Abstract
The objective of this study was to evaluate the fruit development, the quality at harvest and the shelf-life changes of three apricot cultivars—‘Spring Blush’, ‘Robada’, and ‘Kioto’—produced in the conditions of the Southwest Iberian Peninsula. Pomological characteristics and global quality (colour, firmness, total soluble solids, and total acidity) were weekly evaluated during fruit growth and ripening. Apricots were harvested at commercial ripening, and six and three days before, were tested for each harvest on the harvesting day and after three and five days of shelf-life at 20 °C, evaluating chlorophyll and carotenoids content and sensory quality. ‘Spring Blush’ was the earliest cultivar with a small calibre, and the change in colour was found to depend more on the evolution during the shelf-life than on the harvest date; although the panellists rated it well, it presented a significant lack of firmness for successful marketing. ‘Robada’ was the cultivar with the lowest evolution of colour, sugar and acid content, and it was the worst valued by tasters. In ‘Kioto’, differences in soluble solids, acidity, and colour were of high importance in the last days of development on the tree. ‘Kioto’ was the cultivar that showed the largest fruit size and colouring during the ripening on the tree, as well as the one with the highest overall quality that improves during shelf-life.
1. Introduction
The apricot (Prunus armeniaca L.), originally from China and Central Asia, is currently cultivated throughout the world, the largest producers being the countries of the Middle East and the Mediterranean; Spain holds the seventh place at worldwide production [1].
The apricot is a highly perishable climacteric fruit that is susceptible to dehydration, loss of firmness, mechanical damage, and decay, so it is usually harvested prematurely [2,3,4]. The harvest is carried out when the fruit has reached a good colour development and an optimum firmness value of 3–3.5 kg, measured with an 8 mm probe, to ensure that the firmness of the fruit is sufficient to withstand the marketing period (AFRUEX, personal communication). However, the ripening conditions are often not adequate, and the organoleptic characteristics demanded by consumers cannot be achieved [3,5].
According to various studies, consumers appreciate apricots for their flavour, marked by the sugar content, the sugar/acid ratio, and the aroma [6,7,8]; colour and firmness are other relevant aspects. Regarding firmness, consumers prefer an intermediate range between 10 and 20 N [2,5], although these values vary depending on the studied cultivars and the researchers; they are slightly higher compared to the firmness of the ready-to-eat fruit, established between 9 and 13.5 N [9].
Regarding the colour of the apricot, it is an attribute that depends, among other factors, on the cultivar and the stage of maturity of the fruit [10]. During the ripening period, an active synthesis of carotenoids occurs [11] and is responsible for the intense yellow-orange colour of its skin and pulp, with β-carotene being the largest component [12,13,14,15]. Simultaneously, a degradation of the chlorophylls occurs and results, together with the de novo synthesis of carotenoids, in very marked colour changes [16].
Carotenoids, as well as being important in order to establish the optimum harvesting time, have provitamin A capacity and high antioxidant activity. Therefore, we must not only consider the overall quality but also other properties that make apricot consumption highly recommended, such as nutritional and functional values [17].
There have been numerous quality studies that dealt with the different varieties of apricots at harvesting time [6,7,8,11,12,18,19], but few have been carried out during the development of the fruit [20] or at different stages of ripening [2,5,10,16]. Furthermore, to our knowledge, there have been few studies of the effect of apricot ripening at 20 °C [21].
The study of apricot cultivars in new growing regions should consider the evolution of the fruit during development from the setting of the flowers, with special attention to the moments close to commercial harvesting, as well as its evolution after harvest. The objectives of this work were: (1) to characterise three apricot cultivars from a physical-chemical point of view during their development and ripening on the tree and (2) to determine the functional, sensory, and overall quality of the fruits at the time close to commercial maturity and after a period of shelf-life.
2. Materials and Methods
2.1. Plant Material
The trial was conducted on three apricot cultivars (Prunus armeniaca L.)—‘Spring Blush’, ‘Robada’, and ‘Kioto’, which were grown in three different commercial orchards located in Caia (38°54′ N 7°04′ W), Southeastern Portugal.
Five-year-old trees were grown under the same agronomic conditions, grafted onto the ‘Franco Montclar’ rootstock, planted at a distance of 6 × 4 m, and trained in a vase system. The stages of fruit development were based on days after full bloom (DAFB).
2.2. Experimental Design
Weekly samples were taken during the development of the fruit to determine morphological characteristics, skin colour, compression texture, total soluble solids (TSS), and total acidity (TA). Each sample was prepared from fruits harvested randomly from 9 different trees. After each harvest, apricots were immediately transported to the laboratory. The first sampling was done after fruit formation (35, 28, and 42 DAFB in ‘Spring Blush’, ‘Robada’, and ‘Kioto’, respectively).
Apricots were harvested at commercial ripening, and 6 and 3 days before, (0, −6 and −3) in order to ensure the proper determination of optimum harvesting date. The fruits were analysed at harvest (0) and after a shelf-life period of 3–5 days at 20 °C. In addition to assessing weight, colour, and texture loss due to compression, measurements of texture were also made by penetration, TSS, TA, pigments (chlorophyll and carotenoid compounds), and sensory analysis.
2.3. Standard Quality Evaluation
The surface colour of 20 fruits was measured at two different points around their equatorial region using a Minolta CR-400 reflectance Chroma Meter (Minolta, Osaka, Japan), which provided CIE L*, a*, and b* values. These values were then used to calculate hue (H°), chroma (C*), and the a*/b* ratio. In addition, the visual colour of the fruits was determined with CTIFL charts (1–10 scale) defining four stages of maturation: early (CTIFL < 3), mid (CTIFL 3–5), commercial (CTIFL 5–7), and late (CTIFL > 8).
The weight for each individual fruit (n = 20) was recorded using a Sartorius TE412 digital scale (Sartorius AG, Göttingen, Germany), along with its diameter, length, stone diameter and length, and pulp thickness using a digital calibrator (Infoagro Systems, S.L., Madrid, Spain).
Fruit firmness was assessed in 20 fruits, on opposite sides, with a Micro Systems Texture Analyzer TA-XT2i (Aname, Pozuelo, Madrid, Spain) by means of a compression test to 3% of the whole fruit using a 40 mm plate. Six days before commercial harvesting (−6), and during its shelf-life, firmness was also determined by a penetration test with a 6 mm diameter probe. The maximum force (N) was recorded in both tests.
The remaining fruits were carefully pitted, divided into three groups, and homogenized by a food processor; the homogenates (n = 3) were then employed to assess TSS, TA, chlorophyll, and carotenoid compounds. TSS were evaluated at 20 °C with a Mettler Toledo RE40 refractometer (Coslada, Madrid, Spain) and expressed as °Brix. For TA, 3 g of homogenate were diluted up to 60 mL with distilled water and titrated to pH 8.1 using 0.1 N NaOH in a Mettler Toledo DL50 titrator (Coslada, Madrid, Spain).
2.4. Determination of Chlorophyll and Carotenoid Content
An acetonic extract was prepared by performing 3 extractions with 30 mL of acetone on the 10 g of homogenate and stirring for 20 min in the dark. Chlorophylls were determined in this extract according to the method described by Fernández-León et al. [22], expressing the results as µg g−1 of fresh sample. The total carotenoid content was also calculated in the extract by applying the method developed by Dragovic-Uzelac et al. [10], expressing the concentration in µg de β-carotene g−1 of fresh sample. The method of Bohoyo-Gil et al. [23] was used for the chromatographic separation and determination of individual carotenoids, indicating the concentration in µg g−1 of fresh sample. Briefly, an Agilent 1290 Ultra High Performance Liquid Chromatographer (Agilent Technologies, CA, USA) equipped with a Zorbax Eclipse Plus C18 2.1 × 50 mm column (1.8 μm particle size at 28 °C) and coupled to a DAD detector (at 460 nm) was used. The mobile phase was an 85:15 mix of acetonitrile/methanol (solvent A) and a 60:20:20 mix of acetonitrile/methanol/ethyl acetate (solvent B) containing 0.1% BHT and 0.05% TEA. The gradient was 100% A and 0% B for 4 min, 0% A and 100% B for 5 min, and finally 100% A and 0% B for 0.5 min. The flow rate was 0.3 mL/min, and an external standard technique was used for peak identification and quantification.
2.5. Sensory Evaluation
A panel of 12 trained testers carried out the sensory evaluation for the 3 cultivars on the 3 harvesting dates (−6, −3, and 0), only in the samples that had been at 20 °C for 5 days. The evaluation criteria were colour, flavour, sweetness, acidity, juiciness, firmness, and overall score. In summary, the samples at 20 °C were prepared for each tasting day and tested in order to assess firmness by compression (as described above). Subsequently, half was used to measure TSS while the other half was used for tasting. The fruit was placed in trays and submitted to the testers. The evaluation guidelines considered a continuous scale for each parameter, with scores ranging from 0 (the lowest) to 8 (the highest).
2.6. Statistical Analysis
Data were subjected to ANOVA using IBM SPSS Statistics 20 (SSPS Inc., Chicago, IL, USA). When significant differences between mean values were detected by ANOVA, they were further compared using HSD Tukey’s tests. The relationships between variables and samples were assessed by principal component analysis (PCA). The relationships between colour parameters and CTIFL values were also evaluated by Pearson’s correlation at p ≤ 0.01.
3. Results and Discussion
3.1. Morphological Changes during Fruit Growth
The development process of apricot fruits has a highly variable duration that largely depends on the cultivar [20,24]. The complete cycle, from petal fall (or fruit set) to commercial maturity, was 53 days in ‘Spring Blush’ and 65 days in ‘Robada’ and ‘Kioto.’ ‘Spring Blush’ is an early production cultivar and therefore has a shorter cycle than ‘Robada’ and ‘Kioto’, which produce later given the agro-climatic conditions of the southwest of the Iberian Peninsula.
The pattern of fruit development in the three apricot cultivars followed a double sigmoid type curve, differentiating a first phase of rapid growth of the fruit (Phase I), a second phase of growth stop coinciding with the formation of the stone (Phase II), and a third ripening phase (Phase III) in which the fruit can continue increasing in size and acquires the gustatory characteristics (Figure 1).
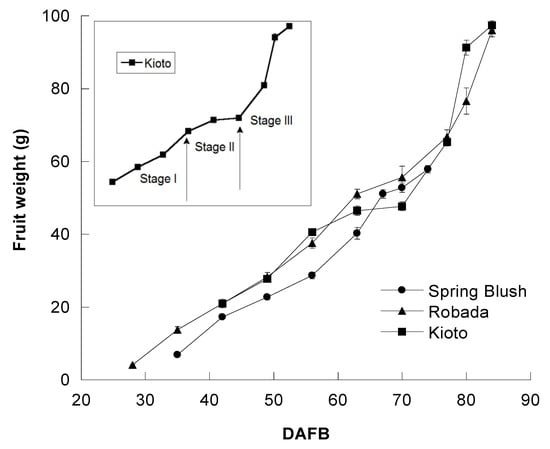
Figure 1.
Fruit growth curve (expressed in g) of the three studied apricot cultivars. Days after full bloom (DAFB) to commercial maturity, indicating stages I, II, and III of the growth for the ‘Kioto’ cultivar. Mean values ± standard error (n = 20).
Stone hardening had started 49 DAFB for the three cultivars. The stone was formed in 50% of the fruits in ‘Spring Blush’, compared to the 13% in ‘Kioto.’ At 56 DAFB, 100% of the fruit had lignified stone in the three apricot cultivars.
The collection of samples for analysis started earlier in ‘Spring Blush’ and ‘Robada’ (35 and 28 DAFB, respectively) than in ‘Kioto’ (42 DAFB). Initially, the average weight of the fruits was 7 and 4 g in ‘Spring Blush’ and ‘Robada’, respectively, compared to 21 g in ‘Kioto.’ As for the characteristics of the stone, ‘Kioto’ showed differentiation and hardening of the tissue surrounding the seed, unlike ‘Spring Blush’ and ‘Robada’, so there were hardly any significant changes on stone length and diameter in ‘Kioto’ between harvesting dates (Table 1).

Table 1.
Evolution of physical parameters of the apricot cultivars during growth and ripening.
‘Spring Blush’ was the smallest fruit cultivar (58 ± 8 g average weight), with commercially harvested sizes of 43/49 mm, which is considered ‘Extra Class’ by apricot marketing standards. ‘Robada’ and ‘Kioto’ were harvested three and four weeks later than ‘Spring Blush’, respectively, and were significantly larger fruit cultivars that reached values close to 100 g in commercial harvest and sizes of 54/59 mm and larger. The mean weight and diameter values of fruits at commercial harvest were higher than those published by Lo Bianco et al. [7] for 16 apricot cultivars produced in Sicily. However, larger fruit sizes (up to 70 g and higher) have been noted in European apricots, especially the newly bred varieties [25].
3.2. Changes in Quality during Growth and Fruit Ripening
3.2.1. Colour
Though skin colour was determined on both sides of the fruit and significant differences were found between the shaded (un-blushed) and sun-exposed (blushed) sides, the evolution of the different colour parameters was very similar on both sides. In general, there were significant increases in L*, a*, and b* with the development and ripening of the fruit resulting from the change in colour from dark green to orange-yellow in all three cultivars (data not shown). For the same reason, there was an increase in the chroma parameter (C*) and a decrease in the hue angle (H°). Out of all the instrumentally determined colour parameters, those that best correlated with the evolution of the visual colour (CTIFL) of the fruits were a*, H°, and the a*/b* ratio, with correlations higher than 0.8 (Figure 2).
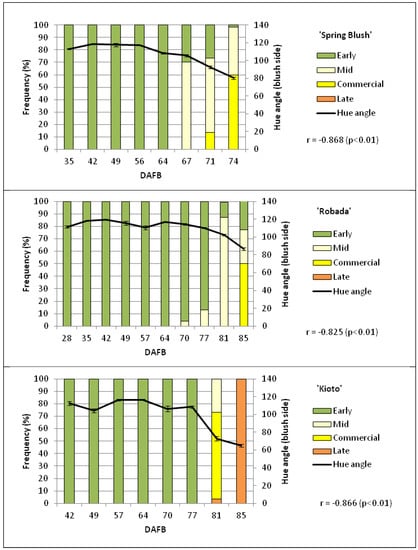
Figure 2.
Relationship between instrumental colour (hue) and the state of visual maturity of the fruit, established by CTIFL colour, for the three apricot cultivars from full bloom to maturity on the tree. Mean values ± standard error (n = 20).
Since it is a widely used index for stone fruit, the a*/b* ratio was calculated [26,27] and showed an upward trend with the development and ripening of the fruit (Figure 3). On the one hand, the a*/b* ratio allowed us to determine the time when the fruit colour change occurred, from green (a*/b* values < 0) in unripe fruits to the pre-commercial pale yellow (a*/b* values ≈ 0) and orange-yellow (a*/b* > 0 values) in ripe fruits. On the other hand, it showed the differences in colour between the three studied apricot cultivars. Thus, ‘Kioto’ was the cultivar with the highest colour intensity (higher a*/b* ratio), while ‘Spring Blush’ and ‘Robada’ showed lighter and similar shades between them. Colour changes during ripening were more subtle in ‘Robada’ (Figure 3).
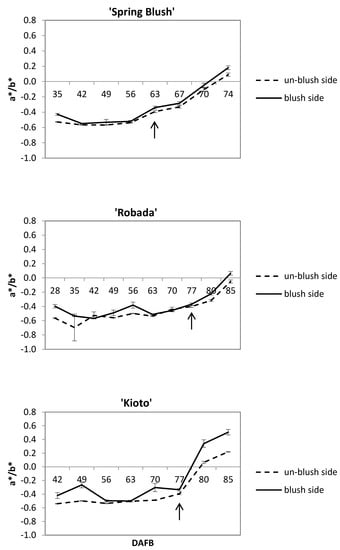
Figure 3.
Evolution of the a*/b* skin colour index of apricot cultivars from full bloom to maturity on the tree. The arrow indicates the time of the initial change in fruit colour. Mean values ± standard error (n = 20).
3.2.2. Firmness
The evolution of fruit firmness, measured by compression, is shown in Figure 4. In ‘Spring Blush’, it remained generally constant up to 67 DAFB, while in ‘Robada’ and ‘Kioto’, a significant increase in firmness was observed from the beginning of the trial up to 63 and 70 DAFB, respectively (Figure 4). These increases in firmness were due to foliar feeding with calcium, which was done to avoid fruit splitting due to abundant rainfall and high temperatures in the production area at that time. In the three apricot cultivars, a significant decrease in fruit firmness was observed from 10 to 15 days before commercial harvest. ‘Spring Blush’ had the lowest firmness values at commercial harvest 14.9 N, compared to 26 and 22 N for ‘Robada’ and ‘Kioto’, respectively. It is difficult to compare our firmness results with those in the literature because the used methodology has been variable among different authors.
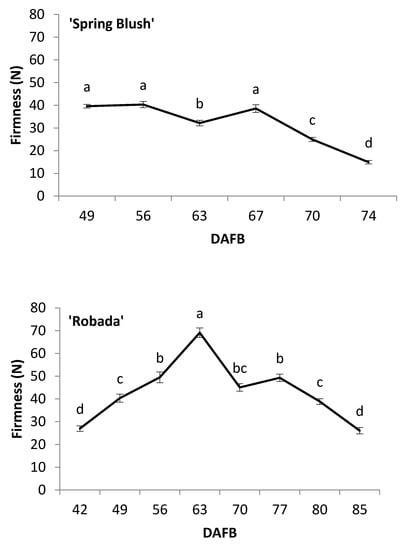
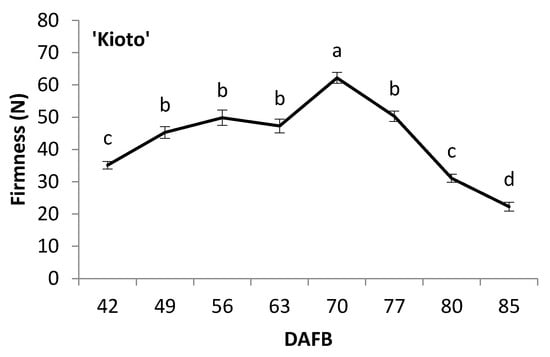
Figure 4.
Evolution of apricot fruit firmness during its development and ripening on the tree. The letters represent significant differences according to Tukey’s test at a level of p < 0.05. Mean values ± standard error (n = 20).
3.2.3. Total Soluble Solids and Total Acidity
The TSS increased during the development and ripening process on all three apricot cultivars (Figure 5). ‘Kioto’ had the highest TSS value (10.9 °Brix), and ‘Robada’ had the lowest (8.3 °Brix). The evolution of acidity was contrary to that of TSS, and a decline was observed as the fruit ripened. ‘Kioto’ was the cultivar with the highest TA at commercial harvest (2% malic acid), and ‘Robada’ had the lowest (1.42% malic acid). As a result of increased TSS and decreased acidity during fruit development and ripening on the tree, the TSS/TA ratio significantly increased at different sampling dates in the three studied apricot cultivars. The high acidity of the ‘Kioto’ cultivar meant that the TSS/TA ratio was not higher than that of the other cultivars despite the higher TSS content, and no significant differences were found between them at the time of commercial harvest (data not shown).
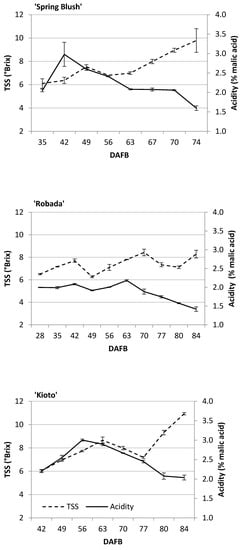
Figure 5.
Evolution of TSS and total acidity of apricot fruits during their development and ripening on the tree. Mean values ± standard error (n = 3).
TSS content values were slightly lower than those shown by other authors for other apricot cultivars, which were found to be in the range of 10–20 °Brix [11,28]. These differences may have been due to both the cultivar and the climatic conditions in the year of production, as well as management practices [5]. Our acidity values, however, were similar to those found by Ayour et al. [29] in twelve apricot cultivars at different stages of maturity.
European apricots, especially the newly bred varieties, have larger fruit with yellow/orange colour and a characteristic apricot aroma; their soluble solids content is lower (around 12–17%) and their acidity is higher (above 1.3–1.5%) compared to Central Asian varieties [25].
3.3. Changes in Quality during Shelf-Life
3.3.1. Weight Loss
Weight losses increased in all three cultivars with shelf-life and ranged between 6 and 12% after five days (Table 2). ‘Kioto’ was the cultivar that experienced the lowest losses, especially in those apricots that were harvested at a more advanced stage of maturity (days −3 and 0). There is very little published information on the effects of ripening at room temperature in apricots; the studies conducted on the ‘Mauricio’ cultivar [30] showed a 20% weight loss after five days, and Quiles-Bernabéu [31] found an 8–12% weight loss after three days of shelf-life in ‘Madison’ apricots that had been previously chilled at 2 °C for 5 or 10 days.

Table 2.
Physico-chemical parameters of the apricot cultivars at harvest and during shelf-life.
3.3.2. Colour
As previously said, colour measurement was assessed on both sides of the fruit (un-blushed and blushed), but only un-blushed side data are shown because they provide more information. The hue angle (H°) in the three apricot cultivars showed a decrease as ripening progressed and during shelf-life (Table 2), in accordance with the results of Quiles-Bernabéu [31], these changes being less evident in the ‘Robada’ cultivar. The development of H° along maturation is a characteristic of the cultivar, according to the results of other authors [16,32]. The L* and C* parameters increased both during ripening and in shelf-life, with variations between cultivars. Regarding the a*/b* ratio, an increase could be seen as ripening and shelf-life progressed (Figure 6). Differences were found between the three cultivars: ‘Spring Blush’ went from negative values at harvest (green colour) to positive values (yellow-orange colour) during the shelf-life for −3 and 0 harvests, ‘Robada’ presented mostly negative values, ‘Kioto’ only registered negative values at harvest day −6, and the remaining samples showed positive values.
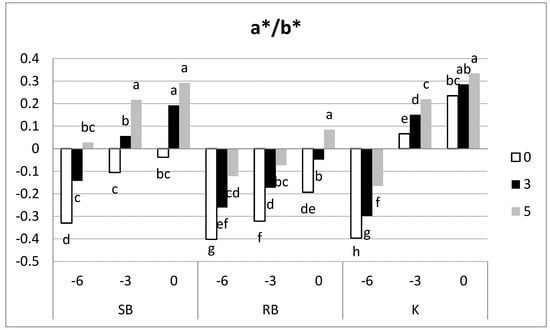
Figure 6.
Evolution of the a*/b* index on skin colour in the un-blushed side of the studied apricot cultivars at all three harvest dates and their shelf-life. Per cultivar, samples with different letters differed at a significance level of 0.05 according to Tukey’s test (n = 20).
At the time of commercial harvest (day 0), the ‘Spring Blush’ cultivar showed a medium stage of maturity, with 70% of the fruits being green-yellow and the rest yellow, while the ratio in ‘Robada’ was 46% green and 54% green-yellow (CTIFL classification), as shown in Figure 2. Fruits with a yellow-green ground colour corresponding to a pre-climacteric stage [21]. On the other hand, the fruits of the ‘Kioto’ cultivar were yellow (positive values of a* on both sides of the fruit; data not shown) from the three days prior to commercial harvest (−3), reaching the characteristic orange tones of the cultivar at later dates.
3.3.3. Firmness
In all three cultivars, there was a decrease in firmness measured by compression, with ‘Spring Blush’ and ‘Kioto’ showing the most significant drop (Figure 7). During shelf-life, firmness values were too low (below 10 N) for ‘Spring Blush’, while the other two cultivars showed acceptable values after three days at 20 °C but not after five days. The lack of firmness in ‘Spring Blush’ could be a limiting factor for its postharvest handling and marketing.
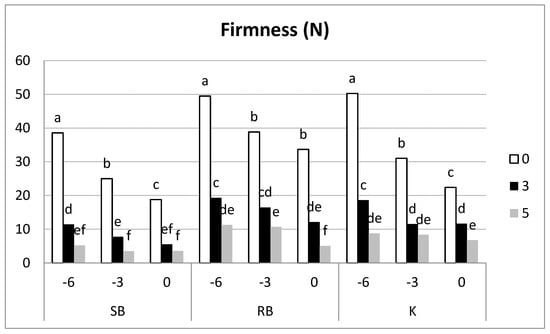
Figure 7.
Evolution of firmness by compression of apricot cultivars at all three harvest dates and subsequent shelf-life. Per cultivar, samples with different letters differ at a significance level of 0.05 according to the Tukey Test (n = 20).
In terms of penetration force (Table 2), a decrease was observed in all three cultivars as ripening progressed, with the greatest decrease in ‘Kioto.’ There was also a decrease in force during shelf-life that was much more evident in the more mature fruits (day 0, commercial harvest), reaching unacceptably low values in the ‘Spring Blush’ cultivar. There was a lower drop in flesh firmness in the ‘Robada’ cultivar during both ripening and shelf-life.
3.3.4. Total Soluble Solids and Total Acidity
The TSS content was low in ‘Spring Blush’ and ‘Robada’, at around 8 °Brix, and, in general, there was no clear effect on their evolution in terms of either harvest date or shelf-life time (Table 2). Fan et al. [33] examined individual sugars during shelf-life, four days at 25 °C, and found no significant variation overall. The ‘Kioto’ cultivar showed a different behaviour with a remarkable increase in TSS from 7 to 11 °Brix as the fruit ripen, as well as a slight tendency to increase during the shelf-life. Values of soluble solids content were consistent with those reported by previous studies [12,29], ranging from 8 to 16 °Brix.
Total acidity decreased as harvest maturity progressed in all three cultivars, though it was much more evident in ‘Kioto’, which was the most acidic (Table 2). These values were somewhat higher than those obtained by Ayour et al. [29]. Regarding the evolution during shelf-life, no clear trend was observed, and there was an increase in shelf-life in some samples. Consequently, there did not seem to be a metabolic consumption of organic acids, contrary to what was described by other authors [31,33] who observed a decrease in the values or as described by Valdenegro et al. [30] who found no significant differences in acidity during shelf-life.
3.3.5. Chlorophyll and Carotenoids
The highest total chlorophyll content (Figure 8) was in all cultivars at harvest day −6; in subsequent harvests or after the shelf-life period, these values always decreased (although differently between cultivars) due to chlorophyll degradation [16,32] (Figure 8 and Table 3). ‘Spring Blush’ showed high levels of chlorophyll on all three harvest dates (−6, −3, and 0), observing a sharp drop in concentration during shelf-life, especially on day 0. ‘Kioto’ showed the highest chlorophyll levels on day −6, and even after five days of shelf-life, the concentration of chlorophyll remained high; for later harvests (−3 and 0), the chlorophyll content was much lower at harvest, but there was little degradation during shelf-life.
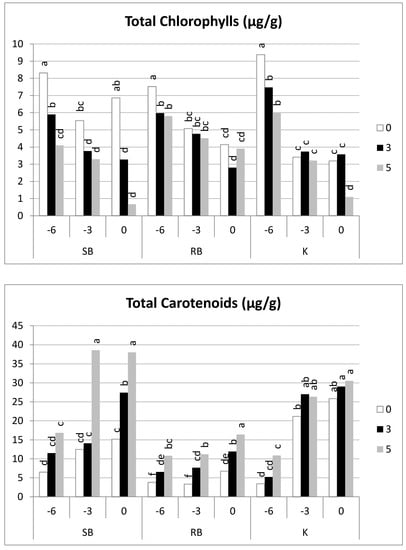
Figure 8.
Apricot pigment (total chlorophyll and total carotenoids) evolution at harvest and during the shelf-life of apricot cultivars at all three harvest dates and subsequent shelf-life. Per cultivar, samples with different letters differ at a significance level of 0.05 according to Tukey’s test (n = 20).

Table 3.
Individual pigment evolution in apricot at harvest and during shelf-life.
Likewise, Huang et al. [32] found very different concentrations of chlorophyll and varied proportions between chlorophyll a and b depending on the cultivar and maturity at harvest.
The relationship between chlorophyll a and chlorophyll b behaved differently depending on the cultivar. Thus, ‘Kioto’ showed the highest ratios in the samples from the earliest harvest (2.83–2.74 μg/g) and the lowest in the later harvest (1.34–0.83 μg/g) due to a much more pronounced degradation of chlorophyll a. For ‘Spring Blush’, there were no significant differences either by date of harvest or by shelf-life, ranging from 2.99 to 1.63 μg/g. The ‘Robada’ cultivar showed an intermediate pattern (Table 3).
As noted by other authors [12,16], the concentration of carotenoids largely depends on the cultivar, harvest time, and shelf-life. In general, there was a significant increase when harvesting was delayed, and for each harvest date, the total carotenoid concentration increased significantly over shelf-life.
When analysing the evolution of total carotenoids (Figure 8), ‘Robada’ showed the lowest concentration, reaching about 15 μg/g at the last date of harvest and after five days of shelf-life. ‘Kioto’ showed very low concentrations of total carotenoids at first harvest (−6) and after appropriate shelf-life. However, concentrations dramatically increased at harvest −3 and 0, with values of 20–25 μg/g at harvest, and they presented no significant differences between the different periods of shelf-life. In contrast to these cultivars, ‘Spring Blush’ always showed an increase in carotenoids, both between harvesting dates and during shelf-life. This total carotenoid synthesis was much higher in the −3 and 0 harvests, reaching a total carotenoid concentration of 38 μg/g after five days of shelf-life.
The dominant carotenoid in the three studied cultivars was β-carotene (Table 3), in accordance with the work of other authors [12,13,14,15]. Though its synthesis at the end of maturation and during shelf-life followed different patterns across cultivars, we can say that its concentration increased in the later harvests. A significant synthesis took place in ‘Kioto’ depending on the harvest date, obtaining concentrations of 2 μg/g on day −6 and increasing to 20 μg/g on days −3 and 0. Regarding the synthesis in shelf-life, there was only a noteworthy increase at the earliest harvest, albeit achieving very low levels (6 μg/g). For the ‘Spring Blush’ and ‘Robada’ cultivars, increases were found when harvesting was delayed and during shelf-life, but these were not significant overall.
As shown in Table 3, the highest concentrations of lutein were found in all cultivars at the earliest harvest dates, and they decreased as the shelf-life progressed, suggesting degradation or transformation into other carotenoids within the synthesis routes. Lutein concentrations were very low, ranging between 0.6 μg/g and 0.05 μg/g. It should be noted that the sum of β-carotene and lutein concentrations produced values similar to the total carotenoid levels in ‘Robada’ and ‘Kioto’ but not for ‘Spring Blush’, which means that this cultivar must have other carotenoids in its composition that are generated in the various reactions of the synthesis route [34] that have not been quantified.
3.3.6. Sensory Analysis
As described in the Material and Methods sections, a sensory analysis was performed on samples from all three apricot cultivars after five days of shelf-life (Figure 9). To compare the tasting results with instrumental parameters, the same fruits to measure the maximum compression force, and they were subsequently divided into two halves; in one, TSS was determined, and the other was offered to the tasters.
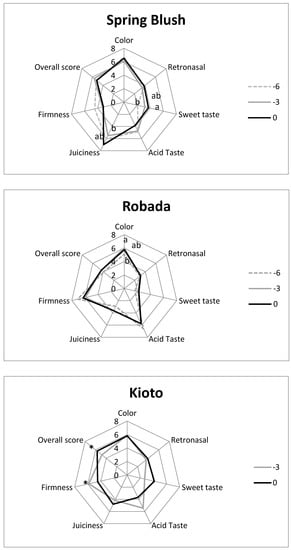
Figure 9.
Sensory evaluation results. For each quality parameter, samples with different letters or marked with asterisks differ at a significance level of 0.05 according to the Tukey test (n = 12).
In ‘Spring Blush’, significant differences were only detected in sweetness and juiciness, which increased as harvesting was delayed, and firmness and acidity scores decreased, although without significant differences. Juiciness was the highest-rated parameter for this cultivar. The TSS did not show notably different values between harvest dates (about 9.5 °Brix), so the significant differences in the sensory evaluation of sweetness could be attributed to a lower acidity. In terms of firmness, by delaying the harvest, tasters detected a slight not significant decrease, but when firmness was instrumentally measured significant differences were found (6.6, 5, and 3.1 N for days −6, −3, and 0).
In the ‘Robada’ cultivar, the tasting results for the three dates were very similar. Colour was the only parameter in which significant differences were detected. This cultivar was considered very acidic and not very juicy by the tasters, presenting the lowest overall score. As mentioned above, this is a highly firm cultivar that was corroborated on both a sensory level (with values close to or above 6 on a scale of 0–8) and an instrumental level (between 29.8 and 12 N). As for sweetness, its sensory evaluation was very poor (approximately 2 on a scale of 0–8), and the TSS were also very low (average of 8.7 °Brix); coupled with the high value of acidic flavour, this explains why the ‘Robada’ cultivar had such a low overall rating at all harvest dates.
The ‘Kioto’ cultivar was not tested on harvest day −6 because its firmness and maturity did not make it suitable for consumption. In general, it was a well rated cultivar at harvest 0, with scores of 4 or higher and reaching 6 on the colour and overall rating parameters. Significant differences were detected in firmness, both in sensory and instrumental measurements (20.5 N at harvest −3 and 8.6 N at day 0). The overall score was also considerably higher for fruits harvested at day 0. In terms of sweetness, the tasters’ evaluation was also higher with no significant differences, although these were detected in the instrumental measure of TSS (9.7–13.9 °Brix).
Based on the obtained results, the sensory quality of the apricots is highly dependent on the cultivar and harvesting date, making it especially necessary to adjust the optimum harvesting time in cultivars such as ‘Robada’ in which there is less evolution of their physical-chemical characteristics during ripening and shelf-life.
According to Stanley et al. [5], the TSS content of apricots was a determining factor in the sensory quality of fruits with higher firmness (over 15 N). This relationship between fruit quality parameters and consumer acceptance has been reported in other stone fruit, where acceptability does not depend on a single parameter but on the interaction of several [26,35].
3.3.7. Principal Component Analysis
In order to explain the connections within the results and provide a better understanding, a principal component analysis was carried out. The first two components accounted for 79.12% of the total variance. The first component, PC1 (67.21% of the total variance), mainly consisted of firmness and chlorophylls on its negative axis and TSS, carotenoids, and colour parameters on its positive axis. The second component, PC2, accounted for 11.91% of the variance and was made up of acidity (positive axis), weight loss, and the colour parameters b* and L* of the blush side (negative axis) (Figure 10).
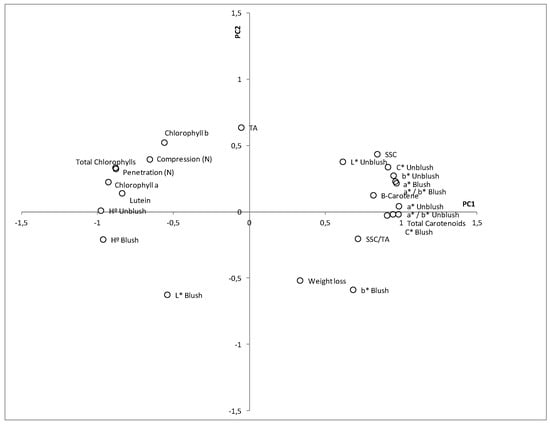
Figure 10.
Parameters on the PCA plane (PC1: 67.2% of total variance; PC2: 11.9% of total variance).
When the samples are represented on the plane defined by the first two principal components (Figure 11), the different positions can be observed, depending on the cultivar, harvesting date, and shelf-life period. ‘Spring Blush’ and ‘Robada’ appear on the lower part of the plane (third and fourth quadrant), while ‘Kioto’ appears in the upper part, always with positive PC2 values (first and second quadrant). As harvesting time progresses, the samples move from negative PC1 values to positive values (from the second to the first quadrant), and, in general, the shelf-life period involves a diagonal downward shift in the PC1–PC2 plane.
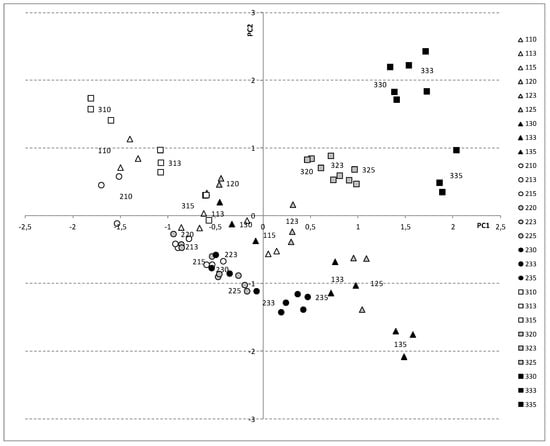
Figure 11.
Samples in the PCA plane (PC1: 67.2% of total variance; PC2: 11.9% of total variance) for apricots. Symbols represent the three cultivars: ‘Spring Blush’ (Δ), ‘Robada’ (○), and ‘Kyoto’ (□). Harvest day −6 (white symbols), harvest day −3 (grey symbols), and harvest day 0 (black symbols).
From day −6 to commercial harvest, ‘Kioto’ showed significant losses in chlorophyll and firmness, increases in carotenoids and TSS, and changes in colour, that explains the location of the samples in PC1-PC2 plane, separated from the other cultivars.
‘Spring Blush’ and ‘Robada’ showed greater weight loss and were therefore in the lower zone of the plane. Colour changes and firmness losses were lower in ‘Robada’ than in ‘Spring Blush’, resulting in the samples being more grouped in the PC1–PC2 plane.
4. Conclusions
The quality characteristics throughout the ripening of the fruit depend on the cultivar. It is necessary to know the performance of each cultivar during development and after harvest, as very different patterns can occur. In this regard, out of the three studied cultivars, ‘Kioto’ was the one that showed a larger fruit and a more intense colour at commercial harvest. The other two cultivars, ‘Spring Blush’ and ‘Robada’, showed very subtle colour changes while on the tree.
During maturation at room temperature (shelf-life), ‘Robada’ hardly showed any change in overall quality compared to ‘Kioto’, which suffered losses of firmness, acidity, and chlorophyll content, along with an increase in fruit colour and carotenoid compounds. ‘Spring Blush’ was somewhere in between ‘Robada’ and ‘Kioto.’
From a sensory point of view, ‘Spring Blush’ and ‘Robada’ were the least appreciated cultivars by tasters, mainly due to their low total soluble solid content or high acidity and to firmness problems. ‘Kioto’ was the most balanced cultivar in all the attributes assessed by the sensory panel.
Author Contributions
B.V.-M.: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Visualization, Writing—review & editing. F.A.-C.: Conceptualization, Methodology, Resources, Writing—review & editing. M.C.A.-Y.: Formal analysis, Investigation, Visualization, Writing—original draft, Writing—review & editing. M.J.B.-G.: Formal analysis, Investigation, Supervision, Visualization, Writing—original draft, Writing—review & editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available in a publicly accessible repository.
Acknowledgments
To the Junta de Extremadura of Spain and FEDER funds for the financial support provided. Thanks to Cristina Miguel Pintado and Laura Peñas Díaz for their help in field and laboratory work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Food and Agriculture Organization of the United Nations. Available online: http://www.fao.org/faostat/en/#data/QL (accessed on 27 February 2021).
- Infante, R.; Meneses, C.; Defilippi, B.G. Effect of harvest maturity stage on the sensory quality of ‘Palsteyn’ apricot (Prunus armeniaca L.) after cold storage. J. Hortic. Sci. Biotechnol. 2008, 83, 828–832. [Google Scholar] [CrossRef]
- Aubert, C.; Bony, P.; Chalot, G.; Hero, V. Changes in physicochemical characteristics and volatile compounds of apricot (Prunus armeniaca L. cv. Bergeron) during storage and post-harvest maturation. Food Chem. 2010, 119, 1386–1398. [Google Scholar] [CrossRef]
- Muftuoğlu, F.; Ayhan, Z.; Eştürk, O. Modified Atmosphere Packaging of Kabaaşı Apricot (Prunus armeniaca L. ‘Kabaaşı’): Effect of Atmosphere, Packaging Material Type and Coating on the Physicochemical Properties and Sensory Quality. Food Bioprocess Technol. 2010, 5, 1601–1611. [Google Scholar] [CrossRef]
- Stanley, J.; Feng, J.; Olsson, S. Crop load and harvest maturity effects on consumer preferences for apricots. J. Sci. Food Agric. 2014, 95, 752–763. [Google Scholar] [CrossRef]
- Ruiz, D.; Egea, J. Phenotypic diversity and relationships of fruit quality traits in apricot (Prunus armeniaca L.) germplasm. Euphytica 2007, 163, 143–158. [Google Scholar] [CrossRef]
- Bianco, R.L.; Farina, V.; Indelicato, S.G.; Filizzola, F.; Agozzino, P. Fruit physical, chemical and aromatic attributes of early, intermediate and late apricot cultivars. J. Sci. Food Agric. 2010, 90, 1008–1019. [Google Scholar] [CrossRef]
- Melgarejo, P.; Calín-Sánchez, Á.; Carbonell-Barrachina, Á.A.; Nicolas, J.J.M.; Legua, P.; Martínez, R.; Hernández, F. Antioxidant activity, volatile composition and sensory profile of four new very-early apricots (Prunus armeniaca L.). J. Sci. Food Agric. 2013, 94, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Crisosto, C.H.; Mitcham, E.J.; Kader, A.A. Recommendations for Maintaining Postharvest Quality. 2017. Available online: http://postharvest.ucdavis.edu/Commodity_Resources/Fact_Sheets/Datastores/Fruit_English/?uid=6&ds=798 (accessed on 10 June 2020).
- Dragovicuzelac, V.; Levaj, B.; Mrkic, V.; Bursac, D.; Boras, M.; Dragovicuzelac, V.; Levaj, B.; Mrkic, V.; Bursac, D.; Boras, M. The content of polyphenols and carotenoids in three apricot cultivars depending on stage of maturity and geographical region. Food Chem. 2007, 102, 966–975. [Google Scholar] [CrossRef]
- Ali, S.; Masud, T.; Abbasi, K.S. Physico-chemical characteristics of apricot (Prunus armeniaca L.) grown in Northern Areas of Pakistan. Sci. Hortic. 2011, 130, 386–392. [Google Scholar] [CrossRef]
- Ruiz, D.; Egea, J.; Tomas-Barberan, F.; Gil, M.I. Carotenoids from New Apricot (Prunus armeniaca L.) Varieties and Their Relationship with Flesh and Skin Color. J. Agric. Food Chem. 2005, 53, 6368–6374. [Google Scholar] [CrossRef] [PubMed]
- Sass-Kiss, A.; Kiss, J.; Milotay, P.; Kerek, M.; Toth-Markus, M. Differences in anthocyanin and carotenoid content of fruits and vegetables. Food Res. Int. 2005, 38, 1023–1029. [Google Scholar] [CrossRef]
- Bureau, S.; Renard, C.M.; Reich, M.; Ginies, C.; Audergon, J.-M. Change in anthocyanin concentrations in red apricot fruits during ripening. LWT 2009, 42, 372–377. [Google Scholar] [CrossRef]
- Zhou, W.; Niu, Y.; Ding, X.; Zhao, S.; Li, Y.; Fan, G.; Zhang, S.; Liao, K. Analysis of carotenoid content and diversity in apricots (Prunus armeniaca L.) grown in China. Food Chem. 2020, 330, 127223. [Google Scholar] [CrossRef] [PubMed]
- Ayour, J.; Sagar, M.; Alfeddy, M.N.; Taourirte, M.; Benichou, M. Evolution of pigments and their relationship with skin color based on ripening in fruits of different Moroccan genotypes of apricots (Prunus armeniaca L.). Sci. Hortic. 2016, 207, 168–175. [Google Scholar] [CrossRef]
- Roussos, P.A.; Sefferou, V.; Denaxa, N.-K.; Tsantili, E.; Stathis, V. Apricot (Prunus armeniaca L.) fruit quality attributes and phytochemicals under different crop load. Sci. Hortic. 2011, 129, 472–478. [Google Scholar] [CrossRef]
- Ruiz, D.; Egea, J.; Gil, M.I.; Tomas-Barberan, F. Characterization and Quantitation of Phenolic Compounds in New Apricot (Prunus armeniaca L.) Varieties. J. Agric. Food Chem. 2005, 53, 9544–9552. [Google Scholar] [CrossRef]
- Akin, E.B.; Karabulut, I.; Topcu, A. Some compositional properties of main Malatya apricot (Prunus armeniaca L.) varieties. Food Chem. 2008, 107, 939–948. [Google Scholar] [CrossRef]
- Milosevic, T.; Milosevic, N.; Glisic, I. Dynamic of Fruit Growth and Internal Fruit Quality of Apricot Trees Grafted on Rootstock or with Interstem. J. Agric. Sci. Tech. 2013, 15, 311–321. [Google Scholar]
- Muñoz-Robredo, P.; Rubio, P.; Infante, R.; Campos-Vargas, R.; Manríquez, D.; González-Agüero, M.; Defilippi, B. Ethylene biosynthesis in apricot: Identification of a ripening-related 1-aminocyclopropane-1-carboxylic acid synthase (ACS) gene. Postharvest Biol. Technol. 2012, 63, 85–90. [Google Scholar] [CrossRef]
- Fernández-León, M.F.; Lozano, M.; Ayuso, M.C.; Fernández-León, A.M.; González-Gómez, D. Fast and accurate alternative UV-chemometric method for the determination of chlorophyll A and B in broccoli (Brassica oleracea Italica) and cabbage (Brassica oleracea Sabauda) plants. J. Food Compos. Anal. 2010, 23, 809–813. [Google Scholar] [CrossRef]
- Bohoyo, D.; Dominguez-Valhondo, D.; García-Parra, J.J.; González-Gómez, D. UHPLC as a suitable methodology for the analysis of carotenoids in food matrix. Eur. Food Res. Technol. 2012, 235, 1055–1061. [Google Scholar] [CrossRef]
- Drogoudi, P.D.; Vemmos, S.; Pantelidis, G.; Petri, E.; Tzoutzoukou, C.; Karayiannis, I. Physical Characters and Antioxidant, Sugar, and Mineral Nutrient Contents in Fruit from 29 Apricot (Prunus armeniaca L.) Cultivars and Hybrids. J. Agric. Food Chem. 2008, 56, 10754–10760. [Google Scholar] [CrossRef] [PubMed]
- Zhebentyayeva, T.; Ledbetter, C.; Burgos, L.; Llacer, G. Apricot. Fruit Breed. 2011, 12, 415–458. [Google Scholar] [CrossRef]
- Iglesias, I.; Echeverría, G. Differential effect of cultivar and harvest date on nectarine colour, quality and consumer acceptance. Sci. Hortic. 2009, 120, 41–50. [Google Scholar] [CrossRef]
- Giménez, M.; Valverde, J.; Valero, D.; Díaz-Mula, H.; Zapata, P.J.; Serrano, M.; Moral, J.; Castillo, S. Methyl salicylate treatments of sweet cherry trees improve fruit quality at harvest and during storage. Sci. Hortic. 2015, 197, 665–673. [Google Scholar] [CrossRef]
- González-Agüero, M.; Troncoso, S.; Gudenschwager, O.; Campos-Vargas, R.; Moya-León, M.; Defilippi, B. Differential expression levels of aroma-related genes during ripening of apricot (Prunus armeniaca L.). Plant Physiol. Biochem. 2009, 47, 435–440. [Google Scholar] [CrossRef]
- Ayour, J.; Sagar, M.; Harrak, H.; Alahyane, A.; Alfeddy, M.N.; Taourirte, M.; Benichou, M. Evolution of some fruit quality criteria during ripening of twelve new Moroccan apricot clones (Prunus armeniaca L.). Sci. Hortic. 2017, 215, 72–79. [Google Scholar] [CrossRef]
- Valdenegro, M.; Egea, I.; Sánchez-Bel, P.; Martínez, M.; Romojaro, F. Respuesta a tratamientos de etileno y 1-Metilciclopropeno del albaricoque var. Mauricio. Hortic. Int. 2006, 51, 72–73. [Google Scholar]
- Quiles-Bernabéu, R. Tratamientos Pre-Cosecha con Elicitores para Mejorar la Calidad de Albaricoques en el Momento de la Recolección; Trabajo; Fin de Grado en Biotecnología, Universidad Miguel Hernández: Elche, Alicante, Spain, 2017. [Google Scholar]
- Huang, Z.; Wang, Q.; Xia, L.; Hui, J.; Li, J.; Feng, Y.; Chen, Y. Preliminarily exploring of the association between sugars and anthocyanin accumulation in apricot fruit during ripening. Sci. Hortic. 2019, 248, 112–117. [Google Scholar] [CrossRef]
- Fan, X.; Shu, C.; Zhao, K.; Wang, X.; Cao, J.; Jiang, W. Regulation of apricot ripening and softening process during shelf life by post-storage treatments of exogenous ethylene and 1-methylcyclopropene. Sci. Hortic. 2018, 232, 63–70. [Google Scholar] [CrossRef]
- Kopsell, D.A.; Kopsell, D.E. Carotenoids in Vegetables. In Bioactive Foods in Promoting Health; Academic Press, 2010; pp. 645–662. Available online: https://www.sciencedirect.com/science/article/pii/B9780123746283000463 (accessed on 5 July 2021).
- Crisosto, C.H.; Garner, D.; Crisosto, G.M.; Bowerman, E. Increasing “Black amber” plum (Prunus salicina Lindell) consumer acceptance. Postharvest Biol. Technol. 2004, 34, 237–244. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).