Abstract
The presented case study illustrates the possibility of adding value to the biological surplus remaining from the mushroom cultivation industry. In essence, the unused mushroom parts were submitted to UV-C irradiation, with the purpose of increasing the vitamin D2 content and validating its extraction. Vitamin D2 concentration in three different mushroom species (Agaricus bisporus, A. bisporus Portobello, and Pleurotus ostreatus) was obtained by high-performance liquid chromatography (HPLC), by means of an ultraviolet (UV) detector. The method was validated using an A. bisporus Portobello sample, and its reproducibility and accuracy were confirmed. Independently of the UV-C irradiation dose, the effect on vitamin D2 concentration was significant, allowing it to increase from less than 4 µg/g dry weight (dw) to more than 100 µg/g dw in all mushroom species. These results are good indicators of the feasibility of industrial surplus mushrooms as sustainable vitamin D2 food sources, besides contributing to strengthen the circularity principals associated to the mushroom production chain.
1. Introduction
The global mushroom market share is dominated by Agaricus bisporus (J.E.Lange) Imbach (button mushroom), Lentinula edodes (Berk.) Pegler (shiitake mushroom) and Pleurotus ostreatus (Jacq.: Fr.) P. Kumm. (oyster mushroom) [1]. The production of mushrooms and truffles is dominated by Asia (78.2%), followed by Europe (14.7%), and then the Americas (6.2%) [2]. During mushroom production, a percentage as high as 20% of surplus might be generated [3]. These mushrooms have low industrial application, because they are in an advanced stage of maturation, or they have deformed lids and/or stems that do not meet the specifications established by retailers, so they are considered mushrooms of low economic value. These unused mushrooms are often discarded, even though their high nutritional compounds (e.g., proteins, carbohydrates) and valuable chemical compounds (e.g., amino acids, polysaccharides, sterols) are not compromised [3,4,5,6].
Currently, the disposal procedures (such as incineration, burying, and landfilling) employed to eliminate these surplus mushrooms generate some cost and may have an environmental impact; these techniques can cause water source contamination, acidification, eutrophication, air pollution, depletion of natural resources, eco-toxicity, among others [3,6].
In this sense, innovative alternatives to add value to this surplus mushroom production need to be explored. The irradiation of surplus mushrooms to obtain vitamin D2 is a sustainable strategy to increase vitamin D availability. In Europe, for example, assessments of vitamin D intake showed that for 77–100% of adults (19–64 years old) and 55–100% of elderly adults (>64 years old), vitamin D intake is inadequate [7,8]. In recent years, with advances in the food industry, in parallel with consumer demand for natural-based options, fortified or enriched foods with natural vitamin D2 are an innovative alternative, particularly for specific groups such as vegans [9,10]. In addition, surplus mushroom production can be used to prepare vitamin D2-enriched extracts that could be applied by the pharmaceutical industry as nutritive supplements [3].
In their natural state, mushrooms present very low concentrations of vitamin D2 [11]. Nonetheless, several researchers have found them to be a rich source of ergosterol (a precursor of vitamin D2), which can be converted into vitamin D2 by artificial UV irradiation [11,12]. Studies assessing the effects of radiation on ergosterol conversion into vitamin D2 using UV light are mostly available for cultivated species namely the ones with high production value [11,13]. There are several examples of mushroom species where some amounts of vitamin D2 have been developed after irradiation [11,13,14,15,16]. To the best of the authors’ knowledge, the present study provides the first report of the use of surplus mushrooms as a sustainable source of vitamin D2.
For natural vitamin D2 to be a real and promising alternative, it is necessary to find suitable methodologies for its extraction, and to develop effective recovery processes. Several technologies are available to extract and quantify vitamin D2 [17]. However, the effectiveness of the employed methodologies is affected by factors such as time, temperature, power, and solvent type. It is therefore important to combine the best operational conditions to achieve the best vitamin D2 recovery indices [3,18].
In view of the growing consumer demand for natural-based ingredients, the objective of this case study was to set the UV-C irradiation and extraction conditions that maximize vitamin D2 contents in the surplus mushrooms production and, meeting the concept reduce-reuse-recycle to minimize the surplus in the mushrooms production sector. The bioactive effects and potential toxicity of vitamin D2-enriched extracts were also evaluated.
2. Materials and Methods
2.1. Samples Information, UV-C Irradiation and Reagents
The surplus production from P. ostreatus and A. bisporus (Portobello and white mushroom) were supplied by Ponto Agrícola, Baião, north of Portugal. Subsequently, the fresh samples were sliced (2 to 3 mm) and divided into the following four groups with twenty specimens in each group: control (non-irradiated, 0.0 mJ/cm2), sample 1 (200 mJ/cm2), sample 2 (800 mJ/cm2) and sample 3 (3200 mJ/cm2) [12,19].
The irradiation was performed at the Centro de Investigação de Montanha of Instituto Politécnico de Bragança, Portugal and took place in an ultraviolet (UV-C) radiation chamber (JP Selecta, Barcelona, Spain) with the following different exposure times: 0, 2, 6 and 10 min. Before analysis, the samples were lyophilized and reduced to a fine, dried powder, and mixed to obtain homogenized samples.
The standard of pure vitamin D2 was purchased from Acrōs Organics (Fair Lawn, NJ, USA). HPLC-grade acetonitrile (99.9%) and n-hexane (95%) were purchased from Fisher Scientific (Lisbon, Portugal). Dimethyl sulfoxide was purchased from Fisher Scientific (Loughborough, UK), sulforhodamine B and ellipticine were acquired from Sigma-Aldrich (St. Louis, MO, USA), four human tumor cell lines were acquired from Leibniz-Institut DSMZ and one non-tumoral cell line was obtained from ATCC, LGC Standards (Middlesex, UK).
Water was treated in a Milli-Q water purification system (TGI Pure Water Systems, Greenville, SC, USA). Methanol and acetonitrile were of HPLC grade, from Lab-Scan (Dublin, Ireland).
2.2. Method Proof Assays
The sensitivity and linearity of the HPLC analysis were determined and the method was validated by the instrumental precision, repeatability, and accuracy, using the best extract obtained. Precision was accessed after six extractions of the same sample; each one being analyzed twice in the same day. The repeatability was accomplished by analyzing the mushroom sample, six times in the same day. The accuracy of the method was evaluated by the standard addition procedure (percentage of recovery), with three additional levels (25%, 50%, and 100% of the peak/area concentration), each one in triplicate. The standard mixture (vitamin D2) was added to the sample and the extraction procedure was carried out [20].
2.3. Extraction Methodology and Chromatographic Analysis
Vitamin D2 was extracted and analyzed according to the method described by Huang et al. [21], with some modifications. Mushroom powder (2.5 g) was mixed with 10 mL of dimethyl sulfoxide and ultrasound-oscillated at 45 °C for 30 min. Then 10 mL of methanol and water (1:1, v/v) and 20 mL of hexane were added, and the mixture was ultrasound-oscillated at 45 °C for 30 min and centrifuged at 5200× g for 5 min (Centurion K24OR, West Sussex, UK). The residue was extracted twice with 20 mL of hexane and centrifuged. The combined filtrate was rotary evaporated (Hei-VAP Advantage, Heidolph, Germany) at 40 °C to dryness, redissolved in 1 mL of methanol (Fisher Scientific, Loughborough, UK), and filtered using a 0.1 µm Whatman nylon filter (Millipore, Billerica, MA, USA) before HPLC injection.
The HPLC system (Knauer system, Smartline 1000, Berlin, Germany) coupled to a UV detector (Knauer Smartline 2500) was used under the same conditions described and optimized by Barreira et al. [22]. Chromatographic separation was performed with an Inertsil 100A ODS-3 reverse phase column (5 μm, 250 × 4.6 mm, BGB Analytik AG, Boeckten, Switzerland) at 35 °C. The mobile phase used was acetonitrile/methanol (70:30, v/v) at a flow rate of 1 mL/min, with an injection volume of 20 µL and the wavelength was 280 nm. Subsequently, the results were analyzed using the Clarity 2.4 software (DataApex, Pod Ohradska, Czech Republic). Vitamin D2 was quantified based on a calibration curve obtained from a commercial standard vitamin D2, and the results were expressed in µg per g of dry weight (dw).
2.4. Bioactivity of the Vitamin D2-Enriched Extract
According to the extraction results, the most potent extract (A. bisporus Portobello irradiated with UV-C, 6 min, 3200 mJ/cm2) was chosen to test the bioactivity in cell lines. In this assay, four human tumor cell lines (MCF-7—breast adenocarcinoma, NCI-H460—non-small cell lung cancer, AGS—gastric cancer, and CaCo-2—colorectal adenocarcinoma) and one non-tumoral cell line of bone origin (h-FOB 1.19—human osteoblasts) were used.
Cell proliferation in the presence and absence of functional extract and pure vitamin D2 was assessed using the sulforhodamine B (SRB) assay. For this assay, the extract prepared above was dissolved in water at a concentration of 8 mg/mL, the procedures were performed as described previously by the authors [23,24], and the final concentrations were 400, 100, 25 and 6.25 mg/mL. Ellipticin was used as a positive control. Absorbance was measured at 495 nm and the results were expressed as GI50 values (sample concentration that inhibited 50% of cell growth) in µg per mL.
2.5. Statistical Analysis
All analyses (extractions) were performed in triplicate and each replicate was also quantified three times. Data were expressed as mean ± standard deviation, presenting the significant numbers in agreement with the magnitude of the corresponding standard deviation.
All statistical tests were applied considering a 5% significance level (IBM SPSS Statistics for Windows, Version 22.0. Armonk; IBM Corp., Armonk, NY, USA). The results were compared through two-way analysis of variance (ANOVA) with type III sums of squares, performed using the general linear model (GLM) procedure. The analyzed statistical factors were “exposure time” (ET) and “ultraviolet radiation” (UV-C) and their effects were classified through the HSD Tukey’s test. The statistical interaction among these two factors was verified in all cases.
3. Results and Discussion
3.1. Method Validation
For this case study, before the surplus mushroom extract analysis, the correlation coefficient (R2), linearity range, and limits of detection and quantification (LOD and LOQ, respectively) of the methodology employed to determine vitamin D2, were fully validated (Table 1). After the linearity check (linearity range: 0.78–50 μg/mL), a seven-level calibration curve (y = 11.909x + 6.9688) was made, using the peak/area ratio versus concentration of the standard concentration (μg/mL), reaching a correlation coefficient of 0.9992. The average of the double determinations for each level was used.

Table 1.
Calibration parameters of the method for vitamin D2 detection and quantification, and method validation parameters using Agaricus bisporus Portobello irradiated with UV-C (6 min, 3200 mJ/cm2).
The LOD, calculated as the concentration corresponding to three times the standard error of the calibration curve, divided by the inclination, was 1.67 μg/mL, while the LOQ, i.d., the concentration corresponding to ten times the calibration error, divided by the inclination, was 5.07 μg/mL.
In order to evaluate the instrumental precision, the mushroom sample (A. bisporus Portobello, irradiated for 6 min at 3200 mJ/cm2) was injected six times, and the chromatographic method proved to be precise, according to the coefficient of variation (CV) of 0.82%. Repeatability was evaluated by applying the whole extraction procedure six times to the same sample, and the CV value obtained was low (1.35%). The method accuracy was evaluated by the standard addition procedure (% of recovery). The standard mixture was added to the samples in three concentration levels (25%, 50% and 100% of the peak/area concentration, each one in duplicate) before the extraction. The method showed good recovery results, with an average of 94% (Table 1).
3.2. Conversion Conditions
The starting point is the use of surplus mushrooms as a sustainable material to obtain vitamin D2, avoiding the use of mushroom suitable for commercialization.
In this sense, Table 2 presents the vitamin D2-enriched extracts content in different mushroom species, exposed to different UV-C radiation doses and exposure times.

Table 2.
Vitamin D2 content in different mushroom species exposed to different UV-C radiation doses and exposure times (ET). The results are presented as mean ± SD 1.
As it is mandatory in any two-way ANOVA, the possible interaction among the assayed factors was verified (ET × UV-C). Since the interaction proved to be significant (p < 0.050) in all the cases, it became obvious that the effect of one factor depends on the level of the second.
Therefore, the variation induced by every single factor could not be classified. Nonetheless, it was possible to observe some evident trends, as confirmed by the individual p-values of each factor. A significant increase (from less than 4 μg/g dw to more than 100 μg/g dw in all the cases) in vitamin D2 concentration was observed with the application of this irradiation type, most likely due to the conversion of ergosterol naturally present in the assayed mushroom. Furthermore, there were no significant differences in the result of using 200, 800 or 3200 mJ/cm2, which indicates that the vitamin D2 increase may be achieved with the least energetic consumption, making this processing approach more competitive and with minimal environmental impact.
With regard to exposure time, there were no significant differences upon irradiating mushrooms during 6 or 10 min, but the intermediate assayed time was better than the 2 min. Hence, the optimal exposure time, considering the results obtained with the surplus of assayed mushroom species, turned out to be 6 min. The origin of the mushroom, applied dose, time after harvest, positioning of the mushrooms to the light source, fresh or dried samples, whole or sliced samples, the method by which vitamin D2 has been extracted, among others, influence the results obtained [12].
3.3. Vitamin D2-Enriched Extracts
As for the mushrooms evaluated in this work (e.g., for 6 min at 3200 mJ/cm2), vitamin D2-enriched extract levels in the Portobello A. bisporus samples reached a maximum concentration of 124 μg/g dw, and in the white A. bisporus and P. ostreatus samples they reached values of 125 μg/g dw (Table 2, Figure 1).
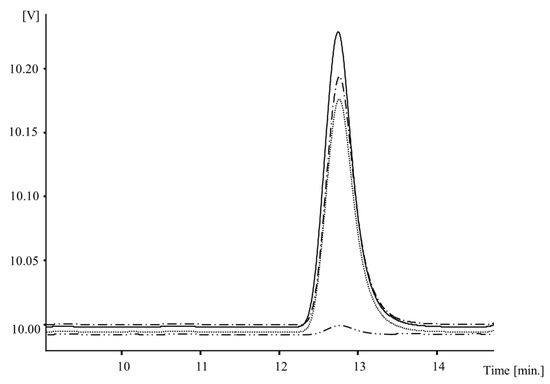
Figure 1.
Vitamin D2-enriched extracts chromatogram profile of Agaricus bisporus Portobello (-), white A. bisporus (.-.-) and Pleurotus ostreatus (...) irradiated with UV-C (6 min at 3200 mJ/cm2), and A. bisporus Portobello control samples (..-..-).
In UV-C-irradiated P. ostreatus samples, Hu et al. [13] reported a maximum concentration of approximately 24 μg/g dw of vitamin D2 content. Teichmann et al. [25] reported 10.14 μg/g dw in white A. bisporus samples, Guan et al. [26] reported 13.4 and 9.5 μg/g dw in white and Portobello A. bisporus samples, respectively, and Jasinghe and Perera [14] reported 34.4 μg/g dw in white button mushrooms. Similarly, for UV-C-irradiated shiitake (Lentinula edodes) mushroom, Xu et al. [27] obtained an increase in vitamin D2 content, until 20.11 μg/g dw.
Concerning UV-B irradiation, Urbain et al. [28] and Urbain et al. [29] obtained 56.8 and 67.1 μg/g dw of vitamin D2, respectively, in button mushrooms; Nölle et al. [30] reported that fresh whole A. bisporus, followed by freeze-drying, obtained 45 μg/g dw of vitamin D2, and slicing before UV-B irradiation led to a ten-fold increase.
There are dissimilarities in the irradiation process and conditions to maximize the photoconversion of ergosterol into vitamin D2 in mushrooms, and most of these cited studies were performed with the whole intact mushroom, with a longer irradiation time (20 min) and higher irradiation dose.
In this sense, based on the case study considered in this work, we make the first attempt to establish the irradiation conditions and extraction procedure needed to maximize ergosterol conversion to vitamin D2 from surplus mushroom production, avoiding the need to use mushroom samples that are suitable to be commercialized.
3.4. Bioactivity of the Vitamin D2-Enriched Extract
The in vitro cytotoxicity of the vitamin D2-enriched extract and pure vitamin D2 was analyzed. The effect of the vitamin D2-enriched extract and pure vitamin D2 in human tumoral cell lines (MCF-7, NCI-H460, AGS and CaCo) and non-tumoral bone cell line (h-FOB 1.19) growth are presented in Table 3. The GI50 values represent the extract concentrations that cause a 50% inhibition of cell growth.

Table 3.
Antiproliferative and cytotoxicity activities of vitamin D2-enriched extracts using Agaricus bisporus Portobello irradiated with UV-C (6 min, 3200 mJ/cm2) and pure vitamin D2 (mean ± SD, n = 9).
The sample of pure vitamin D2 tested did not reveal cytotoxicity at the evaluated concentrations (GI50 values > 400 μg/mL) for all the cell lines tested (tumoral and non-tumoral). However, the vitamin D2-enriched extract presented effective activity in the AGS (82 μg/mL) tumoral cell line, and moderate activity in the NCI-H460 (293 μg/mL) and CaCo (377 μg/mL) tumoral cell lines.
The results obtained indicate that these effects may be related to the compounds (including ergosterol, phenolic compounds, organic acids, etc.) present in the mushroom extract, since the mushrooms are a rich source of bioactive compounds [31]. It is noteworthy that neither vitamin D2-enriched extracts or pure vitamin D2 presented cytotoxicity against the non-tumoral bone cell, h-FOB 1.19 (GI50 > 400 µg/mL).
4. Conclusions
Based on the case study considered, vitamin D2-enriched extracts were obtained by HPLC-UV, using a methodology that proved to be reproducible and accurate. Vitamin D2 was identified and quantified, and A. bisporus Portobello was the species with the highest total content. The recovery of vitamin D2 from surplus mushrooms presents an interesting valorization and sustainable approach.
The use of vitamin D2-enriched extracts from surplus mushroom production could benefit several bio-based industries, since the applications of vitamin D2 from this sustainable material are nonexistent. Accordingly, the development of food applications of mushroom vitamin D2-enriched extract, from surplus mushroom production, can be considered and valued. It could be of added value to promote the agricultural sector or the pharmaceutical industries.
Author Contributions
R.V.C.C.: formal analysis, writing—original draft. Â.F.: methodology, software, validation, investigation, data curation, writing—review and editing. J.C.M.B.: data curation, software, writing—review and editing. R.M.V.A.: data curation, software, writing—review and editing. F.M.: methodology. A.M.G.-P.: visualization, supervision. I.C.F.R.F.: supervision, project administration. L.B.: conceptualization, validation, investigation, writing—review and editing, visualization, supervision, project administration, funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
The authors are grateful to the Foundation for Science and Technology (Fundação para a Ciência e Tecnologia, Lisboa, Portugal) for financial support by national funds FCT/MCTES to CIMO (UIDB/00690/2020), and R.V.C.C.’s PhD grant (SFRH/BD/137436/2018). The authors are also grateful to “Ponto Agricola” for providing the mushroom materials. A.F., J.C.M.B. and L.B. also thank the national funding by FCT, P.I. through the institutional scientific employment program contract. This work is funded by the ERDF through the Regional Operational Program North 2020, within the scope of Project Mobilizador Norte-01-0247-FEDER-024479: ValorNatural®.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fortune Business Insights. Mushroom Market Size, Share & Industry Analysis, by Type, Form, and Regional Forecast 2019–2026. Available online: https://www.fortunebusinessinsights.com/industry-reports/mushroom-market-100197 (accessed on 3 December 2020).
- FAOSTAT. Area Harvested. Food Agric. Organ. United Nations. 2019. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 11 December 2020).
- Papoutsis, K.; Grasso, S.; Menon, A.; Brunton, N.P.; Lyng, J.G.; Jacquier, J.C.; Bhuyan, D.J. Recovery of ergosterol and vitamin D2 from mushroom waste—Potential valorization by food and pharmaceutical industries. Trends Food Sci. Technol. 2020, 99, 351–366. [Google Scholar] [CrossRef]
- Silva, A.R.; Oludemi, T.; Costa, C.; Barros, J.; Ferreira, I.; Nunes, J.; Prieto, M.A.; Simal-Gandara, J.; Barros, L.; Ferreira, I.C.F.R. Mushrooms bio-residues valorisation: Optimisation of ergosterol extraction using response surface methodology. Food Bioprod. Process. 2020, 122, 183–192. [Google Scholar] [CrossRef]
- Antunes, F.; Marçal, S.; Taofiq, O.; Morais, A.M.M.B.; Freitas, A.C.; Ferreira, I.C.F.R.; Pintado, M. Value-added compounds and potential applications. Molecules 2020, 25, 2672. [Google Scholar] [CrossRef] [PubMed]
- Wan Mahari, W.A.; Peng, W.; Nam, W.L.; Yang, H.; Lee, X.Y.; Lee, Y.K.; Liew, R.K.; Ma, N.L.; Mohammad, A.; Sonne, C.; et al. A review on valorization of oyster mushroom and waste generated in the mushroom cultivation industry. J. Hazard. Mater. 2020, 400, 123156. [Google Scholar] [CrossRef] [PubMed]
- Pedrali, D.; Gallotti, F.; Proserpio, C.; Pagliarini, E.; Lavelli, V. Kinetic study of vitamin D2 degradation in mushroom powder to improve its applications in fortified foods. LWT 2020, 125, 109248. [Google Scholar] [CrossRef]
- Spiro, A.; Buttriss, J.L. Vitamin D: An overview of vitamin D status and intake in Europe. Nutr. Bull. 2014, 39, 322–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itkonen, S.T.; Pajula, E.T.; Dowling, K.G.; Hull, G.L.; Cashman, K.D.; Lamberg-Allardt, C.J. Poor bioavailability of vitamin D2 from ultraviolet-irradiated D2-rich yeast in rats. Nutr. Res. 2018, 59, 36–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EFSA. Safety of Vitamin D2 mushroom powder (Agaricus bisporus) as a Novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2021, 19, 6516. [Google Scholar] [CrossRef]
- Taofiq, O.; Fernandes, Â.; Barros, L.; Barreiro, M.F.; Ferreira, I.C.F.R. UV-irradiated mushrooms as a source of vitamin D2: A review. Trends Food Sci. Technol. 2017, 70, 82–94. [Google Scholar] [CrossRef]
- Cardwell, G.; Bornman, J.F.; James, A.P.; Black, L.J. A review of mushrooms as a potential source of dietary vitamin D. Nutrients 2018, 10, 1498. [Google Scholar] [CrossRef] [Green Version]
- Hu, D.; Chen, W.; Li, X.; Yue, T.; Zhang, Z.; Feng, Z.; Li, C.; Bu, X.; Li, Q.X.; Hu, C.Y.; et al. Ultraviolet irradiation increased the concentration of vitamin D2 and decreased the concentration of ergosterol in shiitake mushroom (Lentinus edodes) and oyster mushroom (Pleurotus ostreatus) powder in ethanol suspension. ACS Omega 2020, 5, 7361–7368. [Google Scholar] [CrossRef]
- Jasinghe, V.J.; Perera, C.O. Ultraviolet irradiation: The generator of Vitamin D2 in edible mushrooms. Food Chem. 2006, 95, 638–643. [Google Scholar] [CrossRef]
- Jasinghe, V.J.; Perera, C.O. Distribution of ergosterol in different tissues of mushrooms and its effect on the conversion of ergosterol to vitamin D2 by UV irradiation. Food Chem. 2005, 92, 541–546. [Google Scholar] [CrossRef]
- Sapozhnikova, Y.; Byrdwell, W.C.; Lobato, A.; Romig, B. Effects of UV-B radiation levels on concentrations of phytosterols, ergothioneine, and polyphenolic compounds in mushroom powders used as dietary supplements. J. Agric. Food Chem. 2014, 62, 3034–3042. [Google Scholar] [CrossRef]
- Jäpelt, R.B.; Jakobsen, J. Vitamin D in plants: A review of occurrence, analysis, and biosynthesis. Front. Plant Sci. 2013, 4, 136. [Google Scholar] [CrossRef] [Green Version]
- Pinela, J.; Prieto, M.A.; Pereira, E.; Jabeur, I.; Barreiro, M.F.; Barros, L.; Ferreira, I.C.F.R. Optimization of heat- and ultrasound-assisted extraction of anthocyanins from Hibiscus sabdariffa calyces for natural food colorants. Food Chem. 2019, 275, 309–321. [Google Scholar] [CrossRef] [Green Version]
- Ko, J.A.; Lee, B.H.; Lee, J.S.; Park, H.J. Effect of UV-B exposure on the concentration of vitamin D2 in sliced shiitake mushroom (Lentinus edodes) and white button mushroom (Agaricus bisporus). J. Agric. Food Chem. 2008, 56, 3671–3674. [Google Scholar] [CrossRef]
- Barros, L.; Pereira, C.; Ferreira, I.C.F.R. Optimized analysis of organic acids in edible mushrooms from Portugal by Ultra-Fast Liquid Chromatography and photodiode array detection. Food Anal. Methods 2013, 6, 309–316. [Google Scholar] [CrossRef]
- Huang, S.J.; Lin, C.P.; Tsai, S.Y. Vitamin D2 content and antioxidant properties of fruit body and mycelia of edible mushrooms by UV-B irradiation. J. Food Compos. Anal. 2015, 42, 38–45. [Google Scholar] [CrossRef]
- Barreira, J.C.M.; Oliveira, M.B.P.P.; Ferreira, I.C.F.R. Development of a novel methodology for the analysis of ergosterol in mushrooms. Food Anal. Methods 2014, 7, 217–223. [Google Scholar] [CrossRef]
- Barros, L.; Pereira, E.; Calhelha, R.C.; Dueñas, M.; Carvalho, A.M.; Santos-Buelga, C.; Ferreira, I.C.F.R. Bioactivity and chemical characterization in hydrophilic and lipophilic compounds of Chenopodium ambrosioides L. J. Funct. Foods 2013, 5, 1732–1740. [Google Scholar] [CrossRef]
- Guimarães, R.; Barros, L.; Dueñas, M.; Calhelha, R.C.; Carvalho, A.M.; Santos-Buelga, C.; Queiroz, M.J.R.P.; Ferreira, I.C.F.R. Nutrients, phytochemicals and bioactivity of wild Roman chamomile: A comparison between the herb and its preparations. Food Chem. 2013, 136, 718–725. [Google Scholar] [CrossRef] [Green Version]
- Teichmann, A.; Dutta, P.C.; Staffas, A.; Jägerstad, M. Sterol and vitamin D2 concentrations in cultivated and wild grown mushrooms: Effects of UV irradiation. LWT Food Sci. Technol. 2007, 40, 815–822. [Google Scholar] [CrossRef]
- Guan, W.; Zhang, J.; Yan, R.; Shao, S.; Zhou, T.; Lei, J.; Wang, Z. Effects of UV-C treatment and cold storage on ergosterol and Vitamin D2 contents in different parts of white and brown mushroom (Agaricus bisporus). Food Chem. 2016, 210, 129–134. [Google Scholar] [CrossRef]
- Xu, Z.; Meenu, M.; Xu, B. Effects of UV-C treatment and ultrafine-grinding on the biotransformation of ergosterol to vitamin D2, physiochemical properties, and antioxidant properties of shiitake and Jew’s ear. Food Chem. 2020, 309, 125738. [Google Scholar] [CrossRef]
- Urbain, P.; Singler, F.; Ihorst, G.; Biesalski, H.K.; Bertz, H. Bioavailability of vitamin D2 from UVB-irradiated button mushrooms in healthy adults deficient in serum 25-hydroxyvitamin D: A randomized controlled trial. Eur. J. Clin. Nutr. 2011, 65, 965–971. [Google Scholar] [CrossRef] [Green Version]
- Urbain, P.; Valverde, J.; Jakobsen, J. Impact on Vitamin D2, Vitamin D4 and Agaritine in Agaricus bisporus Mushrooms after Artificial and Natural Solar UV Light Exposure. Plant Foods Hum. Nutr. 2016, 71, 314–321. [Google Scholar] [CrossRef]
- Nölle, N.; Argyropoulos, D.; Ambacher, S.; Müller, J.; Biesalski, H.K. Vitamin D2 enrichment in mushrooms by natural or artificial UV-light during drying. LWT Food Sci. Technol. 2017, 85, 400–404. [Google Scholar] [CrossRef]
- Cardoso, R.V.C.; Fernandes, Â.; Oliveira, M.B.P.P.; Calhelha, R.C.; Barros, L.; Martins, A.; Ferreira, I.C.F.R. Development of nutraceutical formulations based on the mycelium of: Pleurotus ostreatus and Agaricus bisporus. Food Funct. 2017, 8, 2155–2164. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).