Response of Hops to Algae-Based and Nutrient-Rich Foliar Sprays
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Experiments Characterization
2.2. Experimental Design and Treatment Application
2.3. Data Acquisition in the Field and Tissue Sampling
2.4. Laboratory Analyses
2.5. Data Analysis
3. Results
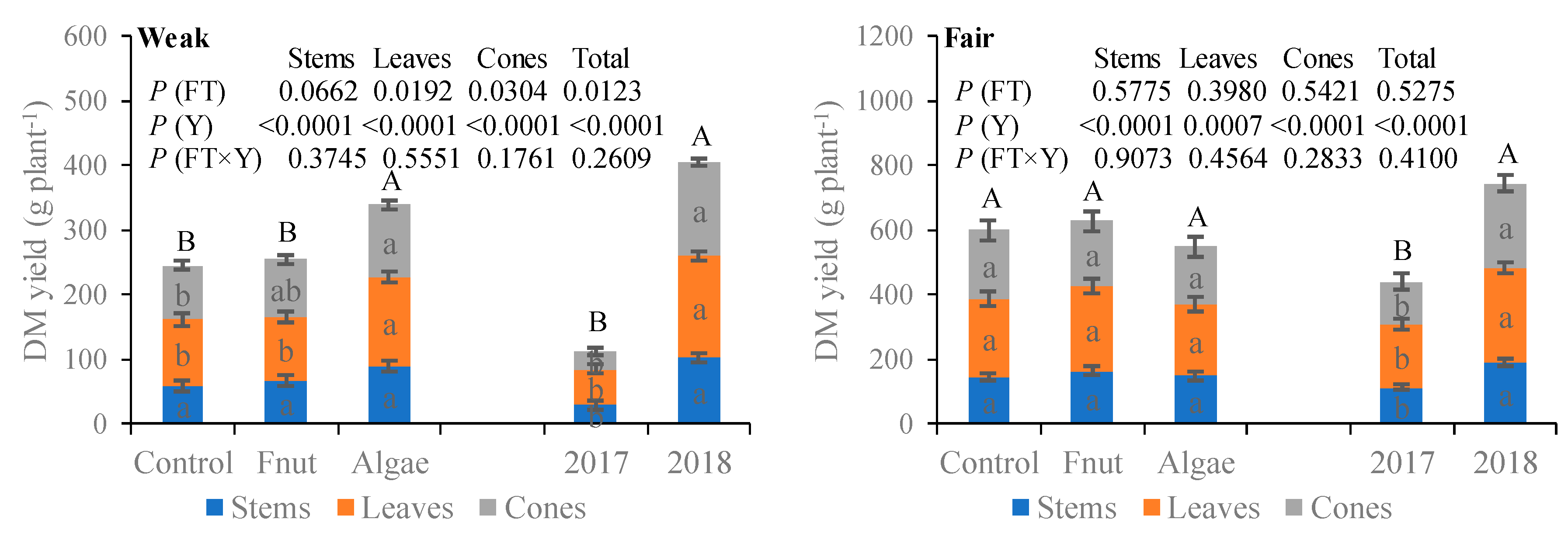
3.1. Plant Dry Matter Yield
3.2. Tissue Nutrient Concentrations
3.3. SPAD Readings, NDVI and Chlorophyll Fluorescence
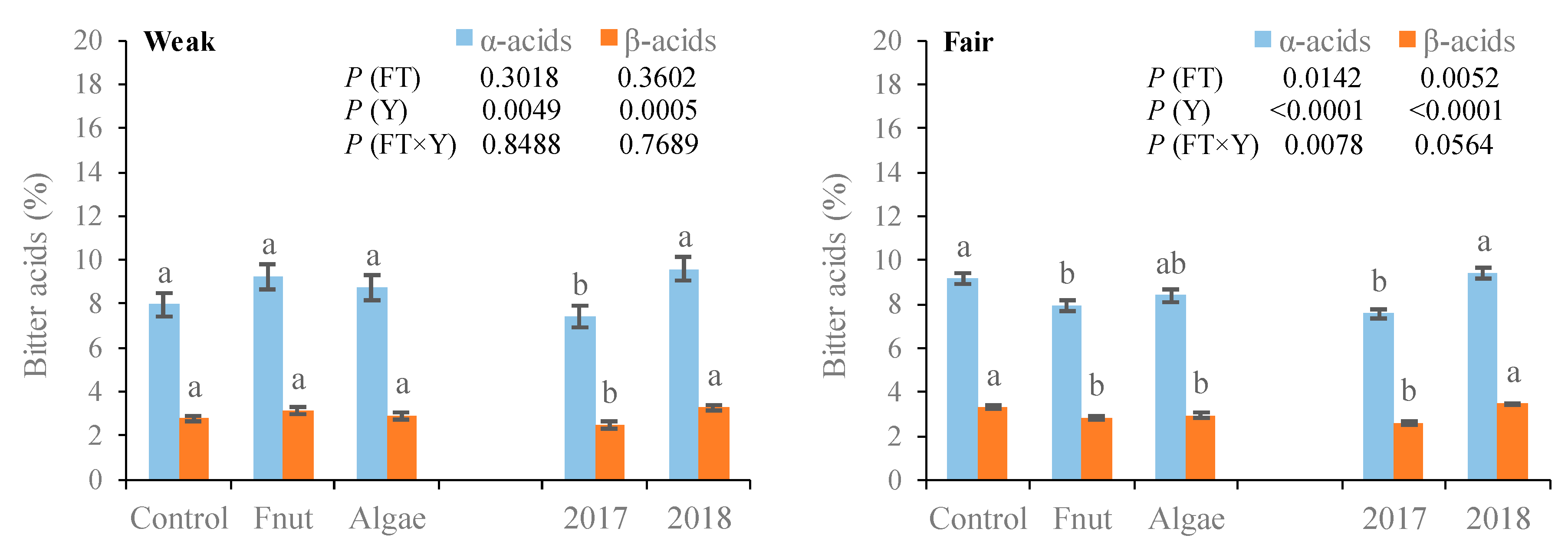
3.4. Concentration of Bitter Acids in the Cones
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weil, R.R.; Brady, N.C. The Nature and Properties of Soils, 15th ed.; Global Edition: London, UK, 2017. [Google Scholar]
- Havlin, J.L.; Tisdale, S.L.; Nelson, W.L.; Beaton, J.D. Soil Fertility and Fertilizers: An Introduction to Nutrient Management, 8th ed.; Pearson: Boston, MA, USA, 2014. [Google Scholar]
- Dean, L. Foliar Fertilizer Market Witnessing Increasing Penetration of Nitrogen-Based Variants. Available online: www.worldfertilizer.com/special-reports/22102019/foliar-fertilizer-market-witnessing-increasing-penetration-of-nitrogen-based-variants/ (accessed on 15 February 2020).
- Colla, G.; Rouphael, Y. Biostimulants in horticulture. Sci. Hortic. 2015, 196, 1–2. [Google Scholar] [CrossRef]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Du Jardin, P.; Xu, L.; Geelen, D. Agricultural functions and action mechanisms of plant biostimulants (PBs). In The Chemical Biology of Plant Biostimulants; John Wiley & Sons: Hoboken, NJ, USA, 2020; pp. 1–30. [Google Scholar]
- Rouphael, Y.; Colla, G. Editorial: Biostimulants in agriculture. Front Plant. Sci. 2020, 11, 40. [Google Scholar] [CrossRef] [Green Version]
- Battacharyya, D.; Babgohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed extracts as biostimulants in horticulture. Sci. Hortic. 2015, 196, 39–48. [Google Scholar] [CrossRef]
- De Saeger, J.; Van Praet, S.; Vereecke, D.; Park, J.; Jacques, S.; Han, T.; Depuydt, S. Toward the molecular understanding of the action mechanism of Ascophyllum nodosum extracts on plants. J. Appl. Phycol. 2020, 32, 573–597. [Google Scholar] [CrossRef] [Green Version]
- Shukla, P.S.; Mantin, E.G.; Adil, M.; Bajpai, S.; Critchley, A.T.; Prithiviraj, B. Ascophyllum nodosum-based biostimulants: Sustainable applications in agriculture for the stimulation of plant growth, stress tolerance, and disease management. Front Plant. Sci. 2019, 10, 655. [Google Scholar] [CrossRef] [Green Version]
- Stirk, W.A.; Rengasamy, K.R.R.; Kulkarni, M.G.; van Staden, J. Plant biostimulants from seaweed. In The Chemical Biology of Plant Biostimulants; John Wiley & Sons: Hoboken, NJ, USA, 2020; pp. 31–55. [Google Scholar]
- Wozniak, E.; Blaszczak, A.; Wiatrak, P.; Canady, M. Biostimulant mode of action. In The Chemical Biology of Plant Biostimulants; John Wiley & Sons: Hoboken, NJ, USA, 2020; pp. 229–243. [Google Scholar]
- Al-Ghamdi, A.A.; Elansary, H.O. Synergetic effects of 5-aminolevulinic acid and Ascophyllum nodosum seaweed extracts on Asparagus phenolics and stress related genes under saline irrigation. Plant. Physiol. Biochem. 2018, 129, 273–284. [Google Scholar] [CrossRef]
- Goñi, O.; Quille, P.; O’Connell, S. Ascophyllum nodosum extract biostimulants and their role in enhancing tolerance to drought stress in tomato plants. Plant. Physiol. Biochem. 2018, 126, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Carmody, N.; Goni, O.; Langowski, L.; O’Connell, S. Ascophyllum nodosum extract biostimulant processing and its impact on enhancing heat stress tolerance during tomato fruit set. Front Plant. Sci. 2020, 11, 807. [Google Scholar] [CrossRef]
- Gunupuru, L.R.; Patel, J.S.; Sumarah, M.W.; Renaud, J.B.; Mantin, E.G.; Prithiviraj, B. A plant biostimulant made from the marine brown algae Ascophyllum nodosum and chitosan reduce Fusarium head blight and mycotoxin contamination in wheat. PLoS ONE 2019, 14, e0220562. [Google Scholar] [CrossRef]
- Patel, J.S.; Selvaraj, V.; Gunupuru, L.R.; Rathor, P.K.; Prithiviraj, B. Combined application of Ascophyllum nodosum extract and chitosan synergistically activates host-defense of peas against powdery mildew. BMC Plant. Biol. 2020, 20, 113. [Google Scholar] [CrossRef]
- Procházka, P.; Štranc, P.; Pazderů, K.; Vostřel, J.; Řehoř, J. Use of biologically active substances in hops. Plant Soil Environ. 2018, 64, 626–632. [Google Scholar] [CrossRef] [Green Version]
- Taskos, D.; Stamatiadis, S.; Yvin, J.-C.; Jamois, F. Effects of an Ascophyllum nodosum (L.) Le Jol. extract on grapevine yield and berry composition of a Merlot vineyard. Sci. Hortic. 2019, 250, 27–32. [Google Scholar] [CrossRef]
- Viencz, T.; Oliari, I.C.R.; Ayub, R.A.; Faria, C.M.D.R.; Botelho, R.V. Postharvest quality and brown rot incidence in plums treated with Ascophyllum nodosum extract Qualidade pós-colheita e incidência de podridão parda em ameixas tratadas com extrato de Ascophyllum nodosum. Cienc. Agrar. 2020, 41, 753–766. [Google Scholar] [CrossRef]
- FAOSAT. Production: Crops. Available online: www.fao.org/faostat/en/#data/QC (accessed on 13 November 2020).
- Ruggeri, R.; Loreti, P.; Rossini, F. Exploring the potential of hop as a dual purpose crop in the Mediterranean environment: Shoot and cone yield from nine commercial cultivars. Eur. J. Agron. 2018, 93, 11–17. [Google Scholar] [CrossRef]
- Marceddu, R.; Carrubba, A.; Sarno, M. Cultivation trials of hop (Humulus lupulus L.) in semi-arid environments. Heliyon 2020, 6, e05114. [Google Scholar] [CrossRef]
- Rossini, F.; Virga, G.; Loreti, P.; Provenzano, M.E.; Danieli, P.P.; Roberto, R. Beyond beer: Hop shoot production and nutritional composition under Mediterranean climatic conditions. Agronomy 2020, 10, 1547. [Google Scholar] [CrossRef]
- Rossini, F.; Loreti, P.; Provenzano, M.E.; De Santis, D.; Ruggeri, R. Agronomic performance and beer quality assessment of twenty hop cultivars grown in Central Italy. Ital. J. Agron. 2016, 11, 180–187. [Google Scholar] [CrossRef] [Green Version]
- Forteschi, M.; Porcu, M.C.; Fanari, M.; Zinellu, M.; Secchi, N.; Buiatti, S.; Passaghe, P.; Bertoli, S.; Pretti, L. Quality assessment of Cascade Hop (Humulus lupulus L.) grown in Sardinia. Eur. Food Res. Technol. 2019, 245, 863–871. [Google Scholar] [CrossRef]
- Rodrigues, M.Â.; Morais, J.; Castro, J.P. O lúpulo: Da cultura ao extrato. Técnica cultural tradicional. In Livro de Atas das Jornadas do Lúpulo e da Cerveja: Novas Oportunidades de Negócio; Instituto Politécnico: Bragança, Portugal, 2015; pp. 1–10. [Google Scholar]
- Afonso, S.; Arrobas, M.; Rodrigues, M.Â. Soil and plant analyses to diagnose hop fields irregular growth. J. Soil Sci. Plant. Nut. 2020, 20, 1999–2013. [Google Scholar] [CrossRef]
- Van Reeuwijk, L. Procedures for Soil Analysis, Technical Paper 9; International Soil Reference and Information Centre: Wageningen, The Netherlands, 2002; p. 120. [Google Scholar]
- Lakanen, E.; Erviö, R. A comparison of eight extractants for the determination of plant available micronutrients in soils. Hels. Yliop. Rehtorin Profr. Erkki Kivisen Juhlajulkaisu Viljo Puustjarvi 1971, 123, 223–232. [Google Scholar]
- Walinga, I.; Van Vark, W.; Houba, V.; Van der Lee, J. Soil and Plant Analysis, Part 7: Plant Analysis Procedures; Wageningen Agricultural University: Wageningen, The Netherlands, 1989. [Google Scholar]
- EBC Analysis Committee, Analytica EBC. Hans Carl Getränke Fachverlag. Nürenberg Method 1998, 7, 7. [Google Scholar]
- Bryson, G.; Mills, H.; Sasseville, D.; Jones, J.B., Jr.; Barker, A. Plant Analysis Handbook III: A Guide to Sampling, Preparation, Analysis and Interpretation for Agronomic and Horticultural Crops; Micro-Macro Publishing: Athens, GA, USA, 2014; Volume VIII. [Google Scholar]
- Jarrell, W.M.; Beverly, R.B. The dilution effect in plant nutrition studies. In Advances in Agronomy; Brady, N.C., Ed.; Academic Press: Cambridge, MA, USA, 1981; Volume 34, pp. 197–224. [Google Scholar]
- Marschner, P.; Rengel, Z. Nutrient availability in soils. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Academic Press: San Diego, CA, USA, 2012; pp. 315–330. [Google Scholar]
- Kalaji, H.M.; Oukarroum, A.; Alexandrov, V.; Kouzmanova, M.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Allakhverdiev, S.I.; Goltsev, V. Identification of nutrient deficiency in maize and tomato plants by in vivo chlorophyll a fluorescence measurements. Plant Physiol. Biochem. 2014, 81, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Afonso, S.; Arrobas, M.; Ferreira, I.Q.; Rodrigues, M.Â. Assessing the potential use of two portable chlorophyll meters in diagnosing the nutritional status of plants. J. Plant Nutr. 2018, 41, 261–271. [Google Scholar] [CrossRef] [Green Version]
- Dinis, L.-T.; Ferreira, H.; Pinto, G.; Correia, C.; Moutinho Pereira, J. Kaolin-based, foliar reflective film protects photosystem II structure and function in grapevine leaves exposed to heat and high solar radiation. Photosynthetica 2016, 54, 47–55. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Łukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol. Plant 2016, 38, 102. [Google Scholar] [CrossRef] [Green Version]
- Arrobas, M.; Ferreira, I.Q.; Afonso, S.; Rodrigues, M.Â. Sufficiency ranges and crop nutrient removals for peppermint (Mentha X piperita L.) established from field and pot fertilizer experiments. Comm. Soil Sci. Plant Anal. 2018, 49, 1719–1730. [Google Scholar] [CrossRef] [Green Version]
- MacKinnon, D.; Pavlovič, V.; Čeh, B.; Naglič, B.; Pavlovič, M. The impact of weather conditions on alpha-acid content in hop (Humulus lupulus L.) cv. Aurora. Plant Soil Environ. 2020, 10, 519–525. [Google Scholar] [CrossRef]
- Mallarino, A.P.; Haq, M.U.; Wittry, D.; Bermudez, M. Variation in soybean response to early season foliar fertilization among and within fields. Agron. J. 2001, 93, 1220–1226. [Google Scholar] [CrossRef]
- Amiri, M.E.; Fallahi, E.; Golchin, A. Influence of foliar and ground fertilization on yield, fruit quality, and soil, leaf, and fruit mineral nutrients in apple. J. Plant Nutr. 2008, 31, 515–525. [Google Scholar] [CrossRef]
- Di Stasio, E.; Van Oosten, M.J.; Silletti, S.; Raimondi, G.; Dell’Aversana, E.; Carillo, P.; Maggio, A. Ascophyllum nodosum-based algal extracts act as enhancers of growth, fruit quality, and adaptation to stress in salinized tomato plants. J. Appl. Phycol. 2018, 30, 2675–2686. [Google Scholar] [CrossRef]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in plant science: A global perspective. Front Plant Sci. 2016, 7, 2049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, C.P.; Leskovar, D.I. Effects of A. nodosum seaweed extracts on spinach growth, physiology and nutrition value under drought stress. Sci. Hortic. 2015, 183, 39–47. [Google Scholar] [CrossRef]
- Frioni, T.; VanderWeide, J.; Palliotti, A.; Tombesi, S.; Poni, S.; Sabbatini, P. Foliar vs. soil application of Ascophyllum nodosum extracts to improve grapevine water stress tolerance. Sci. Hortic. 2021, 277, 109807. [Google Scholar] [CrossRef]
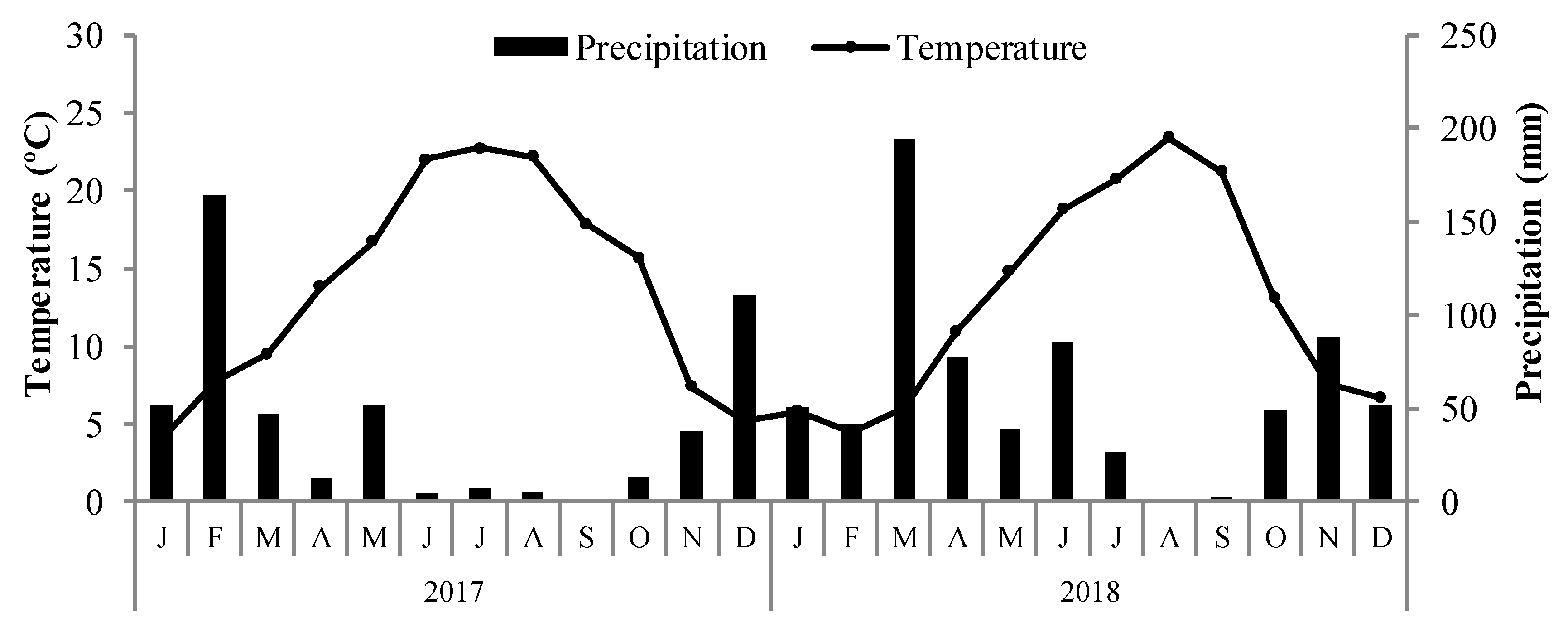



| Plot 1 | Plot 2 | Plot 3 | Plot 4 | ||
|---|---|---|---|---|---|
| Soil Properties | Plant Vigour | Weak | Fair | Good | Very Good |
| pHH2O | 5.8 ± 0.12 | 5.8 ± 0.04 | 5.5 ± 0.10 | 5.1 ± 0.13 | |
| pHKCl | 4.8 ± 0.12 | 4.7 ± 0.04 | 4.4 ± 0.08 | 4.3 ± 0.13 | |
| Organic C (g kg−1) a | 13.4 ± 0.20 | 15.7 ± 0.10 | 7.6 ± 0.04 | 14.5 ± 0.20 | |
| Extract. P (mg P2O5 kg−1) b | 283.0 ± 44.7 | 451.8 ± 33.5 | 191.1 ± 27.9 | 212.6 ± 28.2 | |
| Extract. K (mg K2O kg−1) b | 115.9 ± 7.8 | 193.0 ± 8.6 | 111.0 ± 5.9 | 286.0 ± 5.0 | |
| Exchan. Ca (cmolc kg−1) c | 14.8 ± 1.84 | 23.3 ± 1.39 | 10.7 ± 0.17 | 2.7 ± 0.46 | |
| Exchan. Mg (cmolc kg−1) c | 4.8 ± 0.84 | 9.5 ± 1.22 | 2.7 ± 0.07 | 0.5 ± 0.04 | |
| Exchan. K (cmolc kg−1) c | 0.3 ± 0.02 | 0.5 ± 0.04 | 0.2 ± 0.01 | 0.5 ± 0.08 | |
| Exchan. Na (cmolc kg−1) c | 0.2 ± 0.05 | 0.6 ± 0.05 | 0.1 ± 0.01 | 0.3 ± 0.06 | |
| Exchan. acidity (cmolc kg−1) c | 0.3 ± 0.03 | 0.3 ± 0.02 | 0.2 ± 0.03 | 0.6 ± 0.13 | |
| Cation-exch. capacity (cmolc kg−1) | 20.7 ± 2.64 | 34.4 ± 2.56 | 14.0 ± 0.21 | 5.1 ± 0.37 | |
| Extract. B (mg kg−1) d | 0.7 ± 0.14 | 1.2 ± 0.10 | 0.8 ± 0.13 | 0.6 ± 0.09 | |
| Extract. Fe (mg kg−1) e | 293.5 ± 30.50 | 272.6 ± 29.11 | 114.2 ± 6.75 | 105.7 ± 4.41 | |
| Exctract. Mn (mg kg−1) e | 250.7 ± 28.95 | 179.9 ± 14.02 | 224.1 ± 10.24 | 57.4 ± 7.89 | |
| Extract. Zn (mg kg−1) e | 9.8 ± 0.66 | 11.7 ± 0.80 | 7.2 ± 0.19 | 3.9 ± 0.46 | |
| Extract. Cu (mg kg−1) e | 16.3 ± 1.43 | 16.9 ± 1.09 | 10.1 ± 0.39 | 4.3 ± 0.77 | |
| Nitrogen | Phosphorus | Potassium | Calcium | Magnesium | Iron | Manganese | Copper | Zinc | Boron | |
|---|---|---|---|---|---|---|---|---|---|---|
| (g kg−1) | (mg kg−1) | |||||||||
| Foliar treatment (FT) | Weak | |||||||||
| Control | 30.6 ± 2.15 a | 1.6 ± 0.10 a | 4.3 ± 1.00 a | 12.2 ± 1.70 a | 11.8 ± 3.95 b | 181.7 ± 91.6 a | 49.4 ± 4.4 b | 5.6 ± 0.88 a | 18.5 ± 1.49 a | 34.9 ± 11.86 b |
| Fnut | 29.1 ± 2.09 a | 1.4 ± 0.14 b | 4.0 ± 0.63 a | 12.4 ± 3.18 a | 10.1 ± 1.81 b | 169.0 ± 41.2 a | 43.2 ± 12.5 b | 5.1 ± 0.46 a | 17.2 ± 1.61 a | 30.4 ± 9.86 b |
| Algae | 31.5 ± 1.96 a | 1.6 ± 0.14 a | 4.5 ± 0.85 a | 14.0 ± 1.75 a | 14.8 ± 4.68 a | 127.5 ± 18.9 a | 58.8 ± 8.7 a | 4.8 ± 0.47 a | 17.9 ± 3.11 a | 44.7 ± 7.97 a |
| Year (Y) | ||||||||||
| 2017 | 30.9 ± 1.84 a | 1.6 ± 0.14 a | 3.6 ± 1.51 b | 14.2 ± 2.01 a | 9.2 ± 1.54 b | 195.1 ± 76.3 a | 53.2 ± 3.8 a | 4.9 ± 0.47 a | 16.3 ± 1.85 b | 29.1 ± 9.19 b |
| 2018 | 29.8 ± 2.47 a | 1.5 ± 0.11 b | 4.9 ± 0.43 a | 11.5 ± 1.81 b | 15.2 ± 3.35 a | 123.8 ± 16.0 b | 47.7 ± 14.8 b | 5.5 ± 0.79 a | 19.4 ± 1.01 a | 44.2 ± 7.39 a |
| Prob. (FT) | 0.1282 | 0.0165 | 0.0934 | 0.1362 | 0.0001 | 0.1919 | 0.0008 | 0.0872 | 0.3389 | 0.0030 |
| Prob. (Y) | 0.2555 | 0.0078 | <0.0001 | 0.0028 | <0.0001 | 0.0110 | 0.0424 | 0.0665 | 0.0009 | 0.0001 |
| Prob. (FT×Y) | 0.1167 | 0.5443 | 0.2154 | 0.0402 | 0.0060 | 0.2289 | 0.0005 | 0.4787 | 0.3977 | 0.3045 |
| Foliar treatment (FT) | Very good | |||||||||
| Control | 33.9 ± 2.17 a | 1.4 ± 0.06 a | 24.3 ± 9.48 a | 21.5 ± 7.43 a | 1.19 ± 0.63 a | 100.9 ± 15.2 a | 356.0 ± 88.9 ab | 4.3 ± 1.29 a | 76.7 ± 7.82 a | 70.5 ± 9.50 a |
| Fnut | 35.4 ± 2.43 a | 1.5 ± 0.18 a | 26.1 ± 12.05 a | 17.6 ± 4.54 b | 4.6 ± 1.06 a | 113.2 ± 18.7 a | 367.5 ± 36.3 a | 4.7 ± 1.86 a | 78.9 ± 40.29 a | 64.4 ± 10.20 b |
| Algae | 35.4 ± 1.60 a | 1.6 ± 0.19 a | 24.4 ± 7.20 a | 19.4 ± 6.22 ab | 4.6 ± 0.66 a | 96.6 ± 16.6 a | 285.7 ± 49.4 b | 5.5 ± 1.88 a | 71.8 ± 23.60 a | 62.5 ± 7.63 b |
| Year (Y) | ||||||||||
| 2017 | 33.3 ± 1.69 b | 1.4 ± 0.21 a | 16.5 ± 1.74 b | 24.8 ± 3.73 a | 4.1 ± 0.69 b | 114.9 ± 18.6 a | 309.4 ± 73.7 b | 3.7 ± 1.61 | 64.8 ± 15.86 a | 73.4 ± 6.34 a |
| 2018 | 36.4 ± 1.04 a | 1.5 ± 0.12 a | 33.4 ± 4.12 a | 14.2 ± 1.04 b | 5.6 ± 0.58 a | 92.2 ± 5.7 b | 363.4 ± 51.2 a | 6.0 ± 0.48 | 86.8 ± 29.97 a | 58.2 ± 3.66 b |
| Prob. (FT) | 0.0697 | 0.0570 | 0.4558 | 0.0275 | 0.1925 | 0.0899 | 0.0343 | 0.2364 | 0.8772 | 0.0082 |
| Prob. (Y) | <0.0001 | 0.3468 | <0.0001 | <0.0001 | 0.0002 | 0.0020 | 0.0441 | 0.0009 | 0.0792 | <0.0001 |
| Prob. (FT×Y) | 0.1172 | 0.0285 | 0.0337 | 0.2236 | 0.6847 | 0.3624 | 0.2002 | 0.4213 | 0.2258 | 0.1930 |
| Sufficiency range | [32–56] | [2.7–5.4] | [16–34] | [10–26] | [2.9–6.7] | [44–98] | [45–125] | [8–29] | [23–108] | [18–63] |
| Nitrogen | Phosphorus | Potassium | Calcium | Magnesium | Iron | Manganese | Copper | Zinc | Boron | |
|---|---|---|---|---|---|---|---|---|---|---|
| (g kg−1) | (mg kg−1) | |||||||||
| Foliar treatment (FT) | Weak | |||||||||
| Control | 30.41 ± 5.81 a | 3.63 ± 0.38 a | 9.36 ± 2.01 a | 3.53 ± 1.11 a | 3.49 ± 0.70 a | 236.07 ± 137.4 a | 42.26 ± 7.36 ab | 8.86 ± 1.21 a | 31.99 ± 4.91 b | 18.91 ± 4.59 a |
| Fnut | 27.61 ± 2.58 b | 3.28 ± 0.32 a | 10.73 ± 2.94 a | 3.43 ± 1.23 a | 3.28 ± 0.60 a | 198.64 ± 20.6 a | 36.34 ± 9.99 b | 8.33 ± 1.52 a | 34.53 ± 5.61 ab | 18.62 ± 3.22 a |
| Algae | 28.80 ± 3.98 ab | 3.60 ± 0.32 a | 9.73 ± 2.69 a | 3.71 ± 1.61 a | 3.43 ± 0.78 a | 275.19 ± 132.7 a | 49.99 ± 7.94 a | 9.05 ± 1.54 a | 38.50 ± 11.85 a | 20.53 ± 3.90 a |
| Year (Y) | ||||||||||
| 2017 | 32.92 ± 3.27 a | 3.64 ± 0.39 a | 7.62 ± 1.26 b | 4.89 ± 0.65 a | 3.98 ± 0.49 a | 168.96 ± 27.5 b | 39.33 ± 12.04 a | 8.66 ± 1.57 a | 40.95 ± 8.27 a | 19.10 ± 4.62 a |
| 2018 | 25.96 ± 1.67 b | 3.40 ± 0.31 a | 11.68 ± 1.63 a | 2.56 ± 0.29 b | 2.97 ± 0.39 b | 287.38 ± 122.0 a | 45.51 ± 7.40 a | 8.82 ± 1.32 a | 30.55 ± 4.51 b | 19.55 ± 3.61 a |
| Prob. (FT) | 0.0103 | 0.0598 | 0.2794 | 0.4119 | 0.6423 | 0.4401 | 0.0040 | 0.5315 | 0.0475 | 0.3316 |
| Prob. (Y) | <0.0001 | 0.0803 | <0.0001 | <0.0001 | 0.0001 | 0.0078 | 0.0619 | 0.8059 | 0.0004 | 0.7716 |
| Prob. (FT×Y) | 0.0062 | 0.0691 | 0.5585 | 0.1571 | 0.4437 | 0.1396 | 0.0424 | 0.2594 | 0.0407 | 0.0182 |
| Foliar treatment (FT) | Very good | |||||||||
| Control | 25.5 ± 1.33 ab | 2.9 ± 0.35 a | 20.3 ± 2.75 a | 4.1 ± 1.93 a | 2.0 ± 0.24 a | 151.1 ± 29.6 a | 77.3 ± 15.94 a | 6.7 ± 0.57 a | 33.7 ± 3.80 a | 27.8 ± 2.53 a |
| Fnut | 26.2 ± 1.15 a | 2.8 ± 0.18 a | 21.1 ± 3.24 a | 4.0 ± 1.77 a | 2.2 ± 0.26 a | 144.0 ± 17.8 a | 83.0 ± 12.07 a | 6.4 ± 0.58 a | 32.2 ± 2.51 a | 25.8 ± 1.24 ab |
| Algae | 24.3 ± 1.00 b | 2.8 ± 0.35 a | 19.4 ± 2.48 a | 3.7 ± 1.60 a | 2.0 ± 0.12 a | 128.0 ± 9.3 a | 61.8 ± 5.90 b | 6.3 ± 0.89 a | 30.6 ± 0.94 a | 25.1 ± 1.76 b |
| Year (Y) | ||||||||||
| 2017 | 25.7 ± 1.28 a | 3.0 ± 0.23 a | 18.0 ± 1.47 b | 5.6 ± 0.5 a | 2.0 ± 0.17 b | 138.6 ± 9.0 a | 68.5 ± 11.81 b | 6.5 ± 0.45 a | 32.3 ± 3.21 a | 27.2 ± 2.03 a |
| 2018 | 25.0 ± 1.43 a | 2.6 ± 0.12 b | 22.5 ± 1.81 a | 2.3 ± 0.39 b | 2.1 ± 0.25 a | 143.6 ± 30.4 a | 79.5 ± 15.65 a | 6.4 ± 0.89 a | 32.0 ± 2.62 a | 25.2 ± 1.92 b |
| Prob. (FT) | 0.0112 | 0.4108 | 0.5595 | 0.2999 | 0.0768 | 0.1051 | 0.0040 | 0.5088 | 0.1033 | 0.0125 |
| Prob. (Y) | 0.1509 | <0.0001 | <0.0001 | <0.0001 | 0.0456 | 0.5648 | 0.0269 | 0.7436 | 0.7442 | 0.0097 |
| Prob. (FT×Y) | 0.3497 | 0.0452 | 0.8345 | 0.3995 | 0.7158 | 0.3533 | 0.8485 | 0.3855 | 0.3827 | 0.6418 |
| SPAD | NDVI | O | J | I | P | FV/FM | FV/F0 | |
|---|---|---|---|---|---|---|---|---|
| Foliar treatment (FT) | Weak | |||||||
| Control | 33.5 ± 2.81 b | 0.72 ± 0.08 a | 256 ± 20 a | 362 ± 15 a | 503 ± 56 a | 631 ± 39 b | 0.74 ± 0.02 a | 2795 ± 238 a |
| Fnut | 33.2 ± 3.06 b | 0.73 ± 0.04 a | 265 ± 37 a | 389 ± 54 a | 561 ± 63 a | 714 ± 65 a | 0.76 ± 0.02 a | 3265 ± 295 a |
| Algae | 36.4 ± 2.42 a | 0.74 ± 0.02 a | 256 ± 53 a | 372 ± 81 a | 532 ± 53 a | 697 ± 46 a | 0.77 ± 0.03 a | 3338 ± 598 a |
| Year (Y) | ||||||||
| 2017 | 35.1 ± 1.92 a | 0.69 ± 0.03 b | 273 ± 44 a | 397 ± 71 a | 566 ± 45 a | 708 ± 54 a | 0.75 ± 0.02 a | 3035 ± 354 a |
| 2018 | 33.6 ± 3.78 a | 0.77 ± 0.03 a | 244 ± 22 b | 352 ± 14 b | 498 ± 54 b | 653 ± 57 b | 0.76 ± 0.03 a | 3230 ± 545 a |
| Prob. (FT) | 0.0172 | 0.5864 | 0.9039 | 0.7045 | 0.1160 | 0.0099 | 0.0997 | 0.0840 |
| Prob. (Y) | 0.1029 | <0.0001 | 0.1361 | 0.1113 | 0.0073 | 0.01338 | 0.4274 | 0.3344 |
| Prob. (FT×Y) | 0.0031 | 0.0031 | 0.7454 | 0.6558 | 0.3110 | 0.1990 | 0.4231 | 0.4405 |
| Foliar treatment (FT) | Very good | |||||||
| Control | 42.6 ± 2.75 a | 0.77 ± 0.05 ab | 256 ± 11 a | 402 ± 21 a | 705 ± 64 a | 877 ± 88 a | 0.81 ± 0.02 a | 4291 ± 536 a |
| Fnut | 43.7 ± 1.99 a | 0.77 ± 0.03 a | 250 ± 16 a | 378 ± 16 a | 722 ± 67 a | 900 ± 55 a | 0.82 ± 0.01 a | 4547 ± 272 a |
| Algae | 43.1 ± 2.66 a | 0.75 ± 0.04 b | 252 ± 17 a | 399 ± 25 a | 694 ± 70 a | 886 ± 72 a | 0.82 ± 0.02 a | 4484 ± 454 a |
| Year (Y) | ||||||||
| 2017 | 45.1 ± 1.16 a | 0.73 ± 0.02 b | 245 ± 9 b | 389 ± 18 a | 729 ± 44 a | 922 ± 65 a | 0.82 ± 0.01 a | 4650 ± 407 a |
| 2018 | 41.2 ± 1.51 b | 0.80 ± 0.01 a | 259 ± 15 a | 396 ± 26 a | 691 ± 74 a | 862 ± 64 a | 0.81 ± 0.02 a | 4284 ± 386 b |
| Prob. (FT) | 0.3879 | 0.0188 | 0.7242 | 0.0941 | 0.8514 | 0.8281 | 0.4669 | 0.5198 |
| Prob. (Y) | <0.0001 | <0.0001 | 0.0376 | 0.4652 | 0.2004 | 0.0558 | 0.0591 | 0.0488 |
| Prob. (FT×Y) | 0.4289 | 0.1555 | 0.7781 | 0.6826 | 0.3536 | 0.2864 | 0.2727 | 0.2393 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afonso, S.; Arrobas, M.; Rodrigues, M.Â. Response of Hops to Algae-Based and Nutrient-Rich Foliar Sprays. Agriculture 2021, 11, 798. https://doi.org/10.3390/agriculture11080798
Afonso S, Arrobas M, Rodrigues MÂ. Response of Hops to Algae-Based and Nutrient-Rich Foliar Sprays. Agriculture. 2021; 11(8):798. https://doi.org/10.3390/agriculture11080798
Chicago/Turabian StyleAfonso, Sandra, Margarida Arrobas, and M. Ângelo Rodrigues. 2021. "Response of Hops to Algae-Based and Nutrient-Rich Foliar Sprays" Agriculture 11, no. 8: 798. https://doi.org/10.3390/agriculture11080798
APA StyleAfonso, S., Arrobas, M., & Rodrigues, M. Â. (2021). Response of Hops to Algae-Based and Nutrient-Rich Foliar Sprays. Agriculture, 11(8), 798. https://doi.org/10.3390/agriculture11080798






