Evaluating Genotype × Environment Interactions of Yield Traits and Adaptability in Rice Cultivars Grown under Temperate, Subtropical and Tropical Environments
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Location and Field Experimental Design
2.3. Measurement of Traits
2.4. Statistical Analyses
3. Results
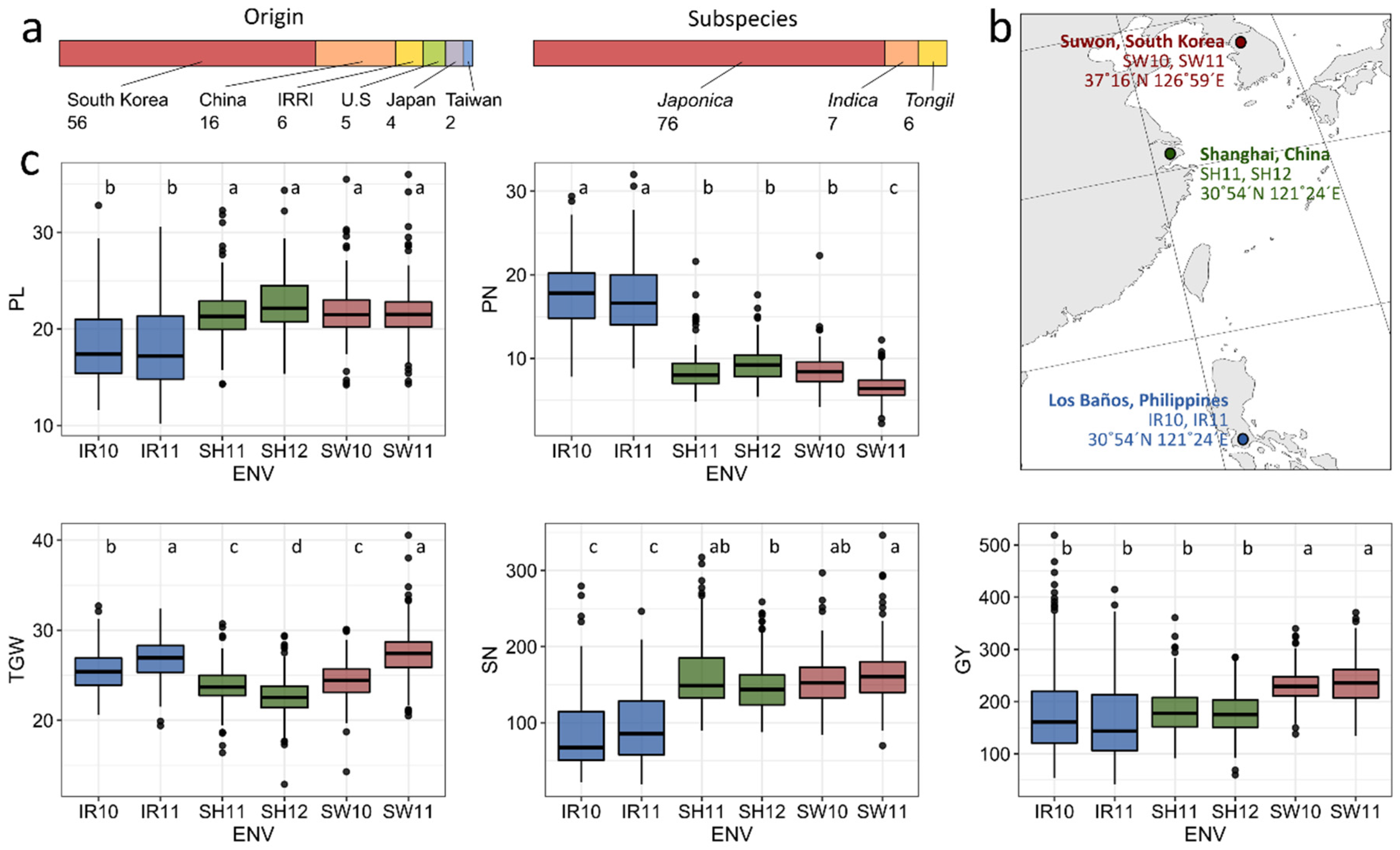
3.1. Variation of Grain Yield and Its Components in Rice Cultivars in Multiple Environments
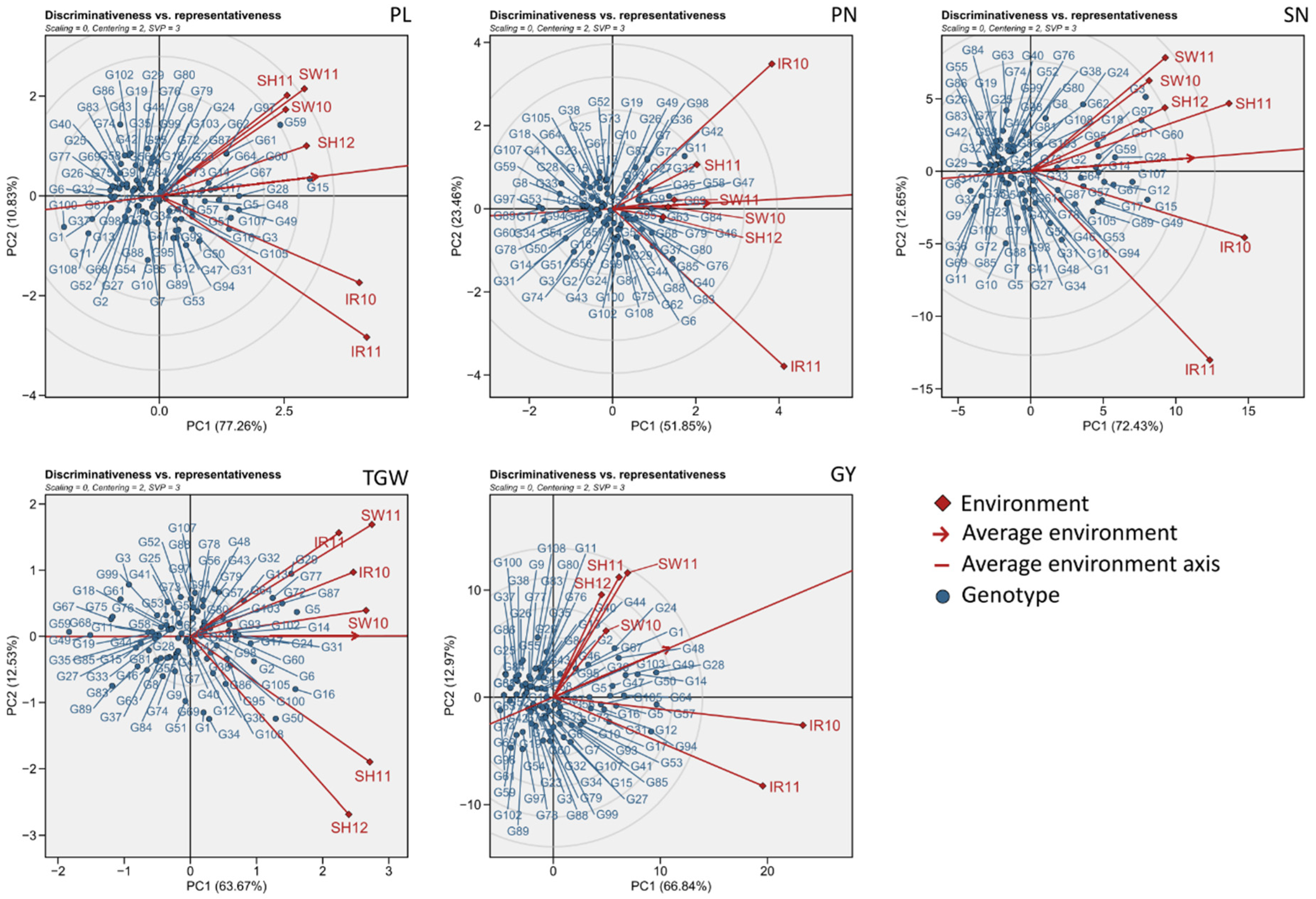
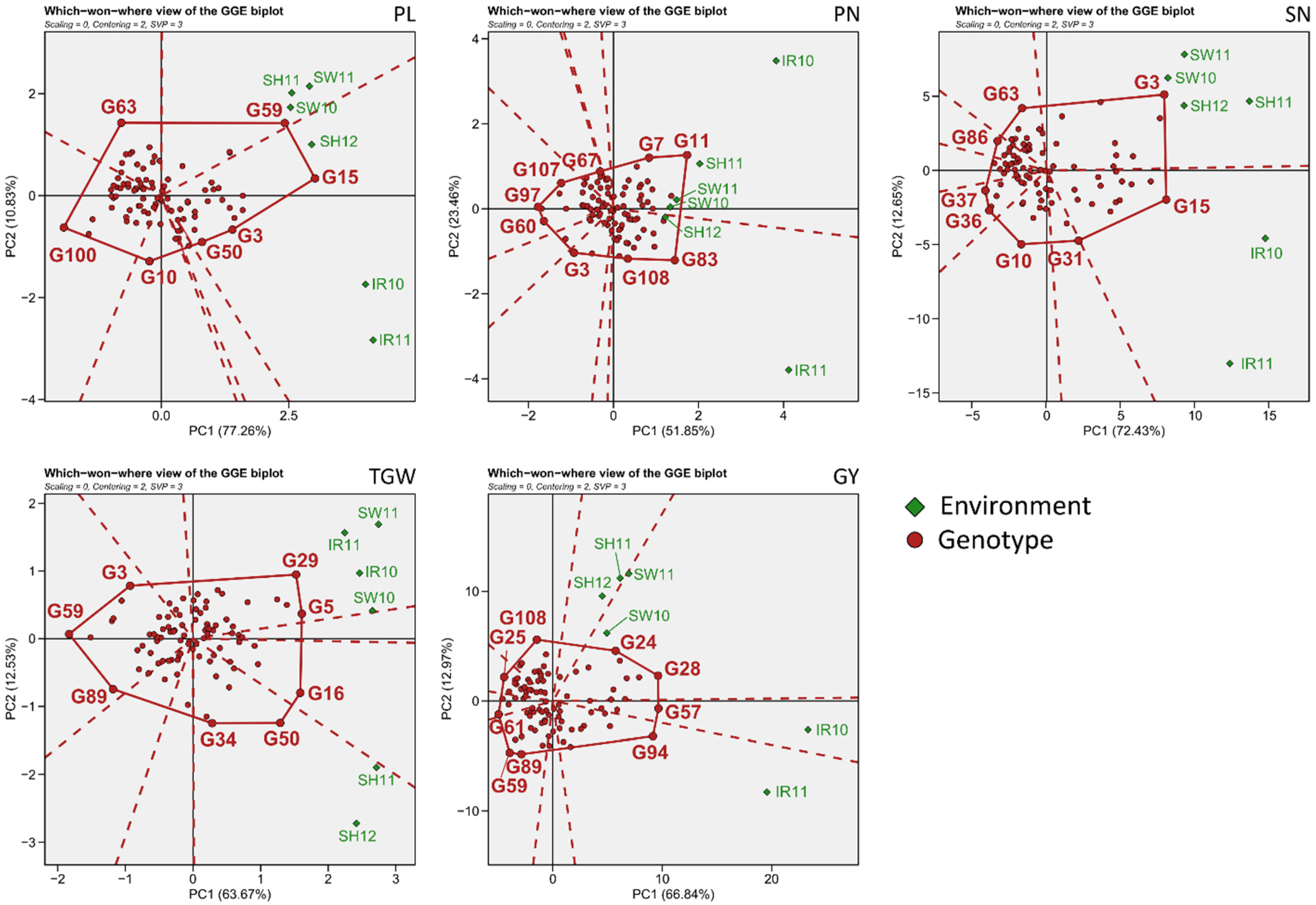
3.2. Environmental Evaluation and Adaptability in Specific Environments
3.3. General Adaptability of Yield Traits
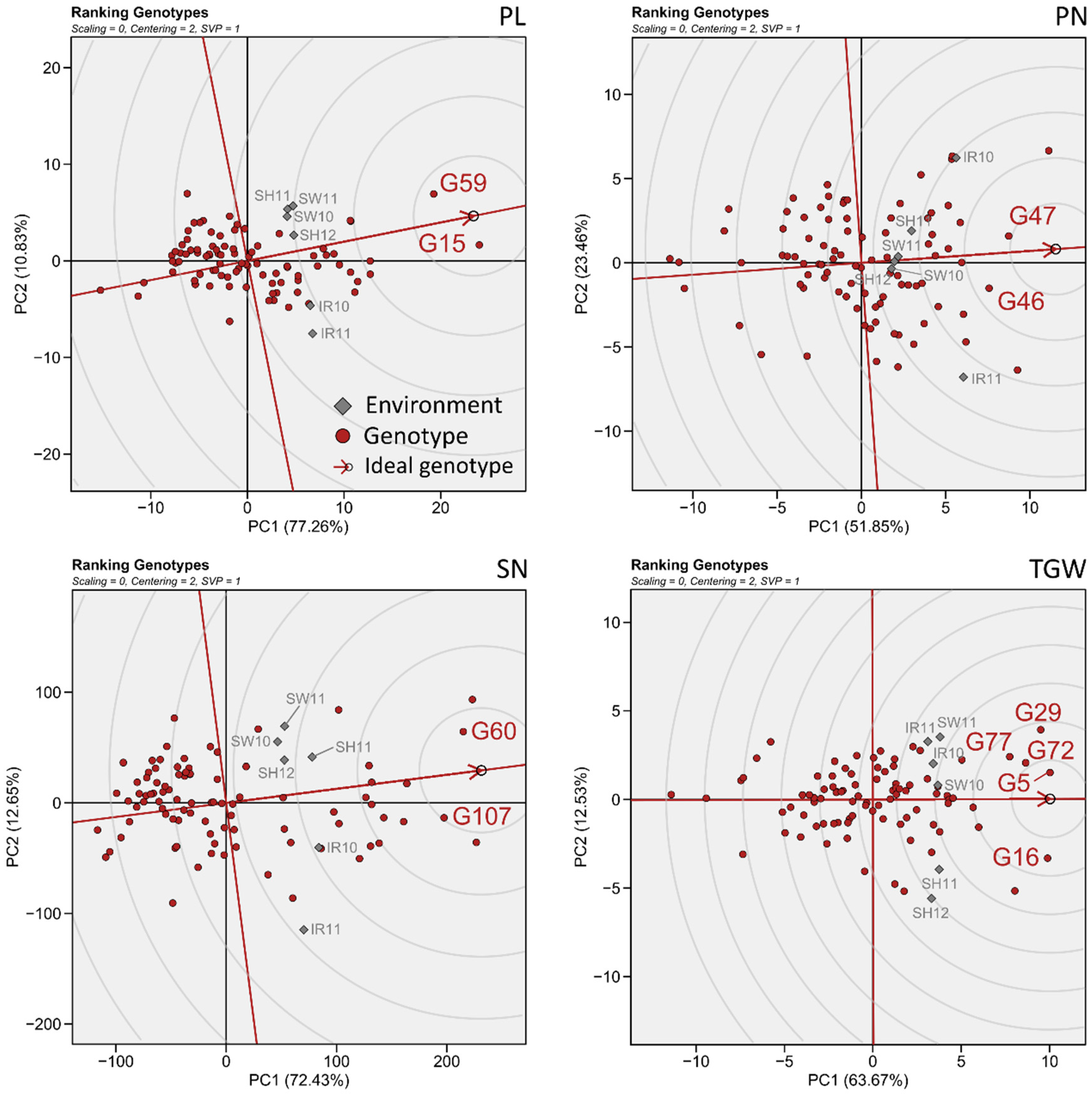
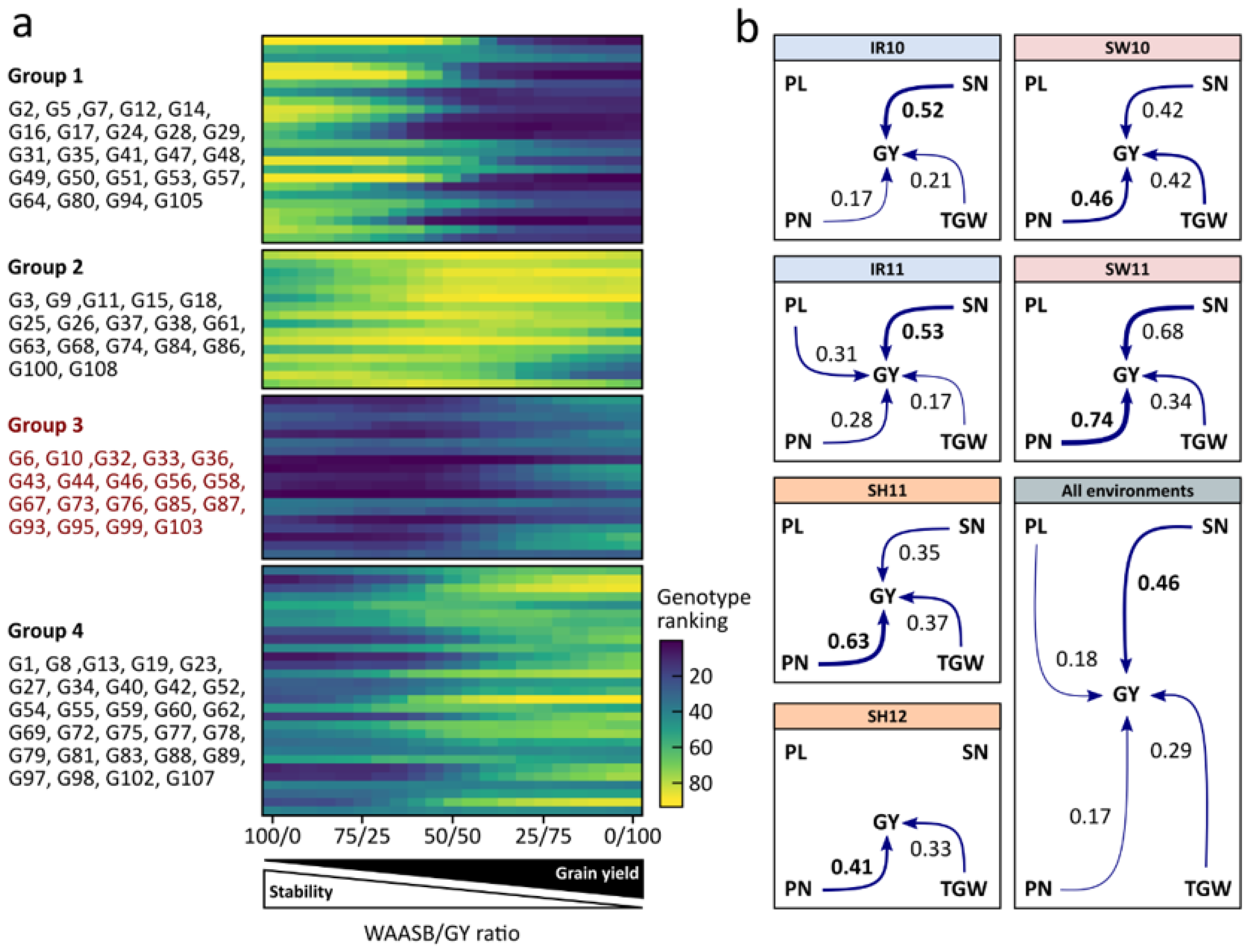
3.4. Simultaneous Selection of High Grain Performance and High Environmental Stability
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- FAOSTAT. FAOSTAT Database; Food and Agriculture Organization (FAO): Rome, Italy, 2015. [Google Scholar]
- Cordero-Lara, K.I. Temperate japonica rice (Oryza sativa L.) breeding: History, present and future challenges. Chil. J. Agric. Res. 2020, 80, 303–314. [Google Scholar] [CrossRef]
- Welch, J.R.; Vincent, J.R.; Auffhammer, M.; Moya, P.F.; Dobermann, A.; Dawe, D. Rice yields in tropical/subtropical Asia exhibit large but opposing sensitivities to minimum and maximum temperatures. Proc. Natl. Acad. Sci. USA 2010, 107, 14562–14567. [Google Scholar] [CrossRef]
- Peng, S.; Huang, J.; Sheehy, J.E.; Laza, R.C.; Visperas, R.M.; Zhong, X.; Centeno, G.S.; Khush, G.S.; Cassman, K.G. Rice yields decline with higher night temperature from global warming. Proc. Natl. Acad. Sci. USA 2004, 101, 9971–9975. [Google Scholar] [CrossRef] [PubMed]
- Wade, L.J.; McLaren, C.G.; Quintana, L.; Harnpichitvitaya, D.; Rajatasereekul, S.; Sarawgi, A.K.; Kumar, A.; Ahmed, H.U.; Sarwoto; Singh, A.K.; et al. Genotype by environment interactions across diverse rainfed lowland rice environments. Field Crops Res. 1999, 64, 35–50. [Google Scholar] [CrossRef]
- Balakrishnan, D.; Subrahmanyam, D.; Badri, J.; Raju, A.K.; Rao, Y.V.; Beerelli, K.; Mesapogu, S.; Surapaneni, M.; Ponnuswamy, R.; Padmavathi, G.; et al. Genotype × Environment Interactions of Yield Traits in Backcross Introgression Lines Derived from Oryza sativa cv. Swarna/Oryza nivara. Front. Plant Sci. 2016, 7, 1530. [Google Scholar] [CrossRef]
- Acuña, T.L.B.; Lafitte, H.R.; Wade, L.J. Genotype×environment interactions for grain yield of upland rice backcross lines in diverse hydrological environments. Field Crops Res. 2008, 108, 117–125. [Google Scholar] [CrossRef]
- Samonte, S.O.P.; Wilson, L.T.; McClung, A.M.; Medley, J.C. Targeting Cultivars onto Rice Growing Environments Using AMMI and SREG GGE Biplot Analyses. Crop Sci. 2005, 45, 2414–2424. [Google Scholar] [CrossRef]
- Olivoto, T.; Lúcio, A.D.C.; da Silva, J.A.G.; Sari, B.G.; Diel, M.I. Mean Performance and Stability in Multi-Environment Trials II: Selection Based on Multiple Traits. Agron. J. 2019, 111, 2961–2969. [Google Scholar] [CrossRef]
- Kempton, R.A.; Fox, P.N.; Cerezo, M. Statistical Methods for Plant Variety Evaluation; Springer Science & Business Media: Berlin, Germany, 2012; ISBN 978-94-009-1503-9. [Google Scholar]
- Gauch, H.G.; Piepho, H.-P.; Annicchiarico, P. Statistical Analysis of Yield Trials by AMMI and GGE: Further Considerations. Crop Sci. 2008, 48, 866–889. [Google Scholar] [CrossRef]
- Yan, W. Analysis and Handling of G × E in a Practical Breeding Program. Crop Sci. 2016, 56, 2106–2118. [Google Scholar] [CrossRef]
- Xu, L.; Yuan, S.; Man, J. Changes in rice yield and yield stability in China during the past six decades. J. Sci. Food Agric. 2020, 100, 3560–3569. [Google Scholar] [CrossRef]
- Yan, W.; Hunt, L.A.; Sheng, Q.; Szlavnics, Z. Cultivar Evaluation and Mega-Environment Investigation Based on the GGE Biplot. Crop Sci. 2000, 40, 597–605. [Google Scholar] [CrossRef]
- Olivoto, T.; Lúcio, A.D.C.; da Silva, J.A.G.; Marchioro, V.S.; de Souza, V.Q.; Jost, E. Mean Performance and Stability in Multi-Environment Trials I: Combining Features of AMMI and BLUP Techniques. Agron. J. 2019, 111, 2949–2960. [Google Scholar] [CrossRef]
- International Rice Research Institute. Standard Evaluation System for Rice; International Rice Research Institute: Manila, Philippines, 1996. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Mendiburu, F.D. Agricolae: Statistical Procedures for Agricultural Research. R Package Version 1.3-3. 2020. Available online: https://www.cran.r-project.org/web/packages/agricolae/index.html (accessed on 7 January 2021).
- Yan, W.; Kang, M.S.; Kang, M.S. GGE Biplot Analysis: A Graphical Tool for Breeders, Geneticists, and Agronomists; CRC Press: Boca Raton, FL, USA, 2002; ISBN 978-0-429-12272-9. [Google Scholar]
- Olivoto, T.; Lúcio, A.D. Metan: An R package for multi-environment trial analysis. Methods Ecol. Evol. 2020, 11, 783–789. [Google Scholar] [CrossRef]
- Rosseel, Y. Lavaan: An R Package for Structural Equation Modeling. J. Stat. Softw. 2012, 48, 1–36. [Google Scholar] [CrossRef]
- Yan, W.; Tinker, N.A. Biplot analysis of multi-environment trial data: Principles and applications. Can. J. Plant Sci. 2006, 86, 623–645. [Google Scholar] [CrossRef]
- Yan, W.; Kang, M.S.; Ma, B.; Woods, S.; Cornelius, P.L. GGE Biplot vs. AMMI Analysis of Genotype-by-Environment Data. Crop Sci. 2007, 47, 643–653. [Google Scholar] [CrossRef]
- Sultan, S.E. Phenotypic plasticity for plant development, function and life history. Trends Plant Sci. 2000, 5, 537–542. [Google Scholar] [CrossRef]
- Dingkuhn, M.; Luquet, D.; Quilot, B.; de Reffye, P. Environmental and genetic control of morphogenesis in crops: Towards models simulating phenotypic plasticity. Aust. J. Agric. Res. 2005, 56, 1289–1302. [Google Scholar] [CrossRef]
- Morishima, H.; Oka, H.-I. Phylogenetic Differentiation of Cultivated Rice, XXII. Numerical Evaluation of the Indica-Japonica Differentiation. Jpn. J. Breed. 1981, 31, 402–413. [Google Scholar] [CrossRef]
- Glaszmann, J.C. Isozymes and classification of Asian rice varieties. Theor. Appl. Genet. 1987, 74, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Jing, Q.; Spiertz, J.H.J.; Hengsdijk, H.; van Keulen, H.; Cao, W.; Dai, T. Adaptation and performance of rice genotypes in tropical and subtropical environments. NJAS-Wagening. J. Life Sci. 2010, 57, 149–157. [Google Scholar] [CrossRef]
- Bocianowski, J.; Niemann, J.; Nowosad, K. Genotype-by-environment interaction for seed quality traits in interspecific cross-derived Brassica lines using additive main effects and multiplicative interaction model. Euphytica 2018, 215, 7. [Google Scholar] [CrossRef]
- Ajay, B.C.; Bera, S.K.; Singh, A.L.; Kumar, N.; Gangadhar, K.; Kona, P. Evaluation of Genotype × Environment Interaction and Yield Stability Analysis in Peanut Under Phosphorus Stress Condition Using Stability Parameters of AMMI Model. Agric. Res. 2020, 9, 477–486. [Google Scholar] [CrossRef]
- Lozada, D.; Carter, A. Insights into the Genetic Architecture of Phenotypic Stability Traits in Winter Wheat. Agronomy 2020, 10, 368. [Google Scholar] [CrossRef]
- Quddus, M.R.; Rahman, M.A.; Jahan, N.; Debsharma, S.K.; Disha, R.F.; Hasan, M.M.; Aditya, T.L.; Iftekharuddaula, K.M.; Collard, B.C.Y. Estimating Pedigree-Based Breeding Values and Stability Parameters of Elite Rice Breeding Lines for Yield under Salt Stress during the Boro Season in Bangladesh. Plant Breed. Biotechnol. 2019, 7, 257–271. [Google Scholar] [CrossRef]
| Source | Df | PL | PN | SN | TGW | GY | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MS | ESS (%) | MS | ESS (%) | MS | ESS (%) | MS | ESS (%) | MS | ESS (%) | ||
| E | 5 | 692.9 *** | 23.6 | 4114.3 *** | 69.6 | 212,686.3 *** | 35.8 | 613.1 *** | 34.8 | 151,970.4 *** | 16.5 |
| Rep (E) | 6 | 5.4 ** | 0.2 | 9.9 * | 0.2 | 1459 *** | 0.3 | 8.5 *** | 0.6 | 955.7 ns | 0.1 |
| G | 88 | 88.1 *** | 52.9 | 32.7 *** | 9.7 | 13,729.7 *** | 40.6 | 34.0 *** | 33.9 | 18,606.4 *** | 35.6 |
| GEI | 440 | 6.0 *** | 18.1 | 8.4 *** | 12.4 | 1210.5 *** | 17.9 | 3.9 *** | 19.6 | 3881.8 *** | 37.1 |
| Residuals | 528 | 1.4 | 5.2 | 4.5 | 8.0 | 302.3 | 5.4 | 1.9 | 11.1 | 925.8 | 10.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Jang, S.; Kim, B.; Piao, Z.; Redona, E.; Koh, H.-J. Evaluating Genotype × Environment Interactions of Yield Traits and Adaptability in Rice Cultivars Grown under Temperate, Subtropical and Tropical Environments. Agriculture 2021, 11, 558. https://doi.org/10.3390/agriculture11060558
Huang X, Jang S, Kim B, Piao Z, Redona E, Koh H-J. Evaluating Genotype × Environment Interactions of Yield Traits and Adaptability in Rice Cultivars Grown under Temperate, Subtropical and Tropical Environments. Agriculture. 2021; 11(6):558. https://doi.org/10.3390/agriculture11060558
Chicago/Turabian StyleHuang, Xing, Su Jang, Backki Kim, Zhongze Piao, Edilberto Redona, and Hee-Jong Koh. 2021. "Evaluating Genotype × Environment Interactions of Yield Traits and Adaptability in Rice Cultivars Grown under Temperate, Subtropical and Tropical Environments" Agriculture 11, no. 6: 558. https://doi.org/10.3390/agriculture11060558
APA StyleHuang, X., Jang, S., Kim, B., Piao, Z., Redona, E., & Koh, H.-J. (2021). Evaluating Genotype × Environment Interactions of Yield Traits and Adaptability in Rice Cultivars Grown under Temperate, Subtropical and Tropical Environments. Agriculture, 11(6), 558. https://doi.org/10.3390/agriculture11060558






