Biopesticide Evaluation from Lab to Greenhouse Scale of Essential Oils Used against Macrosiphum euphorbiae
Abstract
:1. Introduction
2. Material and Methods
2.1. Biological Material
2.1.1. Plants
2.1.2. Insects
2.2. Chemical Materials and Analysis
2.2.1. Gas Chromatography-Mass Spectrometry (GC-MS)
2.2.2. Compound Identification
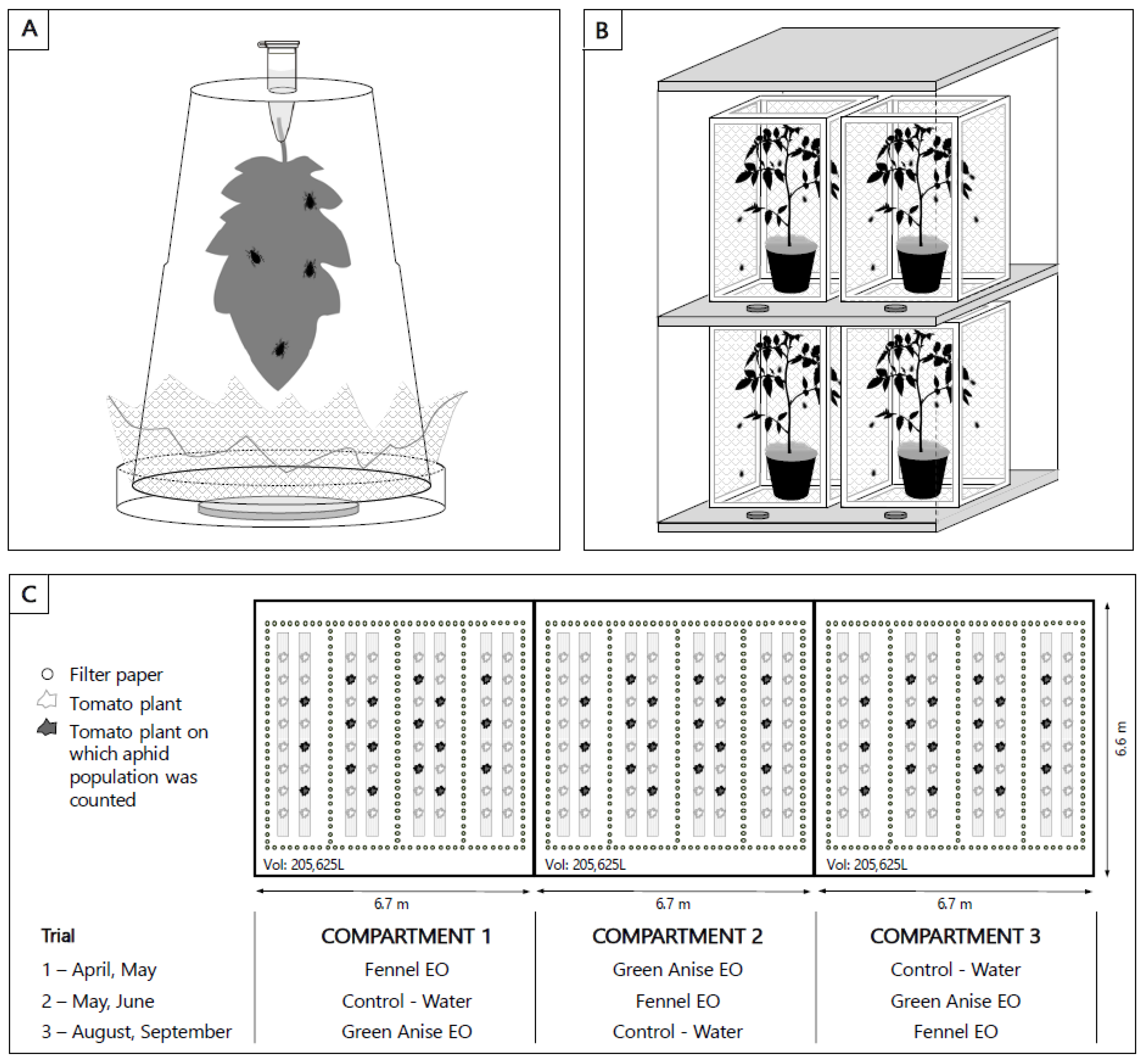
2.3. Experimental Set-Up
2.3.1. Leaflet Bioassays
2.3.2. Plant Experiments
2.3.3. Greenhouse Experiments
2.3.4. Statistical Analysis
3. Results
3.1. Chemical Analysis of the Essential Oil Composition
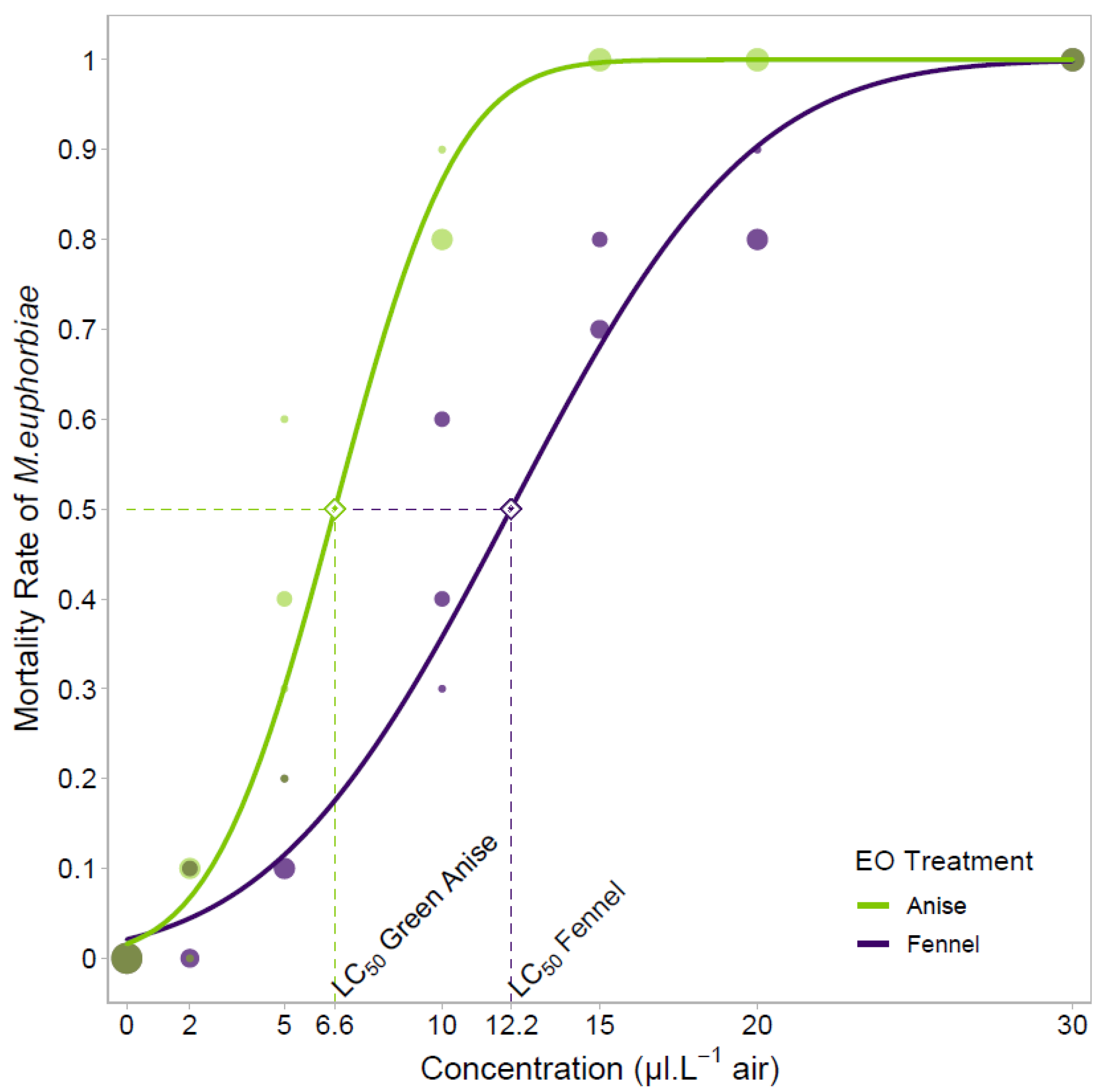
3.2. Fumigation Treatments at Laboratory Scale–Dose-Response Curves and Determination of the LC50
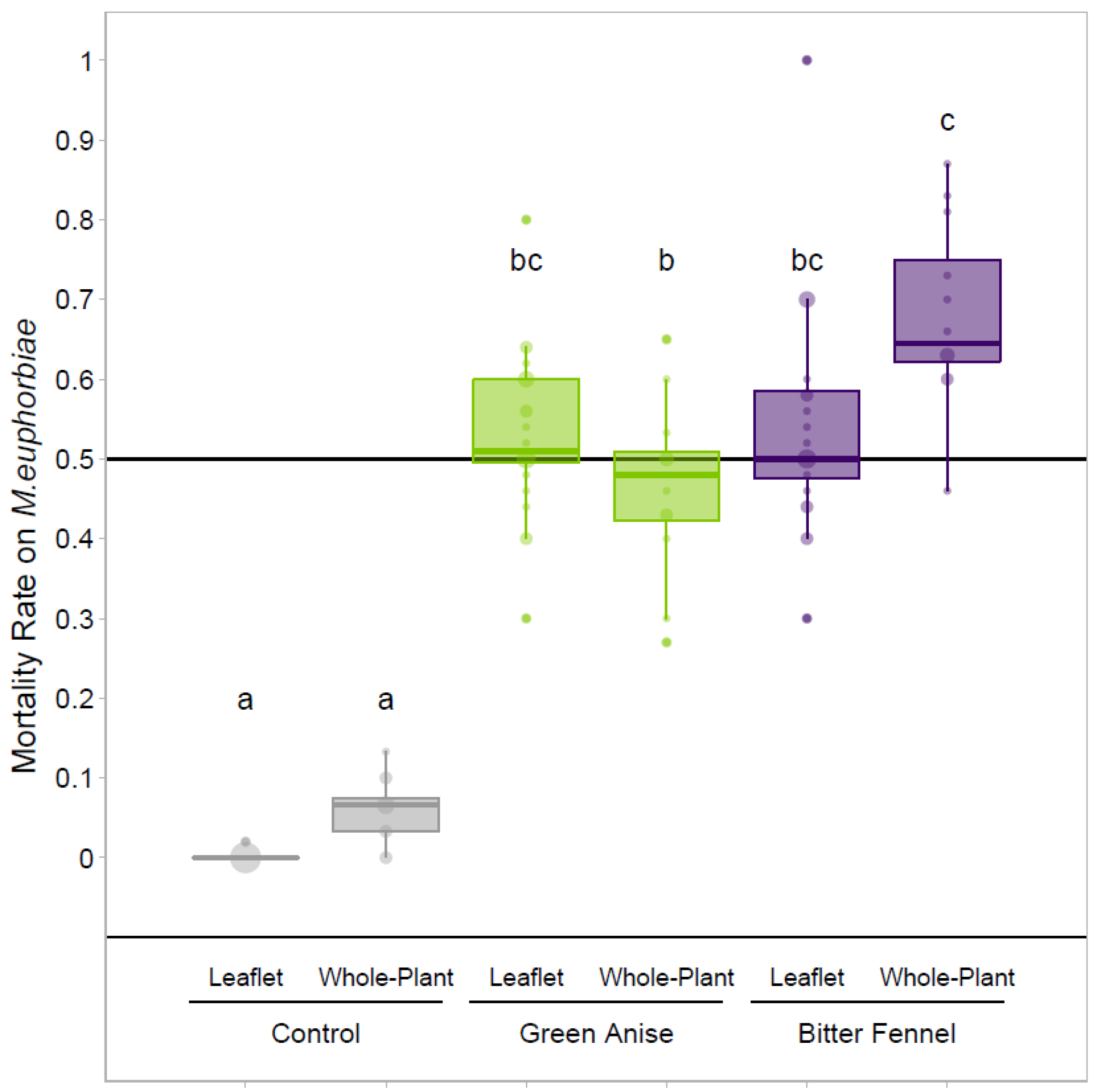
3.3. Fumigation Treatments at the Whole-Plant Scale–Verification of the LC50 at the Whole-Plant Scale
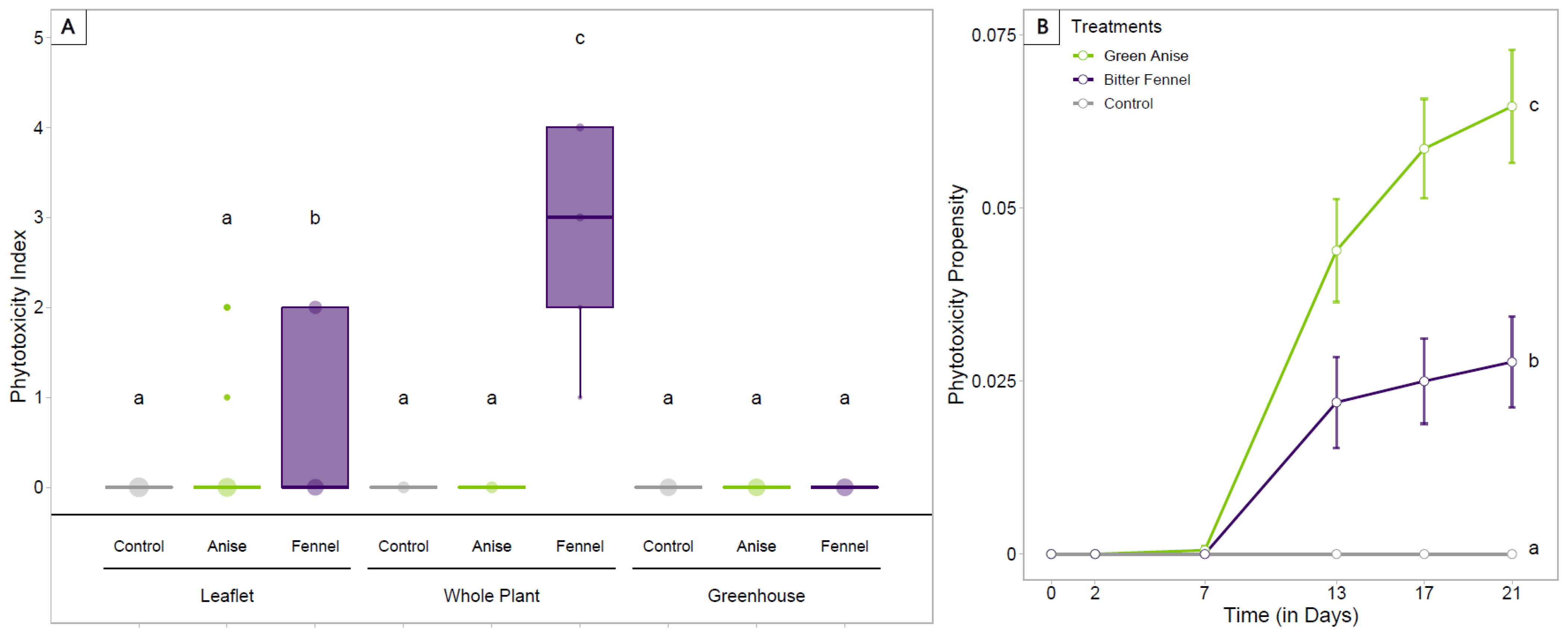
3.4. Fumigation Treatments at the Greenhouse Scale–Population Dynamics
4. Discussion
4.1. Across-Scale Assessment of the Efficiency of EO Treatments
4.2. Relative Efficiency and Variability between Green Anise and Fennel EO Treatments
4.3. Potential Causes of EOs Efficiency Variability
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hullé, M.; Chaubet, B.; Turpeau, E.; Simon, J.C. Encyclop’Aphid: A website on aphids and their natural enemies. Entomol. Gen. 2020, 40, 97–101. [Google Scholar] [CrossRef]
- Blackman, R.L.; Eastop, V.F. Aphids as crop pests. In Aphids as Crop Pests; van Emden, H.F., Harrington, R., Eds.; CABI Publishing: Wallingford, UK, 2007; pp. 1–36. [Google Scholar]
- Isman, M.B. Botanical insecticides: For richer, for poorer. Pest Manag. Sci. 2007, 64, 8–11. [Google Scholar] [CrossRef]
- Desneux, N.; Decourtye, A.; Delpuech, J.-M. The Sublethal Effects of Pesticides on Beneficial Arthropods. Annu. Rev. Ѐntomol. 2007, 52, 81–106. [Google Scholar] [CrossRef]
- Gul, H.; Ullah, F.; Biondi, A.; Desneux, N.; Qian, D.; Gao, X.; Song, D. Resistance against clothianidin and associated fitness costs in the chive maggot, Bradysia odoriphaga. Entomol. Gen. 2019, 39, 81–92. [Google Scholar] [CrossRef]
- Weisenburger, D.D. Human health effects of agrichemical use. Hum. Pathol. 1993, 24, 571–576. [Google Scholar] [CrossRef]
- Decourtye, A.; Henry, M.; Desneux, N. Environment: Overhaul pesticide testing on bees. Nature 2013, 497, 188. [Google Scholar] [CrossRef]
- European Commission Implementing Regulation (EU) 2018/785. Amending Implementing Regulation (EU) No 540/2011 as Regards the Conditions of Approval of the Active Substance Thiamethoxam (Text with EEA Relevance.). Off. J. Eur. Union 2018, 2018, 40–44. [Google Scholar]
- Seiber, J.N.; Coats, J.; Duke, S.O.; Gross, A. Biopesticides: State of the Art and Future Opportunities. J. Agric. Food Chem. 2014, 62, 11613–11619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damalas, C.A.; Koutroubas, S.D. Current Status and Recent Developments in Biopesticide Use Christos. Agriculture 2018, 8, 13. [Google Scholar] [CrossRef] [Green Version]
- Smith, G.H.; Roberts, J.M.; Pope, T.W. Terpene based biopesticides as potential alternatives to synthetic insecticides for control of aphid pests on protected ornamentals. Crop Prot. 2018, 110, 125–130. [Google Scholar] [CrossRef]
- Pavela, R.; Benelli, G. Essential Oils as Ecofriendly Biopesticides? Challenges and Constraints. Trends Plant Sci. 2016, 21, 1000–1007. [Google Scholar] [CrossRef]
- Burger, P.; Plainfossé, H.; Brochet, X.; Chemat, F.; Fernandez, X. Extraction of Natural Fragrance Ingredients: History Overview and Future Trends. Chem. Biodivers. 2019, 16, e1900424. [Google Scholar] [CrossRef] [PubMed]
- Jankowska, M.; Rogalska, J.; Wyszkowska, J.; Stankiewicz, M. Molecular Targets for Components of Essential Oils in the Insect Nervous System—A Review. Molecules 2018, 23, 34. [Google Scholar] [CrossRef] [Green Version]
- Pavela, R.; Morshedloo, M.R.; Mumivand, H.; Khorsand, G.J.; Karami, A.; Maggi, F.; Desneux, N.; Benelli, G. Phenolic monoterpene-rich essential oils from Apiaceae and Lamiaceae species: Insecticidal activity and safety evaluation on non-target earthworms. Ѐntomol. Gen. 2020, 40, 421–435. [Google Scholar] [CrossRef]
- Sousa, R.M.O.F.; Rosa, J.S.; Oliveira, L.; Cunha, A.; Fernandes-Ferreira, M. Activities of Apiaceae Essential Oils against Armyworm, Pseudaletia unipuncta (Lepidoptera: Noctuidae). J. Agric. Food Chem. 2013, 61, 7661–7672. [Google Scholar] [CrossRef] [PubMed]
- Mossa, A.-T. Green Pesticides: Essential Oils as Biopesticides in Insect-pest Management. J. Environ. Sci. Technol. 2016, 9, 354–378. [Google Scholar] [CrossRef] [Green Version]
- Regnault-Roger, C.; Vincent, C.; Arnason, J.T. Essential Oils in Insect Control: Low-Risk Products in a High-Stakes World. Annu. Rev. Ѐntomol. 2012, 57, 405–424. [Google Scholar] [CrossRef]
- Sousa, R.M.O.; Cunha, A.C.; Fernandes-Ferreira, M. The potential of Apiaceae species as sources of singular phytochemicals and plant-based pesticides. Phytochemistry 2021, 187, 112714. [Google Scholar] [CrossRef]
- Ikbal, C.; Pavela, R. Essential oils as active ingredients of botanical insecticides against aphids. J. Pest Sci. 2019, 92, 971–986. [Google Scholar] [CrossRef]
- Soliman, M.M.M. Phytochemical and toxicological studies of Artemisia L. (Compositae) essential oil against some insect pests. Arch. Phytopathol. Plant Prot. 2007, 40, 128–138. [Google Scholar] [CrossRef]
- Isman, M.B.; Miresmailli, S.; MacHial, C. Commercial Opportunities for Pesticides Based on Plant Essential Oils in Agriculture, Industry and Consumer Products. Phytochem. Rev. 2011, 10, 197–204. [Google Scholar] [CrossRef]
- König, W.A.; Joulain, D.; Hochmuth, D.H. Terpenoïds and Related Constituents of Essential Oils; Library of Mass Finder 2.1. Database. Owners: University of Hamburg, Germany; Robertet, S.A., Ed.; Grasse Hochmuth Scientific Consulting: Hamburg, Germany; Available online: https://massfinder.com/wiki/Terpenoids_Library (accessed on 1 September 2021).
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography-Mass Spectroscopy, 2nd ed.; Allured Publishing Corp.: Carol Stream, IL, USA, 1995; pp. 10–469. [Google Scholar]
- Isman, M.B.; Grieneisen, M.L. Botanical insecticide research: Many publications, limited useful data. Trends Plant Sci. 2014, 19, 140–145. [Google Scholar] [CrossRef]
- Digilio, M.C.; Mancini, E.; Voto, E.; De Feo, V. Insecticide activity of Mediterranean essential oils. J. Plant Interact. 2008, 3, 17–23. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils–A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Sharma, A.; Baek, K.-H. Antibacterial mode of action of Cudrania tricuspidata fruit essential oil, affecting membrane permeability and surface characteristics of food-borne pathogens. Food Control. 2013, 32, 582–590. [Google Scholar] [CrossRef]
- Devi, K.P.; Nisha, S.A.; Sakthivel, R.; Pandian, S.K. Eugenol (an essential oil of clove) acts as an antibacterial agent against Salmonella typhi by disrupting the cellular membrane. J. Ethnopharmacol. 2010, 130, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Pavela, R. Acute, synergistic and antagonistic effects of some aromatic compounds on the Spodoptera littoralis Boisd. (Lep., Noctuidae) larvae. Ind. Crop. Prod. 2014, 60, 247–258. [Google Scholar] [CrossRef]
- Vergnes, S.; Ladouce, N.; Fournier, S.; Ferhout, H.; Attia, F.; Dumas, B. Foliar Treatments with Gaultheria Procumbens Essential Oil Induce Defense Responses and Resistance against a Fungal Pathogen in Arabidopsis. Front. Plant Sci. 2014, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Erb, M.; Veyrat, N.; Robert, C.; Xu, H.; Frey, M.; Ton, J.; Turlings, T.C.J. Indole is an essential herbivore-induced volatile priming signal in maize. Nat. Commun. 2015, 6, 6273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben-Jabeur, M.; Ghabri, E.; Myriam, M.; Hamada, W. Thyme essential oil as a defense inducer of tomato against gray mold and Fusarium wilt. Plant Physiol. Biochem. 2015, 94, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Vallat, A.; Gu, H.; Dorn, S. How rainfall, relative humidity and temperature influence volatile emissions from apple trees in situ. Phytochemistry 2005, 66, 1540–1550. [Google Scholar] [CrossRef] [PubMed]
- Qifan, W.; Jun, S.; Yang, Z.; Wanjun, L. Influence of Environmental Factors on Volatile Organic Compound Emissions from Plywood Tested by a Rapid Detection Method. For. Prod. J. 2017, 67, 120–125. [Google Scholar] [CrossRef]
- Pavela, R.; Sedlák, P. Post-application temperature as a factor influencing the insecticidal activity of essential oil from Thymus vulgaris. Ind. Crop. Prod. 2018, 113, 46–49. [Google Scholar] [CrossRef]
- Isman, M.B. Commercial development of plant essential oils and their constituents as active ingredients in bioinsecticides. Phytochem. Rev. 2020, 19, 235–241. [Google Scholar] [CrossRef]
- Soares, M.A.; de Campos, M.R.; Passos, L.C.; Carvalho, G.A.; Haro, M.M.; Lavoir, A.-V.; Biondi, A.; Zappalà, L.; Desneux, N. Botanical insecticide and natural enemies: A potential combination for pest management against Tuta absoluta. J. Pest Sci. 2019, 92, 1433–1443. [Google Scholar] [CrossRef] [Green Version]
- Glare, T.; Caradus, J.; Gelernter, W.; Jackson, T.; Keyhani, N.; Köhl, J.; Marrone, P.; Morin, L.; Stewart, A. Have biopesticides come of age? Trends Biotechnol. 2012, 30, 250–258. [Google Scholar] [CrossRef]
- Campolo, O.; Cherif, A.; Ricupero, M.; Siscaro, G.; Grissa-Lebdi, K.; Russo, A.; Cucci, L.M.; Di Pietro, P.; Satriano, C.; Desneux, N.; et al. Citrus peel essential oil nanoformulations to control the tomato borer, Tuta absoluta: Chemical properties and biological activity. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Campolo, O.; Giunti, G.; Laigle, M.; Michel, T.; Palmeri, V. Essential oil-based nano-emulsions: Effect of different surfactants, sonication and plant species on physicochemical characteristics. Ind. Crop. Prod. 2020, 157, 112935. [Google Scholar] [CrossRef]
- González, J.O.W.; Stefanazzi, N.; Murray, A.P.; Ferrero, A.A.; Band, B.F. Novel nanoinsecticides based on essential oils to control the German cockroach. J. Pest Sci. 2014, 88, 393–404. [Google Scholar] [CrossRef]

| Compounds | Measured RI | Literature RI | Green Anise (%) | Fennel (%) |
|---|---|---|---|---|
| α-pinene | 932 | 932 | 0.58 | 2.38 |
| camphene | 953 | 954 | 0.29 | |
| sabinene | 969 | 967 | 0.04 | 0.12 |
| β-pinene | 974 | 977 | 0.14 | 0.9 |
| myrcene | 988 | 985 | 0.08 | 1.07 |
| α-phellandrene | 1002 | 1003 | 0.29 | 2.73 |
| δ-3-carene | 1008 | 1011 | 0.16 | 0.28 |
| α-terpinene | 1018 | 1017 | 0.37 | |
| p-cymene | 1020 | 1025 | 0.04 | |
| limonene | 1024 | 1028 | 1.14 | 31.53 |
| β-phellandrene | 1025 | 1030 | 0.32 | |
| cis-β-ocimene | 1040 | 1037 | 0.51 | |
| ɣ-terpinene | 1054 | 1058 | 0.16 | 0.18 |
| Unidentified | 1082 | 0.1 | 1.74 | |
| fenchone | 1083 | 1071 | 10.1 | |
| terpinolene | 1086 | 1087 | 0.05 | 0.2 |
| linalool | 1095 | 1098 | 1.86 | 0.61 |
| camphor | 1141 | 1145 | 0.03 | 0.15 |
| terpinene-4-ol | 1174 | 1174 | 0.14 | 0.13 |
| α-phellandrene epoxide | 1179 | 1187 | 0.18 | |
| α-terpineol | 1186 | 1190 | 0.11 | |
| estragol | 1195 | 1197 | 3.91 | 2.45 |
| anisaldehyde | 1247 | 1252 | 0.52 | 0.52 |
| trans-anethole | 1249 | 1264 | 87.3 | 41.49 |
| cis-anethole | 1282 | 1284 | 0.23 | 0.21 |
| α–copaene | 1374 | 1375 | 0.05 | |
| eugenol | 1351 | 1358 | 1.05 | |
| β-caryophyllene | 1407 | 1419 | 0.3 | 0.18 |
| α-bergamotene | 1411 | 1430 | 0.37 | 0.15 |
| β-farnesene | 1454 | 1459 | 0.02 | |
| ɣ-muurolene | 1478 | 1497 | 0.01 | |
| valencene | 1496 | 1496 | 0.02 | |
| α–farnesene | 1505 | 1508 | 0.05 | |
| α–bisabolene | 1506 | 1505 | 0.06 | |
| δ-cadinene associate | 1522 | 1522 | 0.05 | |
| nerolidol | 1561 | 1565 | 0.06 | |
| T-muurolol | 1644 | 1641 | 0.06 | |
| foeniculin | 1677 | 1684 | 1.07 | 0.46 |
| linalyle benzoate | 2157 | na | 0.66 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dunan, L.; Malanga, T.; Bearez, P.; Benhamou, S.; Monticelli, L.S.; Desneux, N.; Michel, T.; Lavoir, A.-V. Biopesticide Evaluation from Lab to Greenhouse Scale of Essential Oils Used against Macrosiphum euphorbiae. Agriculture 2021, 11, 867. https://doi.org/10.3390/agriculture11090867
Dunan L, Malanga T, Bearez P, Benhamou S, Monticelli LS, Desneux N, Michel T, Lavoir A-V. Biopesticide Evaluation from Lab to Greenhouse Scale of Essential Oils Used against Macrosiphum euphorbiae. Agriculture. 2021; 11(9):867. https://doi.org/10.3390/agriculture11090867
Chicago/Turabian StyleDunan, Lana, Tara Malanga, Philippe Bearez, Sylvain Benhamou, Lucie S. Monticelli, Nicolas Desneux, Thomas Michel, and Anne-Violette Lavoir. 2021. "Biopesticide Evaluation from Lab to Greenhouse Scale of Essential Oils Used against Macrosiphum euphorbiae" Agriculture 11, no. 9: 867. https://doi.org/10.3390/agriculture11090867
APA StyleDunan, L., Malanga, T., Bearez, P., Benhamou, S., Monticelli, L. S., Desneux, N., Michel, T., & Lavoir, A.-V. (2021). Biopesticide Evaluation from Lab to Greenhouse Scale of Essential Oils Used against Macrosiphum euphorbiae. Agriculture, 11(9), 867. https://doi.org/10.3390/agriculture11090867






