Spatial Analysis of Agronomic Data and UAV Imagery for Rice Yield Estimation
Abstract
:1. Introduction
2. Materials and Methods
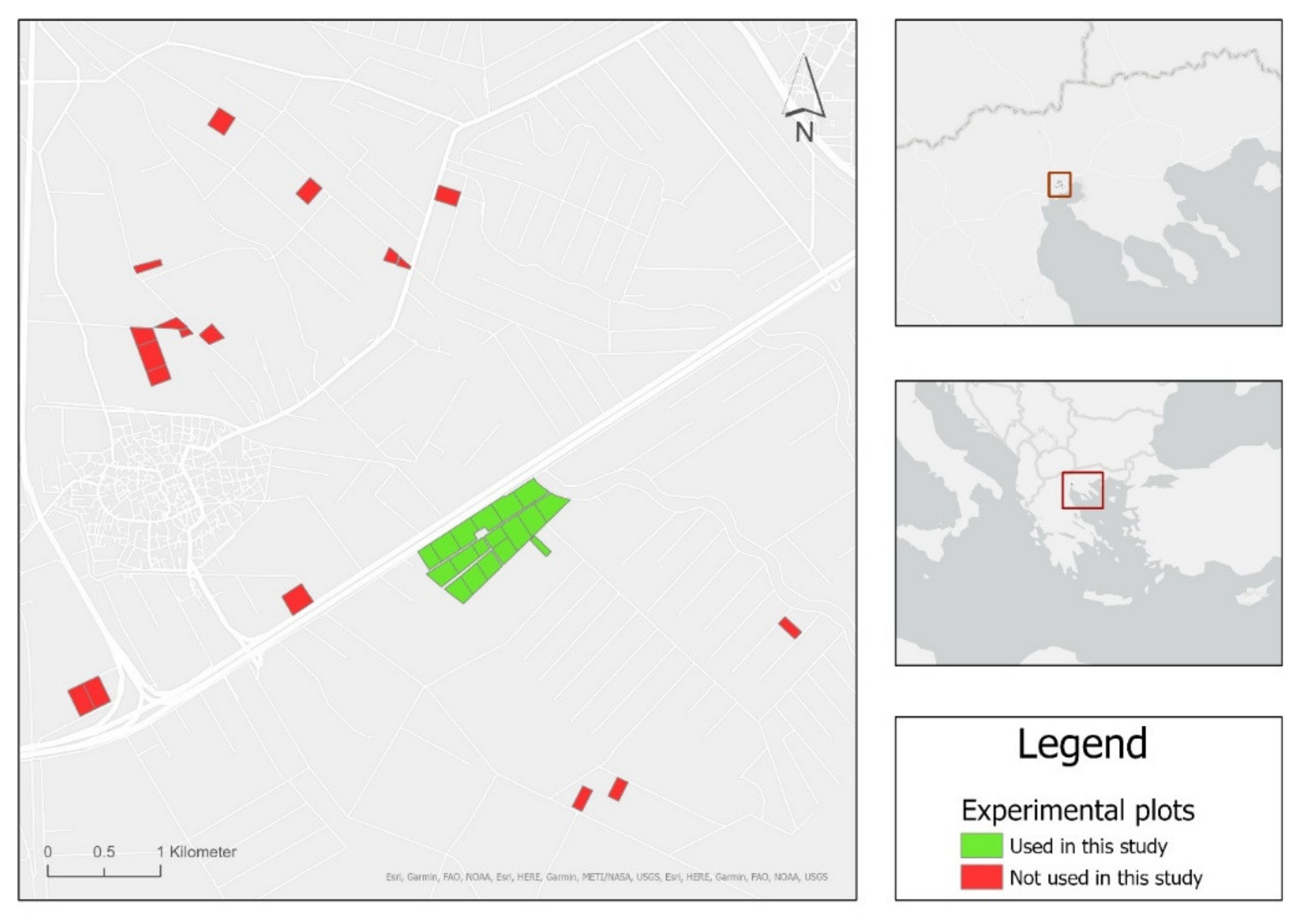
2.1. Study Area
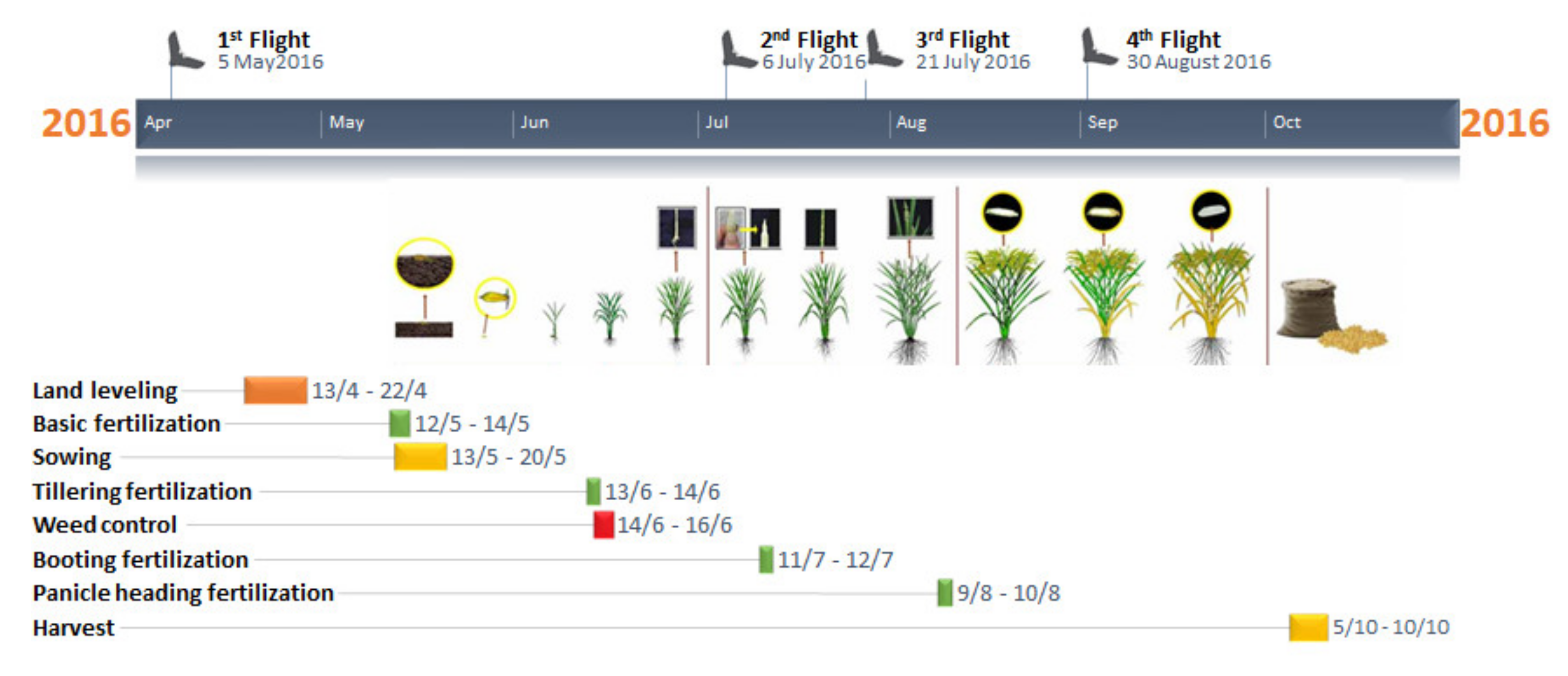
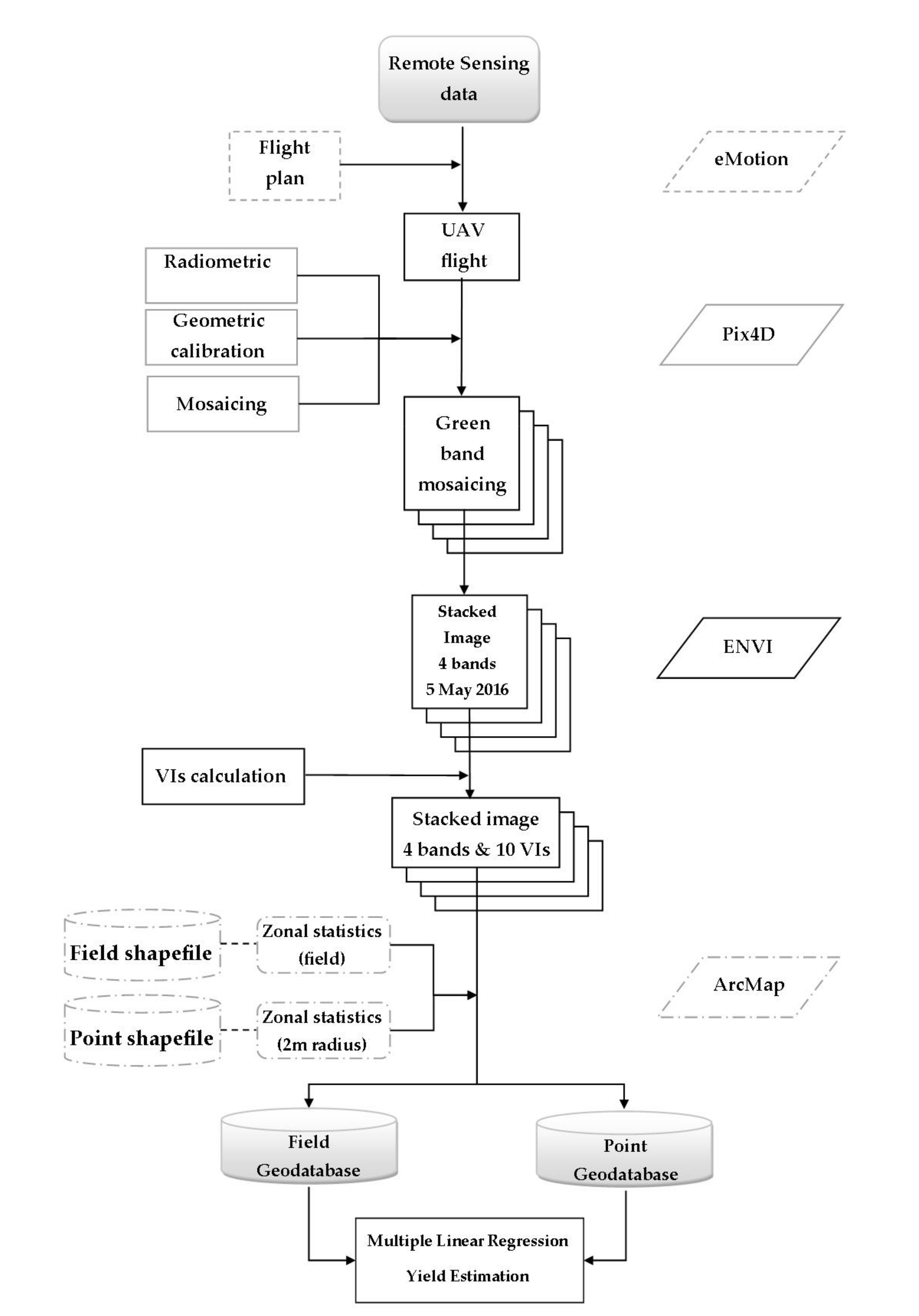
2.2. Remote Sensing and In-Situ Data
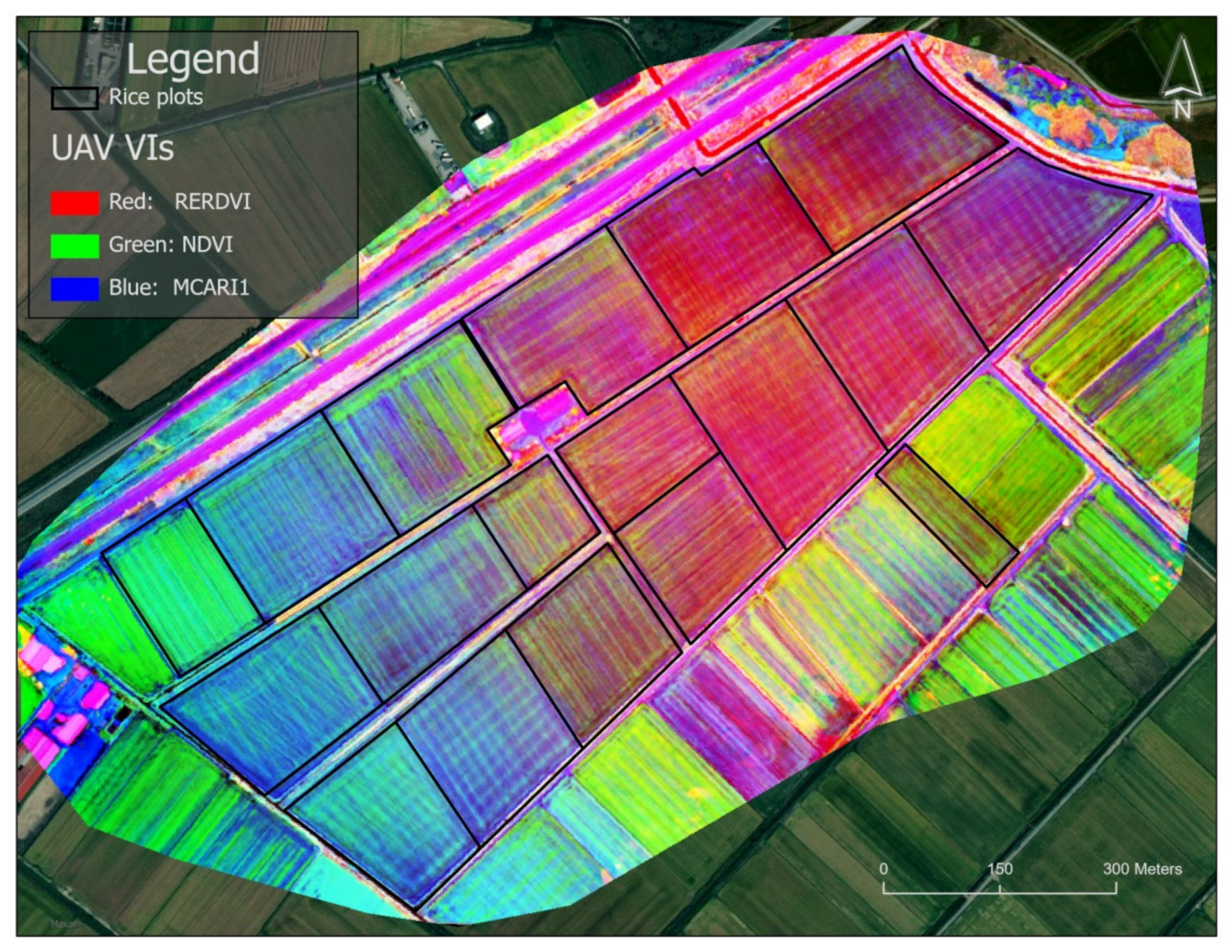
2.3. Image Analysis and Vegetation Indices
- At plot scale.
- At sub-plot scale derived from point data related to yield.
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food Security: The Challenge of Feeding 9 Billion People. Science 2010, 327, 812–818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ricepedia. Available online: https://ricepedia.org/rice-as-food/the-global-staple-rice-consumers (accessed on 8 February 2018).
- Koizumi, T.; Furuhashi, G. Global Rice Market Projections Distinguishing Japonica and Indica Rice under Climate Change. Jpn. Agric. Res. Q. 2020, 54, 63–91. [Google Scholar] [CrossRef] [Green Version]
- FAOSTAT. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 21 July 2021).
- Arable Land (Hectares)—Greece|Data. Available online: https://data.worldbank.org/indicator/AG.LND.ARBL.HA?end=2018&locations=GR&start=1961&view=chart (accessed on 21 July 2021).
- Mosleh, M.; Hassan, Q.; Chowdhury, E. Application of Remote Sensors in Mapping Rice Area and Forecasting Its Production: A Review. Sensors 2015, 15, 769–791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muharam, F.M.; Nurulhuda, K.; Zulkafli, Z.; Tarmizi, M.A.; Abdullah, A.N.H.; Che Hashim, M.F.; Mohd Zad, S.N.; Radhwane, D.; Ismail, M.R. UAV- and Random-Forest-AdaBoost (RFA)-Based Estimation of Rice Plant Traits. Agronomy 2021, 11, 915. [Google Scholar] [CrossRef]
- Reynolds, C.A.; Yitayew, M.; Slack, D.C.; Hutchinson, C.F.; Huete, A.; Petersen, M.S. Estimating Crop Yields and Production by Integrating the FAO Crop Specific Water Balance Model with Real-Time Satellite Data and Ground-Based Ancillary Data. Int. J. Remote Sens. 2000, 21, 3487–3508. [Google Scholar] [CrossRef]
- Curry, B.R. Dynamic Simulation of Plant Growth—Part I. Development of a Model. Trans. ASAE 1971, 14, 946–0949. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Prueger, J.H.; Sauer, T.J.; Dold, C.; O’Brien, P.; Wacha, K. Applications of Vegetative Indices from Remote Sensing to Agriculture: Past and Future. Inventions 2019, 4, 71. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Zheng, H.B.; Xu, X.Q.; He, J.Y.; Ge, X.K.; Yao, X.; Cheng, T.; Zhu, Y.; Cao, W.X.; Tian, Y.C. Predicting Grain Yield in Rice Using Multi-Temporal Vegetation Indices from UAV-Based Multispectral and Digital Imagery. J. Photogramm. Remote Sens. 2017, 130, 246–255. [Google Scholar] [CrossRef]
- Xue, L.-H.; Cao, W.-X.; Yang, L.-Z. Predicting Grain Yield and Protein Content in Winter Wheat at Different N Supply Levels Using Canopy Reflectance Spectra. Pedosphere 2007, 17, 646–653. [Google Scholar] [CrossRef]
- Becker-Reshef, I.; Vermote, E.; Lindeman, M.; Justice, C. A Generalized Regression-Based Model for Forecasting Winter Wheat Yields in Kansas and Ukraine Using MODIS Data. Remote Sens. Environ. 2010, 114, 1312–1323. [Google Scholar] [CrossRef]
- Wang, L.; Tian, Y.; Yao, X.; Zhu, Y.; Cao, W. Predicting Grain Yield and Protein Content in Wheat by Fusing Multi-Sensor and Multi-Temporal Remote-Sensing Images. Field Crops Res. 2014, 164, 178–188. [Google Scholar] [CrossRef]
- Torres-Sánchez, J.; Peña, J.M.; de Castro, A.I.; López-Granados, F. Multi-Temporal Mapping of the Vegetation Fraction in Early-Season Wheat Fields Using Images from UAV. Comput. Electron. Agric. 2014, 103, 104–113. [Google Scholar] [CrossRef]
- Pradhan, S.; Bandyopadhyay, K.K.; Sahoo, R.N.; Sehgal, V.K.; Singh, R.; Gupta, V.K.; Joshi, D.K. Predicting Wheat Grain and Biomass Yield Using Canopy Reflectance of Booting Stage. J. Indian Soc. Remote Sens. 2014, 42, 711–718. [Google Scholar] [CrossRef]
- Lugano Leonardo. Available online: http://www.lugano.it/en/products/ronaldo (accessed on 18 June 2021).
- Almosementi|Gladio; Almo S.p.A.: Mortara, Pavia, Italy.
- EBee X Fixed-Wing Mapping and Surveying Drone—SenseFly. Available online: https://www.sensefly.com/drone/ebee-x-fixed-wing-drone/ (accessed on 16 June 2021).
- User Manual MultiSPEC 4C Camera; senseFly Ltd: Cheseaux, Switzerland, 2014.
- Xu, X.; Gu, X.; Song, X.; Li, C.; Huang, W. Assessing Rice Chlorophyll Content with Vegetation Indices from Hyperspectral Data. In Proceedings of the Computer and Computing Technologies in Agriculture IV, Nanchang, China, 22–25 October 2010; Li, D., Liu, Y., Chen, Y., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 296–303. [Google Scholar]
- Gitelson, A.; Merzlyak, M.N. Spectral Reflectance Changes Associated with Autumn Senescence of Aesculus Hippocastanum L. and Acer Platanoides L. Leaves. Spectral Features and Relation to Chlorophyll Estimation. J. Plant Physiol. 1994, 143, 286–292. [Google Scholar] [CrossRef]
- Rouse, W.J.; Haas, H.R.; Schell, A.J.; Deering, W.D. Monitoring Vegetation Systems in the Great Plains with ERTS. Third ERTS Symp. 1973, 1, 309–317. [Google Scholar]
- Sripada, R.P.; Heiniger, R.W.; White, J.G.; Meijer, A.D. Aerial Color Infrared Photography for Determining Early In-Season Nitrogen Requirements in Corn. Agron. J. 2006, 98, 968–977. [Google Scholar] [CrossRef]
- Roujean, J.-L.; Breon, F.-M. Estimating PAR Absorbed by Vegetation from Bidirectional Reflectance Measurements. Remote Sens. Environ. 1995, 51, 375–384. [Google Scholar] [CrossRef]
- Cao, Q.; Miao, Y.; Wang, H.; Huang, S.; Cheng, S.; Khosla, R.; Jiang, R. Non-Destructive Estimation of Rice Plant Nitrogen Status with Crop Circle Multispectral Active Canopy Sensor. Field Crops Res. 2013, 154, 133–144. [Google Scholar] [CrossRef]
- Barnes, E.; Clarke, T.R.; Richards, S.E.; Colaizzi, P.; Haberland, J.; Kostrzewski, M.; Waller, P.; Choi, C.; Riley, E.; Thompson, T.L. Coincident Detection of Crop Water Stress, Nitrogen Status, and Canopy Density Using Ground Based Multispectral Data. In Proceedings of the Fifth International Conference on Precision Agriculture, Bloomington, MN, USA, 16–19 July 2000; ASA-CSSA-SSSA: Madison, WI, USA, 2000. [Google Scholar]
- Gitelson, A.A. Remote Estimation of Canopy Chlorophyll Content in Crops. Geophys. Res. Lett. 2005, 32, L08403. [Google Scholar] [CrossRef] [Green Version]
- Haboudane, D. Hyperspectral Vegetation Indices and Novel Algorithms for Predicting Green LAI of Crop Canopies: Modeling and Validation in the Context of Precision Agriculture. Remote Sens. Environ. 2004, 90, 337–352. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhao, W.; Zhou, D.; Gong, H. Sensitivity Analysis of Vegetation Reflectance to Biochemical and Biophysical Variables at Leaf, Canopy, and Regional Scales. IEEE Trans. Geosci. Remote Sens. 2014, 52, 4014–4024. [Google Scholar] [CrossRef]
- Huang, S.; Miao, Y.; Zhao, G.; Yuan, F.; Ma, X.; Tan, C.; Yu, W.; Gnyp, M.; Lenz-Wiedemann, V.; Rascher, U.; et al. Satellite Remote Sensing-Based In-Season Diagnosis of Rice Nitrogen Status in Northeast China. Remote Sens. 2015, 7, 10646–10667. [Google Scholar] [CrossRef] [Green Version]
- Cao, Q.; Miao, Y.; Shen, J.; Yu, W.; Yuan, F.; Cheng, S.; Huang, S.; Wang, H.; Yang, W.; Liu, F. Improving In-Season Estimation of Rice Yield Potential and Responsiveness to Topdressing Nitrogen Application with Crop Circle Active Crop Canopy Sensor. Precis. Agric. 2016, 17, 136–154. [Google Scholar] [CrossRef]
- Deering, D.W.; Rouse, J.W.; Haas, R.H.; Schell, J.A. Measuring Forage Production of Grazing Units from Landsat MSS Data. In Proceedings of the 10th International Symposium of Remote Sensing of Environment, Ann Arbor, MI, USA, 6 October 1975; Volume 2, pp. 1169–1178. [Google Scholar]
- Hunt, E.R.; Doraiswamy, P.C.; McMurtrey, J.E.; Daughtry, C.S.T.; Perry, E.M.; Akhmedov, B. A Visible Band Index for Remote Sensing Leaf Chlorophyll Content at the Canopy Scale. Int. J. Appl. Earth Obs. Geoinf. 2013, 21, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Gemtos, T.; Fountas, S.; Tagarakis, A.; Liakos, V. Precision Agriculture Application in Fruit Crops: Experience in Handpicked Fruits. Procedia Technol. 2013, 8, 324–332. [Google Scholar] [CrossRef] [Green Version]
- Nuarsa, I.W.; Nishio, F.; Hongo, C. Spectral Characteristics and Mapping of Rice Plants Using Multi-Temporal Landsat Data. J. Agric. Sci. 2011, 3, 54. [Google Scholar] [CrossRef] [Green Version]
- Angelova, K.; Ilieva, V.; Markova Ruzdik, N.; Karov, I.; Mihajlov, L.; Ilievski, M. Examination on Yield and Some Yield Associated Parameters in Different Rice Genotypes. J. Agric. Plant Sci. 2018, 16, 21–25. [Google Scholar]
- Kanke, Y.; Tubaña, B.; Dalen, M.; Harrell, D. Evaluation of Red and Red-Edge Reflectance-Based Vegetation Indices for Rice Biomass and Grain Yield Prediction Models in Paddy Fields. Precis. Agric. 2016, 17, 507–530. [Google Scholar] [CrossRef]
- Shresthan, R.P.; Naikaset, S. Agro-Spectral Models for Estimating Dry Season Rice Yield in the Bangkok Plain of Thailand. Asian J. Geoinform. 2003, 4, 11–19. [Google Scholar]
- Fountas, S.; Carli, G.; Sørensen, C.G.; Tsiropoulos, Z.; Cavalaris, C.; Vatsanidou, A.; Liakos, B.; Canavari, M.; Wiebensohn, J.; Tisserye, B. Farm Management Information Systems: Current Situation and Future Perspectives. Comput. Electron. Agric. 2015, 115, 40–50. [Google Scholar] [CrossRef] [Green Version]
- Duan, B.; Fang, S.; Gong, Y.; Peng, Y.; Wu, X.; Zhu, R. Remote Estimation of Grain Yield Based on UAV Data in Different Rice Cultivars under Contrasting Climatic Zone. Field Crops Res. 2021, 267, 108148. [Google Scholar] [CrossRef]
- Tamás, J.; Nagy, A.; Farkasné Gálya, B. Beyond NDVI- Spectral Indexing of Biomass. J. Agric. Inform. 2019, 10, 28–36. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Prueger, J.H. Value of Using Different Vegetative Indices to Quantify Agricultural Crop Characteristics at Different Growth Stages under Varying Management Practices. Remote Sens. 2010, 2, 562–578. [Google Scholar] [CrossRef] [Green Version]
- Wan, L.; Cen, H.; Zhu, J.; Zhang, J.; Zhu, Y.; Sun, D.; Du, X.; Zhai, L.; Weng, H.; Li, Y.; et al. Grain Yield Prediction of Rice Using Multi-Temporal UAV-Based RGB and Multispectral Images and Model Transfer—A Case Study of Small Farmlands in the South of China. Agric. Forest Meteorol. 2020, 291, 108096. [Google Scholar] [CrossRef]
- Wang, F.; Wang, F.; Zhang, Y.; Hu, J.; Huang, J.; Xie, J. Rice Yield Estimation Using Parcel-Level Relative Spectral Variables From UAV-Based Hyperspectral Imagery. Front. Plant Sci. 2019, 10, 453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, X.; Fang, S.; Huang, X.; Liu, Y.; Guo, L. Rice Mapping and Growth Monitoring Based on Time Series GF-6 Images and Red-Edge Bands. Remote Sens. 2021, 13, 579. [Google Scholar] [CrossRef]
- Wan, L.; Cen, H.; Zhu, J.; Li, Y.; Zhu, Y.; Sun, D.; Weng, H.; He, Y. Combining UAV-Based Vegetation Indices, Canopy Height and Canopy Coverage to Improve Rice Yield Prediction under Different Nitrogen Levels. In Proceedings of the 2019 ASABE Annual International Meeting, Boston, MA, USA, 7–10 July 2019; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2019. [Google Scholar]
- Duan, B.; Fang, S.; Zhu, R.; Wu, X.; Wang, S.; Gong, Y.; Peng, Y. Remote Estimation of Rice Yield With Unmanned Aerial Vehicle (UAV) Data and Spectral Mixture Analysis. Front. Plant Sci. 2019, 10, 204. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Wang, F.; Zhang, Y.; Hu, J.; Huang, J.; Xie, L.; Xie, J. Rice Yield Estimation at Pixel Scale Using Relative Vegetation Indices from Unmanned Aerial Systems. In Proceedings of the 2019 8th International Conference on Agro-Geoinformatics (Agro-Geoinformatics), Istanbul, Turkey, 16–19 July 2019; pp. 1–4. [Google Scholar]
- Peter, B.G.; Messina, J.P.; Carroll, J.W.; Zhi, J.; Chimonyo, V.; Lin, S.; Snapp, S.S. Multi-Spatial Resolution Satellite and SUAS Imagery for Precision Agriculture on Smallholder Farms in Malawi. Photogramm. Eng. Remote Sens. 2020, 86, 107–119. [Google Scholar] [CrossRef]
- Islam, M.M.; Matsushita, S.; Noguchi, R.; Ahamed, T. Development of Remote Sensing-Based Yield Prediction Models at the Maturity Stage of Boro Rice Using Parametric and Nonparametric Approaches. Remote Sens. Appl. Soc. Environ. 2021, 22, 100494. [Google Scholar] [CrossRef]



| Vegetation Index | Formula | Reference | Scale of Application | Estimated Parameter |
|---|---|---|---|---|
| Normalized difference vegetation index (NDVI) | [23] | Crown | Biomass, vegetation density | |
| Normalized NIR index (NNIR) | [24] | Crown | Vegetation density | |
| Red edge difference vegetation index (REDVI) | [26] | Vegetation coverage | ||
| Normalized difference red edge (NDRE) | [27] | Leaves | Biomass | |
| Red edge chlorophyll index (CIre) | [28] | Chlorophyll | ||
| Modified chlorophyll absorption in reflectance index 1 (MCARI1) | [26] | Leaves | Chlorophyll, LAI | |
| Red edge soil adjusted vegetation index (RESAVI) | [26] | Crown | Biophysical parameters | |
| Red edge re-normalized different vegetation index (RERDVI) | [26] | Chlorophyll | ||
| Transformed vegetation index (TVI) | [33] | Crown | Chlorophyll | |
| Modified transformed vegetation index 2 (MTVI2) | [29] | Crown | Chlorophyll |
| Vegetation Indices | Calculation Methodology |
|---|---|
| VIA | 6 July, booting |
| VIB | 21 July, panicle heading |
| VIC | 30 August, ripening |
| MRL(VI) | Is calculated on a case-by-case based on linear regression of one VI in 2 or 3 dates. |
| SUM (VI AB) | VIA + VIB |
| SUM (VI AC) | VIA + VIC |
| SUM (VI BC) | VIB + VIC |
| MAX (VI) | Max (VIA, VIB, VIC) |
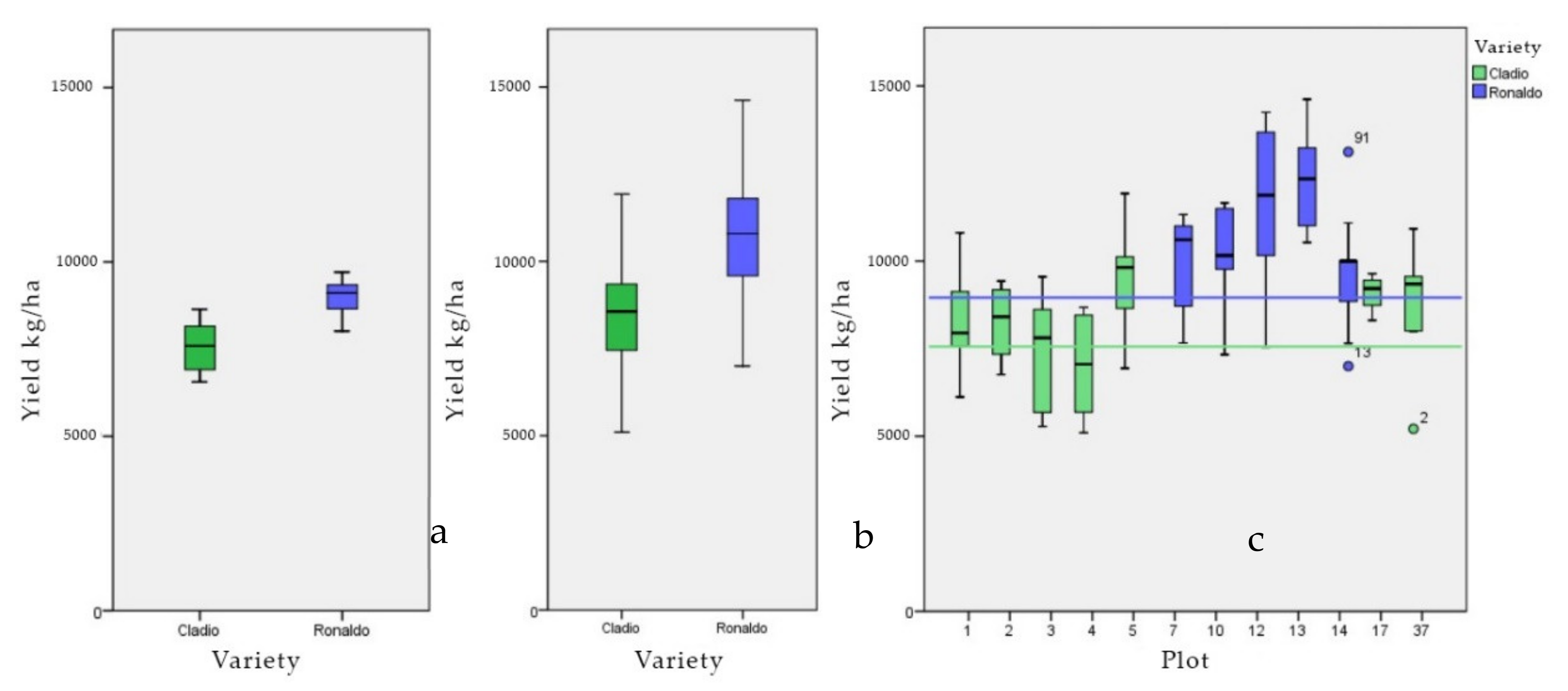
| N | Range | Minimum | Maximum | Mean | Std. Deviation | Variance | CV% | ||
|---|---|---|---|---|---|---|---|---|---|
| Statistic | Std. Error | ||||||||
| General | 18 | 3150 | 6560 | 9710 | 8418.3 | 224.53 | 952.60 | 9074.500 | 11.32 |
| Gladio | 7 | 208 | 6560 | 8640 | 7560 | 304.70 | 806.16 | 6499.000 | 10.66 |
| Ronaldo | 11 | 169 | 8020 | 9710 | 8964.5 | 167.56 | 555.72 | 3088.273 | 6.20 |
| General (Ν = 18) | Ronaldo (Ν = 11) | Gladio (Ν = 7) | |||||||
|---|---|---|---|---|---|---|---|---|---|
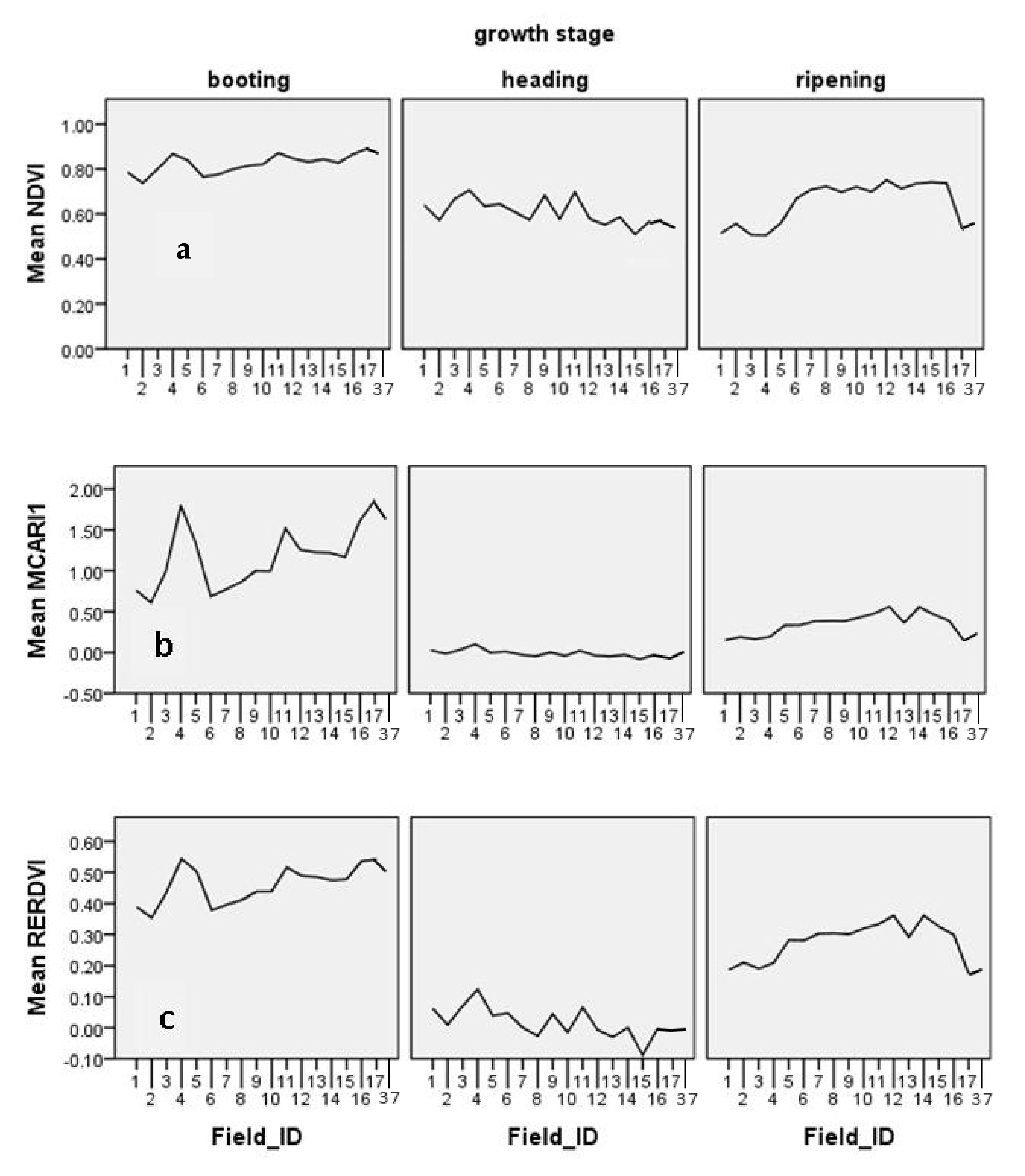
| Vegetation Indices | Booting | Panicle | Ripening | Booting | Panicle Heading | Ripening | Booting | Panicle Heading | Ripening |
| Heading | |||||||||
| NDVI | 0.268 | 0.054 | 0.510 * | 0.781 ** | −0.064 | 0.620 * | 0.939 ** | 0.835 * | −0.370 |
| NNIR | 0.224 | 0.017 | 0.333 | 0.781 ** | −0.098 | 0.668 * | 0.942 ** | 0.874 * | −0.101 |
| REDVI | 0.451 | −0.230 | 0.604 ** | 0.698 * | −0.009 | 0.612 * | 0.921 ** | 0.836 * | 0.178 |
| NDRE | 0.287 | −0.242 | 0.609 ** | 0.731 ** | −0.034 | 0.783 ** | 0.946 ** | 0.846 * | −0.215 |
| CΙre | 0.382 | −0.227 | 0.685 ** | 0.752 ** | −0.059 | 0.735 * | 0.946 ** | 0.855 * | −0.190 |
| MCARI1 | 0.464 | −0.229 | 0.740 ** | 0.722 * | −0.058 | 0.697 ** | 0.975 ** | 0.830 * | 0.009 |
| RESAVI | 0.386 | −0.237 | 0.611 ** | 0.754 ** | −0.024 | 0.784 ** | 0.940 ** | 0.843 * | −0.046 |
| RERDVI | 0.390 | −0.236 | 0.614 ** | 0.744 ** | −0.021 | 0.771 ** | 0.941 ** | 0.842 * | 0.003 |
| TVI | 0.188 | 0.120 | 0.614 ** | 0.783 ** | −0.061 | 0.771 ** | 0.936 ** | 0.816 * | 0.003 |
| MTVI2 | −0.557 * | −0.048 | −0.433 ** | −0.691 * | −0.177 | −0.453 | −0.924 ** | −0.854 * | −0.519 |
| Ronaldo | Gladio | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NDVI | RERDVI | NDVI | MCARI1 | ||||||||||||
| C | B | A | C | B | A | C | B | A | C | B | A | ||||
| A | 0.76 | 0.61 | 0.60 | A | 0.77 | 0.34 | 0.55 | A | 0.01 | 0.01 | 0.88 | A | 0.36 | 0.27 | 0.95 |
| B | 0.67 | 0.00 | 0.61 | B | 0.17 | 0.00 | 0.61 | B | 0.01 | 0.70 | 0.14 | B | 0.10 | 0.69 | 0.54 |
| C | 0.38 | 0.67 | 0.66 | C | 0.59 | 0.50 | 0.80 | C | 0.13 | 0.53 | 0.70 | C | 0.00 | 0.54 | 0.59 |
| Max (NDVI) = 0.60 | Max (RERDVI) = 0.59 | Max (NDVI) = 0.88 | Max (MCARI1) = 0.94 | ||||||||||||
| MRLABC = 0.75 * | MRLABC = 0.81 * | MRLABC = 0.78 * | MRLABC = 0.61 * | ||||||||||||
| * Reference on Supplementary Material | |||||||||||||||
| Legend | |||||||||||||||
| C | B | A | MAX(VI) = MAX(VIA, VIB, VIC) | R2 | |||||||||||
| A | VIA+VIC * | VIA+ VIB * | VIA | MRLABC = aVIA+bVIB+cVIC+d | 1 | A: 06 July 2016 | |||||||||
| B | VIB+ VIC* | VIB | VIBA** | * Reference on Supplementary Material | 0.5 | B: 21 July 2016 | |||||||||
| C | VIC | VI CB ** VI CA ** | ** Reference on Table 2 | 0 | C: 30 August 2016 | ||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perros, N.; Kalivas, D.; Giovos, R. Spatial Analysis of Agronomic Data and UAV Imagery for Rice Yield Estimation. Agriculture 2021, 11, 809. https://doi.org/10.3390/agriculture11090809
Perros N, Kalivas D, Giovos R. Spatial Analysis of Agronomic Data and UAV Imagery for Rice Yield Estimation. Agriculture. 2021; 11(9):809. https://doi.org/10.3390/agriculture11090809
Chicago/Turabian StylePerros, Nikolas, Dionissios Kalivas, and Rigas Giovos. 2021. "Spatial Analysis of Agronomic Data and UAV Imagery for Rice Yield Estimation" Agriculture 11, no. 9: 809. https://doi.org/10.3390/agriculture11090809
APA StylePerros, N., Kalivas, D., & Giovos, R. (2021). Spatial Analysis of Agronomic Data and UAV Imagery for Rice Yield Estimation. Agriculture, 11(9), 809. https://doi.org/10.3390/agriculture11090809






