The Influence of Organic and Conventional Cultivation Patterns on Physicochemical Property, Enzyme Activity and Microbial Community Characteristics of Paddy Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plot Location and Test Materials
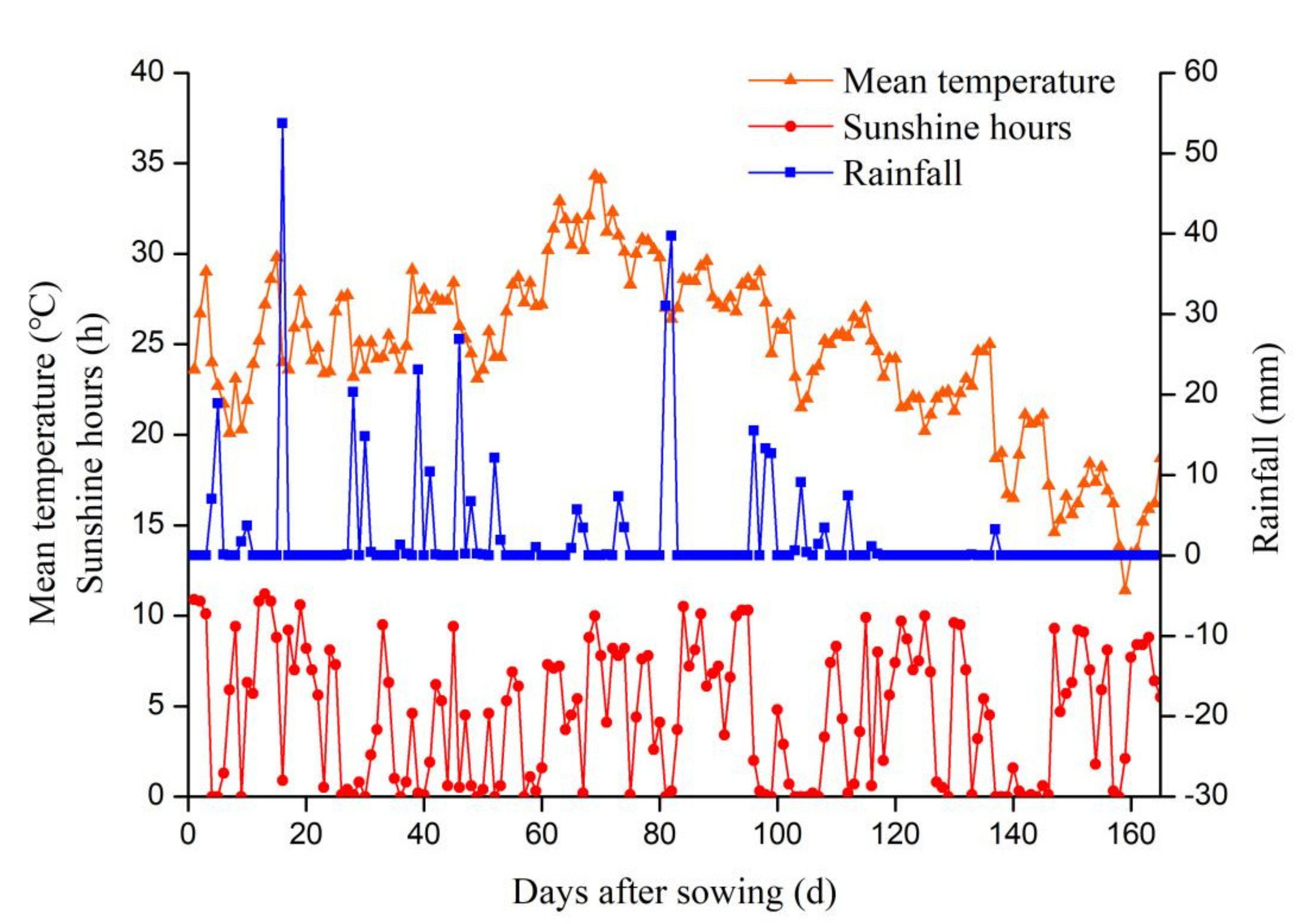
2.2. Experimental Design
2.3. Sample Collection
2.4. Determination of Sample Nutrients and Enzyme Activity
2.5. DNA Extraction, PCR Amplification and High-Throughput Sequencing
2.6. Data Analysis
3. Results
3.1. The Impact of Organic and Conventional Cultivation Patterns on the Physiochemical Property of Topsoil and Undersoil
3.2. The Effect of Organic and Conventional Cultivation Patterns on the Enzyme Activities of the Topsoil and Undersoil
3.3. The Influence of Organic and Conventional Cultivation Patterns on the Microbial Community Characteristics in the Topsoil and Undersoil
3.3.1. α Diversity Analysis
3.3.2. Venn Diagram Analysis Based on OTUs
3.3.3. Analysis of Community Structure Composition
3.3.4. Hierarchical Cluster Analysis
3.4. Correlation Analysis of Physiochemical Property and Enzyme Activity with Microorganisms in Soil
4. Discussion
4.1. The Effects of Both Cultivation Patterns on the Physiochemical Properties and Enzyme Activities of the Topsoil and Undersoil
4.2. The Impact of Both Cultivation Patterns on the Microbial Diversity of the Topsoil and Undersoil
4.3. The Influence of Both Cultivation Patterns on the Composition of Microbial Communities in the Topsoil and Undersoil
4.4. Interrelationship of Nutrient Content, Enzyme Activity and Main Microbial Phyla in the Soil
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sugden, A.M. AGRICULTURAL ECOLOGY: Organic Farming. Science 2001, 293, 17. [Google Scholar] [CrossRef]
- Pankhurst, C.E.; Ophel-Keller, K.; Doube, B.M.; Gupta, V.V.S.R. Biodiversity of soil microbial communities in agricultural systems. Biodivers. Conserv. 1996, 5, 197–209. [Google Scholar] [CrossRef]
- Hartmann, M.; Frey, B.; Mayer, J.; Maeder, P.; Widmer, F. Distinct soil microbial diversity under long-term organic and conventional farming. ISME J. 2015, 9, 1177–1194. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Wang, H.; Feng, Y.; Wang, L.; Xiao, X.; Xi, Y.; Luo, X.; Sun, R.; Ye, X.; Huang, Y.; et al. Consistent responses of the microbial community structure to organic farming along the middle and lower reaches of the Yangtze River. Sci. Rep.-UK 2016, 6, 35046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, H.; Matsushita, Y.; Ito, T.; Nakai, Y.; Nanzyo, M.; Kobayashi, T.; Iwaishi, S.; Hashimoto, T.; Miyashita, S.; Morikawa, T.; et al. Comparative analysis of microbial diversity and bacterial seedling disease-suppressive activity in organic-farmed and standardized commercial conventional soils for rice nursery cultivation. J. Phytopathol. 2018, 166, 249–264. [Google Scholar] [CrossRef]
- Bickel, S.; Or, D. Soil bacterial diversity mediated by microscale aqueous-phase processes across biomes. Nat. Commun. 2020, 11, 116. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Thornton, P.E.; Post, W.M. A global analysis of soil microbial biomass carbon, nitrogen and phosphorus in terrestrial ecosystems. Global Ecol. Biogeogr. 2013, 22, 737–749. [Google Scholar] [CrossRef]
- Fierer, N.; Jackson, R.B. The diversity and biogeography of soil bacterial communities. Proc. Natl. Acad. Sci. USA 2006, 103, 626–631. [Google Scholar] [CrossRef] [Green Version]
- Blume, E.; Bischoff, M.; Reichert, J.M.; Moorman, T.; Konopka, A.; Turco, R.F. Surface and subsurface microbial biomass, community structure and metabolic activity as a function of soil depth and season. Appl. Soil. Ecol. 2002, 20, 171–181. [Google Scholar] [CrossRef]
- Deveau, A.; Bonito, G.; Uehling, J.; Paoletti, M.; Becker, M.; Bindschedler, S.; Hacquard, S.; Herve, V.; Labbe, J.; Lastovetsky, O.A.; et al. Bacterial-fungal interactions: Ecology, mechanisms and challenges. Fems. Microbiol. Rev. 2018, 42, 335–352. [Google Scholar] [CrossRef] [Green Version]
- Gu, Y.; Wang, Y.; Lu, S.; Xiang, Q.; Yu, X.; Zhao, K.; Zou, L.; Chen, Q.; Tu, S.; Zhang, X. Long-term Fertilization Structures Bacterial and Archaeal Communities along Soil Depth Gradient in a Paddy Soil. Front. Microbiol. 2017, 8, 1516. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Xue, D.; Wang, Y.; Zheng, W.; Zhang, G.; Wang, Z. Molecular ecological network analysis of the response of soil microbial communities to depth gradients in farmland soils. Microbiologyopen 2020, 9, e983. [Google Scholar] [CrossRef]
- Ling, Q.; Zhang, H.; Ju, Z.; Dai, Q.; Huo, Z. Effects of different application rates of San’an bio-organic fertilizer on Yield, Quality and Nitrogen Absorption and Utilization of Organic Rice. China Rice 2010, 16, 17–22. [Google Scholar]
- Bao, S. Analysis Method of Soil and Agricultural Chemistry, 3rd ed.; China Agricultural Press: Beijing, China, 2000. [Google Scholar]
- Guan, S. Soil Enzyme and its Study Method; China Agriculture Press: Beijing, China, 1986. [Google Scholar]
- Li, R.; Tao, R.; Ling, N.; Chu, G. Chemical, organic and bio-fertilizer management practices effect on soil physicochemical property and antagonistic bacteria abundance of a cotton field: Implications for soil biological quality. Soil Till. Res. 2017, 167, 30–38. [Google Scholar] [CrossRef]
- Peng, X.; Wang, W. Stoichiometry of soil extracellular enzyme activity along a climatic transect in temperate grasslands of northern China. Soil Biol. Biochem. 2016, 98, 74–84. [Google Scholar] [CrossRef]
- Xun, W.; Zhao, J.; Xue, C.; Zhang, G.; Ran, W.; Wang, B.; Shen, Q.; Zhang, R. Significant alteration of soil bacterial communities and organic carbon decomposition by different long-term fertilization management conditions of extremely low-productivity arable soil in South China. Environ. Microbiol. 2016, 18, 1907–1917. [Google Scholar] [CrossRef]
- Bending, G.D.; Turner, M.K.; Rayns, F.; Marx, M.C.; Wood, M. Microbial and biochemical soil quality indicators and their potential for differentiating areas under contrasting agricultural management regimes. Soil Biol. Biochem. 2004, 36, 1785–1792. [Google Scholar] [CrossRef]
- Li, F.; Chen, L.; Zhang, J.; Yin, J.; Huang, S. Bacterial Community Structure after Long-term Organic and Inorganic Fertilization Reveals Important Associations between Soil Nutrients and Specific Taxa Involved in Nutrient Transformations. Front. Microbiol. 2017, 8, 187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, A.K.; Boral, L.; Tripathi, R.S.; Pandey, H.N. Nitrogen mineralization and microbial biomass-N in a subtropical humid forest of Meghalaya, India. Soil Biol. Biochem. 1997, 29, 1609–1612. [Google Scholar] [CrossRef]
- Bai, J.; Deng, W.; Wang, Q.; Cui, B.; Ding, Q. Spatial distribution of inorganic nitrogen contents of marsh soils in a river floodplain with different flood frequencies from soil-defrozen period. Environ. Monit. Assess. 2007, 134, 421–428. [Google Scholar] [CrossRef]
- Gao, W.; Yang, H.; Kou, L.; Li, S. Effects of nitrogen deposition and fertilization on N transformations in forest soils: A review (vol 15, pg 863, 2015). J. Soil Sediment. 2015, 15, 1233–1234. [Google Scholar] [CrossRef] [Green Version]
- Cai, J.; Luo, W.; Liu, H.; Feng, X.; Zhang, Y.; Wang, R.; Xu, Z.; Zhang, Y.; Jiang, Y. Precipitation-mediated responses of soil acid buffering capacity to long-term nitrogen addition in a semi-arid grassland. Atmos. Environ. 2017, 170, 312–318. [Google Scholar] [CrossRef]
- Taylor, J.P.; Wilson, B.; Mills, M.S.; Burns, R.G. Comparison of microbial numbers and enzymatic activities in surface soils and subsoils using various techniques. Soil. Biol Biochem. 2002, 34, 387–401. [Google Scholar] [CrossRef]
- Lazcano, C.; Gomez-Brandon, M.; Revilla, P.; Dominguez, J. Short-term effects of organic and inorganic fertilizers on soil microbial community structure and function. Biol. Fert. Soils 2013, 49, 723–733. [Google Scholar] [CrossRef]
- Liang, Q.; Chen, H.; Gong, Y.; Yang, H.; Fan, M.; Kuzyakov, Y. Effects of 15 years of manure and mineral fertilizers on enzyme activities in particle-size fractions in a North China Plain soil. Eur. J. Soil Biol. 2014, 60, 112–119. [Google Scholar] [CrossRef]
- Kennedy, A.C.; Smith, K.L. Soil microbial diversity and the sustainability of agricultural soils. Plant Soil 1995, 170, 78–86. [Google Scholar] [CrossRef]
- Innerebner, G.; Knapp, B.; Vasara, T.; Romantschuk, M.; Insam, H. Traceability of ammonia-oxidizing bacteria in compost-treated soils. Soil Biol. Biochem. 2006, 38, 1092–1100. [Google Scholar] [CrossRef]
- Wang, X.; Ma, K.; Wang, Z.; Li, Y.; Wei, C. Effects of no-tillage, mulching and organic fertiliation on soil microbial composition in winter wheat field. Chin. J. Eco-Agric. 2019, 27, 267–276. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Oliverio, A.M.; Brewer, T.E.; Benavent-Gonzalez, A.; Eldridge, D.J.; Bardgett, R.D.; Maestre, F.T.; Singh, B.K.; Fierer, N. A global atlas of the dominant bacteria found in soil. Science 2018, 359, 320. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Wang, H.; Li, X.; Li, X.; Zhang, H. Shifts in bacterial community composition increase with depth in three soil types from paddy fields in China. Pedobiologia 2019, 77, 77. [Google Scholar] [CrossRef]
- Osman, J.R.; Fernandes, G.; DuBow, M.S. Bacterial diversity of the rhizosphere and nearby surface soil of rice (Oryza sativa) growing in the Camargue (France). RHIZOSPHERE 2017, 3, 112–122. [Google Scholar] [CrossRef]
- Chen, D.; Yuan, L.; Liu, Y.; Ji, J.; Hou, H. Long-term application of manures plus chemical fertilizers sustained high rice yield and improved soil chemical and bacterial properties. Eur. J. Agron. 2017, 90, 34–42. [Google Scholar] [CrossRef]
- Zhou, J.; Jiang, X.; Zhou, B.; Zhao, B.; Ma, M.; Guan, D.; Li, J.; Chen, S.; Cao, F.; Shen, D.; et al. Thirty four years of nitrogen fertilization decreases fungal diversity and alters fungal community composition in black soil in northeast China. Soil Biol. Biochem. 2016, 95, 135–143. [Google Scholar] [CrossRef]
- Wang, H.; Wang, S.; Wang, R.; Wang, X.; Li, J. Conservation tillage increased soil bacterial diversity and improved soil nutrient status on the Loess Plateau in China. Arch. Agron. Soil Sci. 2020, 66, 1509–1519. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Polme, S.; Koljalg, U.; Yorou, N.S.; Wijesundera, R.; Villarreal Ruiz, L.; Vasco-Palacios, A.M.; Pham, Q.T.; Suija, A.; et al. Global diversity and geography of soil fungi. Science 2014, 346, 1078. [Google Scholar] [CrossRef] [Green Version]
- Choudhary, M.; Sharma, P.C.; Jat, H.S.; McDonald, A.; Jat, M.L.; Choudhary, S.; Garg, N. Soil biological properties and fungal diversity under conservation agriculture in Indo-Gangetic Plains of India. J. Soil Sci. Plant Nutr. 2018, 18, 1142–1156. [Google Scholar] [CrossRef] [Green Version]
- Ding, J.; Jiang, X.; Guan, D.; Zhao, B.; Ma, M.; Zhou, B.; Cao, F.; Yang, X.; Li, L.; Li, J. Influence of inorganic fertilizer and organic manure application on fungal communities in a long-term field experiment of Chinese Mollisols. Appl. Soil Ecol. 2017, 111, 114–122. [Google Scholar] [CrossRef]
- Wang, J.; Song, Y.; Ma, T.; Raza, W.; Li, J.; Howland, J.G.; Huang, Q.; Shen, Q. Impacts of inorganic and organic fertilization treatments on bacterial and fungal communities in a paddy soil. Appl. Soil Ecol. 2017, 112, 42–50. [Google Scholar] [CrossRef]
- Jiang, Y.; Liang, Y.; Li, C.; Wang, F.; Sui, Y.; Suvannang, N.; Zhou, J.; Sun, B. Crop rotations alter bacterial and fungal diversity in paddy soils across East Asia. Soil Biol. Biochem. 2016, 95, 250–261. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Huo, Y.; Li, H.; Wang, W.; Zhang, A.; Yang, Z. Effect of nitrogen fertilizer reduction on endophytic fungal community composition of summer maize in north China. Trans. Chin. Soc. Agric. Mach. 2018, 49, 312–318. [Google Scholar]
- Fierer, N.; Bradford, M.A.; Jackson, R.B. Toward an ecological classification of soil bacteria. Ecology 2007, 88, 1354–1364. [Google Scholar] [CrossRef]
- Magill, A.H.; Aber, J.D. Variation in soil net mineralization rates with dissolved organic carbon additions. Soil Biol. Biochem. 2000, 32, 597–601. [Google Scholar] [CrossRef] [Green Version]
- Zantua, M.I.; Dumenil, L.C.; Bremner, J.M. Relationships between soil urease activity and other soil properties. Soil Sci. Soc. Am. J. 1977, 41, 350–352. [Google Scholar] [CrossRef]
- Steinweg, J.M.; Dukes, J.S.; Paul, E.A.; Wallenstein, M.D. Microbial responses to multi-factor climate change: Effects on soil enzymes. Front Microbiol. 2013, 4, 146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nahidan, S.; Nourbakhsh, F.; Mosaddeghi, M.R. Variation of soil microbial biomass C and hydrolytic enzyme activities in a rangeland ecosystem: Are slope aspect and position effective? Arch. Agron. Soil Sci. 2015, 61, 797–811. [Google Scholar] [CrossRef]





| Treatment | AN (mg·kg−1) | AP (mg·kg−1) | AK (mg·kg−1) | SOM (g·kg−1) | TN (g·kg−1) | pH |
|---|---|---|---|---|---|---|
| CF_S | 164.50 ± 0.40 Bb | 6.55 ± 0.05 Bb | 96.42 ± 0.57 Bb | 38.69 ± 0.26 Bb | 2.25 ± 0.01 Aa | 8.40 ± 0.01 Aab |
| OF_S | 170.10 ± 0.57 Aa | 8.06 ± 0.05 Aa | 104.40 ± 0.58 Aa | 42.78 ± 0.27 Aa | 2.11 ± 0.01 Bb | 8.31 ± 0.02 Ab |
| CF_X | 91.00 ± 0.48 Cd | 4.72 ± 0.11 Dd | 88.44 ± 0.57 Cd | 20.48 ± 0.38 Dd | 1.38 ± 0.01 Cc | 8.69 ± 0.01 Aa |
| OF_X | 93.10 ± 0.37 Cc | 5.46 ± 0.08 Cc | 91.42 ± 0.58 Cc | 26.85 ± 0.19 Cc | 1.24 ± 0.02 Dd | 8.53 ± 0.01 Aab |
| Treatment | Chao1 | Coverage | Evenness | Shannon | Simpson | |
|---|---|---|---|---|---|---|
| Bacteria | OF_S | 5403.12 | 0.965715 | 0.826086 | 10.0863 | 0.991817 |
| OF_X | 4756.5 | 0.978876 | 0.889906 | 10.7828 | 0.998627 | |
| CF_S | 4684.5 | 0.982093 | 0.892017 | 10.8153 | 0.998645 | |
| CF_X | 3742.45 | 0.984714 | 0.876004 | 10.3057 | 0.997565 | |
| Fungi | OF_S | 720.282 | 0.999896 | 0.732942 | 6.95595 | 0.965398 |
| OF_X | 557.984 | 0.999977 | 0.763078 | 6.96222 | 0.975632 | |
| CF_S | 850.795 | 0.999777 | 0.782208 | 7.60819 | 0.985116 | |
| CF_X | 506.321 | 0.999864 | 0.733932 | 6.58871 | 0.970523 | |
| Phylum | AN | AP | AK | SOM | TN | pH | Sucrase | Urease | |
|---|---|---|---|---|---|---|---|---|---|
| Bacteria | Proteobacteria | −0.011 | 0.554 | −0.416 | −0.107 | 0.140 | 0.128 | 0.131 | −0.248 |
| Acidobacteria | 0.573 | 0.891 ** | 0.142 | 0.401 | 0.720 ** | −0.229 | 0.377 | 0.050 | |
| Chloroflexi | −0.762 ** | −0.559 | −0.641 * | −0.606 * | −0.804 ** | 0.388 | −0.234 | −0.247 | |
| Bacteroidetes | −0.644 * | −0.053 | −0.863 ** | −0.813 ** | −0.475 | 0.739 ** | −0.813 * | −0.961 ** | |
| Thaumarchaeota | 0.608 * | −0.036 | 0.869 ** | 0.675 * | 0.469 | −0.564 | 0.406 | 0.674 | |
| Fungi | Ascomycota | −0.756 ** | −0.418 | −0.755 ** | −0.878 ** | −0.660 * | 0.769 ** | −0.991 ** | −0.935 ** |
| Basidiomycota | 0.739 ** | 0.333 | 0.776 ** | 0.654 * | 0.712 ** | −0.463 | 0.268 | 0.403 | |
| Mucoromycota | −0.402 | 0.066 | −0.619 * | −0.623 * | −0.231 | 0.620 * | −0.790 * | −0.888 ** | |
| Chytridiomycota | 0.392 | 0.683 * | 0.035 | 0.141 | 0.560 | 0.028 | −0.102 | −0.298 | |
| Olpidiomycota | 0.852 ** | 0.905 ** | 0.523 | 0.732 ** | 0.933 ** | −0.518 | 0.651 | 0.411 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, C.; Li, Y.; Hu, X.; Zang, Q.; Zhuang, H.; Huang, L. The Influence of Organic and Conventional Cultivation Patterns on Physicochemical Property, Enzyme Activity and Microbial Community Characteristics of Paddy Soil. Agriculture 2022, 12, 121. https://doi.org/10.3390/agriculture12010121
Xu C, Li Y, Hu X, Zang Q, Zhuang H, Huang L. The Influence of Organic and Conventional Cultivation Patterns on Physicochemical Property, Enzyme Activity and Microbial Community Characteristics of Paddy Soil. Agriculture. 2022; 12(1):121. https://doi.org/10.3390/agriculture12010121
Chicago/Turabian StyleXu, Chengyu, Yulin Li, Xue Hu, Qian Zang, Hengyang Zhuang, and Lifen Huang. 2022. "The Influence of Organic and Conventional Cultivation Patterns on Physicochemical Property, Enzyme Activity and Microbial Community Characteristics of Paddy Soil" Agriculture 12, no. 1: 121. https://doi.org/10.3390/agriculture12010121
APA StyleXu, C., Li, Y., Hu, X., Zang, Q., Zhuang, H., & Huang, L. (2022). The Influence of Organic and Conventional Cultivation Patterns on Physicochemical Property, Enzyme Activity and Microbial Community Characteristics of Paddy Soil. Agriculture, 12(1), 121. https://doi.org/10.3390/agriculture12010121





