Abstract
Probiotics are live microorganisms which, when administered in adequate amounts, confer a health benefit on the host. Traditionally, dairy products are the major and most popular probiotic carriers. At present, there is a growing demand for non-dairy probiotic products. Both fermented and non-fermented non-dairy plant-based food products are becoming highly appealing to both dairy and non-dairy consumers worldwide. Non-dairy plant-based food matrices such as fruits, vegetables, plant-based milk, cereals, and legumes have been used successfully in producing probiotic products with the minimum recommended viable probiotic numbers at the time of consumption. However, due to the exclusion of dairy, whether these food matrices can enhance the functional properties of probiotics such as gastrointestinal survival and immune-enhancing effects needs a thorough investigation. Hence, this review focuses on some of the popular non-dairy plant-based probiotic food products and their microbiological quality characteristics in terms of maintaining probiotic viability during product storage. Their gastrointestinal tolerance in these products, other functional properties, and product qualities have also been briefly discussed.
1. Introduction
The utilization of beneficial microorganisms in health promotion has been practiced in the means of fermented dairy products such as sour milk, yoghurt, and cheese from many thousands of years [1]. The idea that lactic acid bacteria (LAB) prevent intestinal disorders and diseases is as old as the science of microbiology [2]. In 1907, the Nobel Laureate Elie Metchnikoff firstly documented the modern concept of probiotics in his book The Prolongation of Life after observing the Bulgarians’ exceptionally prolonged healthy living was due to their regular consumption of sour milk [3]. The first formula for the deliberate administration of live LAB was a sour milk product based on a culture called la Lactobacilline isolated by Metchnikoff [2]. The culture was comprised of Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus and the product was launched in Paris at the beginning of the 20th century. Thereafter, many different of milk-based probiotic products have been invented and introduced to the market. Probiotics can be defined as live microorganisms which, in adequate amounts, confer a health benefit on the host [4]. These organisms have to be taken regularly at sufficiently higher levels (>106 cfu/mL per day) to avoid washout and assure that their benefits will be accrued in a sustained manner [5].
LAB are common members of the human gut microbiota, which contains trillions of microorganisms belonging to more than 1000 bacterial species. The gut microbiota plays a significant role in the host by influencing the maturation of the immune system, regulating energy metabolism, and affecting brain function and behaviour through the gut-brain axis [3,6,7]. Diet is one of the main factors contributing to the composition and diversity of the human intestinal microbiota. Probiotics and prebiotics have been used as dietary strategies aimed at improving host health by modulating the gut ecosystem which in turn affecting host stress-responses, behaviour, and cognition [6].
Scientists select potential probiotic strains based on their established health benefits, how a strain can tolerate the harsh gastrointestinal tract conditions, safety aspects, and technological properties [7]. Some of these technological and functional properties are depicted in Figure 1.
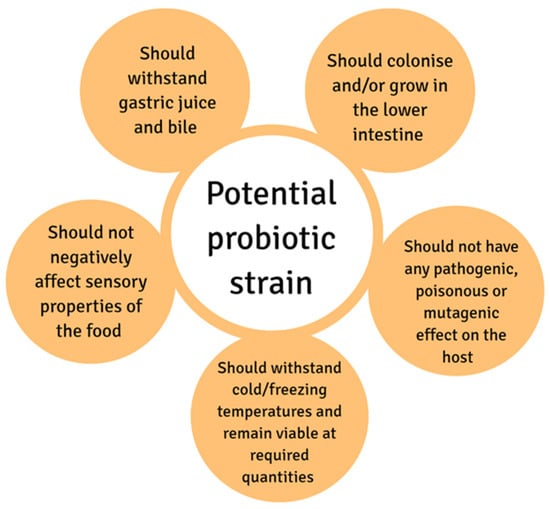
Figure 1.
Technological and functional properties of a potential probiotic strain.
LAB, mainly, Lactobacillus spp. and Bifidobacterium spp. are widely recognized as probiotics since they commonly possess probiotic characteristics [1,3]. Certain strains of probiotics, for example, Lactobacillus acidophilus, Lactobacillus lactis, and Pediococcus acidilactici, have been given the Generally Recognized as Safe (GRAS) status by the U.S. Food and Drug Administration (FDA) to be used as food ingredients [8]. Therefore, these microorganisms can be considered safe to consume with very little or no risk to the host. Probiotics can be obtained from different sources such as human gut microbiota, foods, and other natural environments. They are host specific and, therefore, probiotic bacteria isolated from human intestinal microbiota may be advantageous for use in products intended for human consumption [7]. These probiotic microorganisms have various health-promoting effects, namely preventing intestinal tract infections, improving lactose metabolism, reducing serum cholesterol levels, enhancing immunity, stimulating calcium absorption, improving protein digestibility, synthesizing vitamins (vitamin B including nicotinic acid and folic acid), and counteracting the effect of food-borne pathogens [9]. The health benefits of probiotic-enriched foods are expressed either directly through the interactions of ingested live microorganisms with the host or indirectly as the result of the intake of microbial metabolites synthesized during fermentation [10].
Fermented dairy products have been at the forefront of probiotic carrier foods for many thousands of years. At present, there is an increasing demand for non-dairy plant-based probiotic products due to various reasons including rising vegetarianism and emerging veganism, allergenicity for dairy products, and consumer preferences for various novel tastes. However, probiotic functional efficacy during processing, storage, and after ingestion can be determined by their carrier food substrates. Similarly, probiotic viability in food products is the main factor in determining probiotic efficacy. Therefore, this review aimed to discuss various plant-based food matrices in delivering probiotics, with a special emphasis on probiotic viability and various functions of those probiotic microorganisms in these foods. Since probiotic viability after consumption is mainly determined by their gastrointestinal tolerance, research on plant-based food products in the recent literature has also been briefly discussed. Consumer acceptance of probiotic plant-based food products is largely determined by their quality characteristics. Where relevant, sensory and physico-chemical properties have also been briefly outlined to demonstrate the potential influence of these parameters on probiotic food intake and consequently on survivability during gastrointestinal transit.
2. Plant-Based Matrices and Their Features
Probiotic foods have been largely restricted to dairy products such as yoghurt, fermented sour milk, and cheese that dominate the probiotic food development at present [3,5]. The rising emergence of lactose intolerance, milk allergies, the prevalence of hypercholesterolemia, and the environmental impacts of dairy production such as soil degradation, greenhouse gas emissions, water pollution, and the loss of biodiversity are leading towards a growing demand for dairy alternatives [11,12]. In this context, fermented foods of plant origin are becoming popular as vectors for incorporating probiotic microorganisms [2,5,11]. Consequently, the research interests on probiotic food development have now been diverted towards non-dairy based probiotic products [9,12]. Non-dairy sources such as fruits, vegetables, cereals, and legumes contain a high level of beneficial substances in human nutrition (e.g., antioxidants, vitamins, minerals, dietary fibers) [9,13]. The addition of probiotics into these food matrices may therefore bring additional health benefits. Currently, almost all the probiotic strains employed in commercial food preparation are of human or animal origin. Only a few probiotic cultures used with success in dairy products exhibit acceptable viabilities in plant-based matrices by the time of consumption [5,14]. Therefore, plant-based sources may be an alternative source to screen potential probiotic strains to help overcome such technological challenges.
Fermentation is the oldest method of food preservation and is responsible for certain favorable food features such as texture, flavor, and shelf life. A large variety of fermented non-dairy products exists around the world (Table 1). However, their commercial distribution is limited due to the lack of standard manufacturing protocols coupled with geologically specific raw materials, which are limited in supply [5,15]. The manufacture of fermented non-dairy/plant-based products has traditionally taken advantage of the beneficial microorganisms spontaneously established on the plant materials. LAB responsible for plant material fermentation often belong to the genera Lactobacillus, Leuconostoc, and Pediococcus and some of these are as resistant as animal-derived LAB to gastrointestinal conditions [5,16].
Plant tissues provide conditions favorable to the microbial internalization. Microorganisms tend to be located in pores, holes, and other irregularities naturally occurring on the intact fruits that favor microbial binding and protection [17]. Fruits, vegetables, legumes, and cereals contain non-digestible fibers such as cellulose and may exert a protective effect on the probiotic microorganisms during passage through the intestinal tract. Certain fruit matrices such as apple, guava, banana, and melon have shown strong adhesion to food matrices [13]. Therefore, it is evident that the presence of ridges and natural prebiotic compounds, protect probiotics from the acidic environment of the stomach, and are good sources of nutrients, which positively influences probiotic survival [18]. In contrast, higher contents of proteins and lipids in the milk provide a protective matrix for the survival of probiotics in milk and dairy products. Consequently, dairy products such as sour milk, yoghurt, and cheese dominate as ideal vehicles for probiotic delivery. When non-dairy plant-based matrices are concerned, juices, purees, pickles, snacks, etc., can be identified as ideal vehicles to deliver probiotics to humans.

Table 1.
Different traditional non-dairy probiotic beverages, their principal raw materials and probiotic microorganisms involved in production.
Table 1.
Different traditional non-dairy probiotic beverages, their principal raw materials and probiotic microorganisms involved in production.
| Type | Name | Principal Raw Material | Geographical Regions in Which the Beverage Is Popular | Probiotic Microorganisms Isolated | References |
|---|---|---|---|---|---|
| Cereal and legume-based | Boza | Wheat, rye, millet mixed with sugar/saccharine | Bulgaria, Albania, Turkey, Romania | LABs: Lctobacillus acidophilus, Lb. fermentum, Lb. coprophilus, Lb. brevis, Leuconostoc reffinolactis, Leuconostoc mesenteroides Yeast: Sachharomyces cerevisiae, Candida tropicalis, Candida glabata, Geotrichum penicilliatum, Geotrichum candidum | [19,20] |
| Bushera | Sorghum or millet flour from germinated sorghum or millet grains | Western highlands of Uganda | Lactobacillus sp., Lactococcus sp., Leuconostoc sp., Enterococcus sp., Streptococcus sp. | [21] | |
| Mahewa/amahewa | Corn meal ferment with sorghum, millet malt or wheat flour | Africa and some Arabian Gulf countries | Lc. lactis ssp. lactis | [19,22] | |
| Pozol | Cooking maize in a lime solution (1% w/v) | South-eastern Mexico | Lactococcus lactis | [23] | |
| Togwa | Maize flour and finger millet | Japan & China | Lactobacillus spp., Streptococcus spp., and Lb. plantarum | [2,24] | |
| Ogi | Maize | Nigeria and west Africa | Lb. acidophilus, Lb. plantarum, Lb. brevis, Lb. fermentum, S. cerevisiae, Rhodotorula graminis, Candida krusei, C. tropicalis, Geotrichum candidum, Geotrichum fermentum | [25,26] | |
| Kefir Soy | Soya beans | Greece | Lb. brevis, Lb. kefir, Lb. mesenteroides, Lb. helveticus, Kluyveromyces maxianus and Kluyveromyces lactis | [9] | |
| Ricera | Rice | Lb. acidophilus, Lb. bulgaricus, B. bifidum, S. thermophilus | |||
| Uji | Maize or sorghum | Kenya | Lb. plantarum, Lb. fermentum, Lb. cellobiaosus, Lb. buchneri, Pediococcus nacidilactice, Pediococcus penosaceus, Lb. rhamnosus, S. thermophilus | [27] | |
| Velli | Oat brans and fruits | Russia | B. bifidum, Lb. acidophilus | [9] | |
| Tempeh | Soymilk | Lb. rhamnosus, Bifidobacterium spp. | [9] | ||
| Vegetable-based | Shalgam | black carrot, bulgur flour, sourdough, salt, turnip, and drinkable water | Southern Turkey | Lb. plantarum, Lb. paracasei subsp. paracasei, Lb. brevis, Lb. fermentum | [9,28,29] |
| Kanji | Black carrot and beetroot | Northern India | Lb. plantarum | [30] | |
| Sayurasin | Mustard and cabbage | Indonesia | Lb. farciminis, Lb. fermentum, Lb. namurensis, Lb. plantarum, Lb. helveticus, Lb. brevis, Lb. versmoldensis, Lb. casei Lb. rhamnosus, Lb. fabifermentans, Lb. satsumensis | [31] | |
| Suan-tsai | Mustard | Taiwan | Pediococcus pentosaceus Tetragenococcus halophilus | [32] | |
| Yan-dong-gua | Wax gourd | Taiwan | Weissela paramesenteroides W. cibaria | [33] | |
| Soido | Bamboo shoots (Melocanna bambusoides, Bambusatulda and Dendrocalamus giganteus) | North-eastern states of India | Lc. lactis, Lb. brevis, Leu. fallax | [34] | |
| Jiang-sun | Bamboo shoots | Taiwan | Lb. plantarum | [35] | |
| Dochi | Black beans | Taiwan | Enterococcus faecium | [36] | |
| Jiang-gua | Cucumber | Taiwan | Wissella cibaria, Leuconostoc lactis | [37] | |
| Dua muoi | Mustard and beet | Vietnam | Lb. fermentum, Lb. pentosus, Lb. plantarum | [38] | |
| Ca muoi | Egg plant | Vietnam | Lb. fermentum, Lb. pentosus, Lb. plantarum | [38] | |
| Nozawana-Zuke | Japan | Lb. curvatus | [39] | ||
| Yan-tsai-shin | Broccoli stems | Taiwan | Lb. plantarum, Enterococcus sulphurous, Weissella paramesenteroides, W. minor, Leuconostoc mesenteroides and W. cibaria | [40] | |
| Chinese sauerkraut | Cabbage | China | Lb. mesenteroides, Lb. plantarum, Lb. brevis, Lb. rhamnosus and Lb. plantarum | [41,42] | |
| Kimchi | Napa cabbage and Korean radish | Korea | Leuconostoc mesenteriodes, Leu. carnosum, Lactobacillus curvatus, Lb. pentosus, Weissela kimchi, W. cibaria and Pediococcus pentosaceus | [43] | |
| Dhamuoi | Cabbage | Nepal and India | Leuconostoc mesenteroides and Lb. plantarum, | [44] | |
| Gundruk | Cabbage | Nepal and India | Pediococcus and Lactobacillus spp. | [44] | |
| Paocai | Cabbage, celery, cucumber and radish | China | L. pentosus, L. plantarum, Leuconostoc mesenteroides, L. brevis, L. lactis, and L. fermentum | [45,46] | |
| Kenkey/kormi/kokoe/dorkunu | Maize, millet, sorghum | Africa | Lb. plantarum, Lb. brevis, Lb. fermentum, Lb. reuteri | [47] | |
| Koozh | Rice and millet flour | South India | W. paramesenteroides, Lb. plantarum, Lb. fermentum | [48] | |
| Fruit-based | Pobuzihi | Cummingcordia/glue berry | Taiwan | Lb. plantarum Enterococcus casseliflavus Wissella cibaria | [49] |
| Xi-gua-mian | Water melon | Taiwan | Lb. plantarum Pediococcus pentosaceus | [50] | |
| Hardaliye | Red grape/red grape enriched with crushed mustard seeds and benzoic acid | Turkey | Lb. paracasei ssp. paracasei Lb. casei ssp. pseudoplantarum Lb. brevis, Lb. pontis, Lb. acetotolerans, Lb. sanfransisco, Lb. vaccinostercus | [51] | |
| Tempoyak | Durian fruit | Malaysia | Lb. mali, Lb. brevis, Lb. mesenteroides, Lb. fermentum | [9] |
3. Non-Dairy Plant-Based Probiotic Products
3.1. Cereal-Based Products
Cereals are rich sources of protein, carbohydrates, vitamins, minerals, and fiber. Cereals also contain various prebiotic substances that have several beneficial physiological effects in humans such as selectively stimulating the growth of lactobacilli and bifidobacteria in the colon [52,53]. Cereals are recognized as optimal fermentation substrates for the growth of LAB with probiotic potential and are suggested as matrices for a number of novel food formulations with different health claims [10,54]. In addition to be used as fermentation substrates for probiotic organisms, cereal and cereal components such as water-soluble fibers (e.g., β-glucan and arabinoxylan), oligosaccharides, and resistant starch have been investigated for their potential as novel prebiotics. Therefore, cereal-based ingredients fermented with probiotics would enhance consumer health with the benefits of probiotics, bran fiber and health-promoting bioactive components [10,52,53,55].
Probiotics such as Lb. plantarum, Lb. acidophilus, and Bifidobacterium spp. are predominantly used in the manufacture of cereal-based fermented beverages using maize, millet, barley, oat, rye, wheat, and rice as substrates [56,57]. For example, two strains of Lb. plantarum strains (6E and M6) were successfully employed to manufacture a series of vegetable yoghurt-based beverages from different cereals (rice, barley, emmer, and oat) supplemented with red grape-must (freshly crushed fruit juice) which was used as the main source of ascorbic acid, polyphenolic compounds, and carbohydrates for fermentation by LAB [57].
A large variety of traditional probiotic non-dairy cereal-based beverages has been produced throughout the history of human nutrition. However, the probiotic characteristics of microorganisms involved in these products have been reported recently [53]. Some of these traditional cereal-based beverages are summarized in Table 1.
Apart from these traditional non-dairy probiotic beverages, an ample number of research studies have been conducted on various cereal-based substrates to investigate their suitability as probiotic substrates. The incorporation of probiotics has resulted in additional beneficial features to these products such as achieving greater lactic acid concentrations through mixed-culture fermentations [58] and the production of B-vitamin enriched food [10,59,60].
3.1.1. Oat-Based Products
Oat is a rich source of dietary fiber, both insoluble and soluble, good quality fat, and phytochemicals important for human health. Among different non-digestible dietary fibers, oat β-glucan has been reported to have beneficial effects on insulin resistance, dyslipidemia, hypertension, obesity, enhanced immune response to bacterial infection, and for their applications in cancer treatment and prevention [10]. In the human digestive tract, oat β-glucans act as prebiotics that selectively fermented by butyrate-producing microorganisms [61]. In addition to the benefits of fiber, oat is also a good source of selenium, which works with vitamin E in various antioxidant systems throughout the body. These antioxidative actions reported to have beneficial effects against asthma, heart disease and certain types of cancer [61,62].
Exopolysaccharides (EPS) producing Pediococcus parvulus 2.6 has successfully been employed to improve the viscosity, texture, and mouthfeel of fermented oat-based products [63]. Human trials of the oat-based products fermented by P. parvulus 2.6 showed decreased serum cholesterol levels and increased counts of faecal Bifidobacterium spp. [64]. Moreover, EPS obtained from P. parvulus 2.6 seems to enhance some probiotic properties of LAB strains in vitro. For example, probiotics combined with β-glucan reported enhancing the anti-inflammatory properties of probiotics [59]. Thus, EPS produced by LAB are considered promising molecules in the functional food area as well as prebiotic fermentable substrates able to modulate the intestinal microbiota [65].
In another study, a symbiotic functional drink from oats was manufactured by combining the health benefits of a probiotic culture, Lb. plantarum B28, with oat prebiotic beta-glucan. The levels of starter culture concentration (5%), oat flour (5.5%) and sucrose content (1.5%) were established for completing a controlled fermentation for 8 h. The addition of sweeteners aspartame, sodium cyclamate, saccharine, and Haxol (12% cyclamate and 1.2% saccharine) did not affect either fermentation dynamics or probiotic survivability during 21 days of refrigerated storage. The viable probiotic counts were maintained well above the minimum therapeutic threshold level throughout the storage [56].
3.1.2. Malt-Based Products
Malt can be recognized as an excellent matrix for probiotics to maintain their viability throughout cold storage. The higher viability of the probiotics may be attributed to the presence of sugars in malt substrate such as fructose, glucose, sucrose, maltose, maltotriose, and maltotetraose [66]. The concentration of monosaccharides and disaccharides in malt was reported to be approximately 3 and 12 g/L, respectively [52,54]. Malt-based beverages seem to favor the growth of Bifidobacterium spp. since these food matrices provide ample amounts of metabolizable sugars (3–4 g/L) that stimulate the growth of bifidobacteria. One study revealed that moltotriose and glucose were more preferably utilized by B. adolescentis, B. breve and B. longum whereas fructose consumed all by B. infantis. The authors also demonstrated that high concentrations of growth promoters (yeast extract and peptone; 10 g/L) enhanced the buffering capacity of medium and thereby resulted in higher growth of bifidobacteria. Out of the two growth promoters, yeast extract showed promising effects as it contains substantial levels of vitamins and specific amino acids. However, a combination of both growth promoters had inhibitory effects on bifidobacteria growth [66].
3.1.3. Wheat-Based Products
Flour from emmer, (Triticum dicoccon; hulled wheat; an ancient wheat and an ancestor of modern durum), was successfully utilized to produce probiotic emmer beverages. Coda and colleagues (2011) manufactured a series of probiotic emmer beverages from emmer flour, emmer gelatinized flour, and emmer malt at percentages ranging 5–30% (w/w) using Lb. plantarum 6E as the starter [67]. The results showed that the combination of EPS-producing strain Lb. rhamnosus SP1 with Lb. plantarum 6E during the manufacture of emmer beverage containing 30% of gelatinized flour increased the viability of Lb. plantarum 6E than it is alone. Lb. rhamnosus SP1 with Lb. plantarum 6E showed viability of 8.9 log cfu/mL and 8.1 log cfu/ ml, respectively, during a month-long storage period at 4 °C. Saccharomyces cerevisiae var boulardii has been suggested as a suitable candidate for potential probiotic wheat beer development. The probiotic yeast showed equivalent metabolic efficiency on sugar wort and growth as that of the brewer’s yeast. More importantly, it remained alive during processing, storage (for over 60 days) and GI transit (>106 cfu/mL) [68].
The gastric tolerance of the probiotics delivered in cereal-based products including wheat has already been assayed. In a study conducted by Charalampopouls et al. (2003), the gastric tolerance of Lactobacillus plantarum, Lactobacillus acidophilus, and Lactobacillus reuteri delivered in wheat, malt, and barley extracts were assayed [69]. All strains showed a significant reduction in their cell concentrations in the absence of cereal extracts. In contrast, the viability of Lb. plantarum has been increased by approximately 4 log10 cycles in the presence of malt, and by 3 log10 cycles in the presence of either wheat or barley. The viability of Lb. acidophilus and Lb. reuteri has been increased by more than 1.5 and 0.7 log10 cycles. The results suggest that malt, wheat and barley extracts demonstrate a significant protective effect on the viability of the above probiotics. Moreover, Tian et al. (2021) reported that Lb. rhamnosus GG releases additional phenolic acids including trans-ferulic acid from digestive residues during colonic fermentation of whole wheat products (e.g., bread, cookie and pasta) leading to overall colon health. The incorporation of Lb. rhamnosus GG into wheat-based products would, therefore, provide additional health benefits for health-conscious customers [70].
3.1.4. Rice-Based Products
Rice has given rise to various rice-based fermented beverages and foods in the Asia-Pacific region. Rice beer is one such beverage popular among the ethnic communites in different parts of India [71]. In most cases, probiotics are the predominant strains found in these traditional beverages. Bacillus velezensis strain DU14 and Lactobacillus fermentum KKL1 are two such probiotic candidates isolated from the traditional rice beers Apong and Haria, respectively [71,72]. These probiotics do not only aid in fermentation, but also possess multi functionalities. For instance, Lb. fermentum KKL1 found to improve the accumulation of functional compositions (e.g., minerals), digestibility (due to α-amylase, glucoamylase, and phytase activities) and therapeutic potentials (e.g., antioxidative properties) [71]. Fermented sour rice is another traditional food of the Indian subcontinent which is believed to have therapeutic and prophylactic applications against various disorders. The probiotic candidate, Weissella confusa strain GCC 19R1 was found to be the predominant fermentative bacteria in this product [73]. Interestingly, a probiotic-fermented rice tablet has also been tested recently. The starter composed of Brettanomyces custersii ZSM-001 and Lactobacillus plantarum ZSM-002, and the sensory properties of the resulting tablet was similar to the tablet prepared using commercial starters. The viable bacterial counts for Lb. plantarum ZSM-002 remained >8 log cfu/g after simulated gastric and intestinal digestion [74].
3.1.5. Maize-Based Products
Maize is a widely used raw material in indigenous beverage production [75]. The available literature suggests that the maize matrix has been successfully utilized in the production of fermented probiotic beverages using yeast-lactic fermentation. For example, a novel, functional fermented beverage has been developed using potentially probiotic yeasts (Saccharomyces cerevisiae CCMA 0732, S. cerevisiae CCMA 0731, and Pichia kluyveri CCMA 0615) in combination with commercial probiotic strain Lactobacillus paracasei LBC-81. During fermentation and storage, except P. kluyveri, all tested strains showed viabilities >6 log cfu/mL. Interestingly, the beverages lacked a sweet taste and had no flavoring additive effect [75]. In another study, a series of novel fermented beverages from a blend of maize and rice were developed using Lactobacillus plantarum CCMA 0743, Torulaspora delbrueckii CCMA 0235, and the commercial probiotic Lb. acidophilus LACA 4 in mixed culture. These beverages were supplemented with the prebiotic, fructooligosaccharide (FOS). The results showed that FOS favored the growth of Lb. acidophilus and the yeast T. delbrueckii. The viable probiotic counts were maintained ≥T7 cfu/mL during fermentation and refrigerated storage for 28 d. A sensory analysis showed that >50% of the panellists liked the beverages slightly or extremely [76].
Apart from this, a fermented probiotic maize porridge has been prepared using maize flour and barley. The porridge was fermented with either Lactobacillus reuteri, Lb. acidophilus LA5, Lb. acidophilus 1748, or Lb. rhamnosus GG. Most strains reported to reach maximum cell counts (7.2–8.2 log cfu/g) after 12-h fermentation [77].
3.1.6. Millet-Based Products
The pearl millet substrate is rich in proteins, macro and micro minerals, resistant starch, soluble and insoluble dietary fibers, and dietary antioxidants (e.g., C-glycosylflavones, ferulic acid, β-carotene, etc.) [78]. Millet-based traditional food products are rich sources of potential probiotic microorganisms with various functionalities. For example, Palaniswamy and Govindasamy (2016) isolated five ferulolyl esterase-producing Lactobacillus strains that belonged to Lb. fermentum and Lb. delbrueckii from a traditional pearl millet porridge (kambu koozh) and characterized their probiotic potential. Out of these five strains, Lb. fermentum CFR5 found to be a promising probiotic candidate with the abilities to produce β-galactosidase and glutamate decarboxylase enzymes and demonstrated cholesterol-lowering effects in vitro [78]. Further, five probiotic strains of Lactobacillus (Lb. pentosus SW02; Lb. plantarum subsp. plantarum SW03, SW06 and SW07; Lb. sakei subsp. sakei SW04), and two strains of Pediococcus (P. pentosaceus SW01 and P. acidilactici SW05) were isolated from Omegisool, a traditionally-fermented alcoholic beverage in South Korea, and possessed antioxidative properties [79]. Previous studies showed that probiotic LAB populations were as high as 108 cfu/mL after fermentation [80].
3.2. Legume-Based Products
Legumes contain high amounts of resistant starch and galacto-oligosaccharides which are known as effective metabolites for gut microbiota and probiotics that stimulate the growth and survival of probiotics in low-processed food while positively affecting the microbial quality of the final product [81].
3.2.1. Soya-Based Products
Soybean (Glycine max) provides high-quality protein, fats, and carbohydrates and contains no cholesterol or lactose. It is a good source of nutrients for lactose-intolerant individuals, vegetarians, and those with milk allergy [82]. The production of soy products has been emerging as an interesting alternative to dairy products and their incorporation into human diets is increasing due to their nutritional and functional properties [83].
Several studies have shown that soy products, especially soy yoghurt, is a good vehicle for probiotic delivery [84,85,86,87]. Due to the presence of raffinose and stachyose, soymilk is a good medium for Bifidobacterium spp., as most of the strains belong to this genus can ferment these sugars. Strains of Lb. acidophilus has also been reported to metabolize oligosaccharides present in soymilk during fermentation [83,85,86]. Many probiotic strains possess α-galactosidase activity that allows their growth in soymilk [83,88]. These probiotic strains seem to have no negative effects when they incorporated with traditional yoghurt starter cultures. Farnworth et al. (2007) reported that the presence of probiotic bacteria [Lb. johnsonii NCC533 (La-1), Lb. rhamnosus ATCC 53103 (GG), and human-derived bifidobacteria] did not affect the growth of the yoghurt strains [86]. Approximately 2 log increases in both Lb. rhamnosus GG and Lb. johnsonii La-1 were observed when each was added with yoghurt strains in the soy beverage.
Mounting evidence suggests that soymilk matrix may also provide adequate protection to the probiotics during gastrointestinal transit. For instance, Lb. acidophilus and Bifidobacterium spp. showed resistance to simulated gastrointestinal conditions when delivered through a fermented drink made of a mixed extract of soy and rice by-products with added waxy cornstarch [89]. In another study, fermented soy matrix protected Lb. acidophilus La-5 and Bifidobacterium animalis Bb-12 against gastrointestinal juices, where the Bb-12 showed higher resistance to artificial gastrointestinal juices compared to La-5 [83]. Moreover, Lb. casei Zhang has also shown good tolerance to simulated gastric transit and intestinal juice in the fermented soymilk and maintained high viability (>108 cfu/g) during cold storage (at 4 °C for 28 days) [87].
Soy oligosaccharides, mainly α-galactosides, are prevalently present in soy protein products and can result in unfavorable digestive effects when consumed. Certain probiotic strains are capable of decreasing α-galactoside content due to their high level of α-galactosidase activity while maintaining acceptable viability counts. Lb. acidophilus LA-2 showed greater α-galactosidase activity when induced by raffinose and was able to retain viability over 14 weeks of cold storage (4 °C) when microencapsulated and freeze-dried [90].
Soymilk is an excellent source of bioactive peptides and fermentation is an effective way of generating bioactive peptides. The β-glucosidase-producing probiotic Lb. rhamnosus CRL981 allowed for obtaining a soy beverage with enhanced antioxidant capacity. The higher antioxidant activity was due to increased isoflavone aglycone contents during fermentation because of β-glucosidase activity towards isoflavone glucosides [91]. Lb. plantarum C2 was excellent in terms of growth and peptide generation in soymilk, which showed excellent log count increases, protein hydrolysis, and α-galactosidase activities. Seventeen biofunctional soy peptides showing both antioxidant and ACE-inhibitory activities have been identified from the fermented soymilk produced using Lb. plantarum C2 [92]. ACE inhibitory activity in vitro has also been reported in fermented soy whey produced by using Lb. acidophilus FTCC 0291 in the optimized soy-whey medium [88]. Accordingly, soymilk consumption could improve some oxidative stress factors among patients with diabetic kidney disease [93]. In addition, a regular intake of a soy-based probiotic drink (Enterococcus faecium CRL 183 and Bifidobacterium longum ATCC 15707) was reported to modulate the microbiota and reduce body weight gain in diet-induced obesity in mice [94]. These pieces of evidence suggest that certain probiotics strains can be used for the preparation of soy-based functional fermented foods and bioactive food supplements.
Probiotics in the soy matrix could alter the sensory attributes of the products as well. A synbiotic soy yoghurt prepared using optimized FOS concentration (8.1% w/v) resulted in well-set products with very less whey separation (1.14%). The developed product showed good nutritional, textual, and sensory characteristics [95]. Norouzi et al. (2019) compared the survival rate of Lb. paracasei in fermented and non-fermented frozen soy dessert and their sensory properties over 180 days of storage at −24 °C. Results showed that the colour, mouthfeel and overall acceptability were significantly improved in probiotic products compared to frozen dessert without probiotics. Further, both fermented and non-fermented products reported maintaining viable Lb. paracasei counts well over 106 cfu/mL [96].
Albuquerque et al. (2019) evaluated the effect of passion fruit by-product (PFBP) and fructooligosaccharides (FOS) on the viability of Streptococcus thermophilus TH-4 and Lactobacillus rhamnosus LGG in folate bio-enriched fermented soy products, and on probiotic survival and folate bio-accessibility under in vitro simulated gastrointestinal conditions during storage (at 4 °C for 28 days) [97]. Only Lb. rhamnosus LGG retained the desired viability (>8 log cfu/mL) during storage, whereas St. thermophilus TH-4 populations decreased to 5.5 log cfu/mL by day 28. Therefore, the bio-enriched probiotic fermented soy products present great potential as innovative functional food by delivering probiotic microorganisms and providing 14% of the recommended daily folate intake.
3.2.2. Chick-Pea-Based Products
Chick-peas (Cicer arietinum L.) are an excellent source of essential amino acids, raffinose-family oligosaccharides, resistant starches and fibers, and possess prebiotic effects on the growth and survival of the probiotic microorganisms [98]. A beverage produced with chick-pea and coconut extract at the 9:1 ratio found to be a viable matrix to deliver Lb. paracasei LBC 81, maintaining viable counts of >108 cfu/mL during 10 days of refrigerated storage [99]. Interestingly, roasted chick-peas containing Lb. plantarum 299v and Lb. rhamnosus GG (produced by immersing in probiotic dispersions followed by drying at 42 °C) was also suggested as a viable probiotic carrier matrix. The viability of Lb. plantarum 299v in roasted-chick peas was >109 cfu/g at 4 °C, and >107 cfu/g at 25 °C after a 3-month long storage period [100].
3.2.3. Miscellaneous Legume-Based Products
Recently, there is an increasing trend of utilizing legume sprouts as probiotic carrier foods. A probiotic drink produced from sprouted green gram showed viable Lb. acidophilus NCDC14 counts of 1010–1011 cfu/mL after 8 h of fermentation [101]. Swieca et al. (2019) investigated the effectiveness of lentil and adzuki bean sprouts as carriers for the probiotic yeast S. cerevisiae var. boulardii and found that the sprouts obtained from seeds soaked in the inoculum and further cultivated at 30 °C for 4 days gave the highest probiotic counts (>107 cfu/g). More importantly, the two matrixes effectively protected the probiotic yeasts during digestion in vitro. Further, the sprouts enriched with S. boulardii were characterized by lower mold counts and coliform counts [81]. Chick-pea sprouts fermented with Lactobacillus casei 0979 hinder the growth of pathogenic microorganisms and result in products with safety. Lb. plantarum 299v reported increasing starch digestibility in the lentil and mung bean sprouts [102]. These results suggest that legume sprout-based food matrices are effective probiotic carrier foods and probiotics can be utilized to improve the microbial safety, nutritional composition, and nutrient digestibility of these products.
Lb. plantarum B1-6 has been successfully utilized to produce a probiotic food using the mung bean (Vigna radiata) as a probiotic carrier. Probiotic fermentation resulted in viable counts of >108 cfu/mL and significantly higher ACE inhibitory activity at the end of fermentation [103]. In another study, Romero-Espinoza et al. (2020) successfully used the combination of yeasts S. cerevisiae and S. boulardii and a mix of commercial probiotic bacteria composed of Lb. acidophilus, Lb. casei, Lb. rhamnosus, Lb. plantarum, and Bifidobacterium infantis to ferment whole meal lupin (Lupinus mutabilis var. bola L.). The probiotic fermentation resulted in significant degradation of oligosaccharides (27.3–82.3%), phytic acid (61.9–67%), and alkaloids (25.5–36.7%) which are the antinutritional factors that limit the consumption of lupin [104].
3.3. Vegetable-Based Products
Vegetables are rich in carbohydrates, vitamins, minerals, and health-promoting substances for example phytochemicals and phytonutrients. They do not contain lactose. Hence, vegetable-based probiotic products are well suited for lactose-intolerant consumers. Vegetables such as carrot, cabbage, tomato, and beet are popular substrates used to manufacture probiotic products employing LAB such as Lb. acidophilus, Lb. plantarum, and B. longum. The lactic fermentation of the vegetable substrates improves the nutritional value of the raw material [9].
3.3.1. Carrot-Based Products
Although carrot has been widely utilized in producing traditional probiotic beverages, the probiotic strains involved in such fermentations have recently been studied. Shalgam is a traditional beverage mainly consumed in southern Turkey, which is produced from the lactic acid fermentation of black carrot, bulgur flour, sourdough, salt, turnip, and drinkable water. It is produced on an industrial scale using two methods: the traditional method and direct method. The traditional method comprises sourdough fermentation and carrot fermentation, while the direct method involves only the carrot fermentation [28]. Two recent studies showed that Lactobacillus plantarum and Lb. paracasei subsp. paracasei are the dominant probiotic strains frequently found in all lactic fermentations of Shalgam. Besides, Lactobacillus brevis and Lb. fermentum were also determined in some fermentations. Low populations of Leuconostoc mesenterroides subsp. mesenteroides, Pediococcus pentosaceaceus, and Lactobacillus delbrueckii subsp. delbrueckii were also present at the beginning yet died off during the fermentation. These bacteria are responsible for adding typical taste and flavor compounds during fermentation [9,29]. The viability counts of Lb. plantarum, Lb. paracasei subsp. casei and Lb. brevis were >6 log cfu/mL over a 10-day fermentation period [28].
Kanji is another natural lactic acid fermented probiotic beverage consumed in Northern India during summers. It is consumed as an accompaniment along with meals. Kanji is prepared using black carrot and is well known to help in digestion. Lb. plantarum was identified as the responsible probiotic used in manufacturing Kanji and it was reported to maintain viable counts of more than 106 cfu/mL during refrigerated storage (4 °C). The highest probiotic count could be achieved with 8% salt based on the weight of black carrot [30].
3.3.2. Beetroot-Based Products
Beetroot contains a number of bioactive molecules such as phenolic compounds, carotenoids, betalain, vitamins, and minerals that possess specific physiological effects. Beetroot itself is recognized as one of the most powerful vegetables with antioxidative properties [105]. Recently, considerable attention has been paid in manufacturing non-dairy probiotic beverages using beetroot substrates. Panghal and colleagues (2017) produced a probiotic drink from beetroot juice using the probiotics Lb. rhamnnosus, Lb. plantarum, and Lb. delbrueckii. These three probiotic strains were capable of growing well on pasteurized beetroot juice at 37 °C while elevating levels of total phenolic and flavonoids, and antioxidant activity [105].
In another study, a beetroot beverage containing Lb. casei 431 showed the highest sensory acceptability after 2 h of fermentation at 37 °C compared to 4 and 6 h of fermentation. Lb. casei 431 grew well on beetroot substrate and reached almost 108 cfu/mL after 2 h of fermentation. However, the viability decreased gradually during the refrigerated storage (4 °C) and remained 106–108 cfu/mL at the end of 4 week-long storage [106].
3.3.3. Cabbage/Broccoli-Based Products
A number of studies showed that probiotics, especially Lactobacillus strains such as Lb. plantarum C3, Lb. casei A4, Lb. delbrueckii D7, Lb. brevis, Lb. plantarum, and Lb. rhamnosus grew well on cabbage juice and reached cell counts more than 108 cfu/mL within 48 h of fermentation at their optimal temperatures [107,108]. During cold storage conditions, fermented cabbage juice matrix protected most of the probiotic strains and maintained viable counts over the minimum therapeutic level (Table 2). The only exception was Lb. casei, which did not survive the low pH and high acidic conditions in fermented cabbage juice and lost viability completely after 2 weeks of cold storage [108]. Lactic acid was the major end-product of the fermented cabbage juice attaining concentrations of 6.97, 9.69, and 12.2 g/L lactic acid for Lb. plantarum, Lb. rhamnosus, and Lb. brevis, respectively. LAB fermentation retained more than 75% of the total phenolic and total flavonoid contents, and antioxidant capacity of the initial raw material [107].
One of the major drawbacks associated with the conventional fermentation of cabbage is that the fermentation leads to a complete elimination of glucosinolates. However, applying a thermal treatment (blanching) followed by fermentation (4% brine at 25 °C) by the probiotic strain Lactobacillus paracasei LMG P22043 retained 35% of the original glucosinolates (27.2 μmol/100 g) even after 71 h of fermentation. A higher retention of glucosinolates (23.7 μmol/100 g) was observed even after 30 days of refrigerated vacuum-packed storage [109].
There is a large variety of cabbage-based traditional fermented foods, and these products are important as sources of numerous probiotic strains. Some of these probiotics have potential industrial applications. For instance, Lactobacillus rhamnosus JAAS8 isolated from Chinese sauerkraut is an exopolysaccharide-producing bacteria with potential industrial applications [41,42]. Moreover, Lactobacillus plantarum DKL119 and DKL121 are two LAB strains isolated from Kimchi that have successfully employed as starter cultures in the production of a fermented dairy product. It showed faster acid development than commercial starter culture and remained viable (>9 log cfu/mL) over 15 days of cold storage at 10 °C [110]. Some probiotic strains are involved in altering the sensorial attributes of these products. Sayurasin has been known as traditional fermented mustard that is made by the addition of certain fermentable microorganisms, resulting in sensory changes bearing acidic and unique characteristics to the mustard [31]. Some other strains demonstrated health-promoting effects. For example, LAB isolated from Nozawana-zuke has demonstrated immune-enhancing effects [111]. A recent study revealed that Nozawana-zuke produced with Lactiplantibacillus plantarum K4G4 and K5G3, Lactilactobacillus curvatus #4G2, and Levilactobacillus brevis K4G1 maintained well above 7 log cfu/g throughout a 21-day long fermentation period at 10 °C. The results further showed that the application of these starter cultures improved the quality of fermented Nozawana-zuke mainly through the elevated production levels of taste- and aroma active compounds (e.g., mannitol, isothiocyanates, hexanoic acid, etc.) [112].
3.3.4. Tomato-Based Products
Tomato (Lycopersicon esculentum) is one of the most popular vegetables consumed worldwide, which has high water content, low caloric value, and low fat and protein contents. It is a valuable source of bioactive compounds including minerals, vitamin C, vitamin E, pro-vitamin A, carotenoid, lutein, and lycopene. Lycopene is one of the most important natural antioxidants that is claimed to have numerous health benefits. Tomato is processed into variety of products such as dried tomatoes, ketchups, pastes, sauces, soups, and purees [113].
Many studies have revealed that tomato is an ideal matrix to deliver probiotics in their live form [114,115,116]. For instance, four LAB strains (Lb. acidophilus, Lb. plantarum, Lb casei, Lb. delbrueckii) have been successfully employed to manufacture probiotic fermented beverages for vegetarians and consumers allergic to dairy-based products [116]. Both raw and fried tomatoes have shown a protective effect against the loss of viability of Lb. reuteri as it passes through the stomach and small intestine [114]. Immobilization has been suggested as an effective strategy that could be used to enhance cell viability and improve the sensory quality of fermented tomato juice. For instance, viable cell counts of immobilized Lb. acidophilus were maintained at 107 cfu/mL in the fermented tomato juice during 10 weeks of cold storage at 4 °C, compared with 104 cfu/mL in free cells [117].

Table 2.
Viability of probiotics in non-dairy probiotic products at the end of the storage period.
Table 2.
Viability of probiotics in non-dairy probiotic products at the end of the storage period.
| Product Type | Product | Probiotic Strains | Viability at the End of Storage | Storage Time | References |
|---|---|---|---|---|---|
| Cereal-based | Fermented oat flour | Lb. plantarum | 108 cfu/g | 21 d at 4 °C | [10] |
| Oat-based drink | Lb. plantarum B28 | 106–107 cfu/mL | 24 d at 4 °C | [56] | |
| Oat bars | B. lactis Bb-12 | 109 cfu/25 g | 7–14 d at 4 °C | [118] | |
| Probiotic fermented oat beverage incorporated with guava, orange & passion fruit by-products | Lb. casei Lc-1 | >108 cfu/mL | 28 d at 4 °C | [119] | |
| Emma beverage | Lb. plantarum Lb. acidophilus | 108 cfu/mL | 30 d at 4 °C | [67] | |
| Yoghurt-like beverages from rice, rice and soy, rice and barley, rice and emmer, rice and oat | Lb. plantarum 6E and 6M | 108 cfu/mL | 30 d at 4 °C | [57] | |
| Milk-based maize/rice pudding | Lb. acidophilus La5 and 1748 B. animalis Bb12, and Lb. rhamnosus GG grown separately | 108–109 cfu/g | 21 d at 4–6°C | [120] | |
| Maize-based fermented beverages | Saccharomyces cerevisiae CCMA 0732, S. cerevisiae CCMA 0731, and Pichia kluyveri CCMA 0615 in combination with Lb. paracasei LBC-81 | >106 cfu/mL | 28 d at 4 °C | [75] | |
| Fermented beverage produced from maize and rice supplemented with fructooligosaccharides | Lactobacillus plantarum CCMA 0743, Torulaspora delbrueckii CCMA 0235, and Lb. acidophilus LACA 4 in mixed culture | ≥107 cfu/mL | 28 d at 4 °C | [76] | |
| Legume-based | Soya frozen dessert | Lb. acidophilus Lb. paracasei B. lactis Lb. rhamnosus S. boulardii | 107 cfu/g ~105 cfu/g | 28 d at −18 °C | [121] |
| Probiotic soymilk | B. breve strain Yakult | 109 cfu/mL | 20 d at 10 °C | [122] | |
| Beverage containing chick-pea & coconut extract in 9:1 ratio | Lb. paracasei LBC 81 | >108 cfu/mL | 10 d at 4 °C | [99] | |
| Probiotic roasted chick-peas | Lb. plantarum 299v | >109 cfu/mL | 3 months at 4 °C | [100] | |
| Vegetable-based | Probiotic beetroot juice | Lb. casei 431 | 106–108 cfu/mL | 28 d at 4 °C | [106] |
| Probiotic cabbage juice | Lb. plantarum Lb. rhamnosus Lb. brevis | >109 cfu/mL >1010 cfu/mL >109 cfu/mL | 30 d at 4 °C | [107] | |
| Probiotic blanched cabbage | Lb. paracasei LMG P22043 | >108 cfu/g | 30 d at 4 °C | [109] | |
| Probiotic cabbage juice | Lb. plantarum C3 Lb. delbrueckii D7 | >107 cfu/mL >105 cfu/mL | 28 d at 4 °C | [108] | |
| Probiotic tomato juice | Lb. acidophilus Lb. casei Lb. plantarum Lb. delbrueckii | 109 cfu/mL 108 cfu/mL 106 cfu/mL 108 cfu/mL | 28 d at 4 °C | [116] | |
| Fruit-based | Probiotic apple juice supplemented with oligofructose | Lb. paracasei ssp. paracasei | >106 cfu/mL | 28 d at 4 °C (in a glass container) | [123] |
| Osmotically dehydrated probiotic cut apple | Lb.plantarum 299v | >107 cfu/mL | 6 d at 4 °C | [124] | |
| Probiotic enriched apple snacks | Lb. plantarum SICC | >106 cfu/g | 120 d at 25 °C | [125] | |
| Probiotic pineapple juice | Meyerozyma caribbica 9 D | 107 cfu/g | 21 d at 4 °C | [126] | |
| Probiotic pineapple juice | Lb. casei NRRL B-442 | >106 cfu/mL | 21 d at 4 °C | [127] | |
| Probiotic mixed pineapple and jussara juice | Lb. rhamnosus GG | >107 cfu/mL | 28 d at 8 °C | [128] | |
| Spray-dried or freeze-dried probiotic orange juice powder | Lb. plantarum 299v P.acidilactici HA-6111-2 | 108 cfu/mL | 180 d at 4 °C | [129] | |
| Synbiotic orange juice | Lb. paracasei ssp. paracasei | >106 cfu/mL | 28 d at 4 °C | [130] | |
| Probiotic orange juice fortified with nettle | Lb. rhamnosus ATCC 53103 | ~107 cfu/mL | 28 d at 4 °C | [131] | |
| Probiotic orange juice | P. acidilactici CE51 | >107 cfu/mL | 35 d at 4 °C | [132] | |
| Probiotic cashew apple juice | Lb. casei NRRL B-442 | >108 cfu/mL | 42 d at 4 °C | [133] | |
| Non-fermented probiotic beverage from Jucara fruit blended with banana and strawberry | Lb. plantarum CNPC003 Lb. casei BGP93 | ~106 cfu/mL >107 cfu/mL | 90 d at 4 °C | [134] | |
| Probiotic Sohiong fruit juice powder | Lb. plantarum | >106 cfu/mL | 36 d at 25 °C | [135] | |
| Probiotic cataloupe juice | Lb. casei NRRL B-442 | >108 cfu/mL | 42 d at 4 °C | [136] | |
| Honeydew melon | Lb. casei NCIMB 4114 | >108 cfu/mL | [137] | ||
| Probiotic pomegranate juice | Lb. plantarum Lb. delbrueckii | Not available | 28 d at 4 °C | [138] | |
| Table olives | Lb. rhamnosus Lb. paracasei B. bifidum B. longum | 106–108 cfu/g | 90 d at 4 °C | [139] |
Lb., Lactobacillus; Lc., Lactococcus; P., Pediococcus.
Leuconostoc mesenteroides BD1710 in the tomato juice supplemented with sucrose (15%) could synthesize approximately 32 g/L dextran when cultured at 28 °C for 48 h. Based on these results, the probiotic fermentation of tomato juice has been suggested as an alternative method to manufacture dextran on large scale [140]. On the other hand, probiotic treatment has shown extended the shelf life of certain tomato based products. For instance, the shelf life of a tomato paste has significantly extended to 25–30 days when treated with Lb. plantarum Cs and Lb. acidophilus ATCC 314 suggesting the possible use of these two probiotic strains as bio-preservatives in tomato processing [115].
3.4. Fruit-Based Probiotic Products
Fruit juices may represent an alternative means of delivering probiotics to consumers as they could be considered as healthy and refreshing beverages consumed regularly by people of all ages. They are rich in sugars and bioactive compounds (minerals, vitamins, fiber, and antioxidants) that can be utilized by probiotics. More importantly, these fruit-based probiotic products do not contain starter cultures as those in dairy products, which compete with probiotics for nutrients [123,127].
3.4.1. Apple-Based Products
Due to the high porosity that makes it easy for the incorporation of probiotics, the apple food matrix has been suggested as an excellent matrix for the delivery of probiotics. Furthermore, cellulose in apple is not digestible, and therefore serve as a protective matrix for probiotics during the gastrointestinal transit [124].
Probiotics incorporated into apple juice and other apple-based products resulted in satisfactory viable counts over the refrigerated storage suggesting that apple is an ideal vehicle for the delivery of probiotics (Table 2). Pimentel et al. (2015) evaluated the effect of the supplementation of clarified apple juice with probiotic Lb. paracasei subsp. paracasei and/or oligofructose on the physiochemical characteristics, probiotic viability, and acceptability during refrigerated storage (4 °C) either in plastic or glass packages [123]. The results showed that apple juice is a suitable medium for incorporating the probiotic strain, resulting in products with similar chemical composition, density, acceptability and purchase intention compared to pure juice. However, probiotic products had higher acidity, turbidity, and red color. The addition of oligofructose did not change either the physiochemical characteristics, acceptability, purchase intention, or storage stability of the products; however, it enhanced the probiotic survival during storage. The glass package was more efficient in maintaining the viability of probiotics than the plastic package.
Drying brings several advantages to foods such as increased shelf life, no requirement of refrigeration, and reduction of storage, packaging, and transporting costs [141]. Different drying methods have been tested on a variety of apple-based probiotic products. Out of four common drying methods (air drying, freeze-drying, freeze-drying followed by microwave vacuum drying, and air drying followed by explosion puffing drying), freeze-drying followed by microwave vacuum drying was most suitable drying method in order to develop probiotic enriched apple snack with anticipated quality. The probiotic viability (Lactobacillus plantarum SICC) in this product remained above 106 cfu/g over 120 days of storage at 25 °C. Another study revealed that the viability of the probiotic in the apple snack was similar to that of the commercial probiotic dairy products when the apples were dried 60 °C or when ultrasound-assisted air-drying was applied [141]. Viable counts of apple slices impregnated with Lactobacillus paracasei LL13, dried with either conventional or vacuum drying at 45 °C, and stored at 4 °C for 28 days were maintained above 7 and 6 log cfu/g, respectively over the cold storage. Vacuum dried apple snacks were more pleasing to consumers in terms of sensory evaluation [142]. In another study, free freeze-dried Lactobacillus rhamnosus GG was able to maintain high viability (>106 cfu/mL) in apple juice for an entire week at 4 °C [143]. When Lactobacillus plantarum 299v was incorporated (107–108 cfu/g) into osmotically dehydrated apple cubes (24 h at 37 °C) using sucrose and sorbitol as osmotic agents, the probiotic survived over a period of 6 days at 4 °C maintaining viability counts of >107 cfu/g. Moreover, the viability did not decrease during a simulated gastro-intestinal passage of 2 h [124].
The fermentation of traditional food and beverages by Selenium (Se)-enriched probiotics provides an easy and appropriate alternative to increase daily consumption of both vegetables and fruits as well as selenium. In a recent study, the effect of adding Se-enriched probiotics and the Se-enrichment of probiotic-fermented blended juices were evaluated. Among the probiotics tested (Streptococcus thermophilus, Bifidobacterium breve, and Lactobacillus plantarum), S. thermophilus showed the best Se-tolerance ability, which was then used to produce a Se-enriched probiotic. Addition of 1% Se-enriched S. thermophilus starters resulted in a 13-fold increase in Se content of the fermentation juice. The optimum processing parameters were found to be: liquid ratio of apple juice, orange juice, carrot juice, Chinese jujube juice of 25:35:30:10, a ratio of strains of B.breve, Lb. plantarum, Se-enriched S. thermophilus of 1:1:2, inoculum size of 2%, and a fermentation time of 18 h [144].
3.4.2. Pineapple-Based Products
Pineapple (Ananas cosmosus L. Merril) is a tropical fruit with a good balance between acidity and sugar that makes it one of the most popular fruits in producing regions and in importing countries. Pineapple is widely used to produce juice, jams and wine [126].
Mixed pineapple and jussara (Euterpe edulis Martius) juices have been suggested as an excellent carrier matrix for Lactobacillus rhamnosus GG (LGG). The LGG viability in probiotic juice was maintained well above 7.2 log cfu/mL throughout 28 days of storage at 8 °C. More importantly, LGG in mixed pineapple and jussara juice showed greater resistance to gastrointestinal conditions in vitro and in vivo. A blood analysis of Wistar rats fed the probiotic juice for 10 weeks (1 mL/day) showed that the probiotic juice did not induce hepato- and/or nephrotoxicity, and was capable of regulating the cholesterolemic index [128].
Pineapple juice is also a suitable substrate for the incorporation of probiotic yeast. Two strains of Meyerozyma caribbica (9 C and 9 D) have been shown desirable in vitro probiotic properties similar or superior to the reference probiotic yeast strain, S. cerevisiae var. boulardii. Out of the two strains, M. caribbica 9 D was able to result in a fermented pineapple beverage with good sensorial characteristics. M. caribbica population remained stable during refrigerated storage with cell counts greater than 7 log cfu/g for over 21 days [126].
Costa et al. (2013) analyzed sonication as a pre-treatment for cultivating the probiotic strain Lactobacillus casei NRRL B-442, which was able to ferment sonicated pineapple juice without any nutrient supplementation [127]. Greater viable cells counts were obtained within a shorter fermentation time (12 h) and probiotic viability was maintained above the acceptable range for at least 21 days under cold storage (4 °C).
3.4.3. Orange-Based Products
Orange (Citrus sinensis L. Osbeck) is a fruit with a high water content and is high in protein, sugars, fiber, minerals, and vitamins such as vitamin C (57 mg per 100 mL) and carotene (120 mg per 100 mL). If probiotics are incorporated, the nutrient content in juice can enhance the survivability of the added microorganisms [129]. Orange juice is the most commonly consumed juice worldwide, mainly due to its pleasant taste [132].
Probiotic survivability is probably the most important parameter in probiotic orange juice powder. Barbosa et al. (2015) evaluated the effect of three drying methods (spray-drying, freeze-drying, and convective drying) on the survivability of Lb. plantarum 299v and Pediococcus acidilactici HA-6111-2 [129]. Both probiotic strains reported good survival rates after spray drying and freeze-drying processes (>9 log cfu/g) compared to convective drying (~6 log cfu/g). Furthermore, after 180 days of cold storage at 4 °C, greater probiotic survivability was observed in the products manufactured by spray drying and freeze-drying (108 cfu/g) compared to those manufactured by convective drying (104 cfu/g) [129].
Prebiotics, which are known as nondigestible food ingredients may lead to improve the survival of probiotics in fruit juices [131]. The combined use of nettle (Urtica dioica L.) and probiotic lead to an increase in total phenolic content of the juice samples and slowed down the decline of antioxidative capacity during storage [131]. Research evidence suggest that it is possible to develop symbiotic orange juice beverages by using prebiotics and probiotic cultures without altering physiochemical and sensory attributes of pure juice. For example, a symbiotic orange juice with probiotic culture, ascorbic acid, and/or oligofructose (prebiotic) supplementation showed similar physiochemical and sensory attributes as those of pure juice. Oligofructose or ascorbic acid did not exert a protective effect on probiotic during storage, but the juices showed probiotic viability greater than 106 cfu/mL [89].
It is considered that the orange juice flavanones undergo limited absorption in the upper gastrointestinal tract leaving them to reach the colon where they are transformed and get absorbed. Pereira-Caro et al. (2018) investigated the ability of Bifidobacterium longum R0175 and Lactobacillus rhamnosus subsp. rhamnosus NCTC 10302 to catabolize orange juice flavanones. Results found that both strains were able to transform hesperetin and naringenin suggesting involvement in the colonic catabolism of orange juice flavanones [145].
3.5. Miscellaneous Products
3.5.1. Products from Fruit by-Products
Fruit by-products have been successfully incorporated into a variety of food products including fermented milk and plant-based fermented products. These products are often produced using probiotic strains, making them one of the most profitable and important categories of functional foods. The incorporation of fruit by-products to probiotic-fermented foods is desirable since they may protect the probiotics from harsh conditions found in the human gastrointestinal tract. They may also possess prebiotic potential, acting in synergy with probiotics in the gut after their ingestion [119]. Depending on the food matrix, the addition of fruit by-products may have altered fermentation time. Orange and passion fruit by-products incorporated into oat-based fermented probiotic product resulted in increased tolerance of the probiotics to simulated gastrointestinal conditions. Moreover, fruit by-products also resulted in increased acidification throughout the storage (28 days at 4 °C) without affecting the probiotic counts. The presence of fruit by-products showed a significant increase in fermentation time of rice beverages but did not affect fermentation times of fermented oat beverage or goat-milk beverage [119]. Therefore, it is evident that the presence of fruit by-products may alter fermentation time of the products depending on the food matrix used.
3.5.2. Plant-Based Dairy Alternatives
Several plant-based dairy alternatives such as coconut cream can be considered as suitable vehicle in delivering probiotics and their probiotic carrier potential have been thoroughly evaluated in our recent review [146].
4. Conclusions
Although dairy food matrices remain at the forefront of probiotic delivery, certain non-dairy plant-based food matrices such as fruits, vegetables, cereals, and legumes have been used as successful carriers in delivering probiotics to humans. Probiotics, in particular lactobacilli and bifidobacteria in these products, clearly demonstrated their ability in maintaining adequate viable probiotic numbers (106–108 cfu/mL or g of the carrier food product) during product shelf life. These food matrices can also enhance the gastrointestinal survival of probiotics, one of the important functional properties that should be fulfilled in providing health benefits for the consumers. Therefore, non-dairy plant-based food products play a significant role in delivering probiotics to humans.
Author Contributions
Conceptualization, writing—original draft preparation, writing—review and editing, D.M.D.R. and C.S.R.; writing—review and editing, J.K.V., S.F.L., D.R.P.A. and A.G.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not Applicable.
Informed Consent Statement
Not Applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ranadheera, C.S.; Naumovski, N.; Ajlouni, S. Non-bovine milk products as emerging probiotic carriers: Recent developments and innovations. Curr. Opin. Food Sci. 2018, 22, 109–114. [Google Scholar] [CrossRef]
- Molin, G. Probiotics in foods not containing milk or milk constituents, with special reference to Lactobacillus plantarum 299v. Am. J. Clin. Nutr. 2001, 73. [Google Scholar] [CrossRef] [PubMed]
- Ranadheera, C.S.; Vidanarachchi, J.K.; Rocha, R.S.; Cruz, A.G.; Ajlouni, S. Probiotic delivery through fermentation: Dairy vs. non-dairy beverages. Fermentation 2017, 3, 67. [Google Scholar] [CrossRef]
- Joint FAO/WHO Working Group. Report on Drafting Guidelines for the Evaluation of Probiotics in Food; WHO: London, OT, Canada, 2002. [Google Scholar]
- Peres, C.M.; Peres, C.; Hernández-Mendoza, A.; Malcata, F.X. Review on fermented plant materials as carriers and sources of potentially probiotic lactic acid bacteria—With an emphasis on table olives. Trends Food Sci. Technol. 2012, 26, 31–42. [Google Scholar] [CrossRef]
- Marques, T.M.; Cryan, J.F.; Shanahan, F.; Fitzgerald, G.F.; Ross, R.P.; Dinan, T.G.; Stanton, C. Gut microbiota modulation and implications for host health: Dietary strategies to influence the gut-brain axis. Innov. Food Sci. Emerg. Technol. 2014, 22, 239–247. [Google Scholar] [CrossRef]
- Ranadheera, C.S.; Evans, C.A.; Baines, S.K.; Balthazar, C.F.; Cruz, A.G.; Esmerino, E.A.; Freitas, M.Q.; Pimentel, T.C.; Wittwer, A.E.; Naumovski, N.; et al. Probiotics in Goat Milk Products: Delivery Capacity and Ability to Improve Sensory Attributes. Compr. Rev. Food Sci. Food Saf. 2019. [Google Scholar] [CrossRef]
- Mattia, A.; Merker, R. Regulation of probiotic substances as ingredients in foods: Premarket approval or “generally recognized as safe” notification. Clin. Infect. Dis. 2008, 46. [Google Scholar] [CrossRef]
- Panghal, A.; Janghu, S.; Virkar, K.; Gat, Y.; Kumar, V.; Chhikara, N. Potential non-dairy probiotic products—A healthy approach. Food Biosci. 2018, 21, 80–89. [Google Scholar] [CrossRef]
- Russo, P.; de Chiara, M.L.V.; Capozzi, V.; Arena, M.P.; Amodio, M.L.; Rascón, A.; Dueñas, M.T.; López, P.; Spano, G. Lactobacillus plantarum strains for multifunctional oat-based foods. LWT Food Sci. Technol. 2016, 68, 288–294. [Google Scholar] [CrossRef]
- Haas, R.; Schnepps, A.; Pichler, A.; Meixner, O. Cow milk versus plant-based milk substitutes: A comparison of product image and motivational structure of consumption. Sustainability 2019, 11, 5046. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Domínguez, R.; Budaraju, S.; Roselló-Soto, E.; Barba, F.J.; Mallikarjunan, K.; Roohinejad, S.; Lorenzo, J.M. Effect of innovative food processing technologies on the physicochemical and nutritional properties and quality of non-dairy plant-based beverages. Foods 2020, 9, 288. [Google Scholar] [CrossRef]
- Martins, E.M.F.; Ramos, A.M.; Vanzela, E.S.L.; Stringheta, P.C.; de Oliveira Pinto, C.L.; Martins, J.M. Products of vegetable origin: A new alternative for the consumption of probiotic bacteria. Food Res. Int. 2013, 51, 764–770. [Google Scholar] [CrossRef]
- Rodríguez, H.; Curiel, J.A.; Landete, J.M.; de las Rivas, B.; de Felipe, F.L.; Gómez-Cordovés, C.; Mancheño, J.M.; Muñoz, R. Food phenolics and lactic acid bacteria. Int. J. Food Microbiol. 2009, 132, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Çetin, B. Production of probiotic mixed pickles (turşu) and microbiological properties. Afr. J. Biotechnol. 2011, 10, 14926–14931. [Google Scholar] [CrossRef]
- Gobbetti, M.; De Angelis, M.; Corsetti, A.; Di Cagno, R. Biochemistry and physiology of sourdough lactic acid bacteria. Trends Food Sci. Technol. 2005, 16, 57–69. [Google Scholar] [CrossRef]
- Sapers, G.M. Efficacy of Washing and Sanitizing Methods for Disinfection of Fresh Fruit and Vegetable Products. Food Technol. Biotechnol. 2001, 39, 305–311. [Google Scholar]
- Ranadheera, R.D.C.S.; Baines, S.K.; Adams, M.C. Importance of food in probiotic efficacy. Food Res. Int. 2010, 43, 1–7. [Google Scholar] [CrossRef]
- Blandino, A.; Al-Aseeri, M.E.; Pandiella, S.S.; Cantero, D.; Webb, C. Cereal-based fermented foods and beverages. Food Res. Int. 2003, 36, 527–543. [Google Scholar] [CrossRef]
- Gotcheva, V.; Pandiella, S.S.; Angelov, A.; Roshkova, Z.G.; Webb, C. Microflora identification of the Bulgarian cereal-based fermented beverage boza. Process Biochem. 2000, 36, 127–130. [Google Scholar] [CrossRef]
- Muyanja, C.M.B.K.; Narvhus, J.A.; Treimo, J.; Langsrud, T. Isolation, characterisation and identification of lactic acid bacteria from bushera: A Ugandan traditional fermented beverage. Int. J. Food Microbiol. 2003, 80, 201–210. [Google Scholar] [CrossRef]
- Gadaga, T.H.; Mutukumira, A.N.; Narvhus, J.A.; Feresu, S.B. A review of traditional fermented foods and beverages of Zimbabwe. Int. J. Food Microbiol. 1999, 53, 1–11. [Google Scholar] [CrossRef]
- Wacher, C.; Cañas, A.; Bárzana, E.; Lappe, P.; Ulloa, M.; Owens, J.D. Microbiology of Indian and Mestizo pozol fermentations. Food Microbiol. 2000, 17, 251–256. [Google Scholar] [CrossRef]
- Oi, Y.; Kitabatake, N. Chemical Composition of an East African Traditional Beverage, Togwa. J. Agric. Food Chem. 2003, 51, 7024–7028. [Google Scholar] [CrossRef]
- David, O.M.; Famurewa, O. Prophylactic and bio-therapeutic benefits of ‘ogi’: A lactic acid fermented food. Bull. Biol. Sci. 2010, 2, 72–77. [Google Scholar]
- Omemu, A.M. Fermentation dynamics during production of ogi, a Nigerian fermented cereal porridge. Rep. Opin. 2011, 3, 8–17. [Google Scholar]
- Kort, R.; Westerik, N.; Mariela Serrano, L.; Douillard, F.P.; Gottstein, W.; Mukisa, I.M.; Tuijn, C.J.; Basten, L.; Hafkamp, B.; Meijer, W.C.; et al. A novel consortium of Lactobacillus rhamnosus and Streptococcus thermophilus for increased access to functional fermented foods. Microb. Cell Fact. 2015, 14. [Google Scholar] [CrossRef]
- Erten, H.; Tanguler, H.; Canbaş, A. A traditional Turkish lactic acid fermented beverage: Shalgam (Salgam). Food Rev. Int. 2008, 24, 352–359. [Google Scholar] [CrossRef]
- Tanguler, H.; Erten, H. Occurrence and growth of lactic acid bacteria species during the fermentation of shalgam (salgam), a traditional Turkish fermented beverage. LWT Food Sci. Technol. 2012, 46, 36–41. [Google Scholar] [CrossRef]
- Lamba, J.; Goomer, S.; Saxena, S.K. Study the lactic acid bacteria content in traditional fermented Indian drink: Kanji. Int. J. Gastron. Food Sci. 2019, 16. [Google Scholar] [CrossRef]
- Sukara, E.; Salamah, A.; Dinoto, A.; Mangunwardoyo, W. Identification of lactic acid bacteria in sayur asin from Central Java (Indonesia) based on 16S rDNA sequence. Int. Food Res. J. 2014, 21, 527–532. [Google Scholar]
- Chen, Y.S.; Yanagida, F.; Hsu, J.S. Isolation and characterization of lactic acid bacteria from suan-tsai (fermented mustard), a traditional fermented food in Taiwan. J. Appl. Microbiol. 2006, 101, 125–130. [Google Scholar] [CrossRef]
- Lan, W.T.; Chen, Y.S.; Yanagida, F. Isolation and characterization of lactic acid bacteria from Yan-dong-gua (fermented wax gourd), a traditional fermented food in Taiwan. J. Biosci. Bioeng. 2009, 108, 484–487. [Google Scholar] [CrossRef] [PubMed]
- Tamang, J.P. Himalayan Fermented Foods: Microbiology, Nutrition, and Ethnic Values; CRC Press: Boca Raton, FL, USA, 2009; ISBN 9781420093254. [Google Scholar]
- Chen, Y.S.; Wu, H.C.; Liu, C.H.; Chen, H.C.; Yanagida, F. Isolation and characterization of lactic acid bacteria from jiang-sun (fermented bamboo shoots), a traditional fermented food in Taiwan. J. Sci. Food Agric. 2010, 90, 1977–1982. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.S.; Yanagida, F.; Hsu, J.S. Isolation and characterization of lactic acid bacteria from dochi (fermented black beans), a traditional fermented food in Taiwan. Lett. Appl. Microbiol. 2006, 43, 229–235. [Google Scholar] [CrossRef]
- Chen, Y.S.; Wu, H.C.; Lo, H.Y.; Lin, W.C.; Hsu, W.H.; Lin, C.W.; Lin, P.Y.; Yanagida, F. Isolation and characterisation of lactic acid bacteria from Jiang-gua (fermented cucumbers), a traditional fermented food in Taiwan. J. Sci. Food Agric. 2012, 92, 2069–2075. [Google Scholar] [CrossRef]
- Nguyen, D.T.L.; Van Hoorde, K.; Cnockaert, M.; De Brandt, E.; Aerts, M.; Binh Thanh, L.; Vandamme, P. A description of the lactic acid bacteria microbiota associated with the production of traditional fermented vegetables in Vietnam. Int. J. Food Microbiol. 2013, 163, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, T.; Iida, A.; Toyama, Y.; Fukuda, K. Characterization of the bacteriocinogenic lactic acid bacteria lactobacillus curvatus strain YI08 isolated from nozawana-zuke pickles. Food Sci. Technol. Res. 2010, 16, 253–262. [Google Scholar] [CrossRef][Green Version]
- Chen, Y.S.; Liou, M.S.; Ji, S.H.; Yu, C.R.; Pan, S.F.; Yanagida, F. Isolation and characterization of lactic acid bacteria from Yan-tsai-shin (fermented broccoli stems), a traditional fermented food in Taiwan. J. Appl. Microbiol. 2013, 115, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, X.; Li, D.; Niu, C.; Yang, Z. Study on the identification and resistant properties of Lactobacillus plantarum isolated from sauerkraut. Food Sci. Technol. 2010, 31, 141–144. [Google Scholar]
- Yang, Z.; Li, S.; Zhang, X.; Zeng, X.; Li, D.; Zhao, Y.; Zhang, J. Capsular and slime-polysaccharide production by Lactobacillus rhamnosus JAAS8 isolated from Chinese sauerkraut: Potential application in fermented milk products. J. Biosci. Bioeng. 2010, 110, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Ahn, D.K.; Han, T.W.; Shin, H.Y.; Jin, I.N.; Ghim, S.Y. Diversity and antibacterial activity of lactic acid bacteria isolated from Kimchi. Korean J. Microbiol. Biotechnol. 2003, 31, 191–196. [Google Scholar]
- Dahal, N.R.; Karki, T.B.; Swamylingappa, B.; Li, Q.; Gu, G. Traditional foods and beverages of Nepal-a review. Food Rev. Int. 2005, 21, 1–25. [Google Scholar] [CrossRef]
- Feng, M.; Chen, X.; Li, C.; Nurgul, R.; Dong, M. Isolation and Identification of an Exopolysaccharide-Producing Lactic Acid Bacterium Strain from Chinese Paocai and Biosorption of Pb(II) by Its Exopolysaccharide. J. Food Sci. 2012, 77. [Google Scholar] [CrossRef]
- Yan, P.M.; Xue, W.T.; Tan, S.S.; Zhang, H.; Chang, X.H. Effect of inoculating lactic acid bacteria starter cultures on the nitrite concentration of fermenting Chinese paocai. Food Control 2008, 19, 50–55. [Google Scholar] [CrossRef]
- Nout, M.J.R. Rich nutrition from the poorest—Cereal fermentations in Africa and Asia. Food Microbiol. 2009, 26, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.S.; Varman, D.R.; Kanmani, P.; Yuvaraj, N.; Paari, K.A.; Pattukumar, V.; Arul, V. Isolation, Characterization and Identification of a Potential Probiont from South Indian Fermented Foods (Kallappam, Koozh and Mor Kuzhambu) and Its Use as Biopreservative. Probiotics Antimicrob. Proteins 2010, 2, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-S.; Wu, H.-C.; Wang, C.-M.; Lin, C.-C.; Jhong, Y.-J.; Yanagida, F. Isolation and characterization of lactic acid bacteria from pobuzihi (fermented cummingcordia), a traditional fermented food in Taiwan. Folia Microbiol. 2012, 58, 103–109. [Google Scholar] [CrossRef]
- Chen, Y.S.; Wu, H.C.; Yu, C.R.; Chen, Z.Y.; Lu, Y.C.; Yanagida, F. Isolation and characterization of lactic acid bacteria from xi-gua-mian (fermented watermelon), a traditional fermented food in Taiwan. Ital. J. Food Sci. 2016, 28, 9–14. [Google Scholar] [CrossRef]
- Arici, M.; Coskun, F. Hardaliye: Fermented grape juice as a traditional Turkish beverage. Food Microbiol. 2001, 18, 417–421. [Google Scholar] [CrossRef]
- Shori, A.B. Influence of food matrix on the viability of probiotic bacteria: A review based on dairy and non-dairy beverages. Food Biosci. 2016, 13, 1–8. [Google Scholar] [CrossRef]
- Vasudha, S.; Mishra, H.N. Non-dairy probiotic beverages. Int. Food Res. J. 2013, 20, 7–15. [Google Scholar]
- Charalampopoulos, D.; Wang, R.; Pandiella, S.S.; Webb, C. Application of cereals and cereal components in functional foods: A review. Int. J. Food Microbiol. 2002, 79, 131–141. [Google Scholar] [CrossRef]
- Lamsal, B.P.; Faubion, J.M. The beneficial use of cereal and cereal components in probiotic foods. Food Rev. Int. 2009, 25, 103–114. [Google Scholar] [CrossRef]
- Angelov, A.; Gotcheva, V.; Kuncheva, R.; Hristozova, T. Development of a new oat-based probiotic drink. Int. J. Food Microbiol. 2006, 112, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Coda, R.; Lanera, A.; Trani, A.; Gobbetti, M.; Di Cagno, R. Yogurt-like beverages made of a mixture of cereals, soy and grape must: Microbiology, texture, nutritional and sensory properties. Int. J. Food Microbiol. 2012, 155, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Rathore, S.; Salmerón, I.; Pandiella, S.S. Production of potentially probiotic beverages using single and mixed cereal substrates fermented with lactic acid bacteria cultures. Food Microbiol. 2012, 30, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Russo, P.; López, P.; Capozzi, V.; de Palencia, P.F.; Dueñas, M.T.; Spano, G.; Fiocco, D. Beta-glucans improve growth, viability and colonization of probiotic microorganisms. Int. J. Mol. Sci. 2012, 13, 6026–6039. [Google Scholar] [CrossRef]
- Capozzi, V.; Menga, V.; Digesù, A.M.; De Vita, P.; Van Sinderen, D.; Cattivelli, L.; Fares, C.; Spano, G. Biotechnological production of vitamin B2-enriched bread and pasta. J. Agric. Food Chem. 2011, 59, 8013–8020. [Google Scholar] [CrossRef]
- Kedia, G.; Vázquez, J.A.; Charalampopoulos, D.; Pandiella, S.S. In vitro fermentation of oat bran obtained by debranning with a mixed culture of human fecal bacteria. Curr. Microbiol. 2009, 58, 338–342. [Google Scholar] [CrossRef]
- Bazzano, L.A.; He, J.; Ogden, L.G.; Loria, C.M.; Whelton, P.K. Dietary fiber intake and reduced risk of coronary heart disease in US men and women: The National Health and Nutrition Examination Survey I Epidemiologic Follow-up Study. Arch. Intern. Med. 2003, 163, 1897–1904. [Google Scholar] [CrossRef]
- Martensson, O.; Staaf, J.; Duenas-Chasco, M.; Irastorza, A.; Holst, O. A fermented, ropy, non-dairy oat product based on the exopolysaccharide-producing strain Pediococcus damnosus. Adv. Food Sci. 2002, 24, 4–11. [Google Scholar]
- Mårtensson, O.; Biörklund, M.; Lambo, A.M.; Dueñas-Chasco, M.; Irastorza, A.; Holst, O.; Norin, E.; Welling, G.; Öste, R.; Önning, G. Fermented, ropy, oat-based products reduce cholesterol levels and stimulate the bifidobacteria flora in humans. Nutr. Res. 2005, 25, 429–442. [Google Scholar] [CrossRef]
- Salazar, N.; Gueimonde, M.; de los Reyes-Gavilán, C.G.; Ruas-Madiedo, P. Exopolysaccharides Produced by Lactic Acid Bacteria and Bifidobacteria as Fermentable Substrates by the Intestinal Microbiota. Crit. Rev. Food Sci. Nutr. 2016, 56, 1440–1453. [Google Scholar] [CrossRef]
- Rozada-Sánchez, R.; Sattur, A.P.; Thomas, K.; Pandiella, S.S. Evaluation of Bifidobacterium spp. for the production of a potentially probiotic malt-based beverage. Process Biochem. 2008, 43, 848–854. [Google Scholar] [CrossRef]
- Coda, R.; Rizzello, C.G.; Trani, A.; Gobbetti, M. Manufacture and characterization of functional emmer beverages fermented by selected lactic acid bacteria. Food Microbiol. 2011, 28, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Battistini, C.; Gullón, B.; Ichimura, E.S.; Gomes, A.M.P.; Ribeiro, E.P.; Kunigk, L.; Moreira, J.U.V.; Jurkiewicz, C. Development and characterization of an innovative synbiotic fermented beverage based on vegetable soybean. Braz. J. Microbiol. 2018, 49, 303–309. [Google Scholar] [CrossRef]
- Charalampopoulos, D.; Pandiella, S.S.; Webb, C. Evaluation of the effect of malt, wheat and barley extracts on the viability of potentially probiotic lactic acid bacteria under acidic conditions. Int. J. Food Microbiol. 2003, 82, 133–141. [Google Scholar] [CrossRef]
- Tian, W.; Hu, R.; Chen, G.; Zhang, Y.; Wang, W.; Li, Y. Potential bioaccessibility of phenolic acids in whole wheat products during in vitro gastrointestinal digestion and probiotic fermentation. Food Chem. 2021, 362, 130135. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, K.; Ray, M.; Adak, A.; Halder, S.K.; Das, A.; Jana, A.; Parua (Mondal), S.; Vágvölgyi, C.; Das Mohapatra, P.K.; Pati, B.R.; et al. Role of probiotic Lactobacillus fermentum KKL1 in the preparation of a rice based fermented beverage. Bioresour. Technol. 2015, 188, 161–168. [Google Scholar] [CrossRef]
- Borah, T.; Gogoi, B.; Khataniar, A.; Gogoi, M.; Das, A.; Borah, D. Probiotic characterization of indigenous Bacillus velezensis strain DU14 isolated from Apong, a traditionally fermented rice beer of Assam. Biocatal. Agric. Biotechnol. 2019, 18, 101008. [Google Scholar] [CrossRef]
- Nath, S.; Roy, M.; Sikidar, J.; Deb, B.; Sharma, I.; Guha, A. Characterization and in-vitro screening of probiotic potential of novel Weissella confusa strain GCC_19R1 isolated from fermented sour rice. Curr. Res. Biotechnol. 2021, 3, 99–108. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, H.; Yan, X.; Zhao, S. Preparation of a probiotic rice tablet: Sensory evaluation and antioxidant activity during gastrointestinal digestion. LWT 2020, 124, 108911. [Google Scholar] [CrossRef]
- Menezes, A.G.T.; Ramos, C.L.; Dias, D.R.; Schwan, R.F. Combination of probiotic yeast and lactic acid bacteria as starter culture to produce maize-based beverages. Food Res. Int. 2018, 111, 187–197. [Google Scholar] [CrossRef]
- Freire, A.L.; Ramos, C.L.; Schwan, R.F. Effect of symbiotic interaction between a fructooligosaccharide and probiotic on the kinetic fermentation and chemical profile of maize blended rice beverages. Food Res. Int. 2017, 100, 698–707. [Google Scholar] [CrossRef]
- Helland, M.H.; Wicklund, T.; Narvhus, J.A. Growth and metabolism of selected strains of probiotic bacteria, in maize porridge with added malted barley. Int. J. Food Microbiol. 2004, 91, 305–313. [Google Scholar] [CrossRef]
- Palaniswamy, S.K.; Govindaswamy, V. In-vitro probiotic characteristics assessment of feruloyl esterase and glutamate decarboxylase producing Lactobacillus spp. isolated from traditional fermented millet porridge (kambu koozh). LWT Food Sci. Technol. 2016, 68, 208–216. [Google Scholar] [CrossRef]
- Oh, Y.J.; Jung, D.S. Evaluation of probiotic properties of Lactobacillus and Pediococcus strains isolated from Omegisool, a traditionally fermented milletalcoholic beverage in Korea. LWT Food Sci. Technol. 2015, 63, 437–444. [Google Scholar] [CrossRef]
- Oh, Y.J.; Lee, Y.N.; Hong, C.R.; Moon, G.E.; Jung, D.S. Effect of Aging Temperatures on the Quality of Omegisool, a Traditionally Fermented Alcohol Beverage in Jeju. Natl. Sci. Inst. Seoul Women’s Univ. 2012, 24, 151–158. [Google Scholar]
- Swieca, M.; Kordowska-Wiater, M.; Pytka, M.; Gawlik-Dziki, U.; Seczyk, L.; Złotek, U.; Kapusta, I. Nutritional and pro-health quality of lentil and adzuki bean sprouts enriched with probiotic yeast Saccharomyces cerevisiae var. boulardii. LWT 2019, 100, 220–226. [Google Scholar] [CrossRef]
- Tsai, T.Y.; Chen, L.Y.; Pan, T.M. Effect of probiotic-fermented, genetically modified soy milk on hypercholesterolemia in hamsters. J. Microbiol. Immunol. Infect. 2014, 47, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bedani, R.; Rossi, E.A.; Saad, S.M.I. Impact of inulin and okara on Lactobacillus acidophilus La-5 and Bifidobacterium animalis Bb-12 viability in a fermented soy product and probiotic survival under in vitro simulated gastrointestinal conditions. Food Microbiol. 2013, 34, 382–389. [Google Scholar] [CrossRef]
- Champagne, C.P.; Green-Johnson, J.; Raymond, Y.; Barrette, J.; Buckley, N. Selection of probiotic bacteria for the fermentation of a soy beverage in combination with Streptococcus thermophilus. Food Res. Int. 2009, 42, 612–621. [Google Scholar] [CrossRef]
- Donkor, O.N.; Henriksson, A.; Vasiljevic, T.; Shah, N.P. α-Galactosidase and proteolytic activities of selected probiotic and dairy cultures in fermented soymilk. Food Chem. 2007, 104, 10–20. [Google Scholar] [CrossRef]
- Farnworth, E.R.; Mainville, I.; Desjardins, M.P.; Gardner, N.; Fliss, I.; Champagne, C. Growth of probiotic bacteria and bifidobacteria in a soy yogurt formulation. Int. J. Food Microbiol. 2007, 116, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Guo, Z.; Zhang, Q.; Yan, L.; Chen, W.; Liu, X.M.; Zhang, H.P. Fermentation characteristics and transit tolerance of probiotic Lactobacillus casei Zhang in soymilk and bovine milk during storage. J. Dairy Sci. 2009, 92, 2468–2476. [Google Scholar] [CrossRef]
- WaiYee, F.; MinTze, L. Evaluation of proteolytic and ACE-inhibitory activity of Lactobacillus acidophilus in soy whey growth medium via response surface methodology. LWT Food Sci. Technol. 2010, 43, 563–567. [Google Scholar]
- Costa, K.K.F.D.; Soares Júnior, M.S.; Rosa, S.I.R.; Caliari, M.; Pimentel, T.C. Changes of probiotic fermented drink obtained from soy and rice byproducts during cold storage. LWT Food Sci. Technol. 2017, 78, 23–30. [Google Scholar] [CrossRef]
- Chen, M.; Mustapha, A. Survival of freeze-dried microcapsules of α-galactosidase producing probiotics in a soy bar matrix. Food Microbiol. 2012, 30, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Marazza, J.A.; Nazareno, M.A.; de Giori, G.S.; Garro, M.S. Enhancement of the antioxidant capacity of soymilk by fermentation with Lactobacillus rhamnosus. J. Funct. Foods 2012, 4, 594–601. [Google Scholar] [CrossRef]
- Singh, B.P.; Vij, S. Growth and bioactive peptides production potential of Lactobacillus plantarum strain C2 in soy milk: A LC-MS/MS based revelation for peptides biofunctionality. LWT Food Sci. Technol. 2017, 86, 293–301. [Google Scholar] [CrossRef]
- Miraghajani, M.; Zaghian, N.; Mirlohi, M.; Feizi, A.; Ghiasvand, R. The Impact of Probiotic Soy Milk Consumption on Oxidative Stress Among Type 2 Diabetic Kidney Disease Patients: A Randomized Controlled Clinical Trial. J. Ren. Nutr. 2017, 27, 317–324. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho Marchesin, J.; Celiberto, L.S.; Orlando, A.B.; de Medeiros, A.I.; Pinto, R.A.; Zuanon, J.A.S.; Spolidorio, L.C.; dos Santos, A.; Taranto, M.P.; Cavallini, D.C.U. A soy-based probiotic drink modulates the microbiota and reduces body weight gain in diet-induced obese mice. J. Funct. Foods 2018, 48, 302–313. [Google Scholar] [CrossRef]
- Mishra Pandey, S.; Mishra, H.N. Optimization of the prebiotic & probiotic concentration and incubation temperature for the preparation of synbiotic soy yoghurt using response surface methodology. LWT Food Sci. Technol. 2015, 62, 458–467. [Google Scholar] [CrossRef]
- Norouzi, S.; Pourjafar, H.; Ansari, F.; Homayouni, A. A Survey on the survival of Lactobacillus paracasei in fermented and non-fermented frozen soy dessert. Biocatal. Agric. Biotechnol. 2019, 21. [Google Scholar] [CrossRef]
- Albuquerque, M.A.C.; Yamacita, D.S.; Bedani, R.; LeBlanc, J.G.; Saad, S.M.I. Influence of passion fruit by-product and fructooligosaccharides on the viability of Streptococcus thermophilus TH-4 and Lactobacillus rhamnosus LGG in folate bio-enriched fermented soy products and their effect on probiotic survival and folate bio-accessibility under in vitro simulated gastrointestinal conditions. Int. J. Food Microbiol. 2019, 292, 126–136. [Google Scholar]
- Hussein, H.; Awad, S.; El-Sayed, I.; Ibrahim, A. Impact of chickpea as prebiotic, antioxidant and thickener agent of stirred bio-yoghurt. Ann. Agric. Sci. 2020, 65, 49–58. [Google Scholar] [CrossRef]
- Mesquita, M.C.; Leandro, E.D.S.; de Alencar, E.R.; Botelho, R.B.A. Fermentation of chickpea (Cicer arietinum L.) and coconut (Coccus nucifera L.) beverages by Lactobacillus paracasei subsp paracasei LBC 81: The influence of sugar content on growth and stability during storage. LWT 2020, 132, 109834. [Google Scholar] [CrossRef]
- Paredes-Toledo, J. Roasted chickpeas as a probiotic carrier to improve L. plantarum 299v survival during storage. LWT 2021, 146. [Google Scholar] [CrossRef]
- Mridula, D.; Sharma, M. Development of non-dairy probiotic drink utilizing sprouted cereals, legume and soymilk. LWT Food Sci. Technol. 2015, 62, 482–487. [Google Scholar] [CrossRef]
- Swieca, M.; Gawlik-Dziki, U.; Jakubczyk, A.; Bochnak, J.; Sikora, M.; Suliburska, J. Nutritional quality of fresh and stored legumes sprouts—Effect of Lactobacillus plantarum 299v enrichment. Food Chem. 2019, 288, 325–332. [Google Scholar] [CrossRef]
- Wu, H.; Rui, X.; Li, W.; Chen, X.; Jiang, M.; Dong, M. Mung bean (Vigna radiata) as probiotic food through fermentation with Lactobacillus plantarum B1-6. LWT Food Sci. Technol. 2015, 63, 445–451. [Google Scholar] [CrossRef]
- Romero-Espinoza, A.M.; Serna-Saldivar, S.O.; Vintimilla-Alvarez, M.C.; Briones-García, M.; Lazo-Vélez, M.A. Effects of fermentation with probiotics on anti-nutritional factors and proximate composition of lupin (Lupinus mutabilis sweet). LWT 2020, 130, 109658. [Google Scholar] [CrossRef]
- Panghal, A.; Virkar, K.; Kumar, V.; Dhull, S.B.; Gat, Y.; Chhikara, N. Development of probiotic beetroot drink. Curr. Res. Nutr. Food Sci. 2017, 5, 257–262. [Google Scholar] [CrossRef]
- Gamage, S.M.; Mihirani, M.K.S.; Perera, O.D.A.N.; Weerahewa, H.L.D. Development of synbiotic beverage from beetroot juice using beneficial probiotic Lactobacillus Casei 431. Ruhuna J. Sci. 2016, 7, 64. [Google Scholar] [CrossRef][Green Version]
- Jaiswal, A.K.; Abu-Ghannam, N. Kinetic studies for the preparation of probiotic cabbage juice: Impact on phytochemicals and bioactivity. Ind. Crop. Prod. 2013, 50, 212–218. [Google Scholar] [CrossRef]
- Yoon, K.Y.; Woodams, E.E.; Hang, Y.D. Production of probiotic cabbage juice by lactic acid bacteria. Bioresour. Technol. 2006, 97, 1427–1430. [Google Scholar] [CrossRef]
- Sarvan, I.; Valerio, F.; Lonigro, S.L.; de Candia, S.; Verkerk, R.; Dekker, M.; Lavermicocca, P. Glucosinolate content of blanched cabbage (Brassica oleracea var. capitata) fermented by the probiotic strain Lactobacillus paracasei LMG-P22043. Food Res. Int. 2013, 54, 706–710. [Google Scholar] [CrossRef]
- Cho, Y.H.; Hong, S.M.; Kim, C.H. Isolation and characterization of lactic acid bacteria from Kimchi, Korean traditional fermented food to apply into fermented dairy products. Korean J. Food Sci. Anim. Resour. 2013, 33, 75–82. [Google Scholar] [CrossRef]
- Kawahara, T.; Otani, H. Stimulatory effect of lactic acid bacteria from commercially available Nozawana-zuke pickle on cytokine expression by mouse spleen cells. Biosci. Biotechnol. Biochem. 2006, 70, 411–417. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tomita, S.; Watanabe, J.; Kuribayashi, T.; Tanaka, S.; Kawahara, T. Metabolomic evaluation of different starter culture effects on water-soluble and volatile compound profiles in nozawana pickle fermentation. Food Chem. Mol. Sci. 2021, 2, 100019. [Google Scholar] [CrossRef]
- Demirci, T.; Sert, D.; Aktaş, K.; Atik, D.S.; Öztürk Negiş, H.İ.; Akın, N. Influence of hot and cold break tomato powders on survival of probiotic L. paracasei subsp. paracasei F19, texture profile and antioxidative activity in set-type yoghurts. LWT 2020, 118. [Google Scholar] [CrossRef]
- García-Hernández, J.; Hernández-Pérez, M.; Peinado, I.; Andrés, A.; Heredia, A. Tomato-antioxidants enhance viability of L. reuteri under gastrointestinal conditions while the probiotic negatively affects bioaccessibility of lycopene and phenols. J. Funct. Foods 2018, 43, 1–7. [Google Scholar] [CrossRef]
- George-Okafor, U.; Ozoani, U.; Tasie, F.; Mba-Omeje, K. The efficacy of cell-free supernatants from Lactobacillus plantarum Cs and Lactobacillus acidophilus ATCC 314 for the preservation of home-processed tomato-paste. Sci. Afr. 2020, 8. [Google Scholar] [CrossRef]
- Yoon, K.Y.; Woodams, E.E.; Hang, Y.D. Probiotication of tomato juice by lactic acid bacteria. J. Microbiol. 2004, 42, 315–318. [Google Scholar]
- King, V.A.E.; Huang, H.Y.; Tsen, J.H. Fermentation of tomato juice by cell immobilized Lactobacillus acidophilus. Mid-Taiwan J. Med. 2007, 12, 1–7. [Google Scholar] [CrossRef]
- Ouwehand, A.C.; Kurvinen, T.; Rissanen, P. Use of a probiotic Bifidobacterium in a dry food matrix, an in vivo study. Int. J. Food Microbiol. 2004, 95, 103–106. [Google Scholar] [CrossRef]
- Casarotti, S.N.; Borgonovi, T.F.; Batista, C.L.F.M.; Penna, A.L.B. Guava, orange and passion fruit by-products: Characterization and its impacts on kinetics of acidification and properties of probiotic fermented products. LWT 2018, 98, 69–76. [Google Scholar] [CrossRef]
- Helland, M.H.; Wicklund, T.; Narvhus, J.A. Growth and metabolism of selected strains of probiotic bacteria in milk- and water-based cereal puddings. Int. Dairy J. 2004, 14, 957–965. [Google Scholar] [CrossRef]
- Heenan, C.N.; Adams, M.C.; Hosken, R.W.; Fleet, G.H. Survival and sensory acceptability of probiotic microorganisms in a nonfermented frozen vegetarian dessert. LWT Food Sci. Technol. 2004, 37, 461–466. [Google Scholar] [CrossRef]
- Shimakawa, Y.; Matsubara, S.; Yuki, N.; Ikeda, M.; Ishikawa, F. Evaluation of Bifidobacterium breve strain Yakult-fermented soymilk as a probiotic food. Int. J. Food Microbiol. 2002, 81, 131–136. [Google Scholar] [CrossRef]
- Pimentel, T.C.; Madrona, G.S.; Garcia, S.; Prudencio, S.H. Probiotic viability, physicochemical characteristics and acceptability during refrigerated storage of clarified apple juice supplemented with Lactobacillus paracasei ssp. paracasei and oligofructose in different package type. LWT Food Sci. Technol. 2015, 63, 415–422. [Google Scholar] [CrossRef]
- Emser, K.; Barbosa, J.; Teixeira, P.; Bernardo de Morais, A.M.M. Lactobacillus plantarum survival during the osmotic dehydration and storage of probiotic cut apple. J. Funct. Foods 2017, 38, 519–528. [Google Scholar] [CrossRef]
- Cui, L.; Niu, L.-Y.; Li, D.-J.; Liu, C.-Q.; Liu, Y.-P.; Song, J.-F. Effects of different drying methods on quality, bacterial viability and storage stability of probiotic enriched apple snacks. J. Integr. Agric. 2018, 17, 247–255. [Google Scholar] [CrossRef]
- Amorim, J.C.; Piccoli, R.H.; Duarte, W.F. Probiotic potential of yeasts isolated from pineapple and their use in the elaboration of potentially functional fermented beverages. Food Res. Int. 2018, 107, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.G.M.; Fonteles, T.V.; De Jesus, A.L.T.; Rodrigues, S. Sonicated pineapple juice as substrate for L. casei cultivation for probiotic beverage development: Process optimisation and product stability. Food Chem. 2013, 139, 261–266. [Google Scholar] [CrossRef]
- de Almeida Bianchini Campos, R.C.; Martins, E.M.F.; de Andrade Pires, B.; do Carmo Gouveia Peluzio, M.; da Rocha Campos, A.N.; Ramos, A.M.; de Castro Leite Júnior, B.R.; de Oliveira Martins, A.D.; da Silva, R.R.; Martins, M.L. In vitro and in vivo resistance of Lactobacillus rhamnosus GG carried by a mixed pineapple (Ananas comosus L. Merril) and jussara (Euterpe edulis Martius) juice to the gastrointestinal tract. Food Res. Int. 2019, 116, 1247–1257. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, J.; Borges, S.; Amorim, M.; Pereira, M.J.; Oliveira, A.; Pintado, M.E.; Teixeira, P. Comparison of spray drying, freeze drying and convective hot air drying for the production of a probiotic orange powder. J. Funct. Foods 2015, 17, 340–351. [Google Scholar] [CrossRef]
- da Costa, G.M.; de Carvalho Silva, J.V.; Mingotti, J.D.; Barão, C.E.; Klososki, S.J.; Pimentel, T.C. Effect of ascorbic acid or oligofructose supplementation on L. paracasei viability, physicochemical characteristics and acceptance of probiotic orange juice. LWT Food Sci. Technol. 2017, 75, 195–201. [Google Scholar] [CrossRef]
- Sengun, I.Y.; Kirmizigul, A.; Atlama, K.; Yilmaz, B. The viability of Lactobacillus rhamnosus in orange juice fortified with nettle (Urtica dioica L.) and bioactive properties of the juice during storage. LWT 2020, 118. [Google Scholar] [CrossRef]
- Cristiny de Oliveira Vieira, K.; Da Silva Ferreira, C.; Toso Bueno, E.B.; De Moraes, Y.A.; Campagnolo Gonçalves Toledo, A.C.; Nakagaki, W.R.; Pereira, V.C.; Winkelstroter, L.K. Development and viability of probiotic orange juice supplemented by Pediococcus acidilactici CE51. LWT 2020, 130. [Google Scholar] [CrossRef]
- Pereira, A.L.F.; Maciel, T.C.; Rodrigues, S. Probiotic beverage from cashew apple juice fermented with Lactobacillus casei. Food Res. Int. 2011, 44, 1276–1283. [Google Scholar] [CrossRef]
- Ribeiro, A.P.D.O.; Gomes, F.D.S.; dos Santos, K.M.O.; da Matta, V.M.; Sá, D.D.G.C.F.D.; Santiago, M.C.P.D.A.; Conte, C.; Costa, S.D.D.O.; Ribeiro, L.D.O.; Godoy, R.L.D.O.; et al. Development of a probiotic non-fermented blend beverage with juçara fruit: Effect of the matrix on probiotic viability and survival to the gastrointestinal tract. LWT 2020, 118, 108756. [Google Scholar] [CrossRef]
- Vivek, K.; Mishra, S.; Pradhan, R.C. Characterization of spray dried probiotic Sohiong fruit powder with Lactobacillus plantarum. LWT 2020, 117. [Google Scholar] [CrossRef]
- Fonteles, T.V.; Costa, M.G.M.; de Jesus, A.L.T.; Rodrigues, S. Optimization of the Fermentation of Cantaloupe Juice by Lactobacillus casei NRRL B-442. Food Bioprocess Technol. 2012, 5, 2819–2826. [Google Scholar] [CrossRef]
- Saw, L.K.; Chen, S.; Wong, S.H.; Tan, S.A.; Goh, L.K. Fermentation of tropical fruit juices by lactic acid bacteria. In Proceedings of the 12th Asean Food Conference, Bangkok, Thailand, 16–18 June 2011. [Google Scholar]
- Mousavi, Z.E.; Mousavi, S.M.; Razavi, S.H.; Emam-Djomeh, Z.; Kiani, H. Fermentation of pomegranate juice by probiotic lactic acid bacteria. World J. Microbiol. Biotechnol. 2011, 27, 123–128. [Google Scholar] [CrossRef]
- Lavermicocca, P.; Valerio, F.; Lonigro, S.L.; De Angelis, M.; Morelli, L.; Callegari, M.L.; Rizzello, C.G.; Visconti, A. Study of adhesion and survival of lactobacilli and bifidobacteria on table olives with the aim of formulating a new probiotic food. Appl. Environ. Microbiol. 2005, 71, 4233–4240. [Google Scholar] [CrossRef]
- Han, J.; Hang, F.; Guo, B.; Liu, Z.; You, C.; Wu, Z. Dextran synthesized by Leuconostoc mesenteroides BD1710 in tomato juice supplemented with sucrose. Carbohydr. Polym. 2014, 112, 556–562. [Google Scholar] [CrossRef]
- Rodrigues, S.; Silva, L.C.A.; Mulet, A.; Cárcel, J.A.; Fernandes, F.A.N. Development of dried probiotic apple cubes incorporated with Lactobacillus casei NRRL B-442. J. Funct. Foods 2018, 41, 48–54. [Google Scholar] [CrossRef]
- Akman, P.K.; Uysal, E.; Ozkaya, G.U.; Tornuk, F.; Durak, M.Z. Development of probiotic carrier dried apples for consumption as snack food with the impregnation of Lactobacillus paracasei. LWT 2019, 103, 60–68. [Google Scholar] [CrossRef]
- Haffner, F.B.; Pasc, A. Freeze-dried alginate-silica microparticles as carriers of probiotic bacteria in apple juice and beer. LWT 2018, 91, 175–179. [Google Scholar] [CrossRef]
- Xu, X.; Bao, Y.; Wu, B.; Lao, F.; Hu, X.; Wu, J. Chemical analysis and flavor properties of blended orange, carrot, apple and Chinese jujube juice fermented by selenium-enriched probiotics. Food Chem. 2019, 289, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Caro, G.; Fernández-Quirós, B.; Ludwig, I.A.; Pradas, I.; Crozier, A.; Moreno-Rojas, J.M. Catabolism of citrus flavanones by the probiotics Bifidobacterium longum and Lactobacillus rhamnosus. Eur. J. Nutr. 2018, 57, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Rasika, D.; Vidanarachchi, J.; Rocha, R.; Balthazar, C.; Cruz, A.; Sant’Ana, A.S.; Ranadheera, C. Plant-based milk substitutes as emerging probiotic carriers. Curr. Opin. Food Sci. 2021. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).