Assessment of Different Water Use Efficiency Calculations for Dominant Forage Crops in the Great Lakes Basin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Data Collection
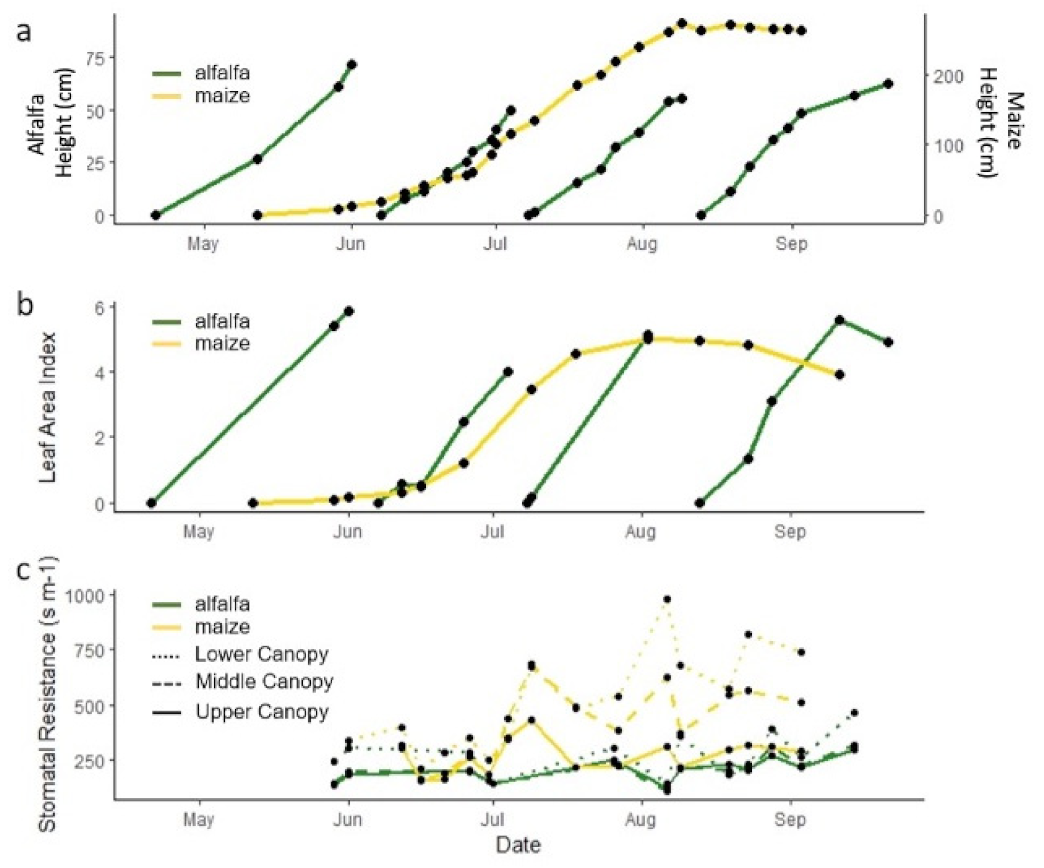
2.2.1. Vegetation
2.2.2. Hydrometric Data Collection
2.2.3. Carbon and Water Flux Processing
2.3. Data Analysis
3. Results and Discussion
3.1. Influence of Approach and Crop Type on WUE Estimates
3.2. Importance of Input Variables and Processing Methods on WUE Estimates
3.2.1. Importance of Water Use Variable
3.2.2. Importance of Carbon Input Variables on Differences between Harvest Water Use Efficiency and Ecosystem Water Use Efficiency
3.2.3. Importance of Processing Method for Ecosystem Water Use Efficiency Method
3.3. Impact of Physiological Stage and Anthropogenic Influences on WUE Methods at Both Seasonal and Shorter Timescales
3.3.1. Impact of Crop Physiology at Different Timescales on Water Use Efficiency Estimate
3.3.2. Impact of Crop Physiology on Water Use Efficiency Estimates
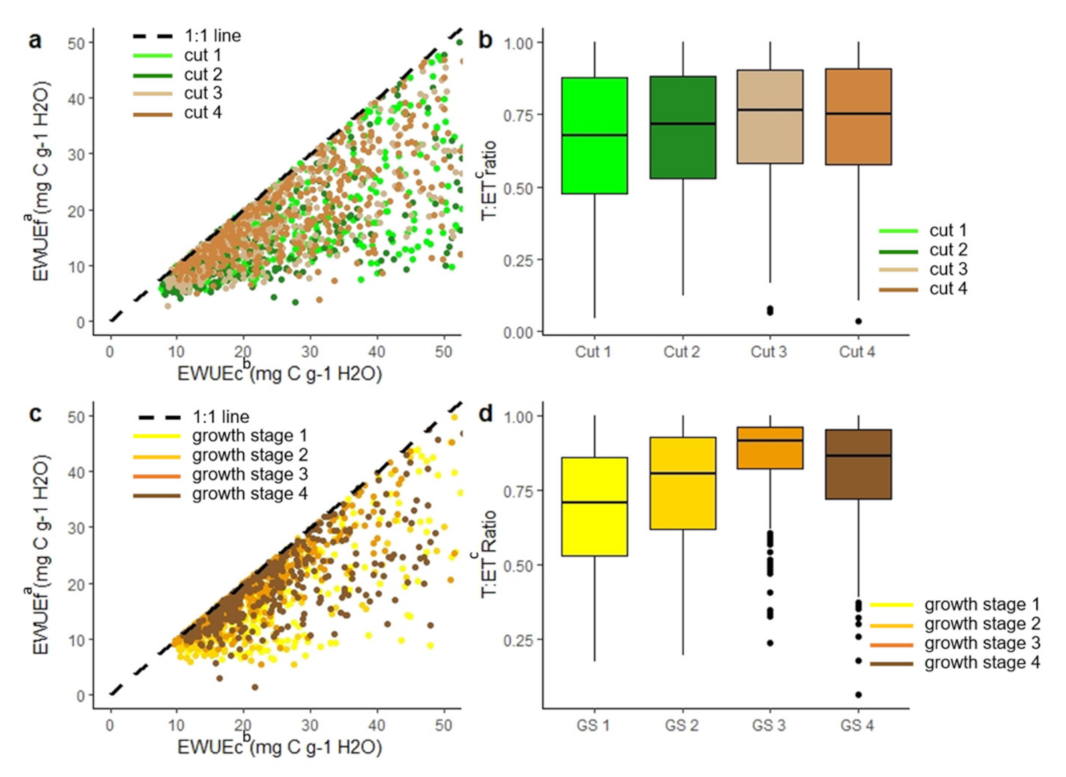
3.3.3. Impact of Crop Physiology on Inconsistencies in Ecosystem Water Use Efficiency Estimates
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ito, A.; Inatomi, M. Water-use efficiency of the terrestrial biosphere: A model analysis focusing on interactions between the global carbon and water cycles. J. Hydrometeorol. 2012, 13, 681–694. [Google Scholar] [CrossRef]
- Knauer, J.; Zaehle, S.; Medlyn, B.E.; Reichstein, M.; Williams, C.A.; Migliavacca, M.; De Kauwe, M.G.; Werner, C.; Keitel, C.; Kolari, P.; et al. Towards physiologically meaningful water-use efficiency estimates from eddy covariance data. Glob. Chang. Biol. 2018, 24, 694–710. [Google Scholar] [CrossRef]
- Beer, C.; Ciais, P.; Reichstein, M.; Baldocchi, D.; Law, B.E.; Papale, D.; Soussana, J.F.; Ammann, C.; Buchmann, N.; Frank, D.; et al. Temporal and among-site variability of inherent water use efficiency at the ecosystem level. Glob. Biogeochem. Cycles 2009, 23, 1–13. [Google Scholar] [CrossRef]
- Kuglitsch, F.G.; Reichstein, M.; Beer, C.; Carrara, A.; Ceulemans, R.; Granier, A.; Janssens, I.A.; Koestner, B.; Lindroth, A.; Loustau, D.; et al. Characterisation of ecosystem water-use efficiency of european forests from eddy covariance measurements. Biogeosci. Discuss. 2008, 5, 4481–4519. [Google Scholar]
- Medlyn, B.E.; De Kauwe, M.G.; Lin, Y.S.; Knauer, J.; Duursma, R.A.; Williams, C.A.; Arneth, A.; Clement, R.; Isaac, P.; Limousin, J.M.; et al. How do leaf and ecosystem measures of water-use efficiency compare? New Phytol. 2017, 216, 758–770. [Google Scholar] [CrossRef] [Green Version]
- Lawson, T.; Blatt, M.R. Stomatal size, speed, and responsiveness impact on photosynthesis and water use efficiency. Plant Physiol. 2014, 164, 1556–1570. [Google Scholar] [CrossRef] [Green Version]
- Baldocchi, D.D. Assessing the eddy covariance technique for evaluating carbon dioxide exchange rates of ecosystems: Past, present and future. Glob. Chang. Biol. 2003, 9, 479–492. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Still, C.J.; Rastogi, B.; Page, G.F.M.; Wharton, S.; Meinzer, F.C.; Voelker, S.; Kim, J.B. Trends and controls on water-use efficiency of an old-growth coniferous forest in the Pacific Northwest. Environ. Res. Lett. 2019. [Google Scholar] [CrossRef] [Green Version]
- Hatfield, J.L.; Dold, C. Water-use efficiency: Advances and challenges in a changing climate. Front. Plant Sci. 2019, 10, 103. [Google Scholar] [CrossRef] [Green Version]
- Albertson, J.D.; Katul, G.G.; Wiberg, P. Relative importance of local and regional controls on coupled water, carbon, and energy Fluxes. Adv. Water 2001, 24, 1103–1118. [Google Scholar] [CrossRef]
- Yu, L.; Gao, X.; Zhao, X. Global synthesis of the impact of droughts on crops’ water-use efficiency (WUE): Towards both high WUE and productivity. Agric. Syst. 2020, 177, 102723. [Google Scholar] [CrossRef]
- Chapin, F.; Matson, P.; Vitousek, P. Principles of Terrestrial Ecosystem Ecology, 2nd ed.; Springer: New York, NY, USA, 2011. [Google Scholar] [CrossRef]
- Kang, W.; Kang, S. On the use of alternative water use efficiency parameters in dryland ecosystems: A review. J. Ecol. Environ. 2019, 43, 24. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Sharkey, T.D. Stomatal Conductance and Photosynthesis. Ann. Rev. Plant Phys. 1982, 33, 317–345. [Google Scholar] [CrossRef]
- Goyal, M.; Harmsen, E. (Eds.) Evapotranspiration: Principles and Applications for Water Management; Apple Academic Press: Toronto, ON, Canada, 2014; ISBN 9781774632864. [Google Scholar]
- Condon, A.G.; Richards, R.A.; Rebetzke, G.J.; Farquhar, G.D. Improving Intrinsic Water-Use Efficiency and Crop Yield. Crop Sci. 2002, 42, 122–131. [Google Scholar] [CrossRef]
- Maleski, J.J.; Bosch, D.D.; Anderson, R.G.; Coffin, A.W.; Anderson, W.F.; Strickland, T.C. Evaluation of miscanthus productivity and water use efficiency in southeastern United States. Sci. Total Environ. 2019, 692, 1125–1134. [Google Scholar] [CrossRef] [PubMed]
- VanLoocke, A.; Twine, T.E.; Zeri, M.; Bernacchi, C.J. A regional comparison of water use efficiency for miscanthus, switchgrass and maize. Agric. For. Meteorol. 2012, 164, 82–95. [Google Scholar] [CrossRef]
- Kimball, B.A.; Boote, K.J.; Hatfield, J.L.; Ahuja, L.R.; Stockle, C.; Archontoulis, S.; Baron, C.; Basso, B.; Bertuzzi, P.; Constantin, J.; et al. Simulation of maize evapotranspiration: An inter-comparison among 29 maize models. Agric. For. Meteorol. 2019, 271, 264–284. [Google Scholar] [CrossRef]
- McMillen, R.T. An eddy correlation technique with extended applicability to non-simple terrain. Boundary-Layer Meteorol. 1988, 43, 231–245. [Google Scholar] [CrossRef]
- Moncrieff, J.B.; Massheder, J.M.; De Bruin, H.; Elbers, J.; Friborg, T.; Heusinkveld, B.; Kabat, P.; Scott, S.; Soegaard, H.; Verhoef, A. A system to measure surface fluxes of momentum, sensible heat, water vapour and carbon dioxide. J. Hydrol. 1997, 188–189, 589–611. [Google Scholar] [CrossRef]
- Skaggs, T.H.; Anderson, R.G.; Al, J.G.; Scanlon, T.M.; Kustas, W.P. Agricultural and Forest Meteorology Fluxpart: Open source software for partitioning carbon dioxide and water vapor fluxes. Agric. For. Meteorol. 2018, 254, 218–224. [Google Scholar] [CrossRef]
- Wutzler, T.; Lucas-Moffat, A.; Migliavacca, M.; Knauer, J.; Sickel, K.; Sigut Menzer, O.; Reichstein, M. Basic and extensible post-processing of eddy covariance flux data with REddyProc. Biogeosciences 2018, 15, 5015–5030. [Google Scholar] [CrossRef] [Green Version]
- Arriga, N.; Rannik, Ü.; Aubinet, M.; Carrara, A.; Vesala, T.; Papale, D. Experimental validation of footprint models for eddy covariance CO2 flux measurements above grassland by means of natural and artificial tracers. Agric. For. Meteorol. 2017, 242, 75–84. [Google Scholar] [CrossRef]
- Foken, T.; Leclerc, M.Y. Methods and limitations in validation of footprint models. Agric. For. Meteorol. 2004, 127, 223–234. [Google Scholar] [CrossRef]
- Vesala, T.; Kljun, N.; Rannik, Ü.; Rinne, J.; Sogachev, A.; Markkanen, T.; Sabelfeld, K.; Foken, T.; Leclerc, M.Y. Flux and concentration footprint modelling: State of the art. Environ. Pollut. 2008, 152, 653–666. [Google Scholar] [CrossRef]
- Falge, E.; Baldocchi, D.; Olson, R.; Anthoni, P.; Aubinet, M.; Bernhofer, C.; Burba, G.; Ceulemans, R.; Clement, R.; Dolman, H.; et al. Gap filling strategies for defensible annual sums of net ecosystem exchange. Agric. For. Meteorol. 2001, 107, 43–69. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Huang, Y. A comparison of three gap filling techniques for eddy covariance net carbon fluxes in short vegetation ecosystems. Adv. Meteorol. 2015, 2015, 260580. [Google Scholar] [CrossRef] [Green Version]
- Eichelmann, E.; Wagner-Riddle, C.; Warland, J.; Deen, B.; Voroney, P. Evapotranspiration, water use efficiency, and energy partitioning of a mature switchgrass stand. Agric. For. Meteorol. 2016, 217, 108–119. [Google Scholar] [CrossRef]
- FAO. Challenges and opportunities for carbon sequestration in grassland systems. A technical report on grassland management and climate change mitigation. Integr. Crop Manag. 2010, 9. Available online: http://www.fao.org/3/i1399e/i1399e.pdf (accessed on 20 July 2021).
- Aguilos, M.; Stahl, C.; Burban, B.; Hérault, B.; Courtois, E. Interannual and Seasonal Variations in Ecosystem Transpiration and Water Use Efficiency in a Tropical Rainforest. Forests 2018, 10, 14. [Google Scholar] [CrossRef] [Green Version]
- Presant, E.W.; Wicklund, R.E. The Soils of Waterloo County, Report No. 44 of the Ontario Soil Survey; Research Branch, Canada Department of Agriculture, Department of Soil Science, University of Guelph and The Ontario Department of Agriculture and Food: Guelph, ON, Canada, 1971; 103p. [Google Scholar]
- Platt, S.; Bassham, J. Photosynthesis and increased production of protein. Adv. Exp. Med. Biol. 1978, 105, 195–247. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.B. C3 or C4? Maize Mutations and the Elaboration of Kranz Anatomy. Plant Cell 1996, 8, 761–762. [Google Scholar] [CrossRef]
- Engels, F.M.; Jung, H.G. Alfalfa stem tissues: Cell-wall development and lignification. Ann. Bot. 1998, 82, 561–568. [Google Scholar] [CrossRef] [Green Version]
- Pittman, J.J.; Arnall, D.B.; Interrante, S.M.; Moffet, C.A.; Butler, T.J. Estimation of biomass and canopy height in bermudagrass, alfalfa, and wheat using ultrasonic, laser, and spectral sensors. Sensors 2015, 15, 2920–2943. [Google Scholar] [CrossRef] [Green Version]
- LI-COR Biosciences; Eddy Covariance Processing Software: Lincoln, NB, USA, 2017; Version # N/A.
- Fratini, G.; Mauder, M. Towards a consistent eddy-covariance processing: An intercomparison of EddyPro and TK3. Atmos. Meas. Tech. 2014, 7, 2273–2281. [Google Scholar] [CrossRef] [Green Version]
- Webb, E.K.; Pearman, G.I.; Leuning, R. Correction of flux measurements for density effects due to heat and water vapour transfer. Q. J. R. Meteorol. Soc. 1980, 106, 85–100. [Google Scholar] [CrossRef]
- Tanner, C.B.; Thurtell, G.W. Anemoclinometer measurements of Reynolds stress and heat transport in the atmospheric surface layer. In US Army Electronics Command; Atmospheric Sciences Laboratory (US): Fort Huachuca, AZ, USA, 1969. [Google Scholar]
- Kljun, N.; Calanca, P.; Rotach, M.W.; Schmid, H.P. A simple two-dimensional parameterisation for Flux Footprint Prediction (FFP). Geosci. Model Dev. 2015, 8, 3695–3713. [Google Scholar] [CrossRef] [Green Version]
- Goulden, M.L.; Munger, J.W.; Fan, S.M.; Daube, B.C.; Wofsy, S.C. Measurements of carbon sequestration by long-term eddy covariance: Methods and a critical evaluation of accuracy. Glob. Chang. Biol. 1996, 2, 169–182. [Google Scholar] [CrossRef] [Green Version]
- Wilson, K. Energy balance closure at FLUXNET sites. Agric. For. Meteorol. 2002, 113, 223–243. [Google Scholar] [CrossRef] [Green Version]
- Priestley, C.H.B.; Taylor, R.J. On the Assessment of Surface Heat Flux and Evaporation Using Large-Scale Parameters. Mon. Weather. Rev. 1972, 100, 81–92. [Google Scholar] [CrossRef]
- Scanlon, T.M.; Kustas, W.P. Partitioning carbon dioxide and water vapor fluxes using correlation analysis. Agric. For. Meteorol. 2010, 150, 89–99. [Google Scholar] [CrossRef]
- Scanlon, T.M.; Sahu, P. On the correlation structure of water vapor and carbon dioxide in the atmospheric surface layer: A basis for flux partitioning. Water Resour. Res. 2008, 44, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Monin, A.S.; Obukhov, A. Basic Laws of Turbulent Mixing in the Surface Layer of the Atmosphere. Contrib. Geophys. Inst. Acad. Sci. USSR 1954, 24, 163–187. [Google Scholar]
- Massman, W.J. A review of the molecular diffusivities of H2O, CO2, CH4, CO, O3, SO2, NH3, N2O, NO, and NO2 in air, O2 and N2 near STP. Atmos. Environ. 1998, 32, 1111–1127. [Google Scholar] [CrossRef]
- Deng, X.-P.; Shan, L.; Zhang, H.; Turner, N. Improving Agricultural Water Use Efficiency in Arid and Semiarid Areas of China. Agric. Water Manag. 2006, 23–40. [Google Scholar] [CrossRef]
- Varvel, G. Precipitation Use Efficiency of Soybean and Grain Sorghum in Monoculture and Rotation. Soil Sci. Soc. Am. J. 1995, 59, 527–531. [Google Scholar] [CrossRef] [Green Version]
- Miranda, J.D.; Armas, C.; Padilla, F.M.; Pugnaire, F.I. Climatic change and rainfall patterns: Effects on semi-arid plant communities of the Iberian Southeast. J. Arid Environ. 2011, 75, 1302–1309. [Google Scholar] [CrossRef]
- Sulman, B.N.; Roman, D.T.; Scanlon, T.M.; Wang, L.; Novick, K.A. Comparing methods for partitioning a decade of carbon dioxide and water vapor fluxes in a temperate forest. Agric. For. Meteorol 2016, 226–227, 229–245. [Google Scholar] [CrossRef] [Green Version]
- Klosterhalfen, A.; Graf, A.; Brüggemann, N.; Drüe, C.; Esser, O.; González-Dugo, M.P.; Heinemann, G.; Jacobs, C.M.J.; Mauder, M.; Moene, A.F.; et al. Source partitioning of H2O and CO2 fluxes based on high-frequency eddy covariance data: A comparison between study sites. Biogeosciences 2019, 16, 1111–1132. [Google Scholar] [CrossRef] [Green Version]
- Shapiro, A.S.S.; Wilk, M.B. An Analysis of Variance Test for Normality (Complete Samples) Published by: Oxford University Press on behalf of Biometrika Trust Stable. Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Hussain, M.Z.; Hamilton, S.K.; Bhardwaj, A.K.; Basso, B.; Thelen, K.D.; Robertson, G.P. Evapotranspiration and water use efficiency of continuous maize and maize and soybean in rotation in the upper Midwest U.S. Agric. Water Manag. 2019, 221, 92–98. [Google Scholar] [CrossRef]
- Zwart, S.J.; Bastiaanssen, W.G.M. Review of measured crop water productivity values for irrigated wheat, rice, cotton and maize. Agric. Water Manag. 2004, 69, 115–133. [Google Scholar] [CrossRef]
- Suyker, A.E.; Verma, S.B. Coupling of carbon dioxide and water vapor exchanges of irrigated and rainfed maize-soybean cropping systems and water productivity. Agric. For. Meteorol. 2010, 150, 553–563. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Tang, X.; Zheng, C.; Gu, Q.; Wei, J.; Ma, M. Differences in ecosystem water-use efficiency among the typical croplands. Agric. Water Manag. 2018, 209, 142–150. [Google Scholar] [CrossRef]
- Jefferson, P.G.; Cutforth, H.W. Comparative forage yield, water use, and water use efficiency of alfalfa, crested wheatgrass and spring wheat in a semiarid climate in southern Saskatchewan. Can. J. Plant Sci. 2005, 85, 877–888. [Google Scholar] [CrossRef] [Green Version]
- Mueller, L.; Behrendt, A.; Schalitz, G.; Schindler, U. Above ground biomass and water use efficiency of crops at shallow water tables in a temperate climate. Agric. Water Manag. 2005, 75, 117–136. [Google Scholar] [CrossRef]
- Wagle, P.; Gowda, P.H.; Northup, B.K. Dynamics of evapotranspiration over a non-irrigated alfalfa field in the Southern Great Plains of the United States. Agric. Water Manag. 2019, 223, 105727. [Google Scholar] [CrossRef]
- Morison, J.; Gifford, R. Stomatal Sensitivity to Carbon Dioxide and Humidity: A Comparison of Two C3 and Two C4 Grass Species. Plant Physiol. 1983, 71, 789–796. [Google Scholar] [CrossRef] [Green Version]
- Ghannoum, O.; Evans, J.R.; von Caemmerer, S. Nitrogen and Water Use Efficiency of C4 Plants. In C4 Photosynthesis and Related CO2 Concentrating Mechanisms; Rowan, S.A., Ed.; Springer Science: Berlin/Heidelberg, Germany, 2011; pp. 129–146. [Google Scholar] [CrossRef]
- Hsiao, T.; Acevedo, E. Plant responses to water deficits, water-use efficiency, and drought resistance. Agric. Meteorol. 1974, 14, 59–84. [Google Scholar] [CrossRef]
- Osborne, C.P.; Sack, L. Evolution of C4 plants: A new hypothesis for an interaction of CO2 and water relations mediated by plant hydraulics. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 583–600. [Google Scholar] [CrossRef] [Green Version]
- Rawson, H.M.; Begg, J.E.; Woodward, R.G. The effect of atmospheric humidity on photosynthesis, transpiration and water use efficiency of leaves of several plant species. Planta 1977, 134, 5–10. [Google Scholar] [CrossRef]
- Way, D.A.; Katul, G.G.; Manzoni, S.; Vico, G. Increasing water use efficiency along the C3 to C4 evolutionary pathway: A stomatal optimization perspective. J. Exp. Bot. 2014, 65, 3683–3693. [Google Scholar] [CrossRef] [Green Version]
- Kocacinar, F.; McKown, A.D.; Sage, T.L.; Sage, R.F. Photosynthetic pathway influences xylem structure and function in Flaveria (Asteraceae). Plant Cell Environ. 2008, 31, 1363–1376. [Google Scholar] [CrossRef]
- Bolinder, M.A.; Angers, D.A.; Bélanger, G.; Michaud, R.; Laverdière, M.R. Root biomass and shoot to root ratios of perennial forage crops in eastern Canada. Can. J. Plant Sci. 2002, 82, 731–737. [Google Scholar] [CrossRef] [Green Version]
- Skuodienė, R.; Tomchuk, D. Root mass and root to shoot ratio of different perennial forage plants under western Lithuania climatic conditions. Rom. Agric. Res. 2015, 32, 1–11. [Google Scholar]
- Li, Q.; Zhou, D.; Denton, M.D.; Cong, S. Alfalfa monocultures promote soil organic carbon accumulation to a greater extent than perennial grass monocultures or grass-alfalfa mixtures. Ecol. Eng. 2019, 131, 53–62. [Google Scholar] [CrossRef]
- Amos, B.; Walters, D. Maize Root Biomass and Net Rhizo deposited Carbon. Soil Sci. Soc. Am. 2006, 70, 1489–1503. [Google Scholar] [CrossRef]
- Hirte, J.; Leifeld, J.; Abiven, S.; Oberholzer, H.R.; Mayer, J. Below ground carbon inputs to soil via root biomass and rhizodeposition of field-grown maize and wheat at harvest are independent of net primary productivity. Agric. Ecosyst. Environ. 2018, 265, 556–566. [Google Scholar] [CrossRef]
- De Haan, K. Progressing Towards Understanding Water Use Efficiency in Southern, Ontario Canada: Quantifying Water Use Efficiency Metrics (WUE) and Investigating Soil and Plant Physiology Influences on WUE. Master’s Thesis, University of Waterloo, Waterloo, ON, Canada, 2020. [Google Scholar]
- Ecoregions Working Group. Ecoclimatic Regions of Canada, First Approximation; Ecological Land Classification Series 23; Ecoregions Working Group: Ottawa, ON, Canada, 1989; p. 118. [Google Scholar]
- Yang, Z.; Zhang, Q.; Hao, X. Evapotranspiration Trend and Its Relationship with Precipitation over the Loess Plateau during the Last Three Decades. Adv. Meteorol. 2016, 2016, 6809749. [Google Scholar] [CrossRef] [Green Version]
- Choudhury, B.J. Modeling radiation- and carbon-use efficiencies of maize, sorghum, and rice. Agric. For. Meteorol. 2001, 106, 317–330. [Google Scholar] [CrossRef]
- Anderson, R.G.; Zhang, X.; Skaggs, T.H. Measurement and Partitioning of Evapotranspiration for Application to Vadose Zone Studies. Vadose Zone J. 2017, 16, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Palatella, L.; Rana, G.; Vitale, D. Towards a Flux-Partitioning Procedure Based on the Direct Use of High-Frequency Eddy-Covariance Data. Boundary-Layer Meteorol. 2014, 153, 327–337. [Google Scholar] [CrossRef]
- Perez-Priego, O.; Katul, G.; Reichstein, M.; El-Madany, T.S.; Ahrens, B.; Carrara, A.; Scanlon, T.M.; Migliavacca, M. Partitioning Eddy Covariance Water Flux Components Using Physiological and Micrometeorological Approaches. J. Geophys. Res. Biogeosci. 2018, 123, 3353–3370. [Google Scholar] [CrossRef]
- Craufurd, P.Q.; Wheeler, T.R.; Ellis, R.H.; Summerfield, R.J.; Williams, J.H. Effect of Temperature and Water Deficit on Water-Use Efficiency, Carbon Isotope Discrimination, and Specific Leaf Area in Peanut. Crop Sci. 1999, 39, 136–142. [Google Scholar] [CrossRef]
- Sinclair, T.R.; Tanner, C.B.; Bennett, J.M. Water-Use Efficiency in Crop Production. Bioscience 1984, 34, 36–40. [Google Scholar] [CrossRef]
- Ehleringer, J.R.; Cerling, T.E.; Helliker, B.R. C4 photosynthesis, atmospheric CO2, and climate. Oecologia 1997, 112, 285–299. Available online: https://www.jstor.org/stable/4221776 (accessed on 14 November 2020). [CrossRef]
- Edwards, E.J.; Osborne, C.P.; Strömberg, C.A.; Smith, S.A.; Bond, W.J.; Christin, P.A.; Cousins, A.B.; Duvall, M.R.; Fox, D.L.; Freckleton, R.P.; et al. The origins of C4 grasslands: Integrating evolutionary and ecosystem science. Science 2010, 328, 587–591. [Google Scholar] [CrossRef] [Green Version]
- Todd, R.W.; Klocke, N.L.; Hergert, G.W.; Parkhurst, A.M. Evaporation from soil influenced by crop shading, crop residue, and wetting regime. Trans. Am. Soc. Agric. Eng. 1991, 34, 461–466. [Google Scholar] [CrossRef]
- Barbieri, P.; Echarte, L.; Della Maggiora, A.; Sadras, V.O.; Echeverria, H.; Andrade, F.H. Maize evapotranspiration and water-use efficiency in response to row spacing. Agron. J. 2012, 104, 939–944. [Google Scholar] [CrossRef]
- Ritchie, J.T. Model for predicting evaporation from a row crop with incomplete cover. Water Resour. Res. 1972, 8, 1204–1213. [Google Scholar] [CrossRef] [Green Version]
- Lin, Z.F.; Ehleringer, J. Effects of leaf age on photosynthesis and water use efficiency of papaya. Photosynthetica 1982, 16, 514–519. [Google Scholar]
- Warren, C.R. Why does photosynthesis decrease with needle age in Pinus pinaster? Trees Struct. Funct. 2006, 20, 157–164. [Google Scholar] [CrossRef]
- Wullschleger, S.D.; Oosterhuis, D.M. Water use efficiency as a function of leaf age and position within the cotton canopy. Plant Soil 1989, 120, 79–85. [Google Scholar] [CrossRef]


| Method | Timescale | Formula | Variables | Advantages | Disadvantages |
|---|---|---|---|---|---|
| HWUEET | Season, Cuts (alfalfa), Growth Stage (maize) | HWUEET = | AGB a carbon content EddyPro/REddyProc ET b | Yield–important to agricultural production, irrigational needs 1 | No below ground carbon storage 2 |
| HWUEP | Season Cuts (alfalfa) Growth Stage (maize) | HWUEP = | AGB a carbon content Precipitation | Yield–important to agricultural production; water use linked only to precipitation 3 Requires minimal equipment. | No below ground carbon storage 2 Evaporation and soil water depletion not measured 3 No frequency and intensity of precipitation 4 |
| EWUES | Season Cuts (alfalfa) Growth Stage (maize) Half-hourly | EWUES | EddyPro/REddyProc GPP c EddyPro/REddyProc ET | Direct measurement of carbon and water exchanges 5 Intra-seasonal variation in WUE e. | Carbon assimilation and transpiration are not directly quantified 5 Requires additional meteorological inputs to partition NEE f to GPP c and Re g,6 |
| EWUEF | Half-hourly | EWUEF | Fluxpart * GPP c Fluxpart * ET b | Minimal equipment to partition NEE f,6 Intra-seasonal variation in WUE e. | Relatively new program requiring broad validation 7 Continuous estimation of leaf scale WUE e required 6 |
| EWUEC | Half-hourly | EWUEC | Fluxpart * GPP c Fluxpart * T d | Stomatal components provide better measure of physiological responses 7 | Relatively new program still requiring broad validation 8 Requires continuous estimation of leaf scale WUE e,6 and stomatal fluxes 6,9 |
| Date | HWUEp a | HWUEET b | EWUES c | Median EWUES d | Median EWUEF e | Median EWUEC f | ||
|---|---|---|---|---|---|---|---|---|
| Alfalfa | Cut 1 | 21 Apr–7 June | 1.51 | 0.81 | 3.11 | 3.79 | 18.17 | 31.46 |
| Cut 2 | 8 June–6 July | 1.80 | 0.66 | 2.84 | 3.35 | 12.87 | 19.31 | |
| Cut 3 | 7 July–13 Aug | 1.14 | 0.65 | 3.02 | 3.66 | 16.65 | 20.54 | |
| Cut 4 | 14 Aug–21 Sept | 1.43 | 1.04 | 3.57 | 4.85 | 19.42 | 27.86 | |
| Growing Season | 21 Apr–21 Sept | 1.45 | 0.78 | 3.11 | 3.91 | 16.81 | 24.48 | |
| Maize | Growth Stage 1 | 3 May–26 June | 0.42 | 0.25 | 1.26 | 2.06 | 15.97 | 21.55 |
| Growth Stage 2 | 27 June–18 July | 5.24 | 1.73 | 3.42 | 4.01 | 12.16 | 16.05 | |
| Growth Stage 3 | 19 July–13 Aug | 4.01 | 2.24 | 3.46 | 5.18 | 17.47 | 20.39 | |
| Growth Stage 4 | 14 Aug–11 Sept | 4.76 | 3.96 | 3.21 | 5.83 | 17.17 | 22.50 | |
| Growing Season | 3 May–11 Sept | 3.01 | 1.73 | 2.58 | 4.05 | 16.18 | 20.57 |
| Date | P a | ETS b | Median ETS b | Median ETF c | Median TF d | BioC e | GPPS f | Median GPPS f | Median GPPF g | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Alfalfa | Cut 1 | 21 Apr–7 June | 0.93 | 1.75 | 2.21 | 1.15 | 0.548 | 1.41 | 5.45 | 1.25 | 3.95 |
| Cut 2 | 8 June–6 July | 0.54 | 1.48 | 3.57 | 2.74 | 1.76 | 0.98 | 4.21 | 2.01 | 5.38 | |
| Cut 3 | 7 July–13 Aug | 0.95 | 1.67 | 3.30 | 2.68 | 1.69 | 1.09 | 5.04 | 1.82 | 4.68 | |
| Cut 4 | 14 Aug–21 Sept | 0.90 | 1.24 | 2.22 | 1.87 | 1.11 | 1.29 | 4.42 | 1.57 | 4.11 | |
| Growing Season | 21 Apr–21 Sept | 3.30 | 6.14 | 2.65 | 1.97 | 1.15 | 4.77 | 19.1 | 1.68 | 4.44 | |
| Maize | Growth Stage 1 | 3 May–26 June | 1.22 | 2.05 | 4.20 | 1.93 | 0.990 | 0.51 | 2.58 | 0.712 | 3.42 |
| Growth Stage 2 | 27 June–18 July | 0.38 | 1.15 | 9.60 | 5.37 | 3.93 | 1.99 | 4.00 | 3.76 | 6.34 | |
| Growth Stage 3 | 19 July–13 Aug | 0.68 | 1.22 | 5.47 | 4.22 | 3.56 | 2.73 | 4.21 | 3.02 | 6.79 | |
| Growth Stage 4 | 14 Aug–11 Sept | 0.89 | 1.07 | 3.46 | 2.54 | 2.12 | 4.24 | 3.44 | 2.17 | 4.59 | |
| Growing Season | 3 May–11 Sept | 3.16 | 5.50 | 4.76 | 3.46 | 2.46 | 9.48 | 14.2 | 1.76 | 5.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Haan, K.; Khomik, M.; Green, A.; Helgason, W.; Macrae, M.L.; Kompanizare, M.; Petrone, R.M. Assessment of Different Water Use Efficiency Calculations for Dominant Forage Crops in the Great Lakes Basin. Agriculture 2021, 11, 739. https://doi.org/10.3390/agriculture11080739
De Haan K, Khomik M, Green A, Helgason W, Macrae ML, Kompanizare M, Petrone RM. Assessment of Different Water Use Efficiency Calculations for Dominant Forage Crops in the Great Lakes Basin. Agriculture. 2021; 11(8):739. https://doi.org/10.3390/agriculture11080739
Chicago/Turabian StyleDe Haan, Kevin, Myroslava Khomik, Adam Green, Warren Helgason, Merrin L. Macrae, Mazda Kompanizare, and Richard M. Petrone. 2021. "Assessment of Different Water Use Efficiency Calculations for Dominant Forage Crops in the Great Lakes Basin" Agriculture 11, no. 8: 739. https://doi.org/10.3390/agriculture11080739
APA StyleDe Haan, K., Khomik, M., Green, A., Helgason, W., Macrae, M. L., Kompanizare, M., & Petrone, R. M. (2021). Assessment of Different Water Use Efficiency Calculations for Dominant Forage Crops in the Great Lakes Basin. Agriculture, 11(8), 739. https://doi.org/10.3390/agriculture11080739






