packaging (Mi) and the fruit mass on the corresponding day of analysis (Mf) as shown in the following formula:
% mass loss = ((Mi − Mf)/Mi) × 100
2.6.2. Total Soluble Solids (TSS)
The determination of TSS was made after filtration of the juice from 25 fruit per batch on each day of analysis with an Atago DBX refractometer (Atago Co., Ltd., Tokyo, Japan). The results were expressed as percentages.
2.6.3. Titratable Acidity (TA)
An aliquot (10 mL) of the filtrate used for TSS determination was diluted 1:10 with distilled water and titrated against a standardized solution 0.1 N of sodium hydroxide until the pH reached 8.1, using a Crison Compact automatic titrator (Hach Lange Spain S.L.U, Barcelona, Spain). Acidity was calculated as mg L−1 of citric acid, considering it is the most abundant carboxylic acid present in raspberries.
2.6.4. Color Determination by Image Digital Analysis
The CIELAB coordinates of 25 fruit per batch and control day were determined by image digital analysis (IDA). The fruit were cut into halves and scanned at a resolution of 1200 dpi in a calibrated HP ScanJet G4010 (HP Inc., Palo Alto, CA, USA) following the color standard UNE 48103:2014 (AENOR, 2014) [43]. The reflectance of the external surfaces was measured in the UV–Vis region (360 to 900 nm). An optical region of interest (ROI) was selected in each fruit half to obtain the RGB values of the samples using the Matrox Inspector 8.0 software (Matrox Electronic Systems Ltd., Dorval, QC, Canada). Equivalences between RGB and CIELAB coordinates were established in base of regression models obtained from the previous calibration of the system with the 300 color standards of the aforementioned UNE regulations.
2.6.5. Fruit Firmness
Firmness was determined on 25 fruit per batch that were free of decay with a TA-XT2i texture analyzer (Stable Micro Systems Ltd., Godalming, UK). The berries were compressed at 10 mm s−1 with a cylindric probe (2.5 cm diameter) until they reached 30% deformation of the initial diameter using a load cell of 5 kg [25]. The results were expressed in N.
2.7. Analysis of Bioactive Compounds
2.7.1. Polyphenolic Extracts Preparation
Phenolic compounds were extracted in two steps to obtain both cytoplasmic and cell membrane-bound compounds in order to achieve the maximum extraction yield [44,45,46,47]. In the first step, free phenolic compounds from 5 g of sample were extracted with ethanol:water (80:20, v:v). The supernatant was collected after homogenization (404 g for 30 s) using an ultra-turrax DI 25 homogenizer (Ika-Werke, Staufen, Germany) and centrifugation (3140 g for 15 min at 4 °C). The residue of this previous step was blended with 10 mL of 10% acidified methanol with HCl 1 N for 1 h at 85 °C in a block heater (Ohaus Europe, Greifensee, Switzerland) with continuous shaking at 1 g [44,45]. The tubes were cooled and centrifugated to obtain the supernatant, which was evaporated to dryness in a rotary evaporator at 40 °C. Then, it was reconstituted with 5 mL ethanol (80%) and combined with the extract containing the free phenolic compounds. Finally, the phenolic extracts were filtered through 0.45 μm acrylamide filters and stored at −18 °C. The extraction procedure was conducted in triplicate.
2.7.2. Total Phenolic Content (TPC)
The total phenolic content was determined by the Folin–Ciocalteu reagent method modified by Singleton, Orthofer and Lamuela-Raventos [48]. Folin reagent was added to test tubes containing 0.5 mL of the phenolic extract and mixed with 0.5 mL of 7.5% sodium carbonate. The solution was measured at 760 nm for colorimetric analysis after shaking and keeping it in darkness for 1 h. The results were obtained from a standard calibration curve (0–250 mg L−1) and expressed as g kg−1 of gallic acid equivalents (GAE) on a fresh mass (f.m.) base. The determination was made in triplicate for each extract obtained as described in Section 2.7.1.
2.7.3. Total Flavonoid Content (TFC)
The total flavonoid content was obtained by reacting phenolic extracts with 0.5% sodium nitrite, 10% aluminum chloride hexahydrate and sodium hydroxide 1 mol L−1 [49]. Flavonoid structures react with aluminum chloride and undergo Al–flavonoid complexation to form a yellow solution that immediately turns red in alkaline conditions [50]. Then, the absorbance was measured at 510 nm and the results were obtained from a catechin standard calibration curve (0–200 mg L−1). TFC was expressed as g kg−1 of catechin equivalents (CE) on f.m. The determination was made in triplicate for each extract obtained as described in Section 2.7.1.
2.7.4. 1,1-Diphenil-2-picrilhidrazil (DPPH) Scavenging Activity
The radical scavenging activity of the extracts was evaluated by modification of the method proposed by Llorach, Tomas-Barberan and Ferreres [51]. Aliquots (900 μL) of 133 μmol L−1 DPPH in methanol solution were added to 900 μL of the fruit extracts in spectrophotometric cuvettes, carefully shaken and incubated for 2 h in darkness at room temperature. Absorbance was measured at 515 nm and the results were expressed as mol kg−1 of Trolox equivalents (TE) on f.m. A standard curve (0–60 μmol L−1) was prepared from a Trolox stock solution. The determination was made in triplicate for each extract obtained as described in Section 2.7.1.
2.7.5. Ferric Reduction Antioxidant Power (FRAP)
A FRAP assay was conducted to evaluate the reducing ability of the extracts following the procedure reported by Benzie and Strain [52] with slight modifications. A 300 mmol L−1 sodium acetate buffer solution was added to 20 mmol L−1 ferric chloride and 10 mmol L−1 2, 4, 6-tripytidyl-s-triazine (TPTZ) solution at a 10:1:1 (v:v:v) ratio. The phenolic extracts (120 μL) were added to 900 μL of the FRAP reagent and incubated at room temperature for 20 min. The absorbance was measured at 595 nm and the concentrations were obtained from a Trolox standard curve made with concentrations ranging from 0 to 1000 μmol L−1. The results were presented as mol kg−1 of TE on f.m. The determination was made in triplicate for each extract obtained as described in Section 2.7.1.
2.7.6. Total Anthocyanin Content (TAC)
The methodology proposed by Dekazos [53] was followed to extract the total anthocyanin content. Extracts were measured at 525 nm, which is the wavelength at which maximum absorbance was observed corresponding to the absorbance band of cyanidin 3-O-glucoside (e = 29,600 L mol−1 cm−1, 449.2 g mol−1). Thus, the results were expressed as g kg−1 of cyanidin 3-O-glucoside equivalents (CGE) on f.m. extrapolated from the Beer–Lambert law. The anthocyanin extraction and determination were conducted in triplicate for each sample batch and control day.
2.8. Statistical Analysis
The graphical user interface R Commander for the R programming language (GNU GPL) was used to conduct two-way ANOVA and to analyze the significance of the differences between the means of the results of the batches (p value < 0.05) and of the storage time after pairwise comparison analysis (Tuckey’s post hoc). Pearson’s correlation coefficient was also determined between TPC or TFC and the antioxidant capacity of the extracts.
3. Results and Discussion
3.1. Determination of Respiratory Activity and Influence of UV-C Radiation
Raspberries have a short postharvest life, mostly due to their relatively high metabolic activity [38]. The use of modified atmosphere packaging (MAP) may be essential for their conservation and distribution. Several authors have concluded that packaging with high CO2 concentrations (15–20%) maintains the firmness of raspberries during their shelf life [7,20,54]. In addition, it slows down their respiratory activity, showing associated beneficial effects, such as maintaining the characteristic red color by inhibiting the degradation of anthocyanins. By matching the film transmission rates for O2 and CO2 (O2TR and CO2TR; mL d−1) with the respiration rate of the packaged raspberries, an optimal atmosphere can be established inside the package. The respiration rates were constant in a wide range of gas compositions, and only if the oxygen concentration decreased did the respiration rate decrease significantly (Figure 1). The CO2-based respiration rates were constant in a wider range of conditions, probably due to the gradual increase in the fermentative metabolism.
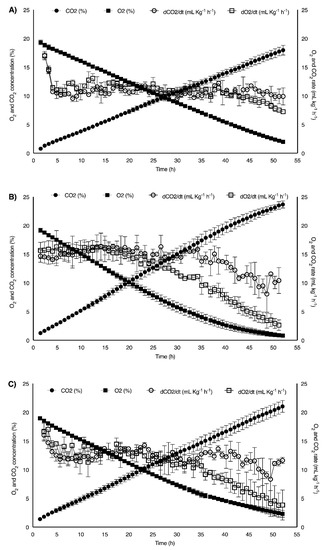
Figure 1.
O2 and CO2 concentration, and calculated respiration rates of: (A) controls, (B) UV1 (2 kJ m−2) and (C) UV2 (4 kJ m−2) treated raspberries. Values represent mean ± standard deviation (n = 3).
The average O2 and CO2 respiration rates determined for the raspberries at 6 °C were 10.66 ± 1.02 mL kg−1 h−1 and 10.03 ± 1.02 mL kg−1 h−1, respectively. These values are in agreement with the CO2 respiration rates found in the literature for raspberries at similar temperatures [55]. The application of UV-C at both doses obviously stressed the fruit, increasing the respiration rates of UV1 (13.72 ± 1.74 mL kg−1 h−1 and 14.67 ± 1.60 mL kg−1 h−1) and UV2 samples (12.46 ± 1.11 mL kg−1 h−1 and 13.30 ± 1.45 mL kg−1 h−1), at least at the beginning of the storage. This behavior has also been described in the case of strawberries or tomatoes treated with different UV-C doses [56,57]. Repetitive measurements of both O2 and CO2 concentrations in a closed system provide useful information about the metabolic activity and for the design of MAP packages. Approximately, when the concentration values of CO2 (15%) and O2 (5%) were reached, the rates of O2 uptake decreased progressively, while those of CO2 release began to exceed O2 uptake, which may indicate the switch to fermentative metabolic routes. Thus, optimal MAP gas compositions should not exceed these limits, at least not for a long-term storage.
3.2. Changes in O2 and CO2 Concentrations in Modified Atmosphere Packages during Shelf Life
Figure 2 shows the changes in the O2 and CO2 concentrations in the packages during storage. The effect of UV treatment on the gas composition inside the package was evident. In high transmission rate packages, the gas composition varied similarly for UV1 and UV2 treated fruit at higher CO2 and lower O2 concentrations, respectively, while it differed from controls (Figure 2A). These differences are due to the higher respiration measured for the UV treatments. Photo-oxidation reactions in plants trigger free radical and superoxide production and may accelerate senescence [56]. In low gas transmission packages (MAP2; Figure 2B) O2 and CO2 concentrations were generally lower and higher, respectively, although the relative changes for these gases were similar. The more restrictive gas composition inside the packages, mainly at the end of the fruit shelf life, may lead to anaerobic metabolism and fermentative processes resulting in off-flavors and a loss of firmness. Thus, films with higher TR should be used. However, the bactericide effect of UV-C light at 4 kJ m−2 (UV2) avoids the proliferation of anaerobic microorganisms, while achieving suitable conditions for raspberry preservation combined with a film with O2TR of 902 mL d−1 and CO2TR of 785 mL d−1.
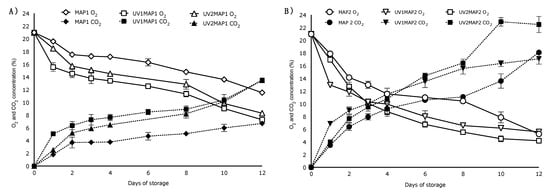
Figure 2.
O2 and CO2 concentrations inside the packages during shelf life. Presented are the means (±S.D.; n = 5) of controls and of raspberry samples, irradiated with UV-C (UV1: 2 kJ m−2; UV2: 4 kJ m−2) and packed using films with two transmission rates (A) MAP1 and (B) MAP2.
Steady state gas compositions were not reached during storage, probably due to a gradual increase in the metabolic activity of the raspberries during storage. The increase in metabolic activity due to physiological stress has been related to the exposure to reduced concentrations of O2 (1% to 10%) and moderate concentrations of CO2 (10% to 15%) in strawberries [58] and raspberries [59], which may intensify the respiration activity in raspberries and, thus, the variation in the gas composition in the headspace. The onset of decay by microorganisms may also increase the respiration activity during storage, as reported in [60].
3.3. Influence of UV-C Radiation, MAP and Combined Treatments on the Microbiological Quality
Rot incidence (Figure 3) increased with the storage time, reaching 58.9% in the control samples and 40.6% and 42.4% in the UV1 and UV2 samples, respectively. Kim and Hung [61] reported similar results in blueberries treated with 4 kJ m−2 of UV-C light, recording approximately 25.0% rotten fruit after only eight days of refrigerated storage. Moreover, the fruit decay was reduced (43.8%) by combining UV-C with 1-MCP [5]; however, this still accounted for 18.0% rotten fruit. Siro et al. [22] reported lower mold development in raspberries packaged in equilibrium-modified atmosphere (3% O2-5% CO2); however, in the present study, MAP1 (36.5%) and UV1MAP1 (39.7%) batches also presented an excessive percentage of rotten fruit. Samples from these batches exhibited severe softening, showing that fruit firmness is correlated with resistance to mold spoilage, mainly to Botrytis cinerea gray mold, which is the predominant cause of raspberry decay [9]. After 12 d of storage, neither MAP2, UV1MAP2, UV2MAP1 nor UV2MAP2 reduced mold incidence below the limits considered acceptable for commercialization. MAP2 and UV2MAP2 showed 15.5% and 10.7%, respectively, rotten fruit at T12. Other authors [8] considered a higher percentage of decay acceptable to claim an increase in the shelf life of raspberries. However, considering the incidence of rot obtained in the present study, the raspberries’ shelf life was set at 8 d. In our opinion, considering the temperature of storage (6 °C), preserving this commodity during this period is a remarkable achievement.
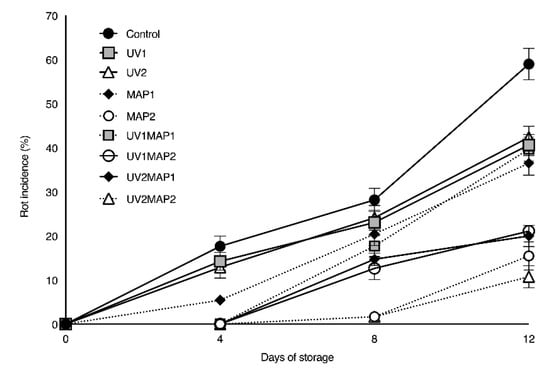
Figure 3.
Evolution of rot incidence during shelf life of red raspberries stored in air (control), treated with UV-C (2 KJ m−2 (UV1) or 4 KJ m−2 (UV2)), packaged in MAP (MAP1 or MAP2) and their combinations. Values represent mean ± standard deviation (n = 5 packages per batch and control day).
Although reduced mold spoilage in UV-C treated fruit has been previously reported, the experimental results demonstrated that the application of 2 and 4 kJ m−2 alone does not delay fungal growth. However, combined treatments showed a greater lag period delaying the appearance of visual mold spoilage by 4 d. MAP2 and UV2MAP2 samples had just 1.7% rotten fruit on day 8 due to the high CO2 concentrations (10% and 16%) inside the packages. A similar behavior should have been noticed in UV1MAP2; however, the raspberries from this batch showed 12.6% decay. MAP2 as well as MAP1 and UV1MAP2 samples had 1 log unit higher total yeast counts than the controls. Total yeast counts also increased two-fold during storage (data not shown), probably related to lower O2 and higher CO2 concentrations due to the film transmission rate.
On the other hand, total yeast counts of UV2MAP2 samples increased by only 0.3 log unit compared to controls at the end of the shelf life. UV-C irradiation has been shown to reduce rot incidences and total microbial loads in blueberries and table grapes, increasing their resistance against pathogens [3,5]. However, the effectiveness depends on the characteristics of the fruit surface [15]. This is relatively irregular in raspberries due to the aggregated drupelets, which makes UV-C light a less effective technology. Thus, it is necessary to combine it with other strategies to delay microbial spoilage during raspberry preservation. Mesophilic aerobic bacteria counts (data not shown) did not reveal much relevant information. Although there are no microbiological criteria available regarding food safety for whole fresh berries, the microbial loads of treated samples closely reflected those of the controls throughout the study without exceeding 4 log CFU cm−2, which may be considered an acceptable limit for their consumption.
3.4. Influence of UV-C Radiation, MAP and Combined Treatments on the Physicochemical Parameters
Table 1 shows that the initial increase in lightness was followed by its decrease in all the samples during storage. This may be related to the accumulation of anthocyanins [27,28], which agrees with the total anthocyanin concentration and with the results obtained by Xu and Liu [5]. The redness (a*) and yellowness (b*) decreased, as observed in various berry fruit [62]; however, these results differed from those obtained from UV-C treated strawberries [26], suggesting the influence of the different anthocyanins accumulated by the various fruits. However, the UV-C treatments did not obviously affect the color of the samples [5]. The lightness of raspberries packaged in MAP2, UV1, UV1MAP1 and UV2MAP1 were higher after simulated shelf life, which may increase customer acceptance [27]. Besides reducing mold development, modified atmospheres maintained a brighter color compared with the control samples [7,24].

Table 1.
Influence of UV radiation, modified atmosphere packaging conditions and combined treatments on the physicochemical quality of raspberries during storage.
The total soluble solids (TSS) (Table 1) decreased in samples of all the batches while varying significantly among samples on each day of analysis. This was especially valid for UV2, MAP2, UV2MAP1 and UV2MAP2 treated raspberries, in which TSS content sharply diminished after day 4. However, raspberries treated with UV1 maintained a higher TSS content until the end of their shelf life. Contrary to the results obtained by Haffner et al. [24], MAP-stored raspberries had lower TSS, which pronouncedly declined on day 4 and day 8 for MAP2 and MAP1 samples, respectively. Significant differences were observed in the pH of the samples on each day of analysis, and also during fruit shelf life. However, these changes were not attributed to the effect of the applied treatments but were assumed to result from the variability of the samples [63,64]. Although fluctuations in TSS and titratable acidity (TA) may be related with fruit dehydration during storage, the acidity decreased from day 4 on, mainly in the control and UV-C treated samples. Non-treated blueberries cultivar ‘Duke’ have also been reported to have lower acidity than those treated with UV light [65].
The ratio of TSS:TA is called the maturity index (MI). It may give a general idea of the degree of fruit senescence because it has been inversely correlated with other quality parameters, such as fruit firmness [65,66]. From day 8, UV treated raspberries showed a higher MI than MAP or UVMAP samples, and a lower MI than the controls. This may indicate that the fruit irradiated with UV-C had a shorter period of storage and in sale. On the other hand, several factors influence the changes in TSS and TA during postharvest preservation, and it is not possible to determine the influence of UV-C radiation alone [67,68].
Raspberry firmness decreased during storage; however, modified atmosphere and combined treatments preserved fruit texture (Table 1). Among them, MAP2 and UV2MAP2 samples maintained higher firmness, which declined by 6.5% and 6.7% at the end of the study, respectively. On the other hand, in the controls and the UV1 and UV2 samples, firmness declined by 44.6%, 37.1% and 57.1%, respectively, indicating that irradiation with 4 kJ m−2 may have accelerated the metabolic activity of the fruit. Fruit firmness was also reduced (37.5%) in raspberries treated with gamma-rays [8]. UV-C inhibits the activity of cell wall degradation enzymes, such as pectinmethylesterase, polygalacturonase and cellulase, during storage [69]. However, the effects of UV-C radiation are dose-dependent (hormesis) and also may be conditioned by the type of fruit. Thus, excessive doses for raspberries, considering our results, maintained higher firmness in strawberries (4.1 kJ m−2) [26,70], mangos (11.7 kJ m−2) [71], and tomatoes (3.7 and 4.2 kJ m−2) [68,72]. The higher firmness may also be closely related with the stimulation of phenylalanine ammonia lyase (PAL) activity, a key enzyme in the synthesis of phenolic compounds and lignin-like polymers. Compounds from the phenylpropanoid pathway act as structural elements and their accumulation reinforces the physical resistance of the cell wall, making it less susceptible to degradation [72]. Consistent with this statement, the UV1 and UV2 treated samples had significantly higher total phenolic content (TPC) compared to the controls on T0 and T4. However, the TPC decreased during the experimental period, with the TPC of the UV2 treated raspberries being markedly lower than that of the control fruit at the end of the study, resulting in a loss of fruit firmness.
The mass losses of the controls and the UV treated raspberries reached 6.9% and 8.1%, respectively (Figure 4); thus, the macroperforated trays were not able to reduce water vapor exchange through the packages, which decreases the attractiveness of samples. Mass loss (1.0%) was satisfactorily reduced in MAP and UVMAP samples due to the reduced water vapor transmission of the MAP packages. Similar values were obtained by Haffner et al. [24] after 7 d of storage.
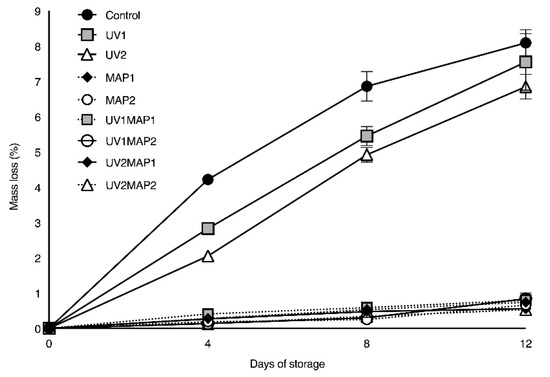
Figure 4.
Mass loss during shelf life of red raspberries stored in air (control), treated with UV-C radiation (UV1 or UV2) and packaged in MAP (MAP1 or MAP2). Values represent mean ± standard deviation (n = 5 packages per batch and control day).
3.5. Influence of UV-C Radiation, MAP and Combined Treatments on Bioactive Compounds
Except UV2 and UV1MAP2, the samples of the other treatments maintained the TPC observed in fresh raspberries (Figure 5) during storage. However, the total flavonoid content (TFC) (Figure 6) significantly increased during storage, mainly in combined treatments (106.5–114.7%), and also in UV-C treated samples (86.9% and 72.1%). On the other hand, the flavonoid concentration in the control also increased to 1.5 g kg−1 of catechin equivalents (CE) after 12 d. UV-C treated samples showed higher TFC on the days after treatment because radiation may promote defense mechanisms, triggering the de novo synthesis of anthocyanins [18,27,28]. Both the TFC and total anthocyanin contents (TAC) of different fruits also increased after the application of UV-radiation in various studies [5,26,63,64,73]. As depicted in Figure 7, the anthocyanin content was correlated with TPC. On day 8, UV1 and UV2 treated samples had higher TAC than the controls. UV-C radiation could induce changes both in TFC and TAC during storage as a defense mechanism [5,27,63,64]. Anthocyanins contribute to the berries’ color; thus, applying UV-radiation, which is involved in the complicated process of their biosynthesis, may maintain fruit appearance.
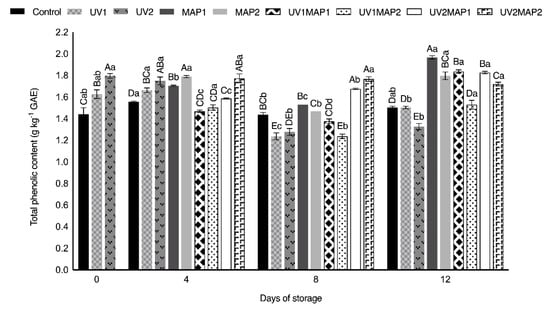
Figure 5.
Total phenolic compounds (TPC) of raspberries stored in air (control), treated with UV-C radiation (UV1 or UV2), packaged in MAP (MAP1 or MAP2) and their combinations during 12 d of storage. Values represent mean ± standard deviation (n = 3). Lower case letters indicate significant differences (p < 0.05) among days within the same batch. Capital letters indicate significant differences (p < 0.05) among batches within the same day.
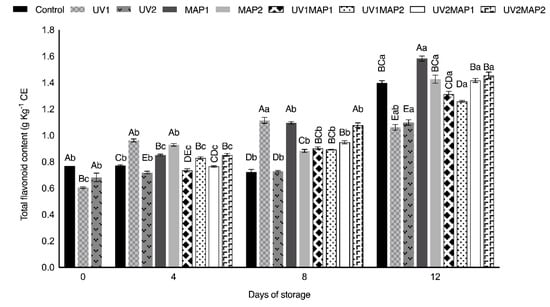
Figure 6.
Total flavonoid content (TFC) of raspberries stored in air (control), treated with UV-C radiation (UV1 or UV2), packaged in MAP (MAP1 or MAP2) and their combinations during 12 d of storage. Values represent mean ± standard deviation (n = 3). Lower case letters indicate significant differences (p < 0.05) among days within the same batch. Capital letters indicate significant differences (p < 0.05) among batches within the same day.
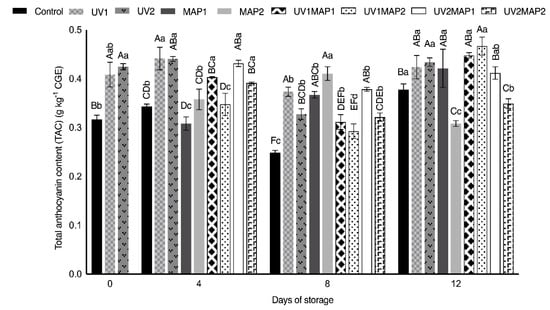
Figure 7.
Concentration of total anthocyanins of raspberries stored in air (control), treated with UV-C radiation (UV1 or UV2), packaged in MAP (MAP1 or MAP2) and their combinations during 12 d of storage. Values represent mean ± standard deviation (n = 3). Lower case letters indicate significant differences (p < 0.05) among days within the same batch. Capital letters indicate significant differences (p < 0.05) among batches within the same day.
MAP has a positive effect on the total phenolic compound content. In MAP1 and MAP2 samples, the TPC concentrations increased by 36.8% and 25.0%, respectively, during storage. The controls also showed higher TPC at the end of their shelf life; however, this effect may be attributed to their considerable mass loss. Moreover, the anthocyanin content of MAP1 samples increased during the study, reaching 0.42 g kg−1 of cyanidin 3-O-glucoside equivalents (CGE). Briano et al. [7] demonstrated that the phenolic and anthocyanin contents and the antioxidant capacity in ‘Grandeur’ raspberries packaged with different films increased, establishing a correlation between polyphenols and antioxidant activity. An increase in the anthocyanin content and antioxidant capacity was also observed in ‘Erika’ raspberries and sea buckthorn drupes packaged under several modified atmospheres when compared with control samples [25,26].
Several authors have reported a correlation between phenolic compounds and antioxidant capacity exhibited by extracts obtained from different plant tissues. For instance, the TPC, TFC and antioxidant capacity of pineapple increased after UV-C treatment [74]. Moreover, positive effects in the antioxidant compounds and shelf life of different green leaves were observed after the application of UV light [75]. The DPPH free radical scavenging activity (Figure 8) and ferric reduction power (Figure 9) increased; however, it did not show good correlation with the TPC and TFC results. The highest Pearson’s correlation coefficient was obtained between FRAP and TPC (0.62) and TFC (0.74), as occurred in coated raspberries [4]. Despite the variability in the results of antioxidant activity determinations, in some cases the differences between the samples were significant. Both FRAP and DPPH scavenging activity significantly increased in all samples during storage. Thus, the antioxidant activity of fresh raspberries was maintained during their storage by combining UV-C and MAP.
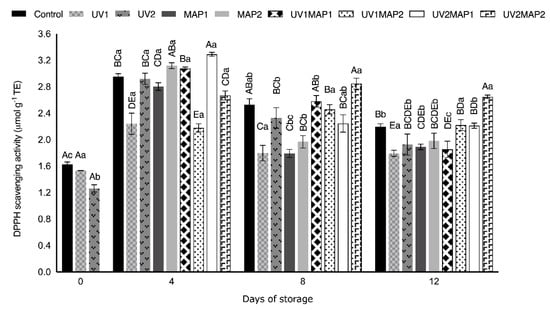
Figure 8.
Antioxidant capacity to inhibit 1,1-diphenil-2-picrilhidrazil (DPPH) free radical of raspberries stored in air (control), treated with UV-C radiation (UV1 or UV2), packaged in MAP (MAP1 or MAP2) and their combinations during 12 d of storage. Values represent mean ± standard deviation (n = 3). Lower case letters indicate significant differences (p < 0.05) among days within the same batch. Capital letters indicate significant differences (p < 0.05) among batches within the same day.
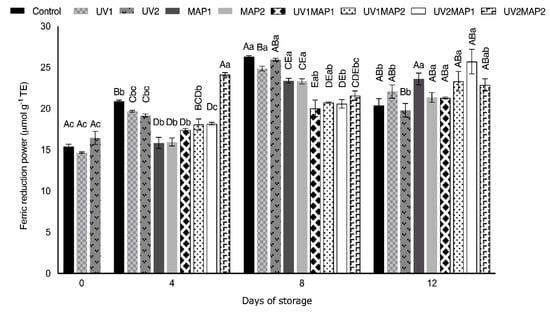
Figure 9.
Ferric reduction antioxidant power (FRAP) of raspberries stored in air (control), treated with UV-C radiation (UV1 or UV2), packaged in MAP (MAP1 or MAP2) and their combinations during 12 d of storage. Values represent mean ± standard deviation (n = 3). Lower case letters indicate significant differences (p < 0.05) among days within the same batch. Capital letters indicate significant differences (p < 0.05) among batches within the same day.
4. Conclusions
This study characterized the parameters involved in quality of red raspberries to evaluate whether the proposed treatments could prolong their storage under refrigeration. The UV-C radiation induced changes in physicochemical and microbiological parameters, and in the concentrations of bioactive compounds. However, a deeper study is needed to comprehend the biochemical processes involved in these changes in order to design other approaches for the applied technologies.
The combination of UV-C radiation and MAP, and not their separate application, improves the quality maintenance of raspberries. However, this is only valid if a suitable MAP film with optimized O2/CO2 permeability effectively controls the gas composition and thus fruit respiration within the packages. This, combined with a pretreatment of 4 kJ m−2 of UV-C radiation, successfully prolongs the shelf life and maintains high contents of bioactive compounds in fruit stored at 6 °C for up to 8 d.
This strategy is appropriate to preserve raspberries and could be an alternative to prolong the shelf life of other perishable fruits. However, further studies will be necessary to optimize the proper combination of UV-C radiation and MAP.
Author Contributions
Conceptualization, D.G., R.O., M.E.V. and E.A.; methodology, D.G., J.G.-B., M.E.V. and E.A.; software, J.G.-B.; validation, D.G., M.E.V. and E.A.; formal analysis, D.G. and J.G.-B.; investigation, D.G. and J.G.-B.; resources, R.O., M.E.V. and E.A.; writing—original draft preparation, D.G. and E.A.; writing—review and editing, D.G., R.O., M.E.V. and E.A.; visualization, D.G., M.E.V. and E.A.; supervision, R.O. and E.A.; project administration, R.O., M.E.V. and E.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Aragon Government and the European Social Found (project A22_20R).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Khanizadeh, S.; Rekika, D.; Ehsani-Moghaddam, B.; Tsao, R.; Yang, R.; Charles, M.T.; Sullivan, J.A.; Gauthier, L.; Gosselin, A.; Potel, A.-M.; et al. Thomas, E. Horticultural Characteristics and Chemical Composition of Advanced Raspberry Lines from Quebec and Ontario. LWT—Food Sci. Technol. 2009, 42, 893–898. [Google Scholar] [CrossRef]
- Barikloo, H.; Ahmadi, E. Shelf Life Extension of Strawberry by Temperatures Conditioning, Chitosan Coating, Modified Atmosphere, and Clay and Silica Nanocomposite Packaging. Sci. Hortic. 2018, 240, 496–508. [Google Scholar] [CrossRef]
- Freitas, P.M.; Lopez-Galvez, F.; Tudela, J.A.; Gil, M.I.; Allende, A. Postharvest Treatment of Table Grapes with Ultraviolet-C and Chitosan Coating Preserves Quality and Increases Stilbene Content. Postharvest Biol. Technol. 2015, 105, 51–57. [Google Scholar] [CrossRef]
- Guerreiro, A.C.; Gago, C.M.L.; Faleiro, M.L.; Miguel, M.G.C.; Antunes, M.D.C. Raspberry Fresh Fruit Quality as Affected by Pectin- and Alginate-Based Edible Coatings Enriched with Essential Oils. Sci. Hortic. 2015, 194, 138–146. [Google Scholar] [CrossRef]
- Xu, F.; Liu, S. Control of Postharvest Quality in Blueberry Fruit by Combined 1-Methylcyclopropene (1-MCP) and UV-C Irradiation. Food Bioprocess Technol. 2017, 10, 1695–1703. [Google Scholar] [CrossRef]
- Elias, M.I.; Madureira, J.; Santos, P.M.P.; Carolino, M.M.; Margaça, F.M.A.; Cabo Verde, S. Preservation Treatment of Fresh Raspberries by E-Beam Irradiation. Innov. Food Sci. Emerg. Technol. 2020, 66, 102487. [Google Scholar] [CrossRef]
- Briano, R.; Giuggioli, N.R.; Girgenti, V.; Peano, C. Biodegradable and Compostable Film and Modified Atmosphere Packaging in Postharvest Supply chain of Raspberry Fruits (cv. Grandeur). J. Food Process Preserv. 2015, 39, 2061–2073. [Google Scholar] [CrossRef]
- Tezotto-Uliana, J.V.; Berno, N.D.; Quispe Saji, F.R.; Kluge, R.A. Gamma Radiation: An Efficient Technology to Conserve the Quality of Fresh Raspberries. Sci. Hortic. 2013, 164, 348–352. [Google Scholar] [CrossRef]
- Forney, C.F.; Jamieson, A.R.; Pennell, K.D.M.; Jordan, M.A.; Fillmore, S.A.E. Relationships between Fruit Composition and Storage Life in Air or Controlled Atmosphere of Red Raspberry. Postharvest Biol. Technol. 2015, 110, 121–130. [Google Scholar] [CrossRef]
- Hirneisen, K.A.; Black, E.P.; Cascarino, J.L.; Fino, V.R.; Hoover, D.G.; Kniel, K.E. Viral Inactivation in Foods: A Review of Traditional and Novel Food-Processing Technologies. Compr. Rev. Food. Sci. Food Saf. 2010, 9, 3–20. [Google Scholar] [CrossRef]
- Janisiewicz, W.J.; Takeda, F.; Glenn, D.M.; Camp, M.J.; Jurick, W.M., II. Dark Period Following UV-C Treatment Enhances Killing of Botrytis Cinerea Conidia and Controls Gray Mold of Strawberries. Phytopathology 2016, 106, 386–394. [Google Scholar] [CrossRef]
- Usall, J.; Ippolito, A.; Sisquella, M.; Neri, F. Physical Treatments to Control Postharvest Diseases of Fresh Fruits and Vegetables. Postharvest Biol. Technol. 2016, 122 (Suppl. I), 30–40. [Google Scholar] [CrossRef]
- Adhikari, A.; Syamaladevi, R.M.; Killinger, K.; Sablani, S.S. Ultraviolet-C Light Inactivation of Escherichia Coli 0157:H7 and Listeria Monocytogenes on Organic Fruit Surfaces. Int. J. Food Microbiol. 2015, 210, 136–142. [Google Scholar] [CrossRef]
- Syamaladevi, R.M.; Lu, X.; Sablani, S.S.; Insan, S.K.; Adhikari, A.; Killinger, K.; Rasco, B.; Dhingra, A.; Bandyopadhyay, A.; Annapure, U. Inactivation of Escherichia Coli Population on Fruit Surfaces Using Ultraviolet-C Light: Influence of Fruit Surface Characteristics. Food Bioprocess Technol. 2013, 6, 2959–2973. [Google Scholar] [CrossRef]
- Syamaladevi, R.M.; Adhikari, A.; Lupien, S.L.; Dugan, F.; Bhunia, K.; Dhingra, A.; Sablani, S.S. Ultraviolet-C Light Inactivation of Penicillium Expansum on Fruit Surfaces. Food Control 2015, 50, 297–303. [Google Scholar] [CrossRef]
- Perkins-Veazie, P.; Collins, J.K.; Howard, L. Blueberry Fruit Response to Postharvest Application of Ultraviolet Radiation. Postharvest Biol. Technol. 2008, 47, 280–285. [Google Scholar] [CrossRef]
- Gonzalez-Barrio, R.; Salmenkallio-Marttila, M.; Tomas-Barberan, F.A.; Cantos, E.; Espin, J.C. Etiology of UV-C-Induced Browning in Var. Superior White Table Grapes. J. Agric. Food Chem. 2005, 53, 5990–5996. [Google Scholar] [CrossRef]
- Rivera-Pastrana, D.M.; Gardea, A.A.; Yahia, E.M.; Martinez-Tellez, M.A.; Gonzalez-Aguilar, G.A. Effect of UV-C Irradiation and Low Temperature Storage on Bioactive Compounds, Antioxidant Enzymes and Radical Scavenging Activity of Papaya Fruit. J. Food Sci. Technol.—MYSORE 2014, 51, 3821–3829. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Teoh, L.S.; Lasekan, O.; Adzahan, N.M.; Hashim, N. The Effect of Combinations of UV-C Exposure with Ascorbate and Calcium Chloride Dips on the Enzymatic Activities and Total Phenolic Content of Minimally Processed Yam Slices. Postharvest Biol. Technol. 2016, 120, 138–144. [Google Scholar] [CrossRef]
- Giuggioli, N.R.; Briano, R.; Baudino, C.; Peano, C. Effects of Packaging and Storage Conditions on Quality and Volatile Compounds of Raspberry Fruits. CyTA—J. Food 2015, 13, 512–521. [Google Scholar] [CrossRef]
- Giuffre, A.M.; Louadj, L.; Rizzo, P.; Poiana, M.; Sicari, V. Packaging and Storage Condition Affect the Physicochemical Properties of Red Raspberries (Rubus Idaeus L., Cv. Erika). Food Control 2019, 97, 105–113. [Google Scholar] [CrossRef]
- Siro, I.; Devlieghere, F.; Jacxsens, L.; Uyttendaele, M.; Debevere, J. The Microbial Safety of Strawberry and Raspberry Fruits Packaged in High-Oxygen and Equilibrium-Modified Atmospheres Compared to Air Storage. Int. J. Food Sci. Technol. 2006, 41, 93–103. [Google Scholar] [CrossRef]
- Arias, E.; Gonzalez, J.; Peiro, J.M.; Oria, R.; Lopez-Buesa, P. Browning Prevention by Ascorbic Acid and 4-Hexylresorcinol: Different Mechanisms of Action on Polyphenol Oxidase in the Presence and in the Absence of Substrates. J. Food Sci. 2007, 72, C464–C470. [Google Scholar] [CrossRef]
- Haffner, K.; Rosenfeld, H.J.; Skrede, G.; Wang, L.X. Quality of Red Raspberry Rubus Idaeus L. Cultivars after Storage in Controlled and Normal Atmospheres. Postharvest Biol. Technol. 2002, 24, 279–289. [Google Scholar] [CrossRef]
- Giovanelli, G.; Limbo, S.; Buratti, S. Effects of New Packaging Solutions on Physico-Chemical, Nutritional and Aromatic Characteristics of Red Raspberries (Rubus Idaeus L.) in Postharvest Storage. Postharvest Biol. Technol. 2014, 98, 72–81. [Google Scholar] [CrossRef]
- Li, D.; Luo, Z.; Mou, W.; Wang, Y.; Ying, T.; Mao, L. ABA and UV-C Effects on Quality, Antioxidant Capacity and Anthocyanin Contents of Strawberry Fruit (Fragaria Ananassa Duch.). Postharvest Biol. Technol. 2014, 90, 56–62. [Google Scholar] [CrossRef]
- Stavang, J.A.; Freitag, S.; Foito, A.; Verrall, S.; Heide, O.M.; Stewart, D.; Sonsteby, A. Raspberry Fruit Quality Changes during Ripening and Storage as Assessed by Colour, Sensory Evaluation and Chemical Analyses. Sci. Hortic. 2015, 195, 216–225. [Google Scholar] [CrossRef]
- Wang, S.Y.; Chen, C.-T.; Wang, C.Y. The Influence of Light and Maturity on Fruit Quality and Flavonoid Content of Red Raspberries. Food Chem. 2009, 112, 676–684. [Google Scholar] [CrossRef]
- Krueger, E.; Dietrich, H.; Schoepplein, E.; Rasim, S.; Kuerbel, P. Cultivar, Storage Conditions and Ripening Effects on Physical and Chemical Qualities of Red Raspberry Fruit. Postharvest Biol. Technol. 2011, 60, 31–37. [Google Scholar] [CrossRef]
- Teng, H.; Fang, T.; Lin, Q.; Song, H.; Liu, B.; Chen, L. Red Raspberry and Its Anthocyanins: Bioactivity beyond Antioxidant Capacity. Trends Food Sci. Technol. 2017, 66, 153–165. [Google Scholar] [CrossRef]
- Butot, S.; Cantergiani, F.; Moser, M.; Jean, J.; Lima, A.; Michot, L.; Putallaz, T.; Stroheker, T.; Zuber, S. UV-C Inactivation of Foodborne Bacterial and Viral Pathogens and Surrogates on Fresh and Frozen Berries. Int. J. Food Microbiol. 2018, 275, 8–16. [Google Scholar] [CrossRef]
- Selcuk, N.; Erkan, M. The Effects of Modified and Palliflex Controlled Atmosphere Storage on Postharvest Quality and Composition of ‘Istanbul’ Medlar Fruit. Postharvest Biol. Technol. 2015, 99, 9–19. [Google Scholar] [CrossRef]
- Wang, L.; Luo, Z.; Li, J.; Yang, M.; Yan, J.; Lu, H.; Li, D.; Chen, C.; Aghdam, M.S.; Wu, B.; et al. Morphological and Quality Characterization of Grape Berry and Rachis in Response to Postharvest 1-Methylcyclopropene and Elevated Oxygen and Carbon Dioxide Atmospheres. Postharvest Biol. Technol. 2019, 153, 107–117. [Google Scholar] [CrossRef]
- Kou, L.; Luo, Y.; Ingram, D.T.; Yan, S.; Jurick, W.M., II. Open-Refrigerated Retail Display Case Temperature Profile and Its Impact on Product Quality and Microbiota of Stored Baby Spinach. Food Control 2015, 47, 686–692. [Google Scholar] [CrossRef]
- Morelli, E.; Noel, V.; Rosset, P.; Poumeyrol, G. Performance and Conditions of Use of Refrigerated Display Cabinets among Producer/Vendors of Foodstuffs. Food Control 2012, 26, 363–368. [Google Scholar] [CrossRef]
- Nunes, M.C.N.; Emond, J.P.; Rauth, M.; Dea, S.; Chau, K.V. Environmental Conditions Encountered during Typical Consumer Retail Display Affect Fruit and Vegetable Quality and Waste. Postharvest Biol. Technol. 2009, 51, 232–241. [Google Scholar] [CrossRef]
- Paula Junqueira-Goncalves, M.; Alarcon, E.; Niranjan, K. The Efficacy of Potassium Sorbate-Coated Packaging to Control Postharvest Gray Mold in Raspberries, Blackberries and Blueberries. Postharvest Biol. Technol. 2016, 111, 205–208. [Google Scholar] [CrossRef]
- Der Steen, C.; Jacxsens, L.; Devlieghere, F.; Debevere, J. Combining High Oxygen Atmospheres with Low Oxygen Modified Atmosphere Packaging to Improve the Keeping Quality of Strawberries and Raspberries. Postharvest Biol. Technol. 2002, 26, 49–58. [Google Scholar] [CrossRef]
- Morales, M.L.; Callejon, R.M.; Ubeda, C.; Guerreiro, A.; Gago, C.; Miguel, M.G.; Antunes, M.D. Effect of Storage Time at Low Temperature on the Volatile Compound Composition of Sevillana and Maravilla Raspberries. Postharvest Biol. Technol. 2014, 96, 128–134. [Google Scholar] [CrossRef]
- Wang, J.-F.; Ma, L.; Xi, H.-F.; Wang, L.-J.; Li, S.-H. Resveratrol Synthesis under Natural Conditions and after UV-C Irradiation in Berry Skin Is Associated with Berry Development Stages in ‘Beihong’ (V. Vinifera × V. Amurensis). Food Chem. 2015, 168, 430–438. [Google Scholar] [CrossRef]
- Gonzalez-Buesa, J.; Ferrer-Mairal, A.; Oria, R.; Salvador, M.L. A Mathematical Model for Packaging with Microperforated Films of Fresh-Cut Fruits and Vegetables. J. Food Eng. 2009, 95, 158–165. [Google Scholar] [CrossRef]
- Gonzalez-Buesa, J.; Salvador, M.L. An Arduino-Based Low Cost Device for the Measurement of the Respiration Rates of Fruits and Vegetables. Comput. Electron. Agric. 2019, 162, 14–20. [Google Scholar] [CrossRef]
- AENOR. UNE 48103:2014. Pinturas y Barnices. Colores Normalizados; AENOR: Madrid, Spain, 2014. [Google Scholar]
- Lei, Z.; Jervis, J.; Helm, R.F. Use of Methanolysis for the Determination of Total Ellagic and Gallic Acid Contents of Wood and Food Products. J. Agric. Food Chem. 2001, 49, 1165–1168. [Google Scholar] [CrossRef]
- Newsome, A.G.; Li, Y.; van Breemen, R.B. Improved Quantification of Free and Ester-Bound Gallic Acid in Foods and Beverages by UHPLC-MS/MS. J. Agric. Food Chem. 2016, 64, 1326–1334. [Google Scholar] [CrossRef]
- Perez-Jimenez, J.; Arranz, S.; Tabernero, M.; Diaz-Rubio, M.E.; Serrano, J.; Goni, I.; Saura-Calixto, F. Updated Methodology to Determine Antioxidant Capacity in Plant Foods, Oils and Beverages: Extraction, Measurement and Expression. Food Res. Int. 2008, 41, 274–285. [Google Scholar] [CrossRef]
- Perez-Ramirez, I.F.; Reynoso-Camacho, R.; Saura-Calixto, F.; Perez-Jimenez, J. Comprehensive Characterization of Extractable and Nonextractable Phenolic Compounds by High-Performance Liquid Chromatography-Electrospray Ionization-Quadrupole Time-of-Flight of a Grape/Pomegranate Pomace Dietary Supplement. J. Agric. Food Chem. 2018, 66, 661–673. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Iacopini, P.; Camangi, F.; Stefani, A.; Sebastiani, L. Antiradical Potential of Ancient Italian Apple Varieties of Malus x Domestica Borkh. in a Peroxynitrite-Induced Oxidative Process. J. Food Compos. Anal. 2010, 23, 518–524. [Google Scholar] [CrossRef]
- Gu, C.; Howell, K.; Dunshea, F.R.; Suleria, H.A.R. LC-ESI-QTOF/MS Characterisation of Phenolic Acids and Flavonoids in Polyphenol-Rich Fruits and Vegetables and Their Potential Antioxidant Activities. Antioxidants 2019, 8, 405. [Google Scholar] [CrossRef]
- Llorach, R.; Tomas-Barberan, F.A.; Ferreres, F. Lettuce and Chicory Byproducts as a Source of Antioxidant Phenolic Extracts. J. Agric. Food Chem. 2004, 52, 5109–5116. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of antioxidant Power: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Dekazos, E.D. Anthocyanin Pigments in Red Tart Cherries. J. Food Sci. 1970, 35, 237–241. [Google Scholar] [CrossRef]
- Peelman, N.; Ragaert, P.; De Meulenaer, B.; Adons, D.; Peeters, R.; Cardon, L.; Van Impe, F.; Devlieghere, F. Application of Bioplastics for Food Packaging. Trends Food Sci. Technol. 2013, 32, 128–141. [Google Scholar] [CrossRef]
- Hardenburg, R.E.; Watada, A.E.; Wang, C.Y. The Commercial Storage of Fruits, Vegetables, and Florist and Nursery Stocks; Unite States Department of Agricultura (USDA): San Jose, Costa Rica, 1986.
- Allende, A.; Marin, A.; Buendia, B.; Tomas-Barberan, F.; Gil, M.I. Impact of Combined Postharvest Treatments (UV-C Light, Gaseous O-3, Superatmospheric O-2 and High CO2) on Health Promoting Compounds and Shelf-Life of Strawberries. Postharvest Biol. Technol. 2007, 46, 201–211. [Google Scholar] [CrossRef]
- Maharaj, R.; Arul, J.; Nadeau, P. Effect of Photochemical Treatment in the Preservation of Fresh Tomato (Lycopersicon esculentum cv. Capello) by Delaying Senescence. Postharvest Biol. Technol. 1999, 15, 13–23. [Google Scholar] [CrossRef]
- Alamar, M.C.; Collings, E.; Cools, K.; Terry, L.A. Impact of Controlled Atmosphere Scheduling on Strawberry and Imported Avocado Fruit. Postharvest Biol. Technol. 2017, 134, 76–86. [Google Scholar] [CrossRef]
- Gonzalez-Orozco, B.D.; Mercado-Silva, E.M.; Castano-Tostado, E.; Vazquez-Barrios, M.E.; Rivera-Pastrana, D.M. Effect of Short-Term Controlled Atmospheres on the Postharvest Quality and Sensory Shelf Life of Red Raspberry (Rubus Idaeus L.). CyTA—J. Food 2020, 18, 352–358. [Google Scholar] [CrossRef]
- Fonseca, S.C.; Oliveira, F.A.R.; Brecht, J.K. Modelling Respiration Rate of Fresh Fruits and Vegetables for Modified Atmosphere Packages: A Review. J. Food Eng. 2002, 52, 99–119. [Google Scholar] [CrossRef]
- Kim, C.; Hung, Y.-C. Inactivation of E. Coli O157:H7 on Blueberries by Electrolyzed Water, Ultraviolet Light, and Ozone. J. Food Sci. 2012, 77, M206–M211. [Google Scholar] [CrossRef] [PubMed]
- Pinto, L.; Palma, A.; Cefola, M.; Pace, B.; D’Aquino, S.; Carboni, C.; Baruzzi, F. Effect of Modified Atmosphere Packaging (MAP) and Gaseous Ozone Pre-Packaging Treatment on the Physico-Chemical, Microbiological and Sensory Quality of Small Berry Fruit. Food Packag. Shelf Life 2020, 26, 100573. [Google Scholar] [CrossRef]
- Sheng, K.; Shui, S.; Yan, L.; Liu, C.; Zheng, L. Effect of Postharvest UV-B or UV-C Irradiation on Phenolic Compounds and Their Transcription of Phenolic Biosynthetic Genes of Table Grapes. J. Food Sci. Technol.—MYSORE 2018, 55, 3292–3302. [Google Scholar] [CrossRef]
- Sheng, K.; Zheng, H.; Shui, S.; Yan, L.; Liu, C.; Zheng, L. Comparison of Postharvest UV-B and UV-C Treatments on Table Grape: Changes in Phenolic Compounds and Their Transcription of Biosynthetic Genes during Storage. Postharvest Biol. Technol. 2018, 138, 74–81. [Google Scholar] [CrossRef]
- Nguyen, C.T.T.; Kim, J.; Yoo, K.S.; Lim, S.; Lee, E.J. Effect of Prestorage UV-A, -B, and -C Radiation on Fruit Quality and Anthocyanin of ‘Duke’ Blueberries during Cold Storage. J. Agric. Food Chem. 2014, 62, 12144–12151. [Google Scholar] [CrossRef]
- Mayorga-Martínez, A.A.; Olvera-Trejo, D.; Elías-Zúñiga, A.; Parra-Saldívar, R.; Chuck-Hernández, C. Non-destructive Assessment of Guava (Psidium guajava L.) Maturity and Firmness Based on Mechanical Vibration Response. Food Bioproc. Technol. 2016, 9, 1471–1480. [Google Scholar] [CrossRef]
- Pan, Y.-G.; Zu, H. Effect of UV-C Radiation on the Quality of Fresh-Cut Pineapples. Second Sree Conference on Engineering Modelling and Simulation (CEMS 2012). Procedia Eng. 2012, 37, 113–119. [Google Scholar] [CrossRef]
- Cote, S.; Rodoni, L.; Miceli, E.; Concellon, A.; Civello, P.M.; Vicente, A.R. Effect of Radiation Intensity on the Outcome of Postharvest UV-C Treatments. Postharvest Biol. Technol. 2013, 83, 83–89. [Google Scholar] [CrossRef]
- Bu, J.; Yu, Y.; Aisikaer, G.; Ying, T. Postharvest UV-C Irradiation Inhibits the Production of Ethylene and the Activity of Cell Wall-Degrading Enzymes during Softening of Tomato (Lycopersicon Esculentum L.) Fruit. Postharvest Biol. Technol. 2013, 86, 337–345. [Google Scholar] [CrossRef]
- Pan, J.; Vicente, A.R.; Martínez, G.A.; Chaves, A.R.; Civello, P.M. Combined Use of UV-C Irradiation and Heat Treatment to Improve Postharvest Life of Strawberry Fruit. J. Sci. Food Agric. 2004, 84. [Google Scholar] [CrossRef]
- Pristijono, P.; Golding, J.B.; Bowyer, M.C. Postharvest UV-C Treatment, Followed by Storage in a Continuous Low-Level Ethylene Atmosphere, Maintains the Quality of ‘Kensington Pride’ Mango Fruit Stored at 20 °C. Horticulturae 2019, 5, 1. [Google Scholar] [CrossRef]
- Charles, M.T.; Goulet, A.; Arul, J. Physiological Basis of UV-C Induced Resistance to Botrytis Cinerea in Tomato Fruit—IV. Biochemical Modification of Structural Barriers. Postharvest Biol. Technol. 2008, 47, 41–53. [Google Scholar] [CrossRef]
- Shen, Y.; Sun, Y.; Qiao, L.; Chen, J.; Liu, D.; Ye, X. Effect of UV-C Treatments on Phenolic Compounds and Antioxidant Capacity of Minimally Processed Satsuma Mandarin during Refrigerated Storage. Postharvest Biol. Technol. 2013, 76, 50–57. [Google Scholar] [CrossRef]
- Sari, L.K.; Setha, S.; Naradisorn, M. Effect of UV-C Irradiation on Postharvest Quality of ‘Phulae’ Pineapple. Sci. Hortic. 2016, 213, 314–320. [Google Scholar] [CrossRef]
- Gogo, E.O.; Opiyo, A.M.; Hassenberg, K.; Ulrichs, C.; Huyskens-Keil, S. Postharvest UV-C Treatment for Extending Shelf Life and Improving Nutritional Quality of African Indigenous Leafy Vegetables. Postharvest Biol. Technol. 2017, 129, 107–117. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).