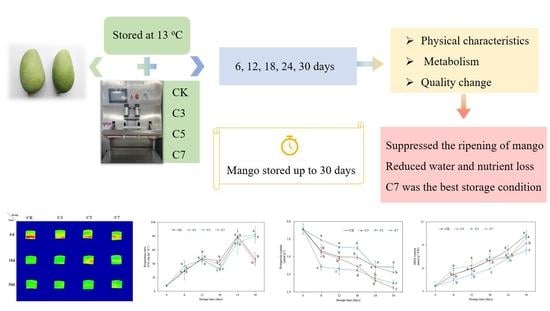
Effects of Different Carbon Dioxide-Modified Atmosphere Packaging and Low-Temperature Storage at 13 °C on the Quality and Metabolism in Mango (Mangifera indica L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials and Treatment
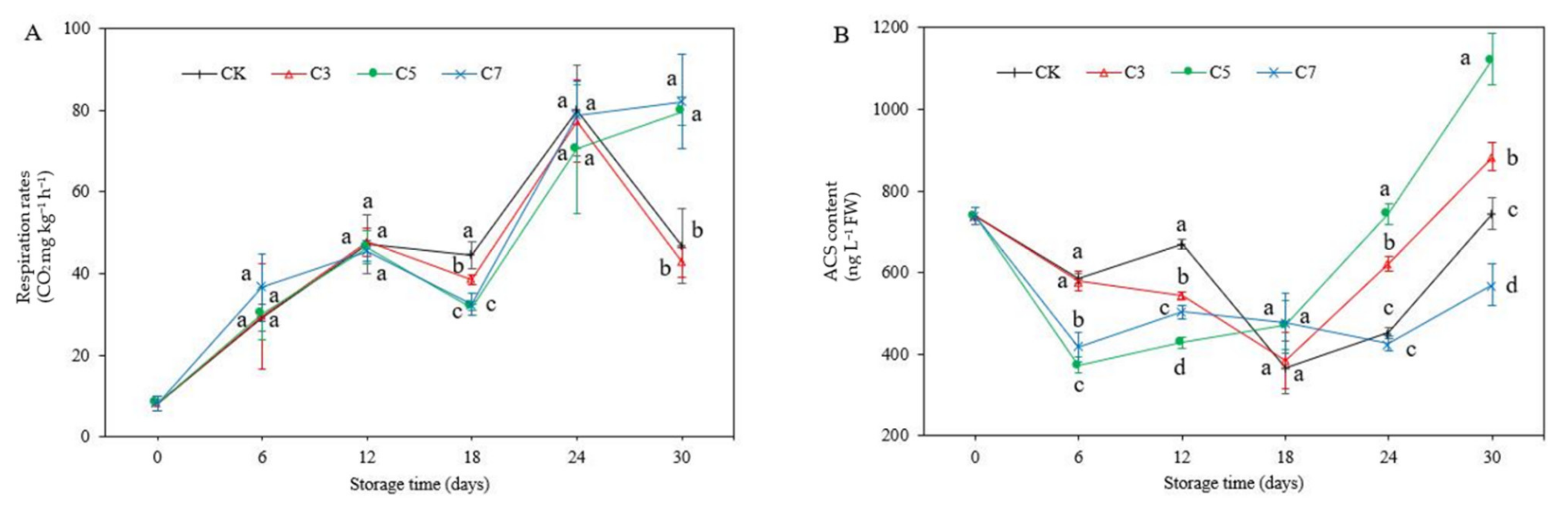
2.2. Respiration Rate
2.3. ACS Content
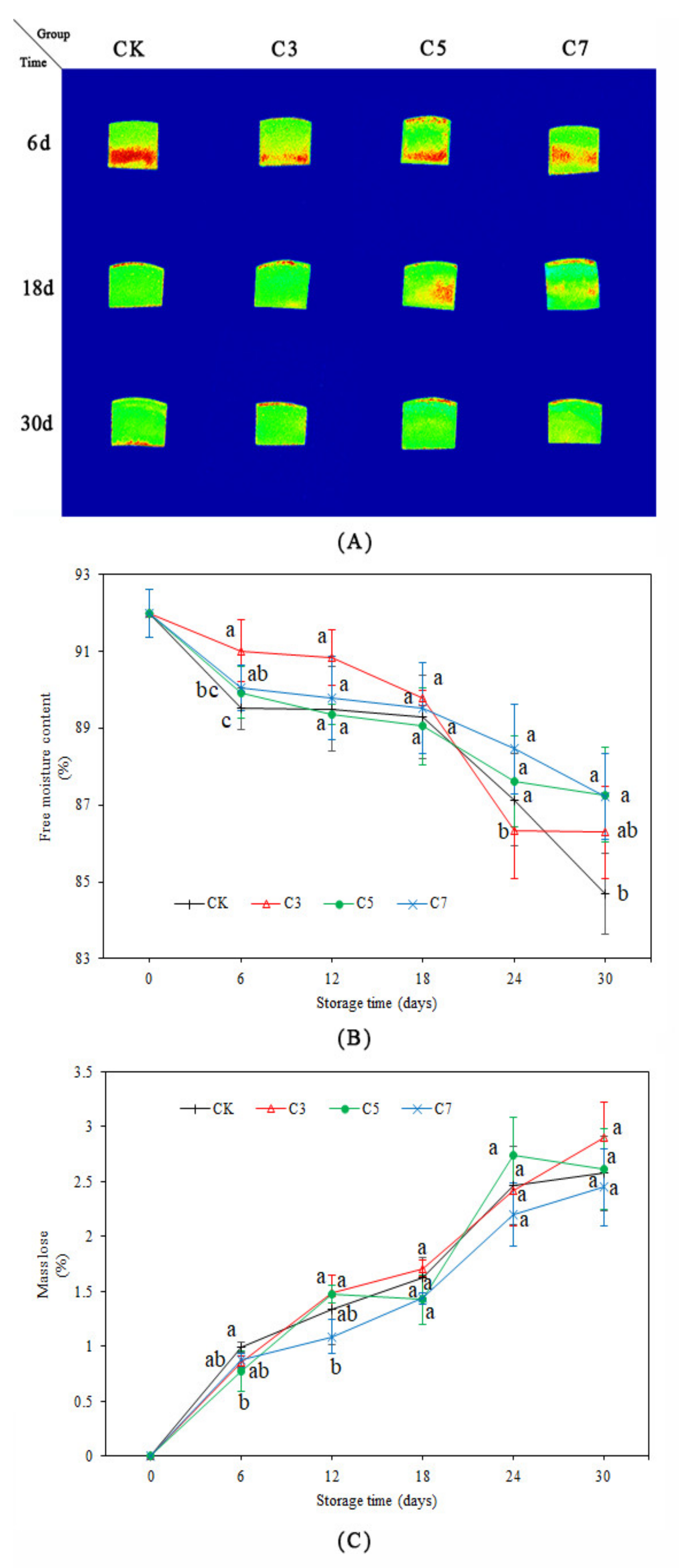
2.4. Magnetic Resonance Imaging (MRI) and Free Moisture Content (FMC)
2.5. Mass Loss (ML)
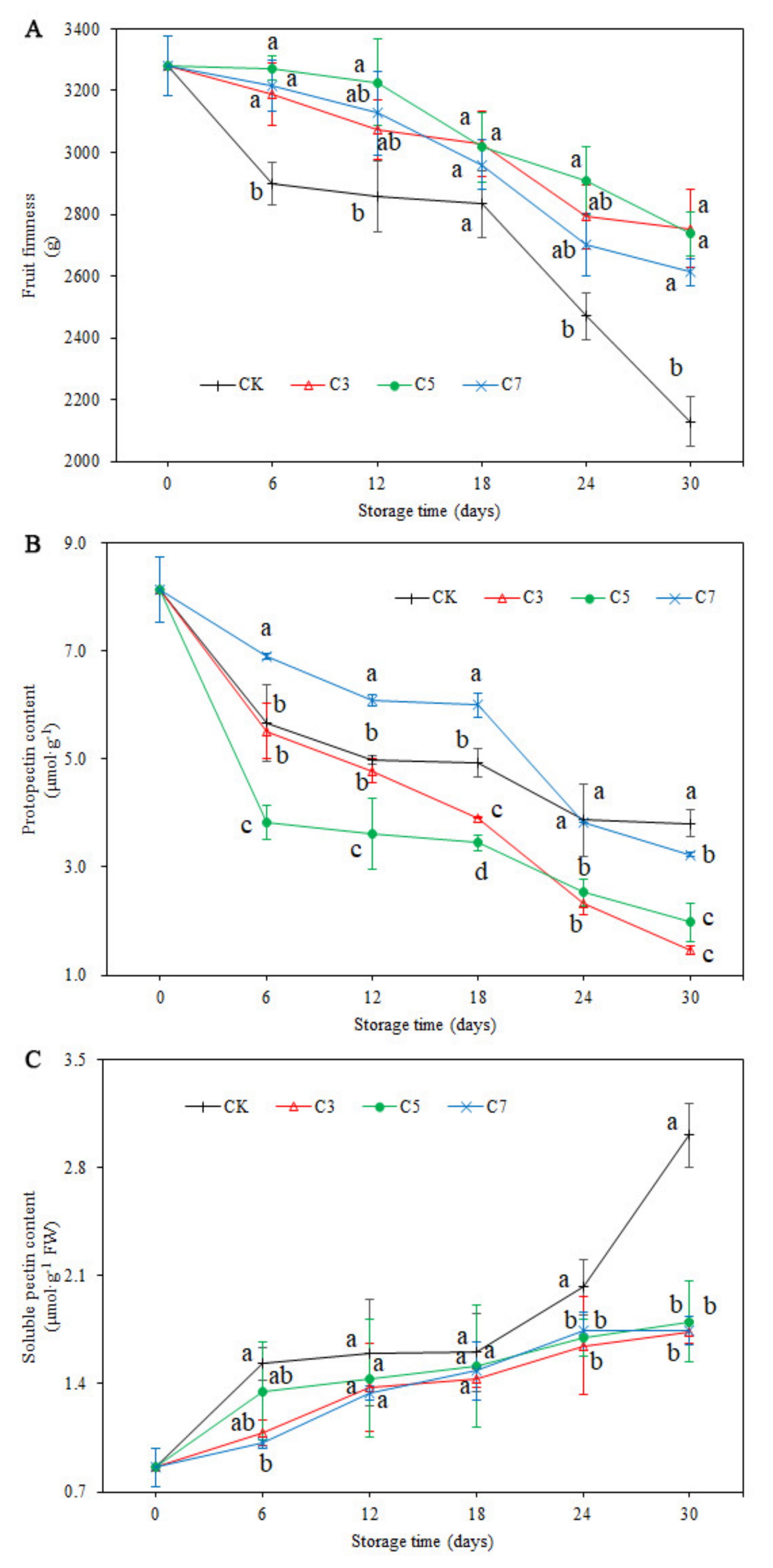
2.6. Fruit Firmness
2.7. Protopectin and Soluble Pectin Content
2.8. Color
2.9. Vitamin C (VC) Content
2.10. Malondialdehyde (MDA) Content
2.11. Statistical Analysis
3. Results and Discussion
3.1. Respiration Rate and ACS Content
3.2. MRI, FMC, and ML
3.3. Fruit Firmness and Pectin Content Analysis
3.4. Color Characteristics
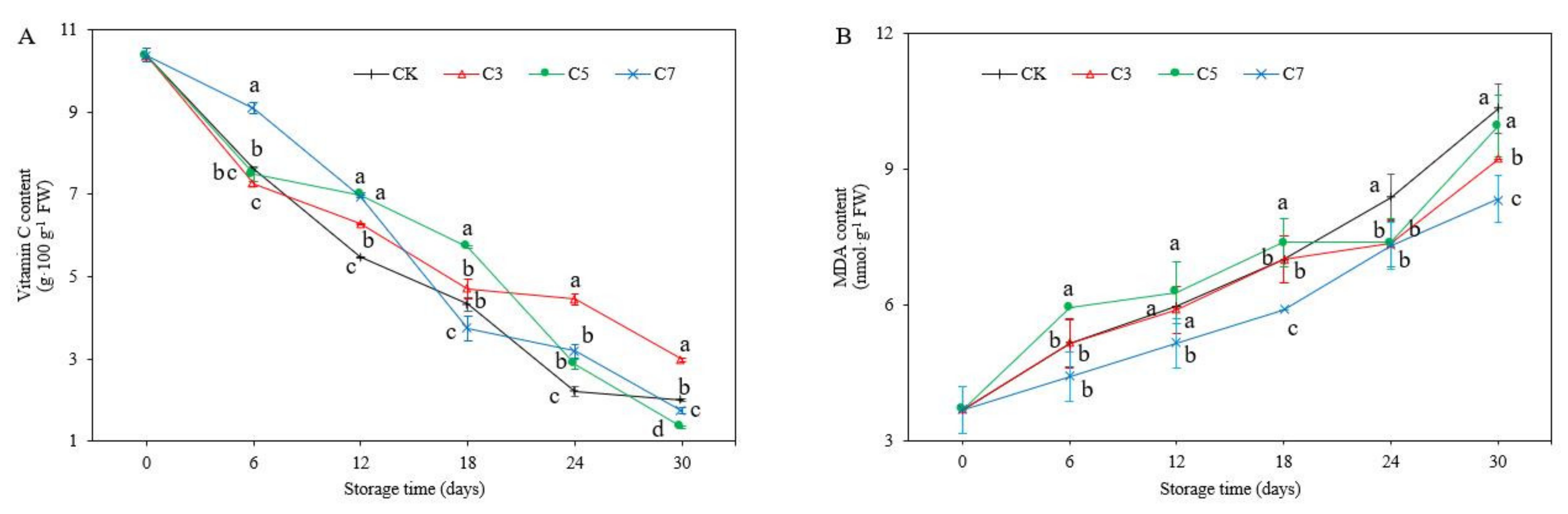
3.5. VC and MDA Content
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ebrahimi, F.; Rastegar, S. Preservation of Mango Fruit with Guar-Based Edible Coatings Enriched with Spirulina Platensis and Aloe Vera Extract During Storage at Ambient Temperature. Sci. Hortic. 2020, 265, 109258. [Google Scholar] [CrossRef]
- De Oliveira, K.a.R.; Berger, L.R.R.; De Araujo, S.A.; Camara, M.P.S.; De Souza, E.L. Synergistic Mixtures of Chitosan and Mentha Piperita L. Essential Oil to Inhibit Colletotrichum Species and Anthracnose Development in Mango Cultivar Tommy Atkins. Food Microbiol. 2017, 66, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.B.; Lei, H.H.; Ma, X.Y.; Lai, T.F.; Song, H.M.; Shi, X.Q.; Li, J.K. Antifungal activity of 1-methylcyclopropene (1-MCP) against anthracnose (Colletotrichum gloeosporioides) in postharvest mango fruit and its possible mechanisms of action. Int. J. Food Microbiol. 2017, 241, 1–6. [Google Scholar] [CrossRef]
- Falagán, N.; Terry, L.A. Recent Advances in Controlled and Modified Atmosphere of Fresh Produce. Johns. Matthey Technol. Rev. 2018, 62, 107–117. [Google Scholar] [CrossRef]
- Reche, J.; García-Pastor, M.E.; Valero, D.; Hernández, F.; Almansa, M.S.; Legua, P.; Amorós, A. Effect of Modified Atmosphere Packaging on the Physiological and Functional Characteristics of Spanish Jujube (Ziziphus Jujuba Mill.) Cv ‘Phoenix’ During Cold Storage. Sci. Hortic. 2019, 258, 108743. [Google Scholar] [CrossRef]
- Liu, H.; Li, D.L.; Xu, W.C.; Fu, Y.B.; Liao, R.J.; Shi, J.Z.; Chen, Y.Z. Application of Passive Modified Atmosphere Packaging in the Preservation of Sweet Corns at Ambient Temperature. Lwt 2021, 136, 110295. [Google Scholar] [CrossRef]
- Ozturk, B.; Uzun, S.; Karakaya, O. Combined Effects of Aminoethoxyvinylglycine and Map on the Fruit Quality of Kiwifruit During Cold Storage and Shelf Life. Sci. Hortic. 2019, 251, 209–214. [Google Scholar] [CrossRef]
- Ntsoane, M.L.; Luca, A.; Zude-Sasse, M.; Sivakumar, D.; Mahajan, P.V. Impact of Low Oxygen Storage on Quality Attributes Including Pigments and Volatile Compounds in ‘Shelly’ Mango. Sci. Hortic. 2019, 250, 174–183. [Google Scholar] [CrossRef]
- Dhalsamant, K.; Mangaraj, S.; Bal, L.M. Modified atmosphere packaging for mango and tomato: An appraisal to improve shelf life. J. Packag. Technol. Res. 2017, 1, 127–133. [Google Scholar] [CrossRef]
- Ramayya, N.; Niranjan, K.; Duncan, E. Effects of modified atmosphere packaging on quality of ‘Alphonso’ Mangoes. J. Food Sci. Technol. 2012, 49, 721–728. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.K.; Zhu, Q.G.; Hu, M.J.; Gao, Z.Y.; An, F.; Li, M.; Jiang, Y.M. Low-Temperature Conditioning Induces Chilling Tolerance in Stored Mango Fruit. Food Chem. 2017, 219, 76–84. [Google Scholar] [CrossRef]
- Zaharah, S.S.; Singh, Z. Postharvest Nitric Oxide Fumigation Alleviates Chilling Injury, Delays Fruit Ripening and Maintains Quality in Cold-Stored ‘Kensington Pride’ Mango. Postharvest Biol. Technol. 2011, 60, 202–210. [Google Scholar] [CrossRef]
- Deng, B.L.; Guo, M.X.; Liu, H.X.; Tian, S.; Zhao, X.S. Inhibition of Autophagy by Hydroxychloroquine Enhances Antioxidant Nutrients and Delays Postharvest Fruit Senescence of Ziziphus Jujuba. Food Chem. 2019, 296, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.S.; Singh, Z.Z.; Tan, S.C. Chilling Injury in Relation to Ethylene Biosynthesis in ‘Kensington Pride’ Mango Fruit. J. Hortic. Sci. Biotechnol. 2004, 79, 82–90. [Google Scholar] [CrossRef]
- Kirtil, E.; Oztop, M.H.; Sirijariyawat, A.; Ngamchuachit, P.; Barrett, D.M.; Mccarthy, M.J. Effect of Pectin Methyl Esterase (Pme) and Cacl2 Infusion on the Cell Integrity of Fresh-Cut and Frozen-Thawed Mangoes: An Nmr Relaxometry Study. Food Res. Int. 2014, 66, 409–416. [Google Scholar] [CrossRef]
- Lo’ay, A.A.; Taher, M.A. Influence of Edible Coatings Chitosan/Pvp Blending with Salicylic Acid on Biochemical Fruit Skin Browning Incidence and Shelf Life of Guava Fruits Cv. ‘Banati’. Sci. Hortic. 2018, 235, 424–436. [Google Scholar] [CrossRef]
- Ren, Y.Y.; Sun, P.P.; Wang, X.X.; Zhu, Z.Y. Degradation of Cell Wall Polysaccharides and Change of Related Enzyme Activities with Fruit Softening in Annona Squamosa During Storage. Postharvest Biol. Technol. 2020, 166, 111203. [Google Scholar] [CrossRef]
- Jongsri, P.; Rojsitthisak, P.; Wangsomboondee, T.; Seraypheap, K. Influence of Chitosan Coating Combined with Spermidine on Anthracnose Disease and Qualities of ‘Nam Dok Mai’ Mango after Harvest. Sci. Hortic. 2017, 224, 180–187. [Google Scholar] [CrossRef]
- Yao, L.Y.; Fan, L.P.; Duan, Z.H. Effects of Different Packaging Systems and Storage Temperatures on the Physical and Chemical Quality of Dried Mango Slices. LWT Food Sci. Technol. 2020, 121, 108981. [Google Scholar] [CrossRef]
- Chen, Q.Q.; Bi, J.F.; Wu, X.Y.; Yi, J.Y.; Zhou, L.Y.; Zhou, Y.H. Drying Kinetics and Quality Attributes of Jujube (Zizyphus Jujuba Miller) Slices Dried by Hot-Air and Short- and Medium-Wave Infrared Radiation. LWT Food Sci. Technol. 2015, 64, 759–766. [Google Scholar] [CrossRef]
- Zheng, X.L.; Tian, S.P. Effect of Oxalic Acid on Control of Postharvest Browning of Litchi Fruit. Food Chem. 2006, 96, 519–523. [Google Scholar] [CrossRef]
- Ho, P.L.; Tran, D.T.; Hertog, M.L.a.T.M.; Nicolaï, B.M. Effect of Controlled Atmosphere Storage on the Quality Attributes and Volatile Organic Compounds Profile of Dragon Fruit (Hylocereus Undatus). Postharvest Biol. Technol. 2021, 173, 111406. [Google Scholar] [CrossRef]
- Vichaiya, T.; Uthaibutra, J.; Saengnil, K. Gaseous Chlorine Dioxide Increases Energy Status and Energy Metabolism-Related Enzyme Activities Leading to Reduction in Pericarp Browning of Longan Fruit During Storage. Sci. Hortic. 2020, 263, 109118. [Google Scholar] [CrossRef]
- Yu, J.; Zhu, M.T.; Bai, M.; Xu, Y.S.; Fan, S.G.; Yang, G.S. Effect of Calcium on Relieving Berry Cracking in Grape (Vitis Vinifera L.) ‘Xiangfei’. PeerJ 2020, 8, e9896. [Google Scholar] [CrossRef] [PubMed]
- Phakdee, N.; Chaiprasart, P. Modified Atmosphere Storage Extends the Shelf Life of ‘Nam Dok Mai Sri Tong’ Mango Fruit. Int. J. Fruit Sci. 2019, 20, 1–11. [Google Scholar] [CrossRef]
- Sheng, K.; Wei, S.C.; Mei, J.; Xie, J. Chilling Injury, Physicochemical Properties, and Antioxidant Enzyme Activities of Red Pitahaya (Hylocereus Polyrhizus) Fruits under Cold Storage Stress. Phyton 2021, 90, 291–305. [Google Scholar] [CrossRef]
- Zerbini, P.E.; Vanoli, M.; Rizzolo, A.; Grassi, M.; Pimentel, R.M.D.A.; Spinelli, L.; Torricelli, A. Optical Properties, Ethylene Production and Softening in Mango Fruit. Postharvest Biol. Technol. 2015, 101, 58–65. [Google Scholar] [CrossRef] [Green Version]
- Zaharah, S.S.; Singh, Z.; Symons, G.M.; Reid, J.B. Mode of action of abscisic acid in triggering ethylene biosynthesis and softening during ripening in mango fruit. Postharvest Biol. Technol. 2013, 75, 37–44. [Google Scholar] [CrossRef]
- Vázquez-Celestino, D.; Ramos-Sotelo, H.; Rivera-Pastrana, D.M.; Vázquez-Barrios, M.E.; Mercado-Silva, E.M. Effects of Waxing, Microperforated Polyethylene Bag, 1-Methylcyclopropene and Nitric Oxide on Firmness and Shrivel and Weight Loss of ‘Manila’ Mango Fruit During Ripening. Postharvest Biol. Technol. 2015, 111, 398–405. [Google Scholar] [CrossRef]
- Khan, A.S.; Singh, Z. 1-Methylcyclopropene Application and Modified Atmosphere Packaging Affect Ethylene Biosynthesis, Fruit Softening, and Quality of ‘Tegan Blue’ Japanese Plum During Cold Storage. J. Am. Soc. Hortic. Sci. 2008, 133, 290–299. [Google Scholar] [CrossRef]
- Bizzani, M.; William Menezes Flores, D.; Bueno Moraes, T.; Alberto Colnago, L.; David Ferreira, M.; Helena Fillet Spoto, M. Non-Invasive Detection of Internal Flesh Breakdown in Intact Palmer Mangoes Using Time-Domain Nuclear Magnetic Resonance Relaxometry. Microchem. J. 2020, 158, 105208. [Google Scholar] [CrossRef]
- Chang, E.-H.; Lee, J.-S.; Kim, J.-G. Cell Wall Degrading Enzymes Activity Is Altered by High Carbon Dioxide Treatment in Postharvest ‘Mihong’ Peach Fruit. Sci. Hortic. 2017, 225, 399–407. [Google Scholar] [CrossRef]
- Lufu, R.; Ambaw, A.; Opara, U.L. Water Loss of Fresh Fruit: Influencing Pre-Harvest, Harvest and Postharvest Factors. Sci. Hortic. 2020, 272, 109519. [Google Scholar] [CrossRef]
- Wei, X.P.; Xie, D.D.; Mao, L.C.; Xu, C.J.; Lu, W.J. Excess Water Loss Induced by Simulated Transport Vibration in Postharvest Kiwifruit. Sci. Hortic. 2019, 250, 113–120. [Google Scholar] [CrossRef]
- Ibarra-Garza, I.P.; Ramos-Parra, P.A.; Hernández-Brenes, C.; Jacobo-Velázquez, D.A. Effects of Postharvest Ripening on the Nutraceutical and Physicochemical Properties of Mango (Mangifera Indica L. Cv Keitt). Postharvest Biol. Technol. 2015, 103, 45–54. [Google Scholar] [CrossRef]
- Wang, Y.P.; Shan, Y.X.; Chen, J.W.; Feng, J.J.; Huang, J.Q.; Jiang, F.; Zheng, S.Q.; Qin, Q.P. Comparison of Practical Methods for Postharvest Preservation of Loquat Fruit. Postharvest Biol. Technol. 2016, 120, 121–126. [Google Scholar] [CrossRef]
- Li, M.; Zhi, H.H.; Dong, Y. Textural Property and Cell Wall Metabolism of ‘Golden Bosc’ and ‘D’anjou’ Pears as Influenced by Oxygen Regimes after Long-Term Controlled Atmosphere Storage. Postharvest Biol. Technol. 2019, 151, 26–35. [Google Scholar] [CrossRef]
- Dziedzic, E.; Błaszczyk, J.; Bieniasz, M.; Dziadek, K.; Kopeć, A. Effect of Modified (Map) and Controlled Atmosphere (Ca) Storage on the Quality and Bioactive Compounds of Blue Honeysuckle Fruits (Lonicera Caerulea L.). Sci. Hortic. 2020, 265, 109226. [Google Scholar] [CrossRef]
- Truc, N.T.; Uthairatanakij, A.; Srilaong, V.; Laohakunjit, N.; Jitareerat, P. Effect of Electron Beam Radiation on Disease Resistance and Quality of Harvested Mangoes. Radiat. Phys. Chem. 2020, 180, 109289. [Google Scholar] [CrossRef]
- Ali, S.; Khan, A.S.; Malik, A.U.; Shahid, M. Effect of Controlled Atmosphere Storage on Pericarp Browning, Bioactive Compounds and Antioxidant Enzymes of Litchi Fruits. Food Chem. 2016, 206, 18–29. [Google Scholar] [CrossRef]
- Rosalie, R.; Joas, J.; Deytieux-Belleau, C.; Vulcain, E.; Payet, B.; Dufosse, L.; Lechaudel, M. Antioxidant and Enzymatic Responses to Oxidative Stress Induced by Pre-Harvest Water Supply Reduction and Ripening on Mango (Mangifera Indica L. Cv. ‘Cogshall’) in Relation to Carotenoid Content. J. Plant Physiol. 2015, 184, 68–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| MAPs | L* | a* | b* | ΔE |
|---|---|---|---|---|
| CK | 53.40 ± 1.95 a | −6.48 ± 0.40 a | 29.68 ± 0.13 b | 47.38 ± 1.82 c |
| C3 | 47.30 ± 0.46 b | −5.83 ± 0.09 b | 29.81 ± 0.18 b | 53.13 ± 0.43 b |
| C5 | 48.76 ± 0.93 b | −6.07 ± 0.06 b | 36.01 ± 0.29 a | 53.82 ± 0.94 b |
| C7 | 41.07 ± 1.21 c | −5.83 ± 0.10 b | 28.08 ± 0.66 c | 58.72 ± 1.03 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, S.; Mei, J.; Xie, J. Effects of Different Carbon Dioxide-Modified Atmosphere Packaging and Low-Temperature Storage at 13 °C on the Quality and Metabolism in Mango (Mangifera indica L.). Agriculture 2021, 11, 636. https://doi.org/10.3390/agriculture11070636
Wei S, Mei J, Xie J. Effects of Different Carbon Dioxide-Modified Atmosphere Packaging and Low-Temperature Storage at 13 °C on the Quality and Metabolism in Mango (Mangifera indica L.). Agriculture. 2021; 11(7):636. https://doi.org/10.3390/agriculture11070636
Chicago/Turabian StyleWei, Saichao, Jun Mei, and Jing Xie. 2021. "Effects of Different Carbon Dioxide-Modified Atmosphere Packaging and Low-Temperature Storage at 13 °C on the Quality and Metabolism in Mango (Mangifera indica L.)" Agriculture 11, no. 7: 636. https://doi.org/10.3390/agriculture11070636
APA StyleWei, S., Mei, J., & Xie, J. (2021). Effects of Different Carbon Dioxide-Modified Atmosphere Packaging and Low-Temperature Storage at 13 °C on the Quality and Metabolism in Mango (Mangifera indica L.). Agriculture, 11(7), 636. https://doi.org/10.3390/agriculture11070636